Summary
Tintinnids (Ciliophora: Spirotricha: Tintinnina) are occasionally the dominant ciliates in the marine plankton. The tintinnid loricae are minute artworks fascinating scientists for more than 230 years, but their chemical composition remained unclear, viz., chitinous or proteinaceous substances were discussed. Since sedimenting loricae contribute to the flux of elements and organic compounds in the oceans, knowledge about their nature is necessary in assessing their ecological role. Previous techniques and new methods, e.g. enzymatic digestion and high-resolution transmission electron microscopy, are applied in the present study. A chitinous nature of the loricae is rejected by the Van-Wisselingh test and failure of chitinase digestion. Only proteins might show a resistance against strong hot bases (KOH at 160°C for ~ 40 min. in tintinnid loricae) similar to that of chitin. Actually, the presence of nitrogen in the EDX analyses and the digestion of at least some loricae by proteinase K strongly indicate a proteinaceous nature. Furthermore, the crystal lattice revealed by high-resolution TEM in Eutintinnus loricae is similar to the proteinaceous surface layer (S-layer) of archaea, and the striation recognizable in transverse sections of Eutintinnus loricae has a periodicity resembling that of the crystalline proteins in the extruded trichocysts of Paramecium and Frontonia. The proteolytic resistance of some loricae does not reject the idea of a proteinaceous nature, as proteins in S-layers of some archaea and in most naturally occurring prions show comparable reactions. The data from the present study and the literature indicate proteins in the loricae of thirteen genera. Differences in the proteolytic resistance and staining properties between genera and congeners are probably due to deviations in the protein composition and the additional substances, e.g. lipids, carbohydrates. At the present state of knowledge, correlations between lorica structure, wall texture, ultrastructure of the lorica forming granules, and the histochemical and enzymatic findings are not evident. Therefore, further studies are required to estimate the taxonomic significance of these features and the ecological role of sedimenting loricae.
Keywords: Chemical composition, crystal lattice, EDX analysis, enzyme digestion, high-resolution transmission electron microscopy, histochemical methods, lorica, tintinnid
INTRODUCTION
Several protists are able to construct tests, shells, or loricae, which are often quite elaborate. The study of these fascinating houses concerned not only their morphology and formation process, but also their chemical composition. In the foraminifera, the test walls and matrixes sticking together the agglomerated particles are of proteinaceous nature (Hedley 1963, Pierce et al. 1968, Hedley and Rudall 1974, Bowser and Bernhard 1993); the material was often called “tectin” or “pseudochitin” (Hyman 1940 and Pokorný 1958; both cited in Hedley 1963). Likewise, the organic tests of Amoebozoa (Moraczewski 1970, 1971a, b) and filose amoebae (Hedley 1960) consist of proteins. Chitin was detected in the loricae of the peritrich ciliate Cothurnia spec. and the heterotrich ciliate Parafolliculina violacea as well as in the resting cysts of the genera Blepharisma, Bursaria, Climacostomum, Cyclogramma, Euplotes, Fabrea, Nassula, Nassulopsis, Phacodinium, Pseudomicrothorax, and Telotrochidium (Bussers and Jeuniaux 1974). In other ciliate species, the resistant cysts contain other polysaccharides, proteins, and/or lipids (Bussers and Jeuniaux 1974); however, proteins are usually among the main components.
Tintinnids are unique among planktonic ciliates in building loricae, which are regarded as the main apomorphy of this taxon. These houses are minute artworks sometimes simply tube- or vase-shaped, sometimes elaborate in a way that we easily forget: the builders are not human architects, but unicellular organisms. After the death of the ciliate, the lorica sediments, transporting chemical compounds to deeper water layers and finally to the bottom of the ocean or lake. As tintinnids occasionally dominate the microzooplankton (heterotrophic organisms of the pelagial 20–2,000 μm in size), the material flux might be considerable, contributing to the benthic food web and nutrient recycling.
There is a long history of investigations into the chemical composition of tintinnid loricae, dating back to Fol (1881). The most comprehensive studies were conducted by Daday (1887), Entz Jr. (1909a, b), and Hofker (1931b). Usually, a chitinous nature of the lorica walls and matrixes was inferred from their resistance against strong bases. However, Entz Jr. (1909b) and Bussers and Jeuniaux (1974) excluded chitin, at least for some species, and the former author suspected a proteinaceous, keratin-like substance. Later studies, even employing energy-dispersive X-ray spectroscopy (EDX analysis; Wasik et al. 1997) or further histochemical methods (Gold 1968, 1980; Gold and Morales 1975a) failed to clearly identify the composition of the tintinnid loricae. Therefore, the subject is addressed here again, applying previous techniques and new methods, e.g. enzymatic digestion and high-resolution transmission electron microscopy, on hyaline and hard, agglomerated (entirely and partially) loricae. The analysis of both kinds of houses and the reassessment of literature data shall provide further insights into the chemical composition of loricae and its variability among tintinnids.
MATERIALS AND METHODS
Collection and preservation
The loricae were collected in Villefranche-sur-mer (Côte d’Azur, France) in May and October 2008 and the Chesapeake Bay (Maryland, USA) in May 2009 and October 2010.
In order to prevent bacterial growth and digestion, the loricae were fixed by different methods: (i) those collected in May 2008 were fixed, following the method of Valbonesi and Luporini (1990; 6 parts of 2% OsO4 in sea water and 1 part of saturated HgCl2), and washed several times with distilled water (marked by “*”); (ii) those collected in October 2008 were fixed with OsO4 plus HgCl2 and washed several times with distilled water (marked by “**”); (iii) those collected in October 2008 were also preserved with Bouin’s solution, following the method of Song and Wilbert (1995), and washed several times with distilled water (marked by “***”); (iv) those collected in the Chesapeake Bay in May 2009 were fixed in 100% ethanol (marked by “****”); (v) those collected in the Chesapeake Bay in May 2009 were also fixed in Bouin’s solution, and washed several times with distilled water (marked with “*****”); and (vi) those collected in the Chesapeake Bay in October 2010 were fixed with 100% ethanol (marked by “******”).
Experiments
Several histochemical and enzymatic tests were performed to recognize carbohydrates, proteins, lipids, and silicate minerals. The reaction of the loricae was followed at 1,000 × magnification under the light microscope. Additionally, EDX analyses and high-resolution transmission electron microscopy were applied.
1. Detection of carbohydrates
1.1. Van-Wisselingh test (chitin/chitinosan test; Tracey 1955, Foissner et al. 2005) The loricae were put in watch glasses filled with saturated aqueous potassium hydroxide and heated to 160°C. Every ten minutes material was taken (finally after 50 min.), allowed to cool down to ~ 21°C, and washed five times with tap water. One drop of 0.2% Lugol’s solution was placed on a slide, to which the loricae were added, and the sample was covered with a coverslip. A drop of 1% sulphuric acid was added from the margin of the coverslip and passed through the preparation to indicate by a red or violet stain the presence of chitin or chitinosan. Subsequently, a drop of 75% sulphuric acid was added from the margin of the coverslip and passed through the preparation to detect cellulose by a swelling and blue staining. Material: loricae of Codonella aspera*, Rhabdonella spiralis**, and Stenosemella ventricosa*. Since resting cysts of Blepharisma americanum contain chitin and dinoflagellates have theca platelets composed of cellulose, they were used as controls. In order to exclude fixation artifacts in the disintegration and staining of the loricae, live and OsO4/HgCl2-fixed cysts of B. americanum, OsO4/HgCl2-fixed cells of B. americana, and OsO4/HgCl2-fixed dinoflagellates were used. Furthermore, unpreserved human hair (= keratin) was tested.
1.2. Chitinase
One millilitre of 0.1 M cacodylate buffer was adjusted to pH 7 by addition of hydrochloric acid and heated to 35°C. Then, 2.2 mg of chitinase from Streptomyces griseus (Sigma-Aldrich; E.C. 3.2.1.14) were dissolved in the buffer. One drop of the enzyme solution was placed on a slide, to which the material was added. The slide was kept in a wet chamber at 37°C for 72 h. Material: loricae of Codonella aspera***, Eutintinnus brandti***, Rhabdonella spiralis***, Tintinnopsis cf. cylindrica***, T. compressa***, and nauplius larvae***.
1.3. Periodic acidic Schiff reaction
(PAS; McManus method in Mulisch and Welsch 2010). The PAS reaction indicates by a pink to violet colouration 1,2-glycols in a wide variety of substances: polysaccharides, neutral mucopolysaccharides, glycoproteins, mucoproteins, glycolipids, phospholipids, and unsaturated fatty acids (Mulisch and Welsch 2010; Merck product information). The loricae were stuck with albumin glycerine on a slide and dried. A drop of 0.5% periodic acid (Merck) was placed for 5 min. on the imbedded cells. Then, the slide was put for 3 min. into distilled water and transferred for 15 min. to the Schiff’s reagent (Merck). Next, the mounted loricae were brought three times for 2 min. each in fresh sulphite water (300 ml of distilled water plus 15 ml of 1 N hydrochloric acid and 18 ml of 10% aqueous sodium disulfide) and rinsed with tap water for at least 10 min. Material: loricae of Rhabdonella spiralis*** and Tintinnopsis cf. cylindrica***.
2. Detection of proteins
2.1. Millon’s solution
Phenols, except for those with double substitutions in the ortho- or meta-positions, react positively by reddish colouration upon Millon’s solution, especially, proteins containing tyrosine (Baker 1956). Millon’s reagent was prepared, following Romeis (1968), i.e. 10 g of mercuric sulphate were dissolved in 100 ml of 10% sulphuric acid while heating. After cooling, the solution was filled up with distilled water to 200 ml. One millilitre of 0.25% sodium nitrite was added to 10 ml of the former solution. The loricae were stuck with albumin glycerine on a slide and dried. A drop of Millon’s solution was put on the loricae and the slide was heated up almost to the boiling point. Then, the slide was washed three time for 2 min. each in distilled water. Material: loricae of Rhabdonella spiralis*** and Tintinnopsis cf. cylindrica***.
2.2. Mercuric bromophenol blue
(Mazia et al. 1953). An alcoholic solution was prepared by dissolving 10 g of mercuric chloride and 100 mg of bromophenol blue in 100 ml of 95% ethanol. Some authors used this histochemical method to detect proteins in tintinnid loricae (Gold 1968, Gold and Morales 1975a, Wasik et al. 1997). According to Baker (1958a), however, mercuric bromophenol blue was not sufficiently tested against lipids, carbohydrates, and other tissue components to use it as a reliable reagent for the histochemical recognition of proteins. So, this dye can merely be applied to indicate basic groups, while it does not stain starch and glycogen. Kanwar (1960) concluded from his experiments, that whatever is stained with this method does not always contain proteins and whatever is not stained is not necessarily devoid of proteins. Despite these shortcomings, the method was applied to elucidate the taxon-specificity of the stain. A drop of mercuric bromophenol blue was added to the loricae in a watch glass. After 45 min., the specimens were transferred to a drop of distilled water on a microscope slide and covered with a coverslip. Material: loricae of Climacocylis spec.***, Codonella aspera***, Eutintinnus brandti***, Rhabdonella spiralis***, and Tintinnopsis cf. cylindrica***, as well as Helicostomella subulata sampled in Trieste (Italy) in May 2002, fixed in Bouin’s solution, and washed with distilled water.
2.3. Alcian blue stain
Alcian blue selectively stains polyanionic glycoproteins (acid mucosubstances; Mulisch and Welsch 2010), i.e. glycosaminoglycans, proteoglycans, glycoproteins, and mucoproteins with carboxyl and/or sulphate groups. At low pH values, mucosubstances with sulphate groups stain, while the carboxyl-containing mucosubstances commence to stain blue when the pH value increases (Lev and Spicer 1964). In the present experiment, a solution of 1% alcian blue 8GS (Serva Feinbiochemica, Heidelberg) in 0.5 N hydrochloric acid (pH ~ 0.5) was prepared and filtered. The loricae were put for 3 min. in a drop of 0.5 N hydrochloric acid on a slide. Subsequently, they were transferred for 30 min. to a drop of the alcian blue solution. Next, distilled water was added to increase the solution’s pH. Material: loricae of Codonella aspera*, Codonellopsis schabi*, Rhabdonella spiralis***, Stenosemella ventricosa*, and Tintinnopsis cf. cylindrica***.
2.4. Protease
The protease of the bacterium Streptomyces griseus (pronase E, actinase E; Sigma-Aldrich, E.C. 3.4.24.31) preferably hydrolyzes peptide bonds on the carboxyl side of glutamic or aspartic acids. It contains at least ten proteolytic components: five serine-type proteases, two Zn2+-endopeptidases, two Zn2+-leucine aminopeptidases, and a Zn2+-carboxypeptidase (Sigma-Aldrich product information). In the present experiment, a solution of 30 mg of protease in 10 ml of 0.1 M cacodylate buffer (pH 6.8) was prepared. The loricae were first put in 100% alcohol, washed in distilled water, put on a microscope slide, and covered with the protease. The slide was kept in a wet chamber at 35°C for ~ 48 h. Material: loricae and cells of Rhabdonella spiralis***.
2.5. Proteinase K
The proteinase K of the microscopic fungus Engyodontium album (formerly Tritirachium album; Sigma-Aldrich, E.C. 3.4.21.64) preferably splits the peptide bonds adjacent to the carboxyl group of aliphatic and aromatic amino acids with blocked alpha amino groups (Sigma-Aldrich product information) and, of course, keratin. In the present experiment, 20 mg of proteinase K were dissolved in 1 ml of 5 mM calcium chloride in distilled water; the calcium ions presumably change the geometry of the substrate-recognition site and thus increase the enzyme activity (Bajorath et al. 1988). The loricae were put in a 1 ml-preparation cap together with the enzyme solution and kept for one week at 60°C and for further five weeks at ~ 21°C. The undigested loricae were transferred to 5% hydrogen peroxide for 10 min. at ~ 21°C and washed three times for 10 min. each in distilled water. Then, they were again transferred to the enzyme solution. Material: loricae of Climacocylis spec.***, Eutintinnus brandti***, Favella arcuata*****, Rhabdonella spiralis***, Stenosemella pacifica*****, Tintinnopsis compressa***, Tintinnopsis cf. cylindrica***, and Tintinnopsis levigata****, and unpreserved human hair.
3. Detection of lipids – Sudan black B
The dye indicates lipids, phospholipids, and lipoproteins by a blue-black colouration. The solution was prepared according to Romeis (1968), i.e. 0.1 g of Sudan black B (Sigma-Aldrich) was added to 100 ml of 70% ethanol and cooked for 1 min. After the solution cooled down to ~ 21°C, it was filtered. In the present experiment, a drop of distilled water containing the loricae was placed on a microscopic slide and covered with a coverslip. A drop of the Sudan black B solution was added from the margin of the coverslip and passed through the preparation. Material: loricae of Codonella aspera* and Stenosemella ventricosa*.
4. Detection of quartz particles – hydrofluoric acid
Hydrofluoric acid is the only acid that dissolves quartz. The loricae were put into a drop of 40% hydrofluoric acid on a microscopic slide for 1 h at ~ 21°C. Material: loricae of Rhabdonella spiralis*** and Stenosemella ventricosa*.
5. Energy-dispersive X-ray spectroscopy (EDX analysis)
Two analyses were performed. In the first study, a drop of distilled water containing the loricae was placed on a scanning electron microscopic stub covered with an adhesive conductive tab of polycarbonate plus very fine graphite (Plano). In an exsiccator, the samples were dried for one week. Subsequently, they were coated with carbonate and analysed in a Philips/FEI XL30 ESEM (Environmental Scanning Electron Microscope). Material: loricae of Codonella aspera*, Codonellopsis schabi***, Rhabdonella spiralis**, ***, Stenosemella ventricosa*, and Tintinnopsis cf. cylindrica**, *** were transferred to 100% ethanol and washed with distilled water; loricae of Eutintinnus brandti** and Tintinnopsis levigata**** as well as nauplius larvae** were washed in distilled water; unpreserved human hair.
The second study was conducted with a scanning electron microscope equipped with an energy-dispersive X-ray spectrometer (EDS or EDX; EDAX Inc., Mahwah, NJ, USA). The investigations were performed by means of an ESEM FEI Quanta 200 FEGi system operated in high vacuum mode (2·10−4 Pa) at an acceleration voltages of 20 kV (FEI company, Eindhoven, NL). The loricae were dried on a grid (300-mesh, copper, covered with holey carbon film; Plano GmbH, Wetzlar, Germany) without any further treatment. Material: loricae of Eutintinnus angustatus******.
6. Middle- and high-resolution transmission electron microscopy
The ethanol-fixed loricae of Eutintinnus angustatus were investigated at low dose with a Tecnai 10 electron microscope with LaB6 source operated at 100 kV and equipped with a F224HD 2k × 2k slow scan CCD camera (Tietz Video and Image Processing Systems GmbH, Gauting, Germany). Additionally, “ultra” high-resolution transmission electron microscopic studies at the atomic scale were performed in order to display the NaCl crystal lattice, using the field emission microscope CM 200 FEG/ST-Lorentz (FEI Company, Eindhoven, Netherlands). It was operating at 200 kV and equipped with a Multiscan 1k × 1k slow-scan CCD camera (Gatan, Pleasanton, CA, USA). The loricae were directly transferred from the ethanol to the transmission electron microscopic grid (300-mesh, copper, covered with holey carbon film, Plano GmbH, Wetzlar, Germany) and dried without any further treatment, such as fixation by OsO4 and heavy metal staining or freeze drying.
RESULTS AND DISCUSSION
1. Detection of carbohydrates
1.1. Van-Wisselingh test
The OsO4/HgCl2-preserved Blepharisma cells dissolved like the unfixed human hair, showing that the fixation did not influence the reaction in the potassium hydroxide. Unfixed and preserved resting cysts of Blepharisma persisted in the strong base at 160°C for 50 min. They stained violet after application of 1% sulphuric acid, while decolourised and dissolved after adding 75% sulphuric acid. Thus, the chitin in the cyst wall was detected despite fixation. The dinoflagellates also survived the potassium hydroxide procedure, but stained only upon 75% sulphuric acid, revealing the cellulose of the theca platelets. Although all loricae were intact after 40 min., they did not stain upon application of 1% and 75% sulphuric acid. Thus, the hard, agglomerated loricae of Codonella aspera and Stenosemella ventricosa and the hyaline lorica of Rhabdonella spiralis do not contain cellulose or chitin in appreciable quantities, which is in accordance with their disintegration after more than 40 min. in the hot potassium hydroxide.
1.2. Chitinase
The chitinase did not digest the loricae, while the also Bouin-fixed nauplius larvae with their chitinous exoskeleton became “ghosts”, i.e. very thin and almost invisible. Thus, it is supposed that the Bouin preservation did not influence the reaction upon chitinase and that the hard, agglomerated loricae of Codonella aspera, Tintinnopsis cf. cylindrica, and T. compressa and the hyaline loricae of Eutintinnus brandti and Rhabdonella spiralis do not contain chitin in appreciable quantities.
1.3. Periodic acidic Schiff reaction
The staining protocol by Sigma-Aldrich and Zakout et al. (2010) recommend Bouin fixation for the material analysed. Accordingly, the preservation of the loricae did not influence the outcome, and the lack of a pink or violet colour in the hyaline lorica of Rhabdonella spiralis and the hard, agglomerated lorica of Tintinnopsis cf. cylindrica indicates that they do not contain polysaccharides, neutral mucopolysaccharides, glycoproteins, mucoproteins, glycolipids, phospholipids, and unsaturated fatty acids with 1,2-glycols in appreciable amounts.
2. Detection of proteins
2.1. Millon’s solution
According to Humason (1972), material preserved with any general fixative can be used, and Nayar (1955) and Gerber (1970) received positive stains with the Millon’s solution in Bouin-fixed tissues. Therefore, the failure of a colouration in the hyaline lorica of Rhabdonella spiralis and the hard, agglomerated lorica of Tintinnopsis cf. cylindrica indicates the absence of phenols (without double substitutions in the ortho- or meta-positions), especially, the amino acid tyrosine.
2.2. Mercuric bromophenol blue
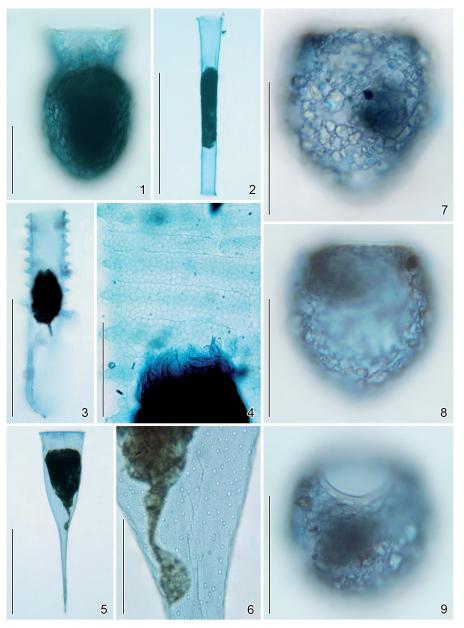
This cytochemical stain was developed, using Bouin-fixed tissue (Mazia et al. 1953); hence, the preservation of the loricae did not influence the outcome. The loricae reacted differently upon the dye: the hyaline ones of Climacocylis spec. and Eutintinnus brandti and the hard, agglomerated lorica of Codonella aspera were faintly stained (Figs 1-4), while the hard, agglomerated houses of Tintinnopsis cf. cylindrica and T. compressa and the hyaline one of Helicostomella subulata did not reveal any colouration. In the hyaline lorica of Rhabdonella spiralis, the staining intensity varied (Figs 5, 6). According to Baker (1958a) and Kanwar (1960), the presence of basic groups was indicated by a colouration, but not necessarily the presence of proteins.
Figs 1–9.
Loricae after mercuric bromophenol blue (1–6) and alcian blue stain (7–9). 1 – Codonella aspera, the staining is restricted to the lorica matrix; 2 – Eutintinnus brandti, the lorica is uniformly stained; 3, 4 – Climacocylis spec., the alveolar texture of the wall is well recognizable; 5, 6 – Rhabdonella spiralis, the alveolar texture, the minute openings, and the spiralled surface ridges are recognizable; 7–9 – Stenosemella ventricosa, lateral (7, 8) and oblique top (9) views. The staining is restricted to the bowl matrix. Scale bars: 50 μm (1, 7–9), 200 μm (2, 3), 40 μm (4), 100 μm (5), and 20 μm (6).
2.3. Alcian blue stain
According to Humason (1972), material treated with any general preservative can be used: Bouin-fixed tissues were analysed by Lai et al. (1975); Santi and Anderson (1986) demonstrated the colourability of osmium tetroxide-fixed tissues; and mercuric chloride preservation was recommended in the original method description (Steedman 1950). Therefore, negative effects of the fixatives on the staining reactions can probably be excluded.
In the acidic solution, the OsO4/HgCl2-fixed loricae of Stenosemella ventricosa stained blue at the margins of the agglomerated particles, where the matrix material was sufficiently thick to elicit a colouration, while the collar was never stained (Figs 7-9). The increase in pH did not change the results, i.e. the stain of S. ventricosa was not intensified and the hard, agglomerated loricae of Codonella aspera, Codonellopsis schabi, and Tintinnopsis cf. cylindrica and the hyaline lorica of Rhabdonella spiralis remained colourless. Accordingly, the substance, which sticks together the agglomerated particles and lines the inner wall of the bowl in S. ventricosa, contains glycoproteins with sulphate groups, while carboxyl groups were not present in appreciable amounts. The failure of a reaction in the collar of S. ventricosa and the other loricae tested indicates the absence of polyanionic glycoproteins.
2.4. Protease
The denaturation of proteins by fixatives does not prevent enzymatic digestion (Geyer 1977), as shown for Bouin-fixed tissues (Bedossa et al. 1987) and Rhabdonella spiralis cells (this study). Thus, the preservation did not prevent the protease digestion of the hyaline R. spiralis lorica. No or only a very slow digestion by pepsin, trypsin, and protease was reported for some proteins, e.g. the protease-resistant ones of prions (Karshan 1930, Gries and Lindner 1960, McKinley et al. 1983, Bermejo-Barrera et al. 1999, Brandelli 2005). Therefore, it is possible that (i) the lorica of R. spiralis consists of protease-resistant proteins or (ii) the incubation period of ~ 48 h was too short for a recognizable digestion; other interpretations are less likely due to the results of the remaining experiments.
2.5. Proteinase K
Gloghini et al. (2004) and Hayat (2005) used proteinase K digestion of Bouin-fixed tissue for the extraction of RNA and in situ hybridization, respectively; Košková et al. (2010) demonstrated the digestion of ethanol-fixed material by proteinase K. Thus, negative preservation effects on the proteolysis can be excluded.
In the proteinase K solution, the unpreserved human hair, the Bouin-fixed hyaline loricae of Climacocylis spec. and Eutintinnus brandti and the ethanol-fixed hard, agglomerated lorica of Tintinnopsis levigata successively dissolved and thus consist of proteins; a dissolution by the calcium chloride can be excluded, as the salt is a component of sea water. Only, the mineral particles of the T. levigata lorica persisted.
The also Bouin-fixed hyaline loricae of Favella arcuata and Rhabdonella spiralis and the hard, agglomerated loricae of Stenosemella pacifica, Tintinnopsis cf. cylindrica, and T. compressa even withstood a second round in the proteinase K solution after exposure to hydrogen peroxide.
In four of the five unaffected loricae, a chitinous substance can be excluded by the failure of the chitinase digestion and/or the Van-Wisselingh test. Their impressive resistance against hot, strong bases (KOH at 160°C for 40 min.) is otherwise only known from some proteins (Krishnan 1954, Moraczewski 1971a). The indigestibility of the loricae by proteinase K does not necessarily reject the idea of a proteinaceous material, as similar reactions were found in proteins of prions (Basu et al. 2007), the surface layer (S-layer) of archaea (Kaminski et al. 2010), and the bacterium Mycoplasma orale (Butler et al. 1991).
3. Detection of lipids – Sudan black B
According to Baker (1958b), osmium tetroxide preservation does not prevent a Sudan black staining and mercuric chloride leaves tissues more receptive of dyes than any other fixative. This is in accordance with the intense colouration of some lipid droplets in the OsO4/HgCl2-fixed cytoplasm of Codonella aspera. The hard, agglomerated loricae of Codonella aspera and Stenosemella ventricosa, however, became only light brown or grey by Sudan black B. Since the preservative obviously did not prevent staining, fatty substances are absent in the loricae tested. This matches the results of the periodic acidic Schiff reaction, which could not detect glycolipids, phospholipids, and unsaturated fatty acids with 1,2-glycols.
4. Detection of quartz particles – hydrofluoric acid
The hydrofluoric acid did not dissolve the lorica material secreted by the ciliate, i.e. it had no effect on the hyaline lorica of Rhabdonella spiralis and the lorica matrix of Stenosemella ventricosa, while the agglomerated mineral particles (clay and silt grains) disappeared in the latter species.
5. Energy-dispersive X-ray spectroscopy (EDX analysis)
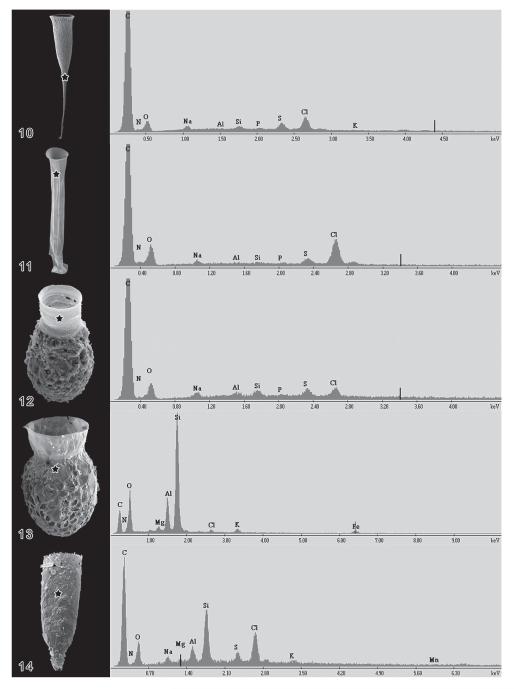
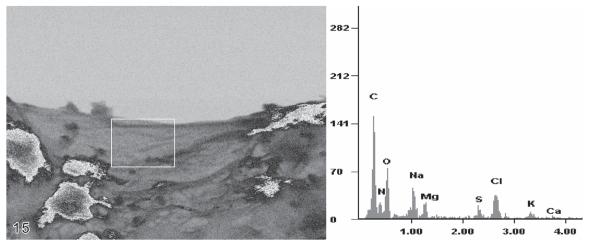
Independent of the fixation, all loricae revealed in both studies carbon, nitrogen, and oxygen, indicating organic lorica walls and matrixes (Figs 10-15). Additionally, inorganic components were detected, such as sodium, potassium, magnesium, and chlorine, which probably stem from sea water traces in the samples. In agglomerated loricae or lorica portions, peaks of further elements occurred, i.e. aluminium and silicon (Figs 13, 14), due to the presence of mineral particles.
Figs 10–14.
Energy-dispersive X-ray spectrometric (EDX) analyses in the scanning electron microscope, using carbon coated material. 10 – hyaline lorica of Rhabdonella spiralis; 11 – hyaline lorica of Eutintinnus brandti; 12 – lorica of Codonellopsis schabi composed of a hyaline collar and a hard, agglomerated bowl; 13 – hard, agglomerated lorica of Codonella aspera; 14 – hard, agglomerated lorica of Tintinnopsis levigata. The asterisks mark the regions analysed.
Fig. 15.
Energy-dispersive X-ray spectrometric (EDX) analysis in the scanning electron microscope, using uncoated material. The analysed area of the Eutintinnus angustatus lorica is marked by a white frame (~ 11 × 9 μm in size). Since this part of the lorica was freely suspended in the vacuum, elemental detection occurred without influence of the carbon substrate.
In contrast to proteins, the polysaccharides cellulose, starch, and glycogen do not exhibit nitrogen (0.392 keV). N-acetyl groups occur as side chains in chitin and chitinosan, which, however, are excluded to be a lorica component by the Van-Wisselingh test and/or the failure of the chitinase digestion in the hard, agglomerated loricae of Codonella aspera, Stenosemella ventricosa, and Tintinnopsis cf. cylindrica and the hyaline loricae of Eutintinnus brandti and Rhabdonella spiralis. Therefore, the data indicate a proteinaceous nature of the lorica walls and matrixes.
The sulphur in the EDX spectrum might originate in the bitter salt on the lorica surface (see below) or in sulphur-linked proteins. A keratinous substance in the loricae can, however, be excluded due to the greater resistance of Codonella aspera, Rhabdonella spiralis, and Stenosemella ventricosa loricae against potassium hydroxide and the persistence of Favella arcuata, Rhabdonella spiralis, Stenosemella pacifica, Tintinnopsis cf. cylindrica, and T. compressa in proteinase K. Furthermore, the human hair with its hard keratin produced a much higher sulphur peak in the EDX analysis (not shown) than the loricae and stains upon Millon’s solution. But a protein with a resistance against strong, hot bases similar to that of chitin was found in the spider Palamneus swammerdami. Like the tintinnid loricae, it did not stain with Millon’s solution, and Krishnan (1954) concluded that the protein is closely related to scleroproteins hardened by sulphur linkages.
6. Middle- and high-resolution transmission electron microscopy
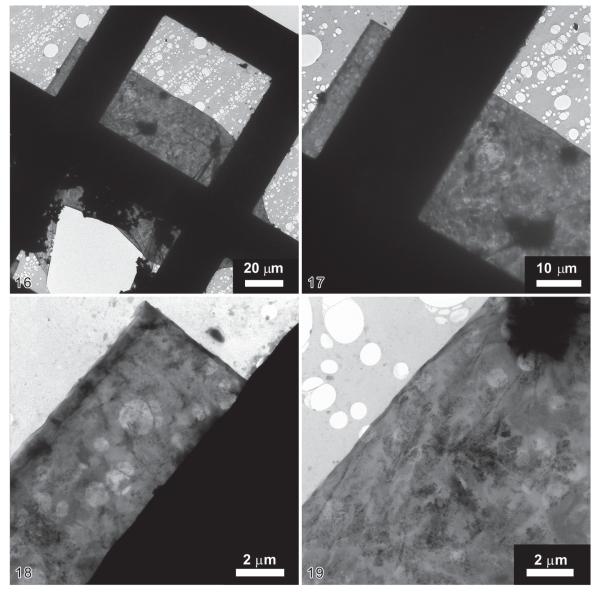
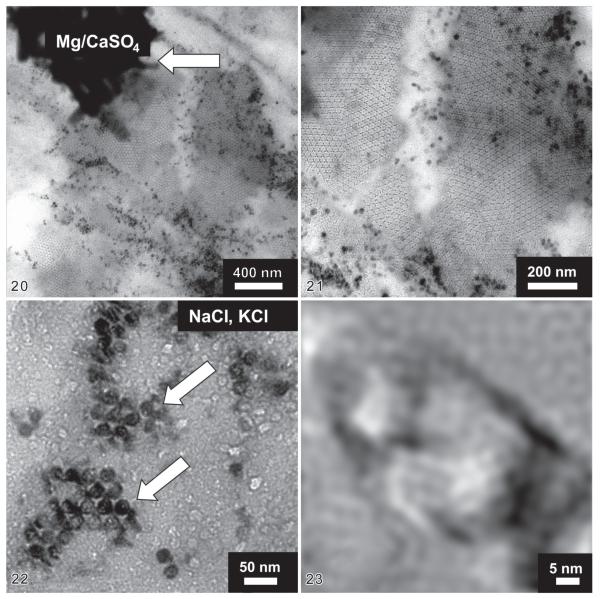
In the ethanol-fixed Eutintinnus angustatus, the wall of the just dried hyaline loricae was slightly wrinkled on the nanometre scale (Figs 16-19) and covered with electron-dense inorganic materials (Fig. 19). Ruptures of the wall were probably caused by the desiccation of the loricae on the TEM grid (Figs 20, 21). At middle resolution, a crystalline structure became recognizable in the lorica surface (Figs 20, 21). By EDX analysis (Fig. 15) and electron diffraction measurements (not shown), the electron-dense aggregates on the walls turned out to be bitter salt (MgSO4; Fig. 20). The nano-sized black spots are sodium and potassium chloride (Fig. 21), as revealed by high-resolution electron microscopy (Fig. 23) and electron diffraction (not shown); the single nanocrystals were similar in size (~ 20 nm) to the unit cells of the crystals composing the lorica wall (Fig. 22).
Figs 16–19.
Transmission electron micrographs of an uncoated lorica surface of Eutintinnus angustatus at different magnifications. 16 – overview of right lorica half. The lorica lies nearly horizontally on the electron transparent holey carbon substrate. The black rectangular structures are the copper bars of the TEM grid; 17 – anterior portion of right lorica half; 18 – apical lorica portion; 19 – lateral lorica portion. The dark crystalline dendritic structures consist of sodium chloride nanocrystals, which probably originate in the sea water.
Figs 20–23.
Transmission electron micrographs of a lorica surface in Eutintinnus angustatus at middle resolution. 20 – a crystalline region ~ 1.5 μm in diameter is shown in the centre of the micrograph. An aggregate of bitter salt (MgSO4) is attached to the wall (arrow); 21 – crystalline area at higher magnification showing dark spots of sodium and potassium chloride on the lorica wall; 22 – each black spot represents a NaCl or KCl nanocrystal (arrows), which has almost the same size as the unit cells of the crystal lattice (~ 20 nm); 23 – Fourier filtered high-resolution micrograph of a sodium chloride nanocrystal.
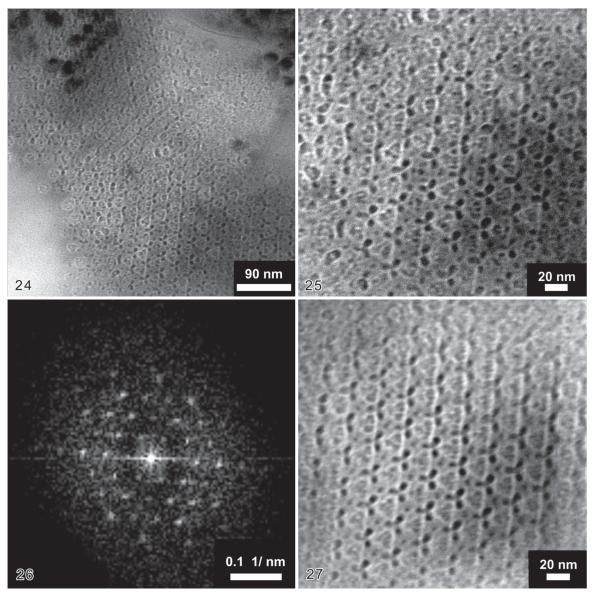
With increasing magnification, more details of the crystal lattice in the surface of the E. angustatus lorica became recognizable (Figs 24-28). The fast Fourier transform (FFT; processed diffraction pattern of the direct image of Fig. 25 at the nano-scale) revealed a hexagonal symmetry of the crystal lattice with a periodicity of ~ 23.7 nm (Fig. 26). This lattice spacing corresponds to a hexagonal unit cell size of ~ 27.4 nm. Finest structural details of the crystal hold a size of ~ 3 nm, as indicated by the highest wide angle reflection up to the 4th order. At this magnification, triangular monomeric motifs became recognizable, enclosing three tiny black and oval structures ~ 3 nm long. The ultrastructure was identical in different regions of the lorica wall, but the resolution and appearance varied according to the defocus of the microscope (Figs 24, 25).
Figs 24–27.
Transmission electron micrographs of a lorica surface in Eutintinnus angustatus at middle (24) and higher (25–27) resolution. 24 – crystal lattice; 25 – digitally enlarged detail of Fig. 24 (bottom part). The periodicity of the hexagonal structures amounts to ~ 23.7 nm with a resolution of ~ 3 nm for the smallest details; 26 – fast Fourier transform of Fig. 25; 27 – noise filtered bright field image after inverse Fourier transform of Fig. 26, using only the diffraction spots. Due to the enhanced contrast, the ultrastructure of the crystal lattice appears more distinct.
Fig. 28.
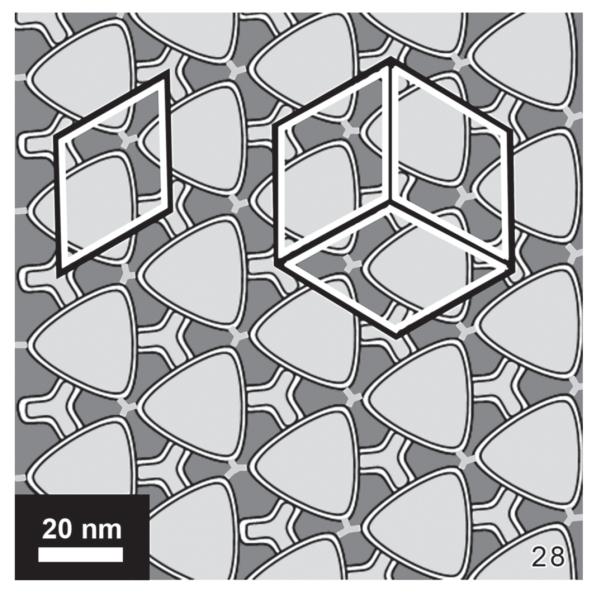
Scheme of the crystal structure in a lorica surface in Eutintinnus angustatus. The primitive unit cell of the crystal is rhombohedral, while three of them form a hexagonal pattern (see white lines). In the simplified model of the crystal structure, the basic units consist of triangles with a diameter of ~ 18 nm, which are interconnected on each side by channels with a diameter of ~ 7 nm. The empty space between the triangles and channels appear as dark areas, having the shape of a cloverleaf, and are ~ 7 nm long. The rim of the triangle and the interconnecting channels visible as bright lines in the image consists of a protein wall, which is ~ 2.5 nm thick. Within the triangles no clear or regular structures could be observed.
The final four-fold magnification of the lorica surface in Eutintinnus revealed that three primitive rhombohedral unit cells each form the hexagonal structures recognizable in the fast Fourier transform (Fig. 27). Figure 28 shows the deduced simple model of the crystal structure, where the main motifs consist of triangles with a diameter of ~ 18 nm. They are interconnected on each side by tiny tubes ~ 7 nm in diameter, which may serve as channels. The empty spaces between the triangles and channels, respectively, appear dark. The rim of the triangle and the interconnecting channels are visible as thin white lines and become more conspicuous at enhanced contrast. Probably, they give rise to an interconnected, proteinaceous wall system with a width of ~ 2.5 nm (Figs 24, 25, 27, 28).
Fibres are not recognizable by high-resolution transmission electron microscopy, indicating that a substance different from chitin and keratin constitutes the lorica. In fact, the crystal lattice is very similar to the proteinaceous surface layers (S-layers) of bacteria and archaea (Schuster and Sleytr 2000, Sleytr et al. 2001), which are occasionally also resistant against proteinase K digestion (see above).
This is the first high-resolution transmission electron microscopic study of a tintinnid lorica and it is restricted to the hyaline Eutintinnus lorica, which has a compact, monolaminar wall texture (Laval-Peuto 1994). Further investigations have to elucidate whether the crystalline composition is typical of tintinnids, namely, shared by the alveolar, monolaminar and the trilaminar textures and all other compact, monolaminar walls.
Longitudinal and transversal wall sections of the hyaline lorica in Eutintinnus show a cross striation due to alternating thin electron-dense and broad electron-lucent stripes (Laval-Peuto 1994). A similar striation and periodicity of the stripes (~ 35 nm vs. 50–55 nm) is found in the “body” of extruded trichocysts in Paramecium and Frontonia, which consists of crystalline proteins (Steers et al. 1969, Hausmann 1978, Sperling et al. 1987, Rosati and Modeo 2003); both the extruded lorica material and the extrusomes remain insoluble upon secretion and undergo a rapid change of state, resulting in an extended extracellular form. Although Paramecium trichocysts show a remarkable stability against “mild” solutions (Tindall et al. 1989), tintinnid loricae are definitely more resistant and thus probably composed of different proteins. A comparable striation also occurs in the proteinaceous kinetodesmal fibres (periodicity 18–40 nm; Pitelka 1965, Hufnagel 1969, Williams et al. 1979).
Comparison with former cytochemical investigations (for details on the species studied, see Table 1 in the Supplementary material available at the journal’s website): For sake of simplicity, the taxonomic acts of Kofoid and Campbell (1929, 1939) are accepted here; thus, the species names and generic affiliations used might deviate from those in the original publications.
Chitin and other polysaccharides
Fol (1881) was the first who performed experiments on the chemical composition of hyaline and hard, agglomerated tintinnid loricae. Due to the resistance of the lorica walls or matrixes against strong, hot, concentrated bases, he supposed a chitinous nature. Daday (1887), Biedermann (1893), Fauré-Fremiet (1908), Entz Jr. (1909a), and Hofker (1931a) drew the same conclusion, which was also supported by Entz Sr. (1885), Kofoid (1930), and Gold and Morales (1975b). Actually, the hyaline and hard, agglomerated loricae analysed in the present study also demonstrated an astonishing resistance against potassium hydroxide at 160°C for 40 min. The absence of a staining by sulphuric acid indicated, however, a substance different from chitin, which was substantiated by the disintegration of the loricae after an elongated exposure in the strong, hot base (this study, Entz Jr. 1909b, Bussers and Jeuniaux 1974) and the failure of the chitinase digestion (this study). Cellulose, as supposed by Schweyer (1909), can also be excluded based on own experiments and those of Entz Jr. (1909b) on hyaline and hard, agglomerated loricae.
The presence of various further polysaccharides is excluded by the periodic acidic Schiff reaction in the hard, agglomerated lorica of Tintinnopsis cf. cylindrica and the hyaline one of Rhabdonella spiralis (this study). After application of iodine alcohol or Lugol’s solutions, the hard, agglomerated loricae of Codonella and Tintinnopsis beroidea and the hyaline ones of Petalotricha, Proplectella subacuta (reported as Undella claparèdei), and R. spiralis became yellow-brown (Entz Jr. 1909b), indicating glycogen (Gomori 1952). This demonstrates a variability in the occurrence of glycogen in R. spiralis and the genus Tintinnopsis. Based on the data available, it can be concluded that polysaccharides are probably never the main component in tintinnid loricae.
Proteins
A proteinaceous, horny, or keratin-like substance in the lorica walls and matrixes was assumed by Kent (1880–1882), Entz Jr. (1909b), and Laval--Peuto and Barria de Cao (1987). Since keratin is easily soluble in potassium hydroxide, its presence can be excluded in the hyaline, the soft, agglomerated, and the hard, agglomerated loricae; additionally, stains with Millon’s solution usually failed in the loricae. However, sulphur-linked proteins are known to persist in hot and concentrated potassium hydroxide for a while (Krishnan 1954, Moraczewski 1971a). Findings of the present study, actually indicate a proteinaceous composition of the lorica walls and matrixes: (i) the digestion of hyaline (Climacocylis, Eutintinnus) and hard, agglomerated (Tintinnopsis levigata) loricae by proteinase K; (ii) the detection of nitrogen in all EDX analyses; (iii) the crystal lattice revealed by high-resolution transmission electron microscopy in the hyaline Eutintinnus lorica resembling the proteinaceous S-layer of bacteria and archaea; and (iv) the striation recognizable in sections of the Eutintinnus lorica wall similar to that produced by crystalline proteins in other ciliate structures (extruded trichocysts, kinetodesmal fibres).
The mercuric bromophenol blue stain was successfully applied in the hyaline loricae of Climacocylis, Cymatocylis, Eutintinnus, Parafavella, and Rhabdonella and the hard, agglomerated ones of Codonella, Codonellopsis, and Laackmanniella (this study, Gold 1968, Gold and Morales 1975a, Wasik et al. 1997). An intrageneric variability was found in Tintinnopsis with a colouration of T. lobiancoi (Wasik et al. 1997), but not of T. compressa and T. cf. cylindrica (this study). For Helicostomella subulata, inconsistent results were obtained, possibly due to the application of different fixatives (formalin or glutaraldehyde vs. Bouin; this study, Wasik et al. 1997). Specimens of Rhabdonella spiralis varied in their staining intensity even in the same experiment (this study). However, mercuric bromophenol blue might not detect all proteins or stain different substances (Baker 1958a, Kanwar 1960). On the other hand, acid fuchsin, clearly indicating proteins, and phosphor-molybdenum acid, indicating proteins and alkaloids (Zimmermann 1892), gave positive results in Tintinnopsis (Merkle 1909). Aqueous and alcoholic solutions of eosin to detect acid proteins (Mulish and Welsch 2010) failed in the hyaline lorica of Amphorella and the hard, agglomerated ones of freshwater Codonella species (all possibly belong to C. cratera) and the marine Codonella galea; the absence of acid proteins or a high pH value might have prevented a colouration (Singer 1952). The congener Codonella cistellula, however, was stained pink-red, like the hard, agglomerated lorica of Dictyocysta (Entz Jr. 1909a, b).
Concentrated sulphuric acid plus sugar (Raspail’s reagent), which probably indicates proteins, alkaloids, and/or glycosides by a pink to violet colouration (Schneider 1874, Poulsen and Trelease 1884), did not stain the hard, agglomerated loricae of Codonella, Codonellopsis, Dictyocysta, and Tintinnopsis and the hyaline one of Petalotricha (Entz Jr. 1909b).
After application of methylene blue, recognizing acid mucopolysaccharides (polyanionic glycoproteins) and lipoids (Adam and Czihak 1964), the soft, agglomerated loricae of Tintinnidium species from freshwater and the hard, agglomerated ones of the marine tintinnids Stenosemella ventricosa and Tintinnopsis campanula were entirely blue. In the marine Tintinnopsis beroidea, only the anterior portion of the hard, agglomerated lorica stained, while the loricae of Codonella, Dictyocysta, Eutintinnus, and Tintinnopsis cylindrica did not show any colouration (Entz Jr. 1909a, b; Foissner and Wilbert 1979). Polyanionic glycoproteins were also indicated by the alcian blue stain in the hyaline lorica of Parafavella and the hard, agglomerated ones of Codonellopsis gaussi and Laackmanniella; no colouration was observed in the hyaline loricae of Cymatocylis and Helicostomella and the hard, agglomerated one of Tintinnopsis lobiancoi (Wasik et al. 1997). The present experiments revealed acid mucopolysaccharides in a single species, viz., in the hard, agglomerated bowl of Stenosemella ventricosa, whereas its hyaline collar as well as the hyaline lorica of Rhabdonella and the hard, agglomerated ones of Codonella, Codonellopsis schabi, and Tintinnopsis cf. cylindrica were not stained. Accordingly, there is a variability in the genera Codonellopsis and Tintinnopsis concerning the presence of polyanionic glycoproteins (acid mucopolysaccharides). Intraspecific inconsistencies were found in the freshwater species Codonella cratera (Entz Jr. 1909a, b; Foissner and Wilbert 1979), and differences in the staining properties occurred even in a single lorica (S. ventricosa; this study).
Experiments recognizing xanthoproteins gave inconsistent results, as they detected only tyrosine by the Millon’s reaction or xanthoproteins in general (the aromatic amino acids tyrosine, tryptophan, histidine, and phenylalanine) plus alkaloids by nitric acid plus potassium hydroxide or ammoniac (Zimmermann 1892). Xanthoproteins were recorded in the hyaline loricae of Favella franciscana, Petalotricha, and Proplectella subacuta (reported as Undella claparèdei), the soft, agglomerated lorica of Leprotintinnus, and the hard, agglomerated one of Tintinnopsis lindeni (reported as Cyttarocylis helix; Entz Jr. 1909b; Kofoid and Campbell 1939). Phenylalanine, tryptophan, histidine, and/or alkaloids were detected in the hard, agglomerated loricae of Codonella aspera, C. cistellula, Codonellopsis lata (reported as Codonella orthoceras), and Dictyocysta (Entz Jr. 1909b). Tyrosine was absent in the hyaline lorica of Amphorella and the hard, agglomerated ones of Tintinnopsis cf. cylindrica and T. campanula (this study, Entz Jr. 1909b), while xanthoproteins in general were absent in the hard, agglomerated loricae of Stenosemella and Tintinnopsis beroidea and the hyaline houses of Coxliella decipiens (phenotype of Favella ehrenbergii; Laval-Peuto 1981), Cyttarocylis cassis, Eutintinnus, Favella adriatica (or possibly F. ehrenbergii), and Rhabdonella (this study, Entz Jr. 1909b). These data demonstrate an intrageneric variability in Favella and Tintinnopsis concerning the presence of xanthoproteins.
Gold and Morales (1975a) found a positive reaction of Parafavella gigantea upon Mallory’s triple connective tissue stain and concluded that the lorica contains carbohydrates forming a pseudochitin; however, the method is not specific for chitin, as the acid fuchsin stains those tissues red which have not been coloured by the other dyes, and possibly indicates proteins (Zimmermann 1892, Humason 1972).
Inter- and intrageneric differences are also demonstrated by Borrel’s stain (probably Borrel’s methylene blue stain; Cold Spring Harbor Protocols 2010). Various shades of red were recognizable in the matrix of the hard, agglomerated loricae in Tintinnopsis and Codonella cistellula and the hyaline loricae of Rhabdonella and Epiplocylis (Hofker 1931b). Shades of blue were found in the hyaline lorica of Dictyocysta mitra and the hard, agglomerated one of D. lepida. Codonella nationalis was extraordinary in that the dye caused different colours in a single lorica: light blue in the areas of agglomerated particles, dark blue between the attached particles, and red in the inner surface of the transition zone between collar and bowl; the lorica sac and its closing apparatus received a pink colour.
Effects of acids
In contrast to the reactions upon bases, acid treatments displayed deviations between and within genera. While the soft, agglomerated lorica of the freshwater species Tintinnidium fluviatile dissolved in nitric acid, the soft, agglomerated one of the freshwater species Tintinnopsis cylindrata (reported as T. cylindrica) was not influenced by the warm solution (Entz Jr. 1909a). The hard, agglomerated lorica of the freshwater species Codonella cratera dissolved in warm nitric acid and warm to hot hydrochloric acid (Entz Jr. 1909b). The similarly structured loricae of marine Tintinnopsis species were, however, not influenced by hydrochloric acid (Merkle 1909), even not at high temperatures and concentrations (Entz Jr. 1909b). Hydrochloric acid merely dissolved the calcium carbonate particles (coccoliths) of hard, agglomerated loricae in, e.g., Codonaria (Gold and Morales 1977). In sulphuric acid, the hard, agglomerated loricae of Tintinnopsis (Entz Jr. 1909b, Merkle 1909) and Stenosemella as well as the hyaline ones of Cyttarocylis and Petalotricha (Entz Jr. 1909b) persisted. The hard, agglomerated house of Tintinnopsis did not dissolve in concentrated acetic acid, but became yellowish-brown (Entz Jr. 1909b), and the hyaline loricae of Petalotricha and Rhabdonella obtained a brown colour due to gallic acid (3,4,5-trihydroxybenzoic acid; Fol 1883).
Hydrofluoric acid removed the siliceous particles from the hard, agglomerated loricae of Codonellopsis, Stenosemella, and Tintinnopsis rapa (this study, Gold and Morales 1977, Gold 1980), while the lorica of Tintinnopsis wangi completely broke down probably due to a very low content of resistant matrix substance (Gold 1980). However, the hyaline lorica of Helicostomella subulata was not influenced (Gold and Morales 1977, Gold 1980). These findings are in accordance with the present study, demonstrating the persistence of the lorica matrix and the hyaline lorica wall of Stenosemella and Rhabdonella, respectively.
EDX analyses
By means of energy-dispersive X-ray spectroscopy (EDX analysis), Gold and Morales (1975a) detected traces of minerals, which probably originated in agglomerated particles. Wasik et al. (1997) analysed the hard, agglomerated loricae of Codonellopsis gaussi, Laackmanniella naviculaefera, and Tintinnopsis lobiancoi and the hyaline ones of Cymatocylis affinis/convallaria, Helicostomella subulata, and Parafavella denticulata. The EDX results were only shown for L. naviculaefera without a detailed description of the findings. Interestingly, the highest EDX peaks observed correspond to copper and zinc, indicating that the lorica of L. naviculaefera consists of brass, which is less likely; thus, the composition of the electron microscopic stub was probably analysed. Recently, Kaulich et al. (2009) performed low-energy X-ray fluorescence microscopy on the hard, agglomerated lorica of Tintinnopsis radix. The results are similar to the present EDX analyses, except for nitrogen, which was not mentioned.
Lorica forming granules
In this study, transmission electron microscopic studies on tintinnid cells were not performed. Therefore, literature data were analysed concerning the lorica forming granules. These granules accumulate in the anterior cell half of dividing cells and are used by the proter (anterior division product) to construct its own lorica, while the opisthe (posterior division product) keeps the old one. Different granule types were reported: (i) the morula type in Cymatocylis affinis/convallaria (Wasik and Mikolajczyk 1992, Wasik 1998), Cyttarocylis brandti (Laval-Peuto 1975), Petalotricha ampulla (Laval 1972), and Parafavella denticulata (Sokolova and Gerassimova 1984); (ii) the compact and granular types in Parafavella gigantea (Hedin 1975) and Tintinnopsis parva (Laval-Peuto et al. 1979); (iii) small granules in Favella; and (iv) a single mass in Dictyocysta (Laval-Peuto 1994). These data demonstrate that different granule types apparently occur in the genus Parafavella, while the same type might occur in taxa with hyaline and hard, agglomerated loricae.
All these lorica forming granules do not show a crystalline structure, but are osmiophilic, indicating lipids, unsaturated –C = C– bonds, and/or proteins, especially, those with lysine, arginine, tryptophane, histidine, and cysteine (Baker 1958b, Mulisch and Welsch 2010). In Favella, the lorica forming granules stained with iron haematoxylin (Campbell 1927), whereas Biernacka (1965) observed in Tintinnopsis a red colouration of the granules after application of Mann’s methyl blue eosin, which recognizes acidophil substances (Gurr 1962). Campbell (1929) characterized the granules as chromophil in Tintinnopsis reflexa.
Conclusion
Although the own and literature data are scattered, there is evidence for a proteinaceous nature of the lorica material.
Rhabdonella spiralis with its hyaline lorica was used in eleven of the fourteen experiments of the present study. Admittedly, the only positive reaction was caused by mercuric bromophenol blue, which is not a reliable indicator of proteins. However, after the exclusion of chitin, cellulose, other polysaccharides, neutral mucopolysaccharides, glycolipids, phospholipids, and unsaturated fatty acids, only proteins remain, some of which are known to possess a resistance against strong, hot bases similar to that of chitin. Actually, the assumption of a proteinaceous substance is supported by the presence of nitrogen in the EDX analysis. These proteins apparently do not comprise glycoproteins, mucoproteins, or phenols, but are apparently similar to the proteolytically resistant ones of prions, bacteria, and archaea.
Additionally, hard, agglomerated loricae and hyaline ones of further genera were analysed in the present study. The presence of nitrogen in the EDX analyses, the exclusion of chitin, cellulose and other polysaccharides, and the proteinase K digestion of some of them again strongly indicated a proteinaceous substance. The high-resolution transmission electron micrographs of the hyaline lorica in Eutintinnus displaying a crystal lattice similar to that in the proteinaceous S-layer of bacteria and archaea and common transmission electron micrographs showing a striation typical of crystalline proteins in ciliate trichocysts pointed into the same direction.
In only few literature studies, the loricae were treated sufficiently long with a very strong base at a very high temperature to observe their disintegration. Accordingly, most studies inferred a chitinous nature of the loricae due to their resistance, which is also displayed by the detection of loricae in sediment traps up to 7000 m deep (Suzuki and Taniguchi 1995). Despite intrageneric and even intraspecific inconsistencies concerning staining properties and enzymatic digestion, the present findings and literature data indicated proteins in loricae of thirteen tintinnid genera: Climacocylis, Codonella, Codonellopsis, Dictyocysta, Eutintinnus, Favella, Laackmanniella, Leprotintinnus, Parafavella, Rhabdonella, Stenosemella, Tintinnidium, and Tintinnopsis. Thus, there is good evidence for a proteinaceous substance in possibly all loricae being responsible for the resistance of the loricae against strong bases. Actually, the occurrence of only one kind of organic macromolecule would support the hypothesis about a single origin of the loricae in the tintinnid evolution. However, the protein composition and also the composition of additional substances vary. To the authors’ best knowledge, none of the proteinaceous shells studied in other protists displayed reactions identical to those of the tintinnid loricae in the histochemical and enzymatic experiments.
Kofoid (1930) predicted taxonomically significant differences in the chemical composition of the loricae. Although histochemical and enzymatic experiments actually demonstrated variability in the reactions, distinctly more data are required for the emergence of taxon-specific traits. General differences between the hyaline, the soft, agglomerated, and the hard, agglomerated loricae as well as between freshwater and marine species were not obvious. Nevertheless, the wall texture (e.g. compact, monolaminar; alveolar, monolaminar; trilaminar) probably depends on the chemical composition of the lorica material (Laval-Peuto 1994), which again might be correlated with certain granule types. Future studies have to elucidate the taxonomic significance of these features.
A quantification of the chemical elements produced by the mother cell and incorporated by the proter into the different kinds of loricae (hard, agglomerated; soft, agglomerated; hyaline; composed a hyaline collar and an agglomerated bowl) is also still missing. However, these data are needed to properly estimate the role of the tintinnids and their loricae in the energy flux and element recycling in the marine and freshwater ecosystems.
Supplementary Material
Acknowledgements
The sampling as well as the histochemical, enzymatic, and EDX analyses of S.A. were supported by the Austrian Science Fund (FWF; Project P20461-B17). The collection of the samples by S.A. was made possible through the hospitality of Dr. Alfred Beran (Department of Biological Oceanography, Istituto Nazionale di Oceanografia e Geofisica Sperimentale, Trieste, Italy), Dr. Wayne Coats (Smithsonian Environmental Research Center, Maryland, USA), and Dr. John Dolan (Laboratoire d’Oceanographie, Station Zoologique, Villefranche-sur-mer, France). We wish to thank Prof. Wilhelm Foissner (University of Salzburg) for discussion and providing the Blepharisma culture as well as Dr. Anke Oertel and Dr. Wolf-Dietrich Krautgartner (University of Salzburg) for their technical assistance. Thanks also go to Prof. Hannes Lichte at the Special Laboratory Triebenberg for Electron Holography of the Technical University of Dresden, providing the facilities for the high-resolution transmission electron microscopic study by P.S. Finally, we thank Dr. Wilder Carrillo-Cabrera (Max Planck Institute for Chemical Physics of Solids, Dresden, Germany) for his help concerning the EDX measurements of Eutintinnus angustatus.
REFERENCES
- Adam H, Czihak G. Arbeitsmethoden der makroskopischen und mikroskopischen Anatomie. Grosses zoologisches Praktikum. Teil 1, Ein Laboratoriumshandbuch für Biologen, Mediziner und technische Hilfskräfte. G. Fischer Verlag; Stuttgart: 1964. [Google Scholar]
- Bajorath J, Hinrichs W, Saenger W. The enzymatic activity of proteinase K is controlled by calcium. Eur. J. Biochem. 1988;176:4341–447. doi: 10.1111/j.1432-1033.1988.tb14301.x. [DOI] [PubMed] [Google Scholar]
- Baker JR. The histochemical recognition of phenols, especially tyrosine. Q. Jl microsc. Sci. 1956;97:161–164. [Google Scholar]
- Baker JR. Note on the use of bromophenol blue for the histochemical recognition of protein. Q. Jl microsc. Sci. 1958a;99:459–460. [Google Scholar]
- Baker JR. Principles of Biological Microtechnique. A Study of Fixation and Dyeing. J. Wiley & Sons Inc.; New York: 1958b. [Google Scholar]
- Basu S, Mohan ML, Luo X, Kundu B, Kong Q, Singh N. Modulation of proteinase K-resistant prion protein in cells and infectious brain homogenate by redox iron: implications for prion replication and disease pathogenesis. Mol. Biol. Cell. 2007;18:3302–3312. doi: 10.1091/mbc.E07-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P, Bacci J, Lemaigre G, Martin E. Effects of fixation and processing on the immunohistochemical visualization of type-I, -III and -IV collagen in paraffin-embedded liver tissue. Histochemistry. 1987;88:85–89. doi: 10.1007/BF00490172. [DOI] [PubMed] [Google Scholar]
- Bermejo-Barrera P, Fernández-Nocelo S, Moreda-Piñeiro A, Bermejo-Barrera A. Usefulness of enzymatic hydrolysis procedures based on the use of pronase E as sample pre-treatment for multi-element determination in biological materials. J. Anal. At. Spectrom. 1999;14:1893–1900. [Google Scholar]
- Biedermann R. Ueber die Structur der Tintinnen-Gehäuse. Univ. Kiel; 1893. 1–38 + Plates 1–3. PhD Thesis. [Google Scholar]
- Biernacka I. Ausscheidung gehäusebildender Substanzen durch reife Formen gewisser Arten der Gattung Tintinnopsis Stein. Acta Protozool. 1965;3:265–268. with Polish summary. [Google Scholar]
- Bowser SS, Bernhard JM. Structure, bioadhesive distribution and elastic properties of the agglutinated test of Astrammina rara (Protozoa: Foraminiferida) J. Eukaryot. Microbiol. 1993;40:121–131. [Google Scholar]
- Brandelli A. Hydrolysis of native proteins by keratinolytic protease of Chryseobacterium sp. Annls Microbiol. 2005;55:47–50. [Google Scholar]
- Bussers JC, Jeuniaux C. Recherche de la chitine dans les productions métaplasmatiques de quelques ciliés. Protistologica. 1974;10:43–46. with English summary. [Google Scholar]
- Butler GH, Kotani H, Kong L, Frick M, Evancho S, Stanbridge EJ, McGarrity GJ. Identification and characterization of proteinase K-resistant proteins in members of the class Mollicutes. Infect. Immun. 1991;59:1037–1042. doi: 10.1128/iai.59.3.1037-1042.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AS. Studies on the marine ciliate Favella (Jörgensen), with special regard to the neuromotor apparatus and its rôle in the formation of the lorica. Univ. Calif. Publ. Zool. 1927;29:429–452. [Google Scholar]
- Campbell AS. House-forming material in a marine ciliate. Anat. Rec. 1929;44:247. [Google Scholar]
- Cold Spring Harbor Protocols Borrel’s methylene blue. 2010 doi:10.1101/pdb.rec11384. [Google Scholar]
- von Daday E. Monographie der Familie der Tintinnodeen. Mitt. zool. Stn Neapel. 1887;7:473–591. + Plates 18–21. [Google Scholar]
- Entz G., Sr. Zur näheren Kenntnis der Tintinnoden. Mitt. zool. Stn Neapel. 1885;6:185–216. + Plates 13, 14. [Google Scholar]
- Entz G., Jr. Die Süsswasser-Tintinniden. Math. naturw. Ber. Ung. 1909a;25:197–225. + Plates 1–4. [Google Scholar]
- Entz G., Jr. Studien über Organisation und Biologie der Tintinniden. Arch. Protistenkd. 1909b;15:93–226. + Plates 8–21. [Google Scholar]
- Fauré-Fremiet E. Le Tintinnidium inquilinum. Arch. Protistenk. 1908;11:225–251. + Plate 12. [Google Scholar]
- Foissner W, Wilbert N. Morphologie, Infraciliatur und Ökologie der limnischen Tintinnina: Tintinnidium fluviatile Stein, Tintinnidium pusillum Entz, Tintinnopsis cylindrata Daday und Codonella cratera (Leidy) (Ciliophora, Polyhymenophora) J. Protozool. 1979;26:90–103. with English summary. [Google Scholar]
- Foissner W, Müller H, Weisse T. The unusual, lepidosome-coated resting cyst of Meseres corlissi (Ciliophora: Oligotrichea): light and scanning electron microscopy, cytochemistry. Acta Protozool. 2005;44:201–215. [Google Scholar]
- Fol H. Contribution a la connaissance de la famille des Tintinnodea. Archs Sci. phys. nat. 1881;5:5–24. + Plate 1. [Google Scholar]
- Fol H. Nouvelle contribution a la connaissance de la famille des Tintinnodea. Archs Sci. phys. nat. 1883;9:554–578. [Google Scholar]
- Gerber GH. Adaptation of the Millon, Sudan Black B, and Periodic Acid-Schiff technics for block staining of insect tissues. Biotech. Histochem. 1970;45:225–229. doi: 10.3109/10520297009067483. [DOI] [PubMed] [Google Scholar]
- Geyer G. Handbuch der Histochemie. I. Allgemeine Methodik. 3. Elektronenmikroskopische Histochemie. 1. Nachweisund Kontrastierungsmethoden für Kohlenhydrate, Proteine, und Aminosäuren, Nucleinsäuren, Lipide und Mineralstoffe. G. Fischer; Stuttgart, New York: 1977. [Google Scholar]
- Gloghini A, Canal B, Klein U, Dal Maso L, Perin T, Dalla-Favera R, Carbone A. RT-PCR analysis of RNA extracted from Bouin-fixed and paraffin-embedded lymphoid tissues. J. Mol. Diagn. 2004;6:290–296. doi: 10.1016/S1525-1578(10)60524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold K. Some observations on the biology of Tintinnopsis sp. J. Protozool. 1968;15:193–194. [Google Scholar]
- Gold K. SEM studies on the lorica of various Tintinnida. Scanning Electron Microsc. 1980;3:537–541. [Google Scholar]
- Gold K, Morales EA. Tintinnida of the New York Bight: loricae of Parafavella gigantea, P. parumdentata, and Ptychocylis obtusa. Trans. Am. microsc. Soc. 1975a;94:142–145. [Google Scholar]
- Gold K, Morales EA. Seasonal changes in lorica sizes and the species of Tintinnida in the New York Bight. J. Protozool. 1975b;22:520–528. [Google Scholar]
- Gold K, Morales EA. Studies on the Tintinnida of Enewetak Atoll. J. Protozool. 1977;24:580–587. [Google Scholar]
- Gomori G. Microscopic Histochemistry – Principles and Practice. University of Chicago Press; Chicago: 1952. [Google Scholar]
- Gries G, Lindner J. Zur Frage des Abbaues von Kollagen. J. Mol. Med. 1960;38:406–407. [Google Scholar]
- Gurr E. Staining Practical and Theoretical. Williams & Wilkins; Baltimore: 1962. [Google Scholar]
- Hausmann K. Extrusive organelles in protists. Int. Rev. Cytol. 1978;52:197–276. doi: 10.1016/s0074-7696(08)60757-3. [DOI] [PubMed] [Google Scholar]
- Hayat MA. Handbook of Immunohistochemistry and in situ Hybridization of Human Carcinomas. Molecular Pathology, Colorectal Carcinoma, and Prostate Carcinoma. Elsevier Academic Press; London: 2005. [Google Scholar]
- Hedin H. On the ultrastructure of Favella ehrenbergii (Claparède & Lachmann) and Parafavella gigantea (Brandt), Protozoa, Ciliata, Tintinnida. Zoon. 1975;3:11–18. [Google Scholar]
- Hedley RH. The iron-containing shell of Gromia oviformis (Rhizopoda) Q. Jl microsc. Sci. 1960;101:279–293. [Google Scholar]
- Hedley RH. Cement and iron in the arenaceous foraminifera. Micropaleontol. 1963;9:433–441. [Google Scholar]
- Hedley RH, Rudall KM. Extracellular silk fibres in Stannophyllum (Rhizopodea: Protozoa) Cell Tiss. Res. 1974;150:107–111. doi: 10.1007/BF00220384. [DOI] [PubMed] [Google Scholar]
- Hofker J. Die Bildung der Tintinnengehäuse. Tijdschr. Ned. Dierk. Vereen. Ser. 1931a;3:144–150. [Google Scholar]
- Hofker J. Studien über Tintinnoidea. Arch. Protistenkd. 1931b;75:315–402. [Google Scholar]
- Hufnagel L. Cortical ultrastructure of Paramecium aurelia. Studies on isolated pellicles. J. Cell Biol. 1969;40:779–801. doi: 10.1083/jcb.40.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humason GL. Animal Tissue Techniques. 3rd ed W. H. Freeman and Company; San Francisco: 1972. [Google Scholar]
- Hyman LH. The Invertebrates: Protozoa through Ctenophora. McGraw-Hill Book Company; New York: 1940. [Google Scholar]
- Kaminski L, Abu-Qarn M, Guan Z, Naparstek S, Ventura VV, Raetz CRH, Hitchen PG, Dell A, Eichler J. AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J. Bacteriol. 2010;192:5572–5579. doi: 10.1128/JB.00705-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar KC. Note on the specificity of mercuric bromophenol blue for the cytochemical detection of proteins. Cell. Mol. Life Sci. 1960;16:355. doi: 10.1007/BF02157899. [DOI] [PubMed] [Google Scholar]
- Karshan M. The chemistry and staining reactions of keratin. J. Dent. Res. 1930;10:181–186. [Google Scholar]
- Kaulich B, Gianoncelli A, Beran A, Eichert D, Kreft I, Pongrac P, Regvar M, Vogel-Mikuš K, Kiskinova M. Lowenergy X-ray fluorescence microscopy opening new opportunities for bio-related research. J. R. Soc. Interface. 2009;6:S641–S647. doi: 10.1098/rsif.2009.0157.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WS. A Manual of the Infusoria: Including a Description of all known Flagellate, Ciliate, and Tentaculiferous Protozoa, British and Foreign, and an Account of the Organization and Affinities of the Sponges. David Bogue; London: 1880-1882. 1880: 1–472 + Plates 1–23; 1881: 473–720 + Plates 24–40; 1882: 721–913 + Plates 41–51. [Google Scholar]
- Kofoid CA. I. Factors in the evolution of the pelagic Ciliata, the Tintinnoinea. Contr. Mar. Biol. 1930:1–39. [Google Scholar]
- Kofoid CA, Campbell AS. A conspectus of the marine and fresh-water Ciliata belonging to the suborder Tintinnoinea, with descriptions of new species principally from the Agassiz Expedition to the eastern tropical Pacific 1904–1905. Univ. Calif. Publs Zool. 1929;34:1–403. [Google Scholar]
- Kofoid CA, Campbell AS. Reports on the scientific results of the expedition to the eastern tropical Pacific, in charge of Alexander Agassiz, by the U. S. Fish Commission Steamer “Albatross,” from October, 1904, to March, 1905, Lieut.-Commander L. M. Garrett, U. S. N. Commanding. XXXVII. The Ciliata: The Tintinnoinea. Bull. Mus. comp. zool., Harv. 1939;84:1–473. + Plates 1–36. [Google Scholar]
- Košková E, Matějusová I, Civáňová K, Koubková B. Ethanol-fixed material used for both classical and molecular identification purposes: Eudiplozoon nipponicum (Monogenea: Diplozoidae) as a case parasite species. Parasitol. Res. 2010;107:909–914. doi: 10.1007/s00436-010-1949-0. [DOI] [PubMed] [Google Scholar]
- Krishnan G. The epicuticle of an arachnid, Palamneus swammerdami. Q. Jl microsc. Sci. 1954;95:371–381. [Google Scholar]
- Lai M, Lampert IA, Lewis PD. The influence of fixation on staining of glycosaminoglycans in glial cells. Histochemistry. 1975;41:275–279. doi: 10.1007/BF00497691. [DOI] [PubMed] [Google Scholar]
- Laval M. Ultrastructure de Petalotricha ampulla (Fol). Comparaison avec d’autres Tintinnides et avec les autres ordres de ciliés. Protistologica. 1972;8:369–386. with English summary. [Google Scholar]
- Laval-Peuto M. Cortex, périlemme et réticulum vésiculeux de Cyttarocylis brandti (Cilié Tintinnide). Les ciliés a périlemme. Protistologica. 1975;11:83–98. with English summary. [Google Scholar]
- Laval-Peuto M. Construction of the lorica in Ciliata Tintinnina – in vivo study of Favella ehrenbergii – variability of the phenotypes during the cycle, biology, statistics, biometry. Protistologica. 1981;17:249–272. [Google Scholar]
- Laval-Peuto M. Classe des Oligotrichea Bütschli, 1887. Ordre des Tintinnida Kofoid et Campbell, 1929. In: de Puytorac P, editor. Traité de Zoologie. Anatomie, Systématique, Biologie. II. Infusoires Ciliés. 2. Systématique. Masson; Paris, Milano, Barcelona: 1994. pp. 181–219. [Google Scholar]
- Laval-Peuto M, Barria de Cao MS. Les capsules, extrusomes caracteristiques des Tintinnina (Ciliophora), permettent une classification evolutive des genres et des familles du sousordre. Ile Réun. Scientif. GRECO 88, Trav. C.R.M. 1987;8:53–60. [Google Scholar]
- Laval-Peuto M, Gold K, Storm ER. The ultrastructure of Tintinnopsis parva. Trans. Am. microsc. Soc. 1979;98:204–212. [Google Scholar]
- Lev R, Spicer SS. Specific staining of sulphate groups with alcian blue at low pH. J. Histochem. Cytochem. 1964;12:309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- Mazia D, Brewer PA, Alfert M. The cytochemical staining and measurement of protein with mercuric bromphenol blue. Biol. Bull. mar. biol. Lab., Woods Hole. 1953;104:57–67. [Google Scholar]
- McKinley MP, Bolton DC, Prusiner SB. A protease-resistant protein is a structural component of the Scrapie prion. Cell. 1983;35:57–62. doi: 10.1016/0092-8674(83)90207-6. [DOI] [PubMed] [Google Scholar]
- Merkle H. Untersuchungen an Tintinnodeen der Ost- und Nordsee. Wiss. Meeresunters., Abt. Kiel. 1909;11:139–186. + Plates 2, 3. [Google Scholar]
- Moraczewski J. Etudes radioautographiques sur l’amibe Arcella; 7th Congr. Int. Microsc. Électron.; Grenoble. 1970.pp. 405–406. [Google Scholar]
- Moraczewski J. La composition chimique de la coque d’Arcella discoides Ehrbg. Acta Protozool. 1971a;8:407–421. + Plate 1 (with Polish summary) [Google Scholar]
- Moraczewski J. Structure et formation de la coque d’Arcella. Acta Protozool. 1971b;8:423–437. + Plates 1–5 (with Polish summary) [Google Scholar]
- Mulisch M, Welsch U. Romeis Mikroskopische Technik. 18th ed Spektrum Akademischer Verlag; Heidelberg: 2010. [Google Scholar]
- Nayar KK. Studies on the neurosecretory system of Iphita limbata Stal. I. Distribution and structure of the neurosecretory cells of the nerve ring. Biol. Bull. mar. biol. Lab., Woods Hole. 1955;108:296–307. [Google Scholar]
- Pierce S, Kossoy V, Valenti R, Smetana DG. Cytochemical studies on the test of Allogromia laticollare. Micropaleontol. 1968;14:242–246. [Google Scholar]
- Pitelka DR. New observations on cortical ultrastructure in Paramecium. J. Microsc., Paris. 1965;4:373–394. [Google Scholar]
- Pokorný V. Grundzüge der zoologischen Mikropaläontologie. Band 1. Dt. Verl. d. Wissenschaften; Berlin: 1958. [Google Scholar]
- Poulsen VA, Trelease W. Botanical Micro-chemistry – An Introduction to the Study of Vegetable Histology Prepared for the Use of Students. S. E. Cassino & Company; Boston: 1884. [Google Scholar]
- Romeis B. Mikroskopische Technik. 16th ed Oldenbourg Verlag; Munich, Vienna: 1968. [Google Scholar]
- Rosati G, Modeo L. Extrusomes in ciliates: diversification, distribution, and phylogenetic implications. J. Eukaryot. Microbiol. 2003;50:383–402. doi: 10.1111/j.1550-7408.2003.tb00260.x. [DOI] [PubMed] [Google Scholar]
- Santi PA, Anderson CB. Alcian blue staining of cochlear hair cell stereocilia and other cochlear tissues. Hearing Res. 1986;23:153–160. doi: 10.1016/0378-5955(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Schneider R. Year-Book of Pharmacy Comprising Abstracts of Papers Relating to Pharmacy, Materia Medica, and Chemistry Contributed to British and Foreign Journals, from July 1, 1873, to June 30, 1874. J. & A. Churchill; London: 1874. Reaction of sugar and sulphuric acid upon some alkaloids; pp. 213–214. [Google Scholar]
- Schuster B, Sleytr UB. S-layer-supported lipid membranes. Rev. Mol. Biotechnol. 2000;74:233–254. doi: 10.1016/s1389-0352(00)00014-3. [DOI] [PubMed] [Google Scholar]
- Schweyer A. Zur Kenntnis des Tintinnodeenweichkörpers, nebst einleitenden Worten über die Hülsenstruktur und die Hülsenbildung. Arch. Protistenkd. 1909;18:134–189. + Plates 10, 11. [Google Scholar]
- Singer M. Factors which control the staining of tissue sections with acid and basic dyes. Int. Rev. Cytol. 1952;1:211–256. [Google Scholar]
- Sleytr UB, Sára M, Pum D, Schuster B. Characterization and use of crystalline bacterial cell surface layers. Progr. Surf. Sci. 2001;68:231–278. [Google Scholar]
- Sokolova YY, Gerassimova ZP. The ultrastructure of the ciliates Parafavella denticulata Ehrenberg, 1840. Tsitologiya. 1984;26:1237–1245. + Plates 1–4 (in Russian with English summary) [Google Scholar]
- Song W, Wilbert N. Benthische Ciliaten des Süßwassers. In: Röttger R, editor. Praktikum der Protozoologie. Gustav Fischer Verlag; Stuttgart: 1995. pp. 156–168. [Google Scholar]
- Sperling L, Tardieu A, Gulik-Krzywicki T. The crystal lattice of Paramecium trichocysts before and after exocytosis by X-ray diffraction and freeze-fracture electron microscopy. J. Cell Biol. 1987;105:1649–1662. doi: 10.1083/jcb.105.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steedman HF. Alcian blue 8GS: a new stain for mucin. Q. Jl microsc. Sci. 1950;91:477–479. [PubMed] [Google Scholar]
- Steers E, Beisson J, Marchesi VT. A structural protein extracted from the trichocyst of Paramecium aurelia. Exp. Cell Res. 1969;57:392–396. doi: 10.1016/0014-4827(69)90165-7. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Taniguchi A. Sinking rate of loricae of some common tintinnid ciliates. Fisheries Oceanography. 1995;4:257–263. [Google Scholar]
- Tindall SH, Devito LD, Nelson DL. Biochemical characterization of the proteins of Paramecium secretory granules. J. Cell Sci. 1989;92:441–447. doi: 10.1242/jcs.92.3.441. [DOI] [PubMed] [Google Scholar]
- Tracey MV. Chitin. In: Paech K, Tracey MV, editors. Moderne Methoden der Pflanzenanalyse 2. Springer-Verlag; Berlin, Göttingen, Heidelberg: 1955. pp. 264–274. [Google Scholar]
- Valbonesi A, Luporini P. A new marine species of Euplotes (Ciliophora, Hypotrichida) from Antarctica. Bull. Br. Mus. (Nat. Hist.) Zool. 1990;56:57–61. [Google Scholar]
- Wasik A. Antarctic tintinnids: their ecology, morphology, ultrastructure and polymorphism. Acta Protozool. 1998;37:5–15. [Google Scholar]
- Wasik A, Mikolajczyk E. The morphology and ultrastructure of the Antarctic ciliate, Cymatocylis convallaria (Tintinnina) Acta Protozool. 1992;31:233–239. [Google Scholar]
- Wasik A, Mikolajczyk E, Gołębiowska M, Sikora J. X-ray analysis and cytochemical staining of some tintinnid loricae. Acta Protozool. 1997;36:153–155. [Google Scholar]
- Williams NE, Vaudaux PE, Skriver L. Cytoskeletal proteins of the cell surface in Tetrahymena: I. Identification and localization of major proteins. Exp. Cell Res. 1979;123:311–320. doi: 10.1016/0014-4827(79)90473-7. [DOI] [PubMed] [Google Scholar]
- Zakout YM, Salih MM, Ahmed HG. The effect of fixatives and temperature on the quality of glycogen demonstration. Biotech. Histochem. 2010;85:93–98. doi: 10.3109/10520290903126883. [DOI] [PubMed] [Google Scholar]
- Zimmermann A. Die botanische Mikrotechnik. Ein Handbuch der mikroskopischen Präparations-, Reaktions- und Tinktions-methoden. Verlag der Laupp’schen Buchhandlung; Tübingen: 1892. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.