Abstract
The orexin/hypocretin system has been implicated in multiple phases of drug addiction. Acute orexin receptor blockade with the orexin-1 receptor (OX1R) antagonist, SB-334867, has been found to reduce cocaine seeking after cocaine self-administration. As repeated drug dosing can have differential effects and is more clinically relevant than acute dosing, in the current study we examined the effects of repeated SB-334867 on cocaine self-administration, extinction, and reinstatement to cocaine seeking in Sprague Dawley rats. We found that repeated SB-334867 (10 mg/kg/day) had no effect on established cocaine self-administration. Repeated SB-334867 (both 10 and 20 mg/kg) attenuated cocaine seeking during extinction; however, this effect was only observed when animals had no prior experience with SB-334867 and when SB-334867 was administered prior to, but not after, daily extinction sessions. Notably, daily treatment with SB-334867 (10 mg/kg) during extinction increased subsequent cue-induced reinstatement, whereas repeated SB-334867 (20 mg/kg) administration during extinction enabled acute SB-334867 to reduce cue-induced reinstatement. Repeated SB-334867 treatment (10 or 20 mg/kg) failed to affect reinstatement induced by priming injections of cocaine (10 mg/kg). These results show that repeated inhibition of OX1R-mediated signaling exerts a lasting and specific role in mediating environmentally activated cocaine seeking.
Keywords: cocaine, extinction, orexin, reinstatement, SB-334867, self-administration
1. Introduction
The orexins (also known as hypocretins) are peptide neurotransmitters expressed in neurons exclusively in hypothalamic nuclei (de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998). The two forms of orexin, orexin-A (OXA) and orexin-B (OXB), bind to two G protein-coupled receptors, orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R), with different affinities – OX1R has 10-fold higher affinity for OXA than for OXB; whereas OX2R has equal affinity for both peptides (Sakurai et al., 1998; Smart et al., 2000). Both the orexin peptides and orexin receptors are widely distributed throughout the brain, including regions involved in drug reward and addiction (reviewed in (Sakurai, 2003)).
The widespread orexin innervation and distinctive expression of orexin receptors in the brain suggest that the orexin system subserves multiple functions. Orexin was initially named for its appetite-enhancing effects, an effect dependent upon OX1R signaling. For example, orexin administration increased feeding behaviors and rewarding properties of food, effects that were reversed by OX1R antagonism (Clegg et al., 2002; Haynes et al., 2000; Nair et al., 2008; Rodgers et al., 2001; Sakurai et al., 1998; Sweet et al., 1999). In addition, orexin has been shown to be involved in arousal and sleep/wake regulation, effects mainly mediated by OX2R or a combination of OX1R and OX2R. Infusion of orexin peptides into the rodent brain increases wakefulness, whereas OX2R and dual orexin receptor antagonist administration has sleep-promoting effects (for a review, see (Scammell and Winrow, 2011).
Over the last few years, accumulating studies have confirmed the involvement of the orexin system in drug addiction. For example, under a progressive ratio (PR) schedule of reinforcement, intracranial OXA administration increased the breakpoint of cocaine self-administration, while blockade of orexin signaling at OX1R via the selective antagonist, SB-334867, significantly reduced the breakpoint of self-administration of cocaine, ethanol, nicotine or heroin (Borgland et al., 2009; Espana et al., 2010; Hollander et al., 2008; Jupp et al., 2011; Smith and Aston-Jones, 2012). Under a fixed ratio (FR) schedule, SB-334867 decreased ethanol, nicotine or heroin self-administration, although it was not effective in cocaine self-administration (Espana et al., 2011; Hollander et al., 2008; Jupp et al., 2011; Lawrence et al., 2006; LeSage et al., 2010; Smith et al., 2009; Smith and Aston-Jones, 2012). However, SB-334867 dose-dependently reduced context-driven cocaine seeking after various lengths of abstinence (Smith et al., 2010). Direct activation of the orexin system can produce reinstatement of a previously extinguished response. For example, intracranial administration of OXA reinstated extinguished cocaine and nicotine seeking in animals with a history of drug self-administration, an effect blocked by OX1R antagonism (Boutrel et al., 2005; Plaza-Zabala et al., 2010; Wang et al., 2009). Systemic SB-334867 dose-dependently attenuated cue- and stress-induced reinstatement of cocaine seeking, but did not affect cocaine-primed reinstatement (Boutrel et al., 2005; Smith et al., 2009; Zhou et al., 2012). Similar effects were obtained with local SB-334867 given into VTA (Mahler et al., in press). The selective actions of orexin system in different forms of reinstatement suggest that orexin circuitry is involved in cue- and stress-induced reinstatement, but is not necessary for cocaine-primed reinstatement.
Previous studies have focused primarily on the effects of acute OX1R antagonism, while repeated OX1R blockade has not been systematically assessed. Because of the potential neuroadaptations to repeated drug exposure, repeated treatment can lead to profoundly different effects than acute treatment (e.g., sensitization or tolerance). In order to evaluate the potential clinical utility of OX1R antagonism in relapse prevention, it is important to assess cocaine seeking following prolonged drug treatment. Here, we examined the effects of repeated OX1R antagonism by SB-334867 at two different doses on established cocaine self-administration, extinction, as well as reinstatement to cocaine seeking induced by acute cocaine or previously cocaine-paired cues.
2. Materials and methods
2.1. Subjects
Adult male (initial weight 250–300 g) Sprague Dawley rats (Charles River Laboratories, Wilmington, NC, USA) were single housed in a temperature- and humidity-controlled animal facility on a reversed 12-h light–dark cycle (lights off at 06:00). All experimental procedures occurred during the dark cycle. Animals were given ad libitum access to water and standard rat chow (Harlan, Indianapolis, IN, USA) for the duration of each experiment. The experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and complied with federal guidelines in the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources on Life Sciences, 1996). All efforts were made to minimize animal suffering and to reduce the number of animals used.
2.2. Surgery
Rats were anesthetized (IP) using a mixture of ketamine hydrochloride (Vedco Inc., St. Joseph, MO) and xylazine (Lloyd Laboratories, Shenandoah, IA) at 66 and 1.33 mg/kg, followed by equithesin (4 mg/kg sodium pentobarbital, 17 mg/kg chloral hydrate, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol and 10% ethanol solution) at 0.5 ml/kg. Ketorolac (Sigma Aldrich) was administered (2 mg/kg, IP) as a preoperative analgesic. One end of a silastic catheter was implanted into the right jugular vein and the other end ran subcutaneously and exited from a small incision just below the scapula and was attached to an infusion harness (Instech Solomon, Plymouth Meeting, PA) that provided access to an external port for IV drug delivery. To help maintain patency, daily IV infusion of 0.1 ml antibiotic cefazolin (100 mg/ml) and 0.1 ml heparinized saline (70 U/ml) was given after surgery and maintained throughout the length of self-administration (given after each session). An additional 0.1 ml heparinized saline (10 U/ml) was infused immediately prior to each self-administration session. Catheter patency was periodically verified with 0.1 ml (10 mg/ml, IV) methohexital sodium (Eli Lilly & Co., Indianapolis, IN), a short-acting barbiturate that produces a rapid loss of muscle tone. Caps were placed onto the catheter ports when the rats were not connected to the infusion pumps.
2.3. Cocaine self-administration
Self-administration sessions were carried out in standard operant conditioning chambers housed in sound-attenuating cubicles and controlled by a computerized data collection program (MED-PC, Med-Associates, St Albans, VT). The chambers were equipped with two retractable levers, two stimulus lights (one above each lever), a tone generator, and a house light. Additionally, each chamber had a balanced metal arm and spring leash attached to a swivel (Instech Solomon, Plymouth Meeting, PA). Tygon tubing extended through the leash and was connected to a 10 ml syringe mounted on an infusion pump located outside the cubicle. Cocaine hydrochloride (National Institute on Drug Abuse, Research Triangle Park, NC) was dissolved in 0.9% sterile saline. Prior to the start of each session, the catheter was connected to the tubing and the house light signaled the initiation of the session and remained on throughout the entire session. Presses on the active (i.e., cocaine-paired) lever resulted in a 2-s activation of the infusion pump (0.2 mg/50 μl infusion) and a 5-s presentation of a stimulus light and tone (4.5 kHz, 78 dB) complex. After each infusion, a 20-s time-out period occurred, during which responses on the active lever were recorded, but resulted in no programmed consequences. Presses on the inactive lever had no consequences. Rats self-administered cocaine during daily 2-h sessions along an FR1 schedule of reinforcement. Self-administration sessions continued for a total period of 15 (experiment 1) or 10 (experiment 2) sessions, with the criterion of ≥10 infusions per session.
2.4. Extinction and reinstatement of cocaine seeking
Following self-administration and abstinence (experiment 1) or directly after self-administration (experiment 2) and between reinstatement tests, animals underwent daily 2-h extinction sessions, during which responses on either lever had no consequences. Extinction sessions continued for 7 sessions prior to the first reinstatement test, and 3 sessions between reinstatement tests. Reinstatement tests consisted of cue-induced reinstatement, whereby responses on the previously cocaine-paired lever resulted in a 5-s presentation of the light + tone cues, and cocaine-primed reinstatement, whereby animals received an injection of cocaine (10 mg/kg, IP) prior to the test and active lever presses had no programmed consequences.
2.5. Experiments
2.5.1. Experiment 1: Repeated SB-334867 (10 mg/kg) treatment during self-administration, abstinence, and extinction
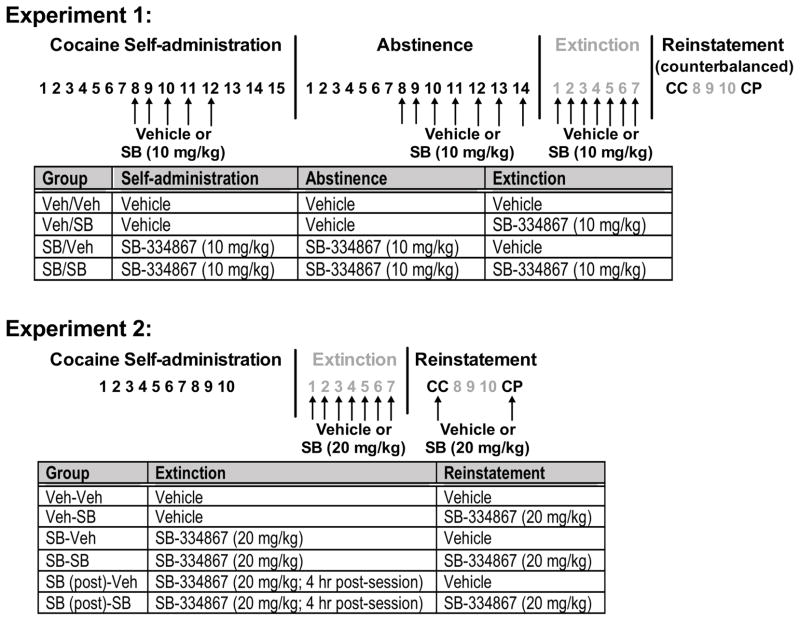
In this experiment, we first tested the effects of repeated SB-334867 treatment on established cocaine self-administration. SB-334867 (RTI International, Research Triangle Park, NC; generously provided by the National Institute on Drug Abuse) was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-beta-cyclodextrin (Sigma-Aldrich, St. Louis, MO) in double-distilled water and injected at the volume of 4 ml/kg (IP). We chose a repeated dose of 10 mg/kg/day, as this dose has minimal effects on basal locomotor function when given both acutely and repeatedly (Borgland et al., 2006; Zhou et al., 2012), but does inhibit some forms of motivated behavior (Borgland et al., 2009). A total of 29 rats initially self-administered cocaine and were then pretreated with daily vehicle or SB-334867 (10 mg/kg) 30 min prior to the 8th-12th self-administration sessions. Animals then underwent three additional self-administration sessions to test potential residual effects of repeated SB-334867 and maintain stable cocaine self-administration prior to 14 days of abstinence in their home cages. The length of abstinence was chosen based on a previous study (Smith et al., 2010) that showed increased sensitivity to SB-334867 treatment on first day of extinction after 2 weeks of abstinence. In the first 7 days of abstinence, no treatment was given. However, during the last 7 days, animals received the same daily administration of vehicle or SB-334867 as during self-administration. We utilized this SB-334867 injection paradigm during abstinence in order to produce sustained OX1R blockade prior to evaluation of the effects on subsequent extinction sessions. Rats underwent 7 extinction sessions, during which animals with the same treatment history during abstinence received either vehicle or SB-334867 prior to each extinction session. Thus, rats in the Veh/Veh or SB/SB groups received either vehicle or SB-334867 treatment in both abstinence and extinction phases, while rats in the Veh/SB group received vehicle treatment during abstinence, but SB-334867 during extinction, and vice versa for rats in the SB/Veh group. Finally, to test for any lasting effects of prior SB-334867 exposure, rats were tested for cocaine seeking induced by conditioned cues or a cocaine prime (10 mg/kg) without any pretreatment in a counterbalanced order, with 3 extinction sessions conducted between tests to re-establish baseline responding. A timeline and treatment groups for experiment 1 are shown in Fig. 1.
Fig. 1. Timeline for experiments 1 and 2.
Phases of cocaine self-administration, abstinence, extinction, and conditioned cue-induced (CC) and cocaine-primed (CP) reinstatement are shown. Arrows indicate administration of vehicle or SB-334867.
2.5.2. Experiment 2: Repeated SB-334867 (20 mg/kg) treatment during extinction
Based on the results from experiment 1, we further explored the impact of repeated SB-334867 treatment during extinction, and its influence on the response to subsequent acute SB-334867 at the time of reinstatement. Since a previous study found that SB-334867 at 20 mg/kg was the lowest effective dose to reduce cue-induced cocaine seeking (Smith et al., 2009), we utilized the 20 mg/kg dose in this experiment. A total of 44 rats first experienced 10 cocaine self-administration sessions with >10 infusions/session. They then underwent 7 daily extinction sessions, during which rats received repeated vehicle or SB-334867 (20 mg/kg) treatment 30 min prior to each extinction session. To determine whether the effect of repeated treatment is performance dependent, we administered the same SB-334867 treatment in another set of rats at 4 hr after each extinction session. Following extinction, rats were tested for reinstatement induced by conditioned cues or cocaine prime (10 mg/kg), with either acute vehicle or SB-334867 (20 mg/kg) pretreatment 30 min before each test. Each rat received the same acute treatment prior to the cue-induced and cocaine-primed reinstatement. Since we found no order effects for reinstatement tests in experiment 1, rats were tested with cue-induced, followed by cocaine-primed reinstatement. Between the two tests, rats underwent three additional extinction sessions with no treatment to re-establish baseline responding. A timeline and detailed treatment conditions for each group are shown in Fig. 1.
2.6. Data analysis
To evaluate the effect of repeated SB-334867 treatment on cocaine self-administration and extinction, mixed-factors, repeated-measures analysis of variance (ANOVA) was used to analyze active lever responses or cocaine intake with group and session as the between- and within-subjects factors, respectively. In addition, paired sample t-tests were used to compare the active lever pressing on the first extinction session and their average response for the last 3 self-administration sessions, as well as responding between the last extinction session and reinstatement tests. In experiment 1, one-way ANOVAs were used to compare active lever responses to cue-induced or cocaine-primed reinstatement in different groups with different repeated treatment history. In experiment 2, two-way ANOVAs were used to evaluate the effects of repeated SB-334867 (20 mg/kg) treatment history during extinction and acute SB-334867 (20 mg/kg) pretreatment on active lever responses during the two reinstatement conditions. Post hoc analyses were conducted using Student-Newman-Keuls tests. All data were presented as the mean ± S.E.M., and α was set at p<0.05. A box plot method was used to detect outliers. Data points that were greater than 3 interquartile range (IQR) from the upper quartile, or smaller than 3 IQR from the lower quartile were considered as significant outliers. If more than one outlier was detected in the same rat with repeated measurements, the entire data set was removed from analysis. Using this approach, one outlier from the vehicle group in experiment 1, and one from the SB (post)-SB group in experiment 2 were excluded from analysis.
3. Results
3.1. Experiment 1 - Repeated SB-334867 (10 mg/kg) treatment during self-administration, abstinence, and extinction
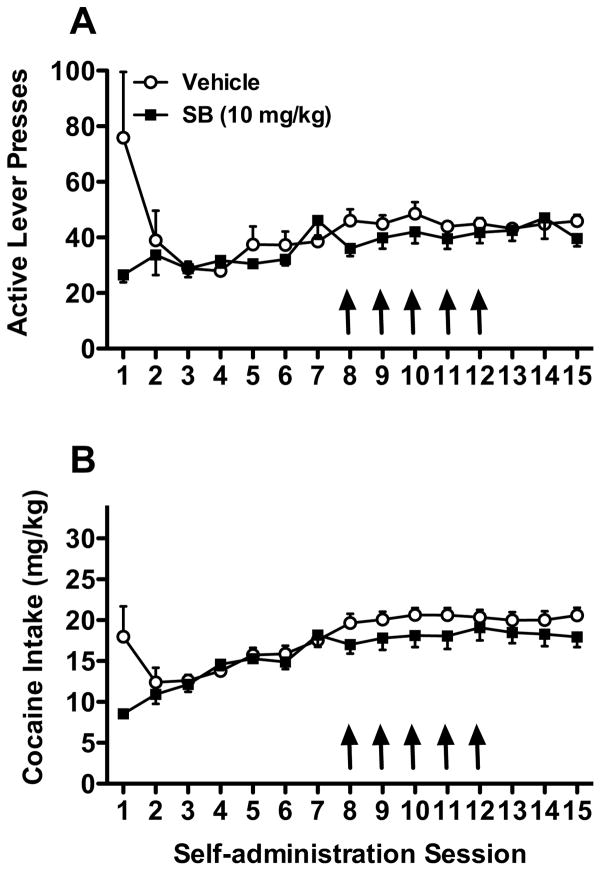
As shown in Fig. 2, rats in both groups showed robust and similar active lever responding and cocaine intake prior to drug treatment. Mixed-model ANOVAs for active lever pressing and cocaine intake across self-administration sessions 8–12 revealed no significant main effect of group or session, or group x session interaction (Fig. 2). These results indicated that repeated SB-334867 (10 mg/kg) treatment before daily sessions failed to affect the maintenance of stabilized cocaine self-administration. Also, mixed-model ANOVAs for active lever pressing and cocaine intake across sessions after the repeated treatment revealed that during sessions 13–15, cocaine self-administration did not differ between rats with previous repeated vehicle or SB-334867 treatment (p>0.05).
Fig. 2. Repeated OX1R blockade fails to affect cocaine seeking or cocaine intake during self-administration.
Rats received daily vehicle or SB-334867 (10 mg/kg) 30 min before the 8th–12th self-administration sessions. Active lever presses (A) and cocaine intake (B) are shown. (n = 14–15/group). Arrows indicate administration of vehicle or SB-334867.
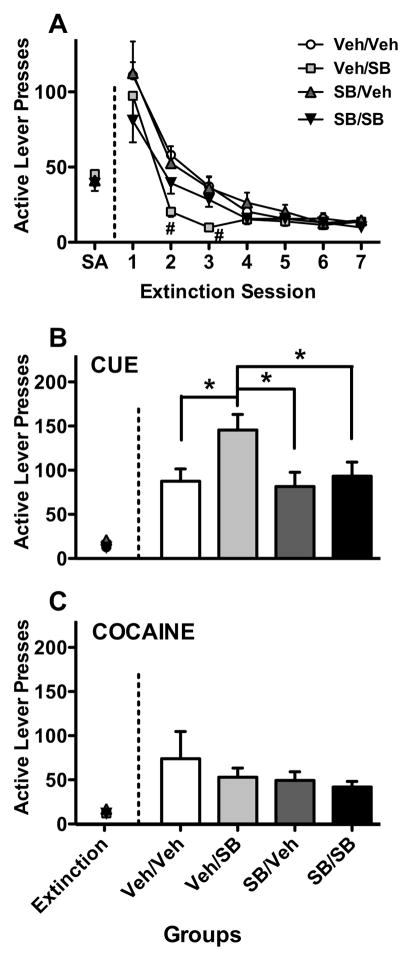
As shown in Fig. 3A, compared to their cocaine seeking during self-administration, all subjects showed higher responding on their first day returning to the self-administration chamber after the two week abstinence period (p<0.05 for all groups). A typical extinction curve developed in all groups across the 7 sessions of extinction. Mixed-model ANOVA for active lever pressing across extinction sessions revealed a significant main effect for session (F(6,150)=110.90, p<0.001), and group x session interaction (F(18, 150)=1.83, p<0.05). However, the main effect of group failed to reach statistical significance. Post-hoc analyses revealed that the Veh/SB group (i.e., animals that received SB for the first time during extinction) showed significantly reduced cocaine seeking as compared to Veh/Veh and SB/Veh groups on the second and third extinction sessions. However, this effect was not observed in the SB/SB group.
Fig. 3. Effects of repeated SB-334867 (10 mg/kg) on cocaine seeking during extinction and reinstatement.
Rats received daily vehicle or SB-334867 (10 mg/kg) on sessions 8–14 of abstinence and prior to the 1st–7th extinction sessions, while no treatment was given on the day of reinstatement tests. Average active lever responding is shown for: A) the average of the last 3 sessions of self-administration (SA) and over the 7 sessions of extinction, B) the last session of extinction and cue-induced reinstatement, and C) the last session of extinction and cocaine primed-reinstatement. #indicates significant differences between Veh/SB group and Veh/Veh, SB/Veh groups (p < 0.05); *indicates significant differences between groups (p < 0.05). (n = 7–8/group)
Both cue-induced and cocaine-primed reinstatement resulted in significant increases in cocaine seeking as compared to the last extinction sessions (p<0.05 for all comparisons; Fig. 3B and 3C). One-way ANOVA of active lever presses during cue-induced reinstatement indicated a significant main effect of group (F(3,28)=3.55, p<0.05), with post hoc analysis showing that the Veh/SB group had significantly higher cocaine seeking than the other three groups (Fig. 3B). In contrast, no between-group differences were seen for cocaine-induced reinstatement (Fig. 3C).
Inactive lever responding for each group across different stages is indicated in Table 1, with no significant differences seen between groups.
Table 1. Inactive lever responding during self-administration, extinction, and reinstatement.
The inactive lever presses for the average of the last 3 sessions of self-administration, sessions 1 and 7 of extinction, and cue- and cocaine-induced reinstatement are shown as the mean±SEM for each group.
| Group | Self-administration | Extinction | Reinstatement | ||
|---|---|---|---|---|---|
| Last 3 sessions | Session 1 | Session 7 | Cue | Cocaine | |
| Veh/Veh | 0.2 ± 0.1 | 27.0 ± 3.4 | 8.3 ± 1.9 | 9.7 ± 2.7 | 6.8 ± 1.1 |
| Veh/SB | 18.8 ± 5.5 | 9.5 ± 4.0 | 20.0 ± 5.6 | 5.0 ± 1.1 | |
| SB/Veh | 0.9 ± 0.4 | 17.1 ± 5.4 | 6.3 ± 1.5 | 7.7 ± 1.1 | 3.1 ± 1.1 |
| SB/SB | 19.1 ± 2.7 | 7.6 ± 2.3 | 10.0 ± 2.7 | 4.0 ± 0.8 | |
| Veh-Veh | 0.1 ± 0.02 | 21.9 ± 1.8 | 6.1 ± 1.0 | 6.1 ± 1.1 | 6.3 ± 2.0 |
| Veh-SB | 7.8 ± 1.6 | 3.9 ± 1.9 | |||
| SB-Veh | 12.6 ± 3.5 | 3.8 ± 0.8 | 6.4 ± 2.5 | 6.4 ± 3.7 | |
| SB-SB | 4.1 ± 1.1 | 2.8 ± 1.2 | |||
| SB (post)-Veh | 19.6 ± 4.5 | 6.6 ± 1.7 | 6.9 ± 2.7 | 5.6 ± 2.0 | |
| SB (post)-SB | 2.1 ± 1.4 | 3.7 ± 1.7 | |||
3.2. Experiment 2 - Repeated SB (20 mg/kg) treatment during extinction
All rats showed robust active lever responding and cocaine intake across the 10 sessions of cocaine self-administration (data not shown). As shown in Fig. 4A, on the first extinction test day, only vehicle (t14=4.40, p<0.01) or post-session SB-334867 (t13=3.77, p<0.01) groups showed significantly higher responding when compared to responding during self-administration. Mixed-model ANOVA for active lever pressing across extinction sessions revealed a significant main effect for group (F(2,41)=5.48, p<0.01), and post hoc analyses indicated that repeated SB-334867 treatment before extinction trials significantly lowered cocaine seeking during extinction as compared to repeated vehicle pre-treatment and post-session SB-334867 administration (4 hr after each extinction trial). However, SB-334867 treatment after extinction did not affect cocaine seeking. There was also a significant effect for session (F(6,246)=83.89, p<0.001), as seen by a steady decrease in cocaine seeking across extinction sessions. However, no group x session interaction was observed.
Fig. 4. Effects of repeated SB-334867 (20 mg/kg) treatment on cocaine seeking during extinction and reinstatement.
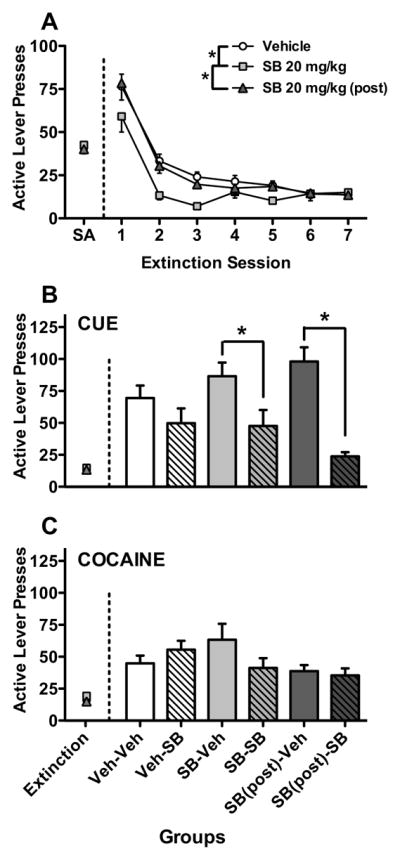
Rats received daily vehicle or SB-334867 (20 mg/kg) 30 min prior to or 4 hr after the 1st–7th extinction sessions. Rats received acute vehicle or SB-334867 (20 mg/kg) 30 min prior to the reinstatement tests. Average active lever responding is shown for: A) the average of the last 3 sessions of self-administration (SA) and over the 7 sessions of extinction, B) the last session of extinction and cue-induced reinstatement, and C) the last session of extinction and cocaine primed-reinstatement. *indicates significant differences between groups (p < 0.05). (n = 14–15/group in panel A; n = 7–8/group in panels B and C).
Rats given acute vehicle treatment showed significant reinstatement to cocaine seeking induced by either conditioned cues or cocaine priming (p<0.05 for all comparisons, Fig 4B and 4C). For cue-induced reinstatement (Fig. 4B), two-way ANOVA on acute treatment and previous repeated treatment history indicated a significant main effect of acute treatment (F(1,37)=25.05, p<0.001), but the interaction between acute treatment and repeated treatment history on active lever responding just failed to reach statistical significance (F(2,37)=3.14, p=0.055). Post-hoc analyses showed that acute SB-334867 prior to cue-induced reinstatement reduced cocaine seeking in rats with repeated SB-334867 treatment history, regardless of whether it was administered before or after extinction trials. Acute SB-334867 failed to significantly reduce cocaine seeking in rats that received SB-334867 for the first time. No differences were observed between any groups for cocaine-primed reinstatement (Fig. 4C).
Inactive lever responding in each group across different stages is indicated in Table 1, with no significant differences seen between groups.
4. Discussion
Several key findings emerged from the current study regarding the effects of repeated OX1R blockade on cocaine seeking. In agreement with previous data using acute SB-334867 administration (Smith et al., 2009; Zhou et al., 2011), repeated OX1R antagonism did not affect cocaine seeking or intake during the maintenance of self-administration, but reduced cocaine seeking during extinction. Interestingly, SB-334867 treatment only attenuated responding during extinction in rats that had no prior SB-334867 history. Moreover, repeated OX1R blockade in this group during extinction subsequently resulted in an enhanced behavioral response during cue-induced reinstatement. Data from experiment 2 further demonstrates that repeated SB-334867 during extinction increased the response to subsequent SB-334867 at the time of cue-induced reinstatement, regardless of whether the daily SB-334867 treatment occurred before or after active extinction responding. Finally, in contrast to environmentally driven cocaine seeking, data from both experiments shows that repeated SB-334867 failed to affect cocaine-primed reinstatement.
In experiment 1, daily SB-334867 treatment at the dose of 10 mg/kg failed to affect the maintenance of cocaine self-administration, consistent with a recent finding that repeated SB-334867 treatment (10 mg/kg daily) had no effect on the acquisition of cocaine self-administration (Hutcheson et al., 2011). Together with the ineffectiveness of acute OX1R manipulation on cocaine self-administration (Boutrel et al., 2005; Espana et al., 2011; Smith et al., 2009; Zhou et al., 2012), this evidence suggests that the reinforcing properties of cocaine are not under the influence of orexin system. However, it should be noted that unlike the FR1 schedule of reinforcement used in the above studies, the same low dose of SB-334867 (10 mg/kg) significantly reduced the breakpoint of cocaine self-administration on a PR schedule, indicating a reduction in motivated drug-seeking (Borgland et al., 2009; Espana et al., 2010). Different SB-334867 effects on cocaine self-administration under an FR and PR schedule of reinforcement indicate that the involvement of OX1R-mediated signaling for motivated drug seeking may vary under different task demands.
Consistent with previous studies, the current results from experiment 1 demonstrate that SB-334867 (10 mg/kg) pretreatment across extinction sessions significantly reduced cocaine seeking as compared to vehicle treatment. In a previous study (Smith et al., 2010), the increased responding on the first day of extinction was attenuated by acute SB-334867 administration, and the sensitivity to SB-334867 treatment was increased after 14 days of abstinence, indicating that the orexin system may be involved in the incubation of drug seeking during abstinence. Thus, disrupting this incubation process by repeated blockade of OX1R during abstinence may have decreased the effect of OX1R antagonism on reducing cocaine seeking in extinction, which is supported by the finding that decreased cocaine seeking was only observed in rats that received SB-334867 treatment in extinction for the first time (Veh/SB group), but not in rats with prior repeated SB-334867 treatment during the late phase of abstinence (SB/SB group).
In the current study, repeated SB-334867 treatment during extinction subsequently resulted in a tendency for higher cue-induced cocaine seeking when no treatment was administrated before the test. This effect is unlikely to be the result of a simple rebound in responding, as cue-induced and cocaine-primed reinstatement tests were counterbalanced, and the effect was only observed during cue-induced reinstatement. Furthermore, cocaine seeking during extinction between the two tests was similar between all groups (data not shown). Several plausible mechanisms may underlie this increased cocaine seeking behavior. First, repeated SB-334867 may have resulted in increased surface expression of OX1Rs. It has been shown that OX1R antagonists (including SB-334867) dose-dependently inhibited OXA-induced receptor internalization (Ward et al., 2011). Potentially elevated OX1R levels or changes in OX1R mediated signaling after long-term SB-334867 treatment may have produced an enhanced response to orexin stimulation induced by cues (Dayas et al., 2008; Mieda et al., 2004). In addition, repeated OX1R antagonism may have exerted a cascade of effects on related neurotransmitter systems. It has been shown that cannabinoid receptor 1 (CB1R) can form heterodimers with OX1R, and hypersensitize OX1R-mediated signaling (Hilairet et al., 2003). Thus, potentially increased CB1R membrane expression after repeated OX1R antagonism (Ward et al., 2011) could further strengthen orexin mediated signaling.
Consistent with our results from experiment 1, daily SB-334867 treatment at 20 mg/kg/day prior to extinction sessions (SB group) significantly reduced cocaine seeking in experiment 2. However, the same dose of SB-334867 administrated 4 hr after each extinction trial (SB (post) group) had no effect on cocaine seeking. This result is congruent with the fact that attenuation of cocaine seeking during extinction was only seen on the first extinction session when acute SB-334867 was given, but not during the following sessions (Smith et al., 2010; Zhou et al., 2012). As a previous study has shown that the half-life of SB-334867 is about 4 hr (Ishii et al., 2005), it is unlikely that sufficient SB-334867 levels would remain 18 hr later during the subsequent extinction session. However, repeated SB-334867 during extinction increased the inhibitory response to acute SB-334867 on cue-induced reinstatement, regardless of whether the daily SB-334867 treatment occurred before (SB-SB group) or after (SB(post)-SB group) each extinction trial. The augmented response to acute SB-334867 may well be related to the aforementioned potential increase in OX1R expression and/or signaling after repeated OX1R inhibition. Another possibility might be a change in the pharmacokinetics of SB-334867 (e.g. increased absorption and/or brain distribution) caused by repeated exposure, which could alter the amount of bioavailable SB-334867 after acute treatment. The finding that acute 20 mg/kg SB-334867 treatment failed to significantly attenuate cue-induced cocaine seeking in rats with prior repeated vehicle treatment is not surprising, as both significant attenuation (Smith et al., 2009) or no effect (Zhou et al., 2012) has been previously reported at this dose. On the other hand, unlike the significant increase in Veh/SB group in experiment 1, repeated OX1R antagonism during extinction in experiment 2 tended to increase responding to conditioned cues after acute vehicle treatment (SB-Veh and SB(post)-Veh groups) as compared to repeated vehicle treatment (Veh-Veh group), but failed to reach statistical significance. These differences might be due to the different experimental designs (i.e. with/without abstinence, with/without acute treatment, or the dosage of SB-334867).
The lack of effect of repeated OX1R inhibition on cocaine-primed reinstatement is consistent with previous findings that acute SB-334867 failed to affect cocaine-primed reinstatement (Zhou et al., 2012; Mahler et al., in press). The selective involvement of the orexin system in cue-induced, but not cocaine-primed, reinstatement further emphasizes that distinct neuronal circuitries are involved in these two forms of reinstatement. Recent studies showed that intra-VTA SB-334867 reduced reinstatement of cocaine seeking elicited by cues, but not by a cocaine prime (James et al., 2011; Mahler et al., in press), suggesting that orexin signaling plays a role in VTA regulation of cue-induced but not cocaine-primed reinstatement. Future studies with site-selective OX1R manipulations in other brain regions critical for cue-induced reinstatement (e.g., amygdala) will help to clarify their roles in orexin-mediated reinstatement to cocaine seeking (Meil and See, 1997).
In summary, repeated OX1R antagonism has some similar effects as acute OX1R antagonism in that it attenuates extinction responding and cue-induced reinstatement to cocaine seeking. Furthermore, both acute and repeated SB-334867 treatment fails to alter responding for cocaine self-administration on an FR schedule of reinforcement or cocaine seeking during cocaine-primed reinstatement. However, three notable effects of repeated SB-334867 stand out in the current study. One is the potential rebound in the reinstatement of cocaine seeking that can occur after repeated SB-334867 treatment when the drug is no longer biologically available. Second is the enhanced response during re-exposure to acute SB-334867 after repeated dosing during extinction. Finally, this enhanced response to acute SB-334867 following repeated dosing appears to be independent of its pairing with the behavioral extinction task. These repeated SB-334867-induced effects may reflect pharmacodynamic changes to OX1R signaling, as well as potential pharmacokinetic changes to SB-334867 disposition. As repeated drug administration is a more relevant paradigm to the clinical treatment of addiction, this study demonstrates the importance of assessing repeated dosing regimens in testing targets for anti-relapse therapeutic interventions. However, for several reasons, the potential of OX1R-based treatments for addiction requires further investigation. First, the potential rebound in reinstatement when the drug is no longer administered might require careful treatment cessation parameters. Second, compared to human application, the drug treatment length in the current study was relatively short; thus, the effects of prolonged chronic OX1R antagonism remain to be determined. Third, we recently reported sex differences in the effects of OX1R inhibition on cue-induced cocaine seeking (Zhou et al., 2012), so gender-specific treatment approaches need to be considered. Finally, development of more selective and efficacious OX1R antagonists will improve targeting and reduce potential side effects.
Highlights.
Cocaine seeking during extinction is attenuated by repeated SB-334867.
Cue-induced reinstatement is altered by repeated SB-334867 during extinction.
SB-334867 fails to alter cocaine self-administration or cocaine-primed reinstatement.
Acknowledgments
This research was supported by NIH grants DA015369, DA016511, DA06214 and C06 RR015455. We thank Dr. Wei-Lun Sun for his comments on this manuscript and Dr. Nathaniel Baker for statistical consultation. We also thank Andrew Novak, Eleni Bucuvalas, Amanda King, and John Yang for technical assistance.
Abbreviations
- OXA
orexin-A
- OXB
orexin-B
- OX1R
orexin-1 receptor
- OX2R
orexin-2 receptor
- FR
fixed ratio
- PR
progressive ratio
- CB1R
cannabinoid receptor 1
Footnotes
Disclosure: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JR. A selective orexin-1 receptor antagonist reduces food consumption in male and female rats. Regul Pept. 2000;96:45–51. doi: 10.1016/s0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- Hilairet S, Bouaboula M, Carriere D, Le Fur G, Casellas P. Hypersensitization of the Orexin 1 receptor by the CB1 receptor: evidence for cross-talk blocked by the specific CB1 antagonist, SR141716. J Biol Chem. 2003;278:23731–23737. doi: 10.1074/jbc.M212369200. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci. 2008;105:19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, Heidbreder C. Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol. 2011;22:173–181. doi: 10.1097/FBP.0b013e328343d761. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, Johns A, Jeffrey P, Summerfield S, Rodgers RJ. Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res. 2005;157:331–341. doi: 10.1016/j.bbr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, Dayas CV. Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:684–690. doi: 10.1017/S1461145711000423. [DOI] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res. 2011;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA. Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 2010;209:203–212. doi: 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler S, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) doi: 10.1007/s00213-012-2681-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Golden SA, Shaham Y. Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol. 2008;154:406–416. doi: 10.1038/bjp.2008.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 2010;30:2300–2310. doi: 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T. Orexin: a link between energy homeostasis and adaptive behaviour. Curr Opin Clin Nutr Metab Care. 2003;6:353–360. doi: 10.1097/01.mco.0000078995.96795.91. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–266. doi: 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart D, Jerman JC, Brough SJ, Neville WA, Jewitt F, Porter RA. The hypocretins are weak agonists at recombinant human orexin-1 and orexin-2 receptors. Br J Pharmacol. 2000;129:1289–1291. doi: 10.1038/sj.bjp.0703257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. Orexin/hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci. 2012;35:798–804. doi: 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- Wang B, You ZB, Wise RA. Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry. 2009;65:857–862. doi: 10.1016/j.biopsych.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RJ, Pediani JD, Milligan G. Heteromultimerization of cannabinoid CB(1) receptor and orexin OX(1) receptor generates a unique complex in which both protomers are regulated by orexin A. J Biol Chem. 2011;286:37414–37428. doi: 10.1074/jbc.M111.287649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ghee SM, Chan C, Lin L, Cameron MD, Kenny PJ, See RE. Orexin-1 receptor mediation of cocaine seeking in male and female rats. J Pharmacol Exp Ther. 2012;340:801–809. doi: 10.1124/jpet.111.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]