Abstract
Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate were discovered >2 decades ago. That they are second messengers for mobilizing Ca2+ stores has since been firmly established. Separate stores and distinct Ca2+ channels are targeted, with cyclic ADP-ribose acting on the ryanodine receptors in the endoplasmic reticulum, whereas nicotinic acid adenine dinucleotide phosphate mobilizes the endolysosomes via the two-pore channels. Despite the structural and functional differences, both messengers are synthesized by a ubiquitous enzyme, CD38, whose crystal structure and catalytic mechanism have now been well elucidated. How this novel signaling enzyme is regulated remains largely unknown and is the focus of this minireview.
Keywords: ADP-ribosyl Cyclase; Calcium; Calcium Intracellular Release; Calcium Signaling; CD38; Cyclic ADP-ribose; Enzyme Catalysis; Enzyme Structure; Inositol 1,4,5-Trisphosphate; NAADP
Introduction
A wide range of physiological functions are signaled and regulated by mobilization of intracellular Ca2+ stores. The generally accepted view is that cells possess multiple types of stores that can be mobilized by specific messenger molecules. The first such molecule identified was inositol trisphosphate (IP3),2 and it is a messenger for the Ca2+ stores in the endoplasmic reticulum (ER) (1). This was soon followed by the discovery of two unrelated nucleotides with Ca2+-mobilizing activity, cyclic ADP-ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP) (2–4). NAADP is derived from NADP, whereas cADPR is a metabolite of NAD. That these well known coenzymes of cellular redox reactions are linked to Ca2+ mobilization as well is unexpected. Results accumulated in the past 2 decades establish that these two nucleotide messengers regulate diverse cell functions ranging from abscisic acid signaling in plants and sponges (5, 6) and cell fission in dinoflagellates (7) to social behavior in mice (Ref. 8; reviewed in Refs. 9–12).
Structure and Function of cADPR
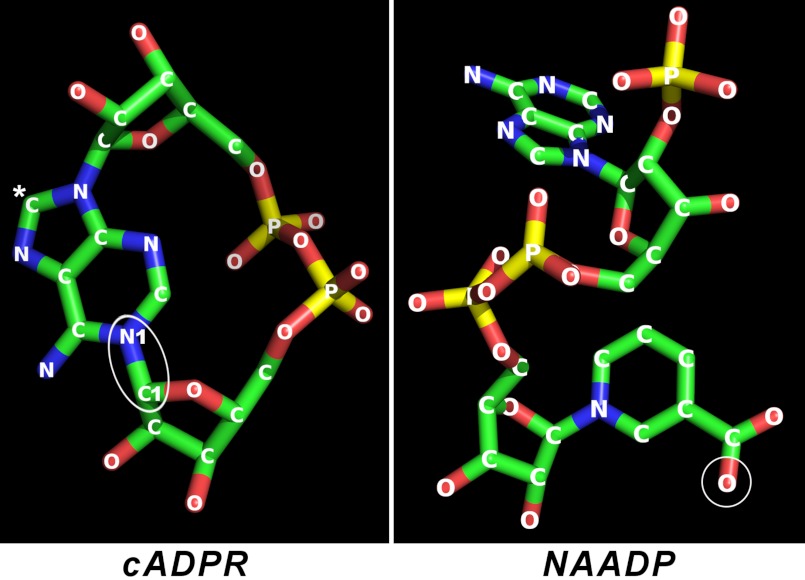
NAD is a linear molecule, and many of the NAD-utilizing enzymes, including redox enzymes, such as lactate dehydrogenase, bind it to their active sites in its linear form as well (13). It is thus remarkable that cADPR, a metabolite of NAD, is a cyclic molecule with the adenine ring linked back to the terminal ribose, forming a complete circle (Fig. 1) (3, 4), foretelling the uniqueness of its catalytic synthesis by CD38.
FIGURE 1.
Crystal structures of cADPR and NAADP. The structure of cADPR was obtained from crystals of its free acid, and that of NAADP was from the crystals of the complex of NAADP and CD38. The cyclization site in cADPR is indicated by an ellipse. The C8 of cADPR is indicated by a white asterisk. Attachment of a bromo (8-bromo-cADPR) or an amino (8-NH2-cADPR) group at this position converts the compound to a specific antagonist of cADPR. The structure of NAADP is identical to that of its parent NADP, except that the amide nitrogen of the nicotinamide group of NADP is changed to oxygen as indicated by the circle. Blue, nitrogen; red, oxygen; yellow, phosphorus; green, carbon.
The Ca2+-mobilizing activity of cADPR was first demonstrated in sea urchin eggs and shown to be independent of the IP3 mechanism (14, 15) but acting instead via the potentiation of the Ca2+-induced Ca2+ release process (16, 17). Pharmacological and single-channel reconstitution studies subsequently confirmed that the target of cADPR is the ryanodine receptor (reviewed in Refs. 11 and 18). In effect, cADPR sensitizes the receptor to Ca2+ in a manner similar to caffeine but with much higher potency (17). The same Ca2+ stores in the ER can thus be mobilized by two distinct messengers, IP3 and cADPR. There is, however, one major difference: the action of cADPR on the ryanodine receptor requires additional protein factors, such as calmodulin (19–21) and FK506-binding protein. Indeed, there are results suggesting that the actual receptor for cADPR is FKBP12.6 (22–25).
There is growing evidence that in addition to mobilizing intracellular Ca2+ stores, cADPR can also activate a Ca2+ influx channel, TRPM2, at the cell surface. The activating effect of cADPR on TRPM2 is synergistic with ADP-ribose, the hydrolysis product of cADPR (26). The action of cADPR on the channel is temperature-dependent, may function as a temperature-sensing mechanism in cells, and may be involved in regulating insulin secretion (27).
Structure and Function of NAADP
That cADPR is a metabolite of NAD prompted the testing of NADP as well and led to the first detection of the Ca2+-mobilizing activity of NAADP as a contaminant in commercial NADP (2). Structure determination shows that it is a derivative of NADP with its nicotinamide group replaced with a nicotinic acid group (Fig. 1). Intuition would suggest that deamidation of NADP should produce NAADP. This is not the case. Instead, as described later, the same enzyme (CD38) that cyclizes NAD to produce cADPR surprisingly is also responsible for forming NAADP (28).
It was apparent early on that NAADP activates a Ca2+ release mechanism pharmacologically distinct from the ryanodine and IP3 receptors (2). Its action also exhibits a remarkable self-sensitization behavior (29, 30). The Ca2+ stores that NAADP mobilizes are separable from those activated by cADPR and IP3. This was first shown by fractionation of sea urchin egg homogenates (31) and by Ca2+ imaging of live eggs with their organelles stratified by centrifugation (32). The targeted organelles were later identified as the acidic reserve granules in the eggs that are related to lysosomes (33). That NAADP mobilizes the endolysosomes has since been confirmed in a variety of cell systems (reviewed in Refs. 34 and 35), establishing the concept that selective organellar Ca2+ stores are regulated by specific messengers.
The actual Ca2+ release channels targeted by NAADP have recently been identified as the lysosomal two-pore channels (TPCs) (36, 37). Although these channels have been documented for some time based mainly on sequence analyses, their functions were largely unknown until now. Three isoforms have been reported, and both types 1 and 2 are responsive to NAADP (36, 37). Thus, cellular response to NAADP can be enhanced by overexpression of TPC and reduced by channel knockdown. In smooth muscle cells, ablation of TPCs depressed both the NAADP- and carbachol-induced contraction, linking the physiological function of TPCs and NAADP (38). Similar results have been reported for differentiation of skeletal muscle cells (39), autophagy in astrocytes (40), and histamine receptor-mediated secretion of von Willebrand factor in endothelial cells (41).
Similar to cADPR, there is evidence that NAADP may also activate Ca2+ influx. In starfish oocytes, intracellular release of NAADP by photolysis of caged NAADP was observed to activate an inwardly rectifying Ca2+ current that requires an intact F-actin cytoskeleton (42).
Separate but Interacting Ca2+ Stores
Although physically separable, the Ca2+ stores in the ER and the endolysosomes can still interact in at least two ways. First, the Ca2+ released from the endolysosomal stores can be sequestered by the ER stores, priming the latter for enhanced release. This mechanism is proposed to account for the NAADP-induced Ca2+ oscillation observed in sea urchin eggs (12, 43) and the excitation-contraction coupling in atrial myocytes (44).
Alternatively, the Ca2+ release from the endolysosomal stores can activate further release from the ER stores via the Ca2+-induced Ca2+ release mechanism. In this manner, NAADP acts as a trigger, whose Ca2+ signal is then amplified through the Ca2+ release regulated by cADPR and IP3. This mechanism was first proposed to account for the blockage of the cholecystokinin (CCK)-activated and NAADP-dependent Ca2+ release in pancreatic acinar cells by antagonists of the cADPR and IP3 pathways (45). Measurements indeed show that the cellular NAADP level elevates immediately after CCK addition, preceding those of cADPR and IP3 (46). Similar conclusions have been obtained in a variety of cells, from sea urchin eggs (47) to human Jurkat cells (Ref. 48; reviewed in Ref. 10).
Despite having three structurally and functionally distinct Ca2+ messengers operating in cells, remarkable specificity is observed in pancreatic acinar cells. Both acetylcholine (ACh) and CCK can trigger Ca2+ oscillations in these cells, but only CCK can elevate NAADP. On the other hand, ACh activates the production of cADPR but not NAADP. CCK can also elevate cADPR but with a much slower time course than NAADP, consistent with NAADP being the triggering signal (46, 49). Similarly, in human sperm, progesterone stimulates elevation only of endogenous cADPR but not NAADP (50).
Dissecting the interaction between the multiple Ca2+ stores and messengers has been greatly aided by the development of specific inhibitors. The derivatives of cADPR modified at the 8-position of the adenine, 8-amino-cADPR and 8-bromo-cADPR (Fig. 1), were the first specific antagonists of cADPR developed (51). 8-Bromo-cADPR is commercially available and is the most widely used diagnostic antagonist for cADPR. Modulators of the ryanodine receptor, ryanodine and caffeine, can also be used to assess the cADPR mechanism. A specific antagonist for NAADP, NED-19, has been developed based on virtual screening of chemical libraries for compounds with structural similarity to NAADP (52). NED-19 specifically blocks both NAADP-dependent Ca2+ signaling and NAADP binding to the receptor. As NAADP mobilizes the endolysosomal Ca2+ stores, inhibitors that target their Ca2+ transport, such as bafilomycin, nigericin, etc., have also been used to suppress selectively the NAADP-dependent Ca2+ signaling (49). Likewise, thapsigargin, an inhibitor of the Ca2+-ATPase in the ER, can be used to selectively block the cADPR or IP3 signaling.
Stimulus-induced Elevation of cADPR and NAADP
That cADPR and NAADP can activate Ca2+ signaling in cells and affect biological functions fulfills only partially the criteria for second messengers. Their endogenous levels need to be shown to change in a stimulus-dependent manner. The first measurement of the endogenous levels of cADPR was done in various rat tissues using a bioassay based on Ca2+ release in sea urchin egg homogenates (53). A more convenient and sensitive fluorometric assay based on signal amplification via the coupling of specific enzymes was later developed and is widely used (54). Stimulus-induced changes in cADPR levels have since been documented in a variety of cells, including plants responding to abscisic acid (5), a dinoflagellate during the cell cycle (7), sea urchin eggs responding to fertilization (55), and human sperm responding to progesterone (50).
That NAADP is endogenously present in cells was first shown in sea urchin sperm using a radioreceptor assay based on the egg homogenates (56). A fluorometric assay similar to that for cADPR has also been developed (48, 57). Diverse agonists are known to elevate cellular NAADP levels, including CCK (46), glucagon-like peptide-1 (58), thrombin (59), and insulin (60). These results establish that cADPR and NAADP are both second messengers operating in a wide range of cells regulating an equally wide range of cell functions.
Structures of CD38 and ADP-ribosyl Cyclase
As a newly discovered molecule, the enzymatic synthesis of cADPR was completely unknown. Remarkably, it was its enzymatic conversion from NAD, unmasking its Ca2+-mobilizing activity, that had led to its discovery (4, 14). However, the first purified enzyme shown to synthesize cADPR was not from sea urchin but from Aplysia, another marine animal. The Aplysia ADP-ribosyl cyclase (referred to hereafter as the cyclase) is a 29-kDa protein with a turnover rate of 580 s−1 for cADPR production (61), making it one of the most efficient enzymes.
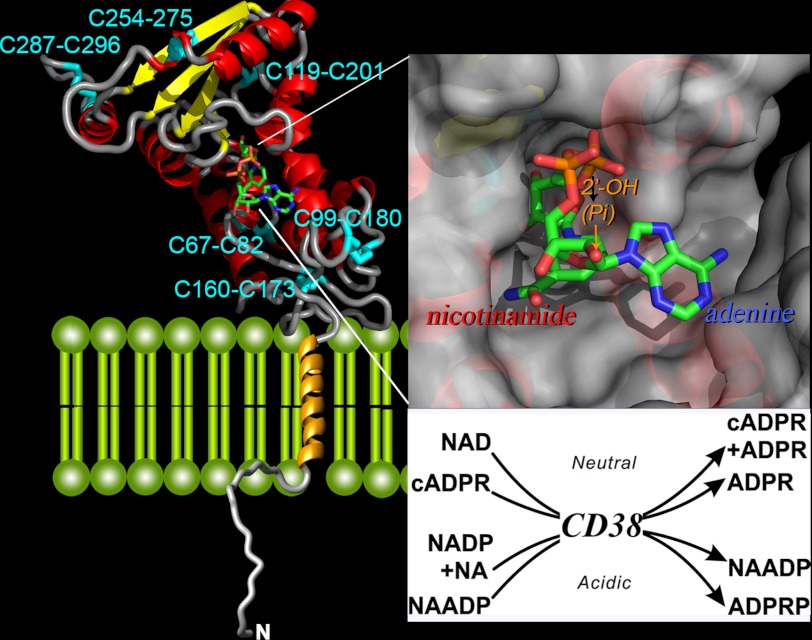
CD38, the mammalian homolog of the cyclase, was identified by sequence comparison (62) and shown to possess cADPR-synthesizing activity as well (63–65). Unlike the soluble cyclase, CD38 has a single-transmembrane segment, a short N-terminal tail, and a C-terminal domain that possesses all of the enzymatic activities (Fig. 2) (28, 66). The sequence identity between the two proteins is only ∼23%, but the crystal structure of the C-terminal domain of CD38 (67) is virtually identical to that of the cyclase (68). Also, the 10 cysteine residues in the cyclase are perfectly aligned with those in CD38, and all of them paired as disulfide linkages. There are five disulfides in the cyclase and six in CD38 (Fig. 2).
FIGURE 2.
Crystal structure of CD38. The secondary structure of the C-terminal domain of CD38 is shown, with helices in red, β-sheets in yellow, and coils in gray. The disulfides are labeled and colored cyan. The transmembrane segment is modeled as a gold helix, whereas the N-terminal tail as a random coil. A molecule of NAD is bound at the active site near the middle of the C-terminal domain and is rendered in stick configuration. Red, oxygen; orange, phosphorus; green, carbon; blue, nitrogen. The upper inset shows the active site in surface view. The nicotinamide end of the bound NAD enters the site first, whereas the adenine end is positioned toward the opening of the site. The lower inset lists the multiple reactions catalyzed by CD38. The synthesis and hydrolysis of cADPR occur at neutral pH, whereas those of NAADP occur at acidic pH. NA, nicotinic acid, ADPRP, ADP-ribose phosphate.
The active sites are located in essentially identical pockets near the middle of both proteins (Fig. 2), with a highly conserved segment forming the bottom of both pockets (69, 70). The conserved catalytic residues Glu-226 in CD38 and Glu-179 in the cyclase are located near the bottom of the active sites (69, 70).
Catalytic Mechanisms of CD38 and the Cyclase
Despite the structural similarity, there is one major catalytic difference between CD38 and the cyclase. Using NAD as substrate, the cyclase produces essentially all the product as cADPR, whereas CD38 produces mainly ADP-ribose, with only a small amount of cADPR (63–65). As noted above, ADP-ribose, as an activator of the TRPM2 channels, is a Ca2+-signaling messenger in its own right. The molecular determinants that control the NAD-cyclizing and NAD-hydrolyzing activities have been shown to be Glu-146 and Thr-221 in CD38 and Phe-174 in the cyclase (71). Mutating Glu-146 to Ala and Thr-221 to Phe converts CD38 to a cyclase-like enzyme producing predominantly cADPR from NAD, whereas changing Phe-174 to Thr correspondingly converts the cyclase to a CD38-like enzyme.
NAADP and cADPR are structurally and functionally distinct, and their parent compounds are also different. It is thus remarkable that NAADP is produced by the same enzymes. Both CD38 and the cyclase catalyze a base-exchange reaction that replaces the nicotinamide group of NADP with nicotinic acid (28). This reaction is unusual in that it requires acidic pH, which, in fact, was the first clue suggesting that NAADP could be targeting the acidic organelles (72). This pH dependence is dictated by the acidic residues Glu-146 and Asp-155 at the active site of CD38, whose negative charges at neutrality repel the approach of the negatively charged substrate nicotinic acid (73).
The multifunctionality of CD38 and the cyclase goes beyond synthesizing cADPR and NAADP. Indeed, CD38 can also hydrolyze NAADP to ADP-ribose phosphate (73), whereas both enzymes can hydrolyze cADPR to ADP-ribose as well (Fig. 2, inset) (63, 74). It thus appears that CD38 is specifically equipped to metabolize these two novel Ca2+ messengers.
Using crystallography in combination with site-directed mutagenesis, the mechanism of this novel multicatalytic property of CD38 and the cyclase has been well elucidated (74–78). It has been shown that NAD enters the active site pocket with the nicotinamide end first (74, 76). The adenine end is extended out of the site and has high degree of freedom to move and rotate. The 2′-OH of the adenylyl ribose of NAD points toward the opening of the active site pocket (Fig. 2, inset), and thus, its phosphorylation does not interfere with the substrate binding, accounting for why both NAD and NADP can serve as substrates. The catalytic residues Glu-226 in CD38 and Glu-179 in the cyclase form hydrogen bonds with the –OH groups of the terminal ribose, destabilizing the glycosidic bond and leading to its breakage and the release of the nicotinamide. An intermediate is formed. Both noncovalent and covalent types have been observed, depending on the substrate used (77).
The final catalytic product formed depends, however, on which nucleophile is available. With nicotinic acid attacking the anomeric carbon of the intermediate, NAADP is produced. On the other hand, cyclization occurs by intramolecular attack by the N1 of the adenine and produces cADPR instead. If the access of water to the site is predominating, hydrolysis results, and ADP-ribose is formed.
The active site also binds cADPR readily, forming hydrogen bonds between the catalytic residues and the –OH groups of the terminal ribose, in a manner similar to that observed for NAD (76). This can lead to similar hydrolysis of the cycling bond between the anomeric carbon of the terminal ribose and the N1 of the adenine ring (Fig. 1), producing ADP-ribose.
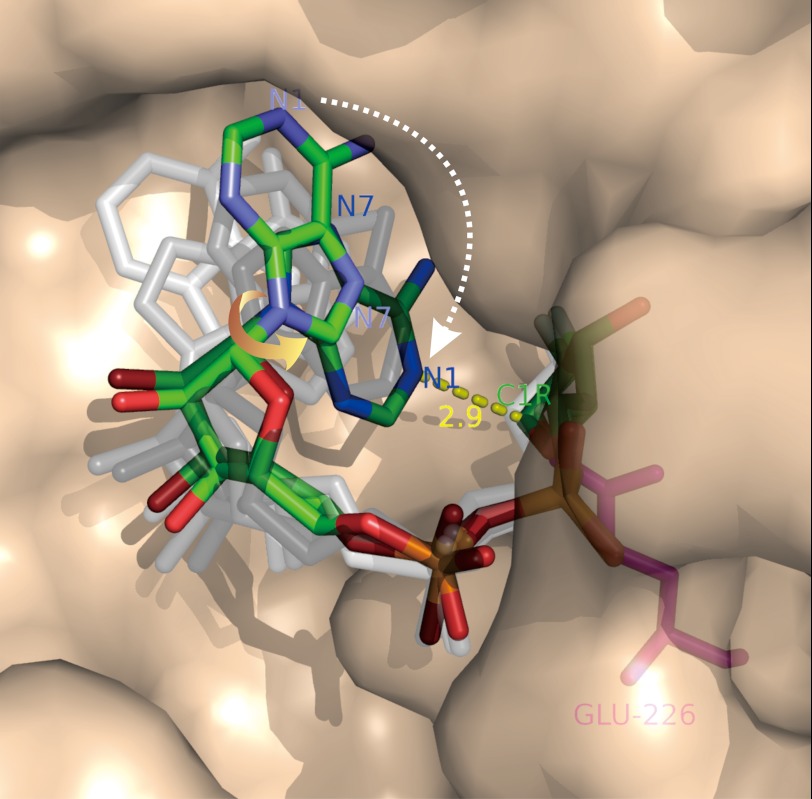
The most novel of these catalytic reactions, the cyclization of NAD, has recently been visualized (79, 80). When a close analog of NAD, 2′-deoxy-2′-fluoroarabinoside NAD, is used as substrate, it is hydrolyzed like NAD to release the nicotinamide group, but its anomeric carbon of the terminal ribose then forms a covalent linkage with the catalytic residue Glu-226 (80). One end of the linear intermediate is thus anchored to the active site, whereas the adenylyl end is free to fold back. Crystallography of the complex shows that there are six molecules of CD38 in each of the asymmetric units. The polypeptide backbones of all of these molecules superimpose well, but each has the covalent intermediate in a different conformation (80). Similar results were obtained with the cyclase, and eight molecules are seen in each unit (79). All of the conformations of the intermediate can be mapped to each other by single-bond rotation (71, 79, 80), indicating they are substates of the cyclization process. A movie of the process can be made by ordering these substates (see supplemental data in Ref. 79), revealing that the folding of the linear substrate involves a major rotation of the adenine ring around its ribosyl bond, such that its N1 position is oriented toward the cyclization site, the C1 of the terminal ribose (Fig. 3). This is an amazing finding, as crystallography is intrinsically a static measurement, and yet it has been used to reveal details of a dynamic process.
FIGURE 3.
Imaging the NAD cyclization by CD38. A mutant of CD38 with Glu-146 changed to alanine (described in text) was co-crystallized with a close analog of NAD, 2′-deoxy-2′-fluoroarabinoside NAD, which forms a covalent linkage between its ribosyl anomeric carbon (C1R) and the catalytic residue Glu-226. Six molecules of the complex are found in each of the asymmetric crystal units, each containing a covalent intermediate in a different conformation. The conformations relate to each other by single-bond rotation, indicating that they represent substates of the cyclization process. Ordering the conformations shows that the cyclization requires a 180° rotation of the adenine ring around its glycosidic bond (yellow arrow), such that the N1 of the adenine is put into close proximity to the ribosyl anomeric carbon (2.9 Å; yellow dashed line), the site of cyclization. Rendered in colored sticks are the starting state, where the adenine ring is farthest from the ribosyl anomeric carbon, and the ending state, where the two are closest. The substates representing various degrees of single-bond rotation of the intermediate between the starting and ending states are colored in shades of gray. Red, oxygen; orange, phosphorus; green, carbon; blue, nitrogen.
Physiological Functions of CD38
The identification of CD38 as a mammalian cADPR-synthesizing enzyme allows the use of gene knock-out to assess its physiological importance. Various type of cells isolated from CD38 knock-out mice have been found to show impairment in the stimulus-induced elevation of cellular NAADP as well as NAADP-mediated Ca2+ signaling (81–83). Likewise, the endogenous cADPR contents decrease greatly in many tissues of the knock-out mice, indicating that CD38 is the dominant enzyme for synthesizing cADPR as well (84). However, in some tissues, particular the brain, a significant level of cADPR remains, suggesting that other unknown enzymes may be able to substitute for CD38 to produce cADPR.
Multiple defects are detected in the knock-out mice, including impairment in insulin secretion (85), increased susceptibility to bacterial infection (84), and altered social behavior (Ref. 8; reviewed in Ref. 11). It is also noteworthy that the ubiquitous “NADase” activity observed in tissues that had no known function is greatly depressed after CD38 ablation (84), clarifying a long-time enigma.
Before the Ca2+-signaling function of CD38 was elucidated, it had long been thought of as a lymphocyte antigen. Indeed, its ligation by antibody can result in activation of cells (reviewed in Ref. 86). More clinically relevant of its antigenic function is the recent finding that CD38 can protect cells from HIV infection by competing with the virus for binding to CD4 (87). CD38 may indeed be critical for human survival because no CD38-negative individual has been detected in a screening of a rather large sample (86).
Regulation of CD38
As described above, CD38 can single-handedly catalyze multiple reactions in the metabolism of two novel Ca2+ messengers. This is a rather unique case, as sequential reactions are normally carried out by a cluster of enzymes in a complex. How this novel Ca2+-signaling enzyme is regulated is a question whose answer is still emerging. Soon after the discovery of cADPR, it was reported that its synthesis in sea urchin eggs is activated by a cGMP-dependent process (88). A similar mechanism also operates in rat hippocampus (89). More recently, agonist-induced and cAMP-dependent activation and phosphorylation of CD38 have also been observed in human granulocytes (90).
However, the membrane topology of CD38 poses a problem. In lymphocytes, CD38 is expressed on the surface as a type II protein, with its catalytic C-terminal domain facing outside (66). On the other hand, phosphorylation generally occurs inside cells, which is the location of the substrates NAD and NADP and the Ca2+ stores that the products cADPR and NAADP target.
It is true that it is now well recognized that CD38 is not exclusively a surface protein but is present in intracellular organelles as well. It is also known that CD38 is not expressed only in lymphocytes but is, in fact, a ubiquitous protein found in virtually all mammalian cells and tissues examined (reviewed in Refs. 11 and 72). However, its type II membrane orientation would put the catalytic domain of the organellar CD38 inside the lumen, away from its substrate and the Ca2+ stores as well.
Currently, there are two actively investigated proposals to resolve this topological paradox. The first one posits that transporters are present in the plasma and organellar membranes to facilitate the movement of the substrate and products of CD38 (91). Evidence indicates that connexin-43 can mediate the transmembrane movement of NAD (92), whereas the nucleoside transporter can transport cADPR (93). Mediated transport of NAADP across membranes has also been observed, which is found to be also inhibited by an inhibitor of the nucleoside transporter, dipyridamole (58). Moreover, the activity of these transporters can be regulated. For example, the transport of NAD by connexin-43 is Ca2+-dependent and suppressed by phosphorylation (91, 94). Consistent with substrate transport being involved in regulating the CD38 activity, knockdown of connexin-43 has been shown to impair the cADPR-mediated signaling of the Fcγ receptor (95).
In essence, the scheme proposes that CD38 itself is not regulated at all but is constitutively active. Because it is a type II protein, its activity is, however, suppressed by the membrane that limits its access to its substrates in the cytosol. The regulation of its activity is therefore not on the enzyme itself but is instead through the availability of the substrate. This is a remarkably unconventional mechanism for regulating an enzyme (Fig. 4).
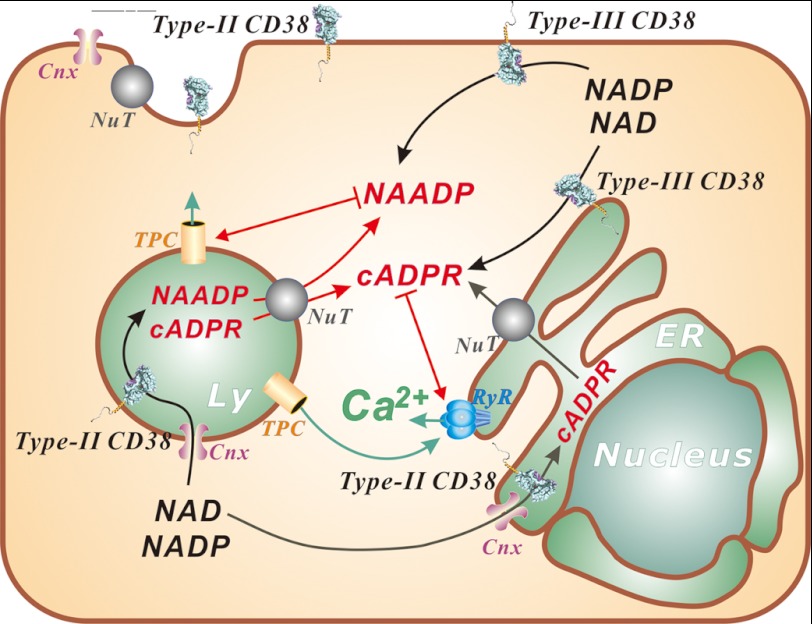
FIGURE 4.
Emerging mechanisms of regulating the signaling function of CD38. NAADP activates the TPCs in the endolysosomes (Ly), whereas cADPR targets the ryanodine receptors (RyR) in the ER. Ca2+ released by NAADP from the endolysosomal stores can be amplified Ca2+-induced Ca2+ release from ER stores. This process is potentiated by cADPR through its sensitization of the ryanodine receptors to Ca2+. CD38 can be coexpressed with both type II and III membrane orientations. Type II CD38 has its catalytic domain facing either outside of the cell or inside the lumen of organelles. Regulation of type II CD38 is proposed to be through substrate limitation, as both NAD and NADP are in the cytosol. Their transport into the organelles is mediated by connexin-43 (Cnx), whereas the products cADPR and NAADP, produced in the lumen, can likewise be transported out to the cytosol by the nucleoside transporters (NuT). Both nucleoside transporters and connexin-43 are also present on the cell surface to serve a similar function for signaling by the ecto-expressed CD38. Type III CD38 is amenable to many common regulation mechanisms, such as phosphorylation.
As an alternative to this scheme of type II CD38 signaling, it has been proposed that CD38 can be expressed as both type II and III proteins, with opposite membrane orientations (Fig. 4) (11). It is generally recognized that one of the important factors that govern the membrane orientation of a protein is the net positive charges on the opposite ends flanking the transmembrane segment, with the more positive end being cytosolic: the “positive inside rule.” Examination of the CD38 sequence shows that the net positive charges on both ends are similar: three on the N-terminal end and two on the C-terminal end. This suggests that both membrane orientations are possible, but with a preference for the N terminus being in the cytosol or type II orientation.
Coexpression of a membrane protein with two opposite orientations has previously been observed. The prion protein is one example and is found to be expressed as both type II and III proteins as well as a glycosylphosphatidylinositol-linked ectoprotein (96). The bacterial multidrug transporter is another example. It is a homodimer, but the two monomers are present in the membrane in opposite orientation (97). In the case of CD38, transfection of a CD38 construct, with a C-terminal tag for detection, into MIN6 cells shows that type III CD38 is expressed and that the ligation of the ecto domain tag of the CD38 can stimulate glucose-induced insulin secretion (98).
The existence of type III CD38 poses, however, yet another fundamental problem. CD38 has six disulfides, and all are important for its enzymatic activity (Fig. 2) (67, 99). It is a generally accepted dogma that disulfide formation does not occur in the cytosol but mainly inside the ER. It is thus not clear whether type III CD38, with its C-terminal domain inside the cytosol, can actually form the important disulfides and, if not, whether it is still enzymatically functional. The question has been directly addressed by making a soluble CD38 construct containing the C-terminal domain (99). The soluble CD38 directed to express in the cytosol is found to be biologically active in elevating the intracellular cADPR level. It is also shown to contain all six disulfides fully formed. This is a remarkable result that challenges the conventional thinking.
Clearly, the type II and III signaling pathways are not mutually exclusive but can operate side by side in cells. The type III mechanism, with the catalytic domain in the cytosol, is amenable to many known types of regulation, such as phosphorylation. It can be involved in fast cellular responses, such as stimulation by hormonal agonists. On the other hand, the type II mechanism, requiring substrate and product transport, may be more suitable for slower and long-term responses, such as cellular differentiation, which has been shown to be regulated by both cADPR (100) and NAADP (39).
More than 2 decades have passed since the discovery of cADPR and NAADP. The physiological importance of these signaling molecules has now been well established by gene ablation studies. It is remarkable that the field began with the identification of a new and hitherto unknown cyclic molecule. It has since progressed to yield even more novel results. Notable is the elucidation of the multicatalytic mechanism of CD38. That endolysosomes are fully functional Ca2+ stores regulated by NAADP likewise opens a new frontier of investigation into the involvement of the pathway in lysosomal storage diseases (101). The emerging mechanisms for the regulation of CD38, both type II and III signaling, are likely to set yet more important precedents for cell signaling in general.
Acknowledgment
I thank Rich Graeff for editing the manuscript.
This work was supported by Research Grants Council of Hong Kong Grants 768408, 769309, 770610, and 771011 and Natural Science Foundation of China (NSFC)/Research Grants Council (RGC) of Hong Kong Grant N_HKU 722/08. This article is part of the Thematic Minireview Series on Ins and Outs of Calcium Transport.
- IP3
- inositol trisphosphate
- ER
- endoplasmic reticulum
- cADPR
- cyclic ADP-ribose
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- TPC
- two-pore channel
- CCK
- cholecystokinin
- ACh
- acetylcholine.
REFERENCES
- 1. Streb H., Irvine R. F., Berridge M. J., Schulz I. (1983) Release of Ca2+ from a non-mitochondrial intracellular store in pancreatic acinar cells by inositol 1,4,5-trisphosphate. Nature 306, 67–69 [DOI] [PubMed] [Google Scholar]
- 2. Lee H. C., Aarhus R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 3. Lee H. C., Aarhus R., Levitt D. (1994) The crystal structure of cyclic ADP-ribose. Nat. Struct. Biol. 1, 143–144 [DOI] [PubMed] [Google Scholar]
- 4. Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., Clapper D. L. (1989) Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 264, 1608–1615 [PubMed] [Google Scholar]
- 5. Wu Y., Kuzma J., Maréchal E., Graeff R., Lee H. C., Foster R., Chua N. H. (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278, 2126–2130 [DOI] [PubMed] [Google Scholar]
- 6. Zocchi E., Basile G., Cerrano C., Bavestrello G., Giovine M., Bruzzone S., Guida L., Carpaneto A., Magrassi R., Usai C. (2003) ABA- and cADPR-mediated effects on respiration and filtration downstream of the temperature-signaling cascade in sponges. J. Cell Sci. 116, 629–636 [DOI] [PubMed] [Google Scholar]
- 7. Lam C. M., Yeung P. K., Lee H. C., Wong J. T. (2009) Cyclic ADP-ribose links metabolism to multiple fission in the dinoflagellate Crypthecodinium cohnii. Cell Calcium 45, 346–357 [DOI] [PubMed] [Google Scholar]
- 8. Jin D., Liu H. X., Hirai H., Torashima T., Nagai T., Lopatina O., Shnayder N. A., Yamada K., Noda M., Seike T., Fujita K., Takasawa S., Yokoyama S., Koizumi K., Shiraishi Y., Tanaka S., Hashii M., Yoshihara T., Higashida K., Islam M. S., Yamada N., Hayashi K., Noguchi N., Kato I., Okamoto H., Matsushima A., Salmina A., Munesue T., Shimizu N., Mochida S., Asano M., Higashida H. (2007) CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 446, 41–45 [DOI] [PubMed] [Google Scholar]
- 9. Galione A., Parrington J., Funnell T. (2011) Physiological roles of NAADP-mediated Ca2+ signaling. Sci. China Life Sci. 54, 725–732 [DOI] [PubMed] [Google Scholar]
- 10. Guse A. H., Lee H. C. (2008) NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10. [DOI] [PubMed] [Google Scholar]
- 11. Lee H. C. (2011) Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling. Sci. China Life Sci. 54, 699–711 [DOI] [PubMed] [Google Scholar]
- 12. Lee H. C. (1997) Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol. Rev. 77, 1133–1164 [DOI] [PubMed] [Google Scholar]
- 13. Grau U. M., Trommer W. E., Rossmann M. G. (1981) Structure of the active ternary complex of pig heart lactate dehydrogenase with S-lac-NAD at 2.7 Å resolution. J. Mole. Biol. 151, 289–307 [DOI] [PubMed] [Google Scholar]
- 14. Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. (1987) Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 262, 9561–9568 [PubMed] [Google Scholar]
- 15. Dargie P. J., Agre M. C., Lee H. C. (1990) Comparison of Ca2+-mobilizing activities of cyclic ADP-ribose and inositol trisphosphate. Cell Regul. 1, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galione A., Lee H. C., Busa W. B. (1991) Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 253, 1143–1146 [DOI] [PubMed] [Google Scholar]
- 17. Lee H. C. (1993) Potentiation of calcium- and caffeine-induced calcium release by cyclic ADP-ribose. J. Biol. Chem. 268, 293–299 [PubMed] [Google Scholar]
- 18. Lee H. C. (2001) Physiological functions of cyclic ADP-ribose and NAADP as calcium messengers. Annu. Rev. Pharmacol. Toxicol. 41, 317–345 [DOI] [PubMed] [Google Scholar]
- 19. Lee H. C., Aarhus R., Graeff R., Gurnack M. E., Walseth T. F. (1994) Cyclic ADP ribose activation of the ryanodine receptor is mediated by calmodulin. Nature 370, 307–309 [DOI] [PubMed] [Google Scholar]
- 20. Lee H. C., Aarhus R., Graeff R. M. (1995) Sensitization of calcium-induced calcium release by cyclic ADP-ribose and calmodulin. J. Biol. Chem. 270, 9060–9066 [DOI] [PubMed] [Google Scholar]
- 21. Tanaka Y., Tashjian A. H., Jr. (1995) Calmodulin is a selective mediator of Ca2+-induced Ca2+ release via the ryanodine receptor-like Ca2+ channel triggered by cyclic ADP-ribose. Proc. Natl. Acad. Sci. U.S.A. 92, 3244–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Noguchi N., Takasawa S., Nata K., Tohgo A., Kato I., Ikehata F., Yonekura H., Okamoto H. (1997) Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. J. Biol. Chem. 272, 3133–3136 [DOI] [PubMed] [Google Scholar]
- 23. Wang Y. X., Zheng Y. M., Mei Q. B., Wang Q. S., Collier M. L., Fleischer S., Xin H. B., Kotlikoff M. (2004) FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells. Am. J. Physiol. Cell Physiol. 286, C538–C546 [DOI] [PubMed] [Google Scholar]
- 24. Zheng J., Wenzhi B., Miao L., Hao Y., Zhang X., Yin W., Pan J., Yuan Z., Song B., Ji G. (2010) Ca2+ release induced by cADP-ribose is mediated by FKBP12.6 proteins in mouse bladder smooth muscle. Cell Calcium 47, 449–457 [DOI] [PubMed] [Google Scholar]
- 25. Tang W. X., Chen Y. F., Zou A. P., Campbell W. B., Li P. L. (2002) Role of FKBP12.6 in cADPR-induced activation of reconstituted ryanodine receptors from arterial smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 282, H1304–H1310 [DOI] [PubMed] [Google Scholar]
- 26. Lange I., Penner R., Fleig A., Beck A. (2008) Synergistic regulation of endogenous TRPM2 channels by adenine dinucleotides in primary human neutrophils. Cell Calcium 44, 604–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Togashi K., Hara Y., Tominaga T., Higashi T., Konishi Y., Mori Y., Tominaga M. (2006) TRPM2 activation by cyclic ADP-ribose at body temperature is involved in insulin secretion. EMBO J. 25, 1804–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., Lee H. C. (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 270, 30327–30333 [DOI] [PubMed] [Google Scholar]
- 29. Aarhus R., Dickey D. M., Graeff R. M., Gee K. R., Walseth T. F., Lee H. C. (1996) Activation and inactivation of Ca2+ release by NAADP+. J. Biol. Chem. 271, 8513–8516 [DOI] [PubMed] [Google Scholar]
- 30. Genazzani A. A., Empson R. M., Galione A. (1996) Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem. 271, 11599–11602 [DOI] [PubMed] [Google Scholar]
- 31. Lee H. C. (1996) Modulator and messenger functions of cyclic ADP-ribose in calcium signaling. Recent Prog. Horm. Res. 51, 355–388; discussion 389 [PubMed] [Google Scholar]
- 32. Lee H. C., Aarhus R. (2000) Functional visualization of the separate but interacting calcium stores sensitive to NAADP and cyclic ADP-ribose. J. Cell Sci. 113, 4413–4420 [DOI] [PubMed] [Google Scholar]
- 33. Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 34. Galione A., Morgan A. J., Arredouani A., Davis L. C., Rietdorf K., Ruas M., Parrington J. (2010) NAADP as an intracellular messenger regulating lysosomal calcium release channels. Biochem. Soc. Trans. 38, 1424–1431 [DOI] [PubMed] [Google Scholar]
- 35. Patel S., Ramakrishnan L., Rahman T., Hamdoun A., Marchant J. S., Taylor C. W., Brailoiu E. (2011) The endolysosomal system as an NAADP-sensitive acidic Ca2+ store: role for the two-pore channels. Cell Calcium 50, 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tugba Durlu-Kandilci N., Ruas M., Chuang K. T., Brading A., Parrington J., Galione A. (2010) TPC2 proteins mediate nicotinic acid adenine dinucleotide phosphate (NAADP)- and agonist-evoked contractions of smooth muscle. J. Biol. Chem. 285, 24925–24932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Aley P. K., Mikolajczyk A. M., Munz B., Churchill G. C., Galione A., Berger F. (2010) Nicotinic acid adenine dinucleotide phosphate regulates skeletal muscle differentiation via action at two-pore channels. Proc. Natl. Acad. Sci. U.S.A. 107, 19927–19932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pereira G. J., Hirata H., Fimia G. M., do Carmo L. G., Bincoletto C., Han S. W., Stilhano R. S., Ureshino R. P., Bloor-Young D., Churchill G., Piacentini M., Patel S., Smaili S. S. (2011) Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 286, 27875–27881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Esposito B., Gambara G., Lewis A. M., Palombi F., D'Alessio A., Taylor L. X., Genazzani A. A., Ziparo E., Galione A., Churchill G. C., Filippini A. (2011) NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood 117, 4968–4977 [DOI] [PubMed] [Google Scholar]
- 42. Moccia F., Lim D., Nusco G. A., Ercolano E., Santella L. (2003) NAADP activates a Ca2+ current that is dependent on F-actin cytoskeleton. FASEB J. 17, 1907–1909 [DOI] [PubMed] [Google Scholar]
- 43. Churchill G. C., Galione A. (2001) NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 20, 2666–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Collins T. P., Bayliss R., Churchill G. C., Galione A., Terrar D. A. (2011) NAADP influences excitation-contraction coupling by releasing calcium from lysosomes in atrial myocytes. Cell Calcium 50, 449–458 [DOI] [PubMed] [Google Scholar]
- 45. Cancela J. M., Churchill G. C., Galione A. (1999) Coordination of agonist-induced Ca2+-signaling patterns by NAADP in pancreatic acinar cells. Nature 398, 74–76 [DOI] [PubMed] [Google Scholar]
- 46. Yamasaki M., Thomas J. M., Churchill G. C., Garnham C., Lewis A. M., Cancela J. M., Patel S., Galione A. (2005) Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr. Biol. 15, 874–878 [DOI] [PubMed] [Google Scholar]
- 47. Churchill G. C., O'Neill J. S., Masgrau R., Patel S., Thomas J. M., Genazzani A. A., Galione A. (2003) Sperm deliver a new second messenger: NAADP. Curr. Biol. 13, 125–128 [DOI] [PubMed] [Google Scholar]
- 48. Gasser A., Bruhn S., Guse A. H. (2006) Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J. Biol. Chem. 281, 16906–16913 [DOI] [PubMed] [Google Scholar]
- 49. Yamasaki M., Masgrau R., Morgan A. J., Churchill G. C., Patel S., Ashcroft S. J., Galione A. (2004) Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J. Biol. Chem. 279, 7234–7240 [DOI] [PubMed] [Google Scholar]
- 50. Park K. H., Kim B. J., Kang J., Nam T. S., Lim J. M., Kim H. T., Park J. K., Kim Y. G., Chae S. W., Kim U. H. (2011) Ca2+-signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 4, ra31. [DOI] [PubMed] [Google Scholar]
- 51. Walseth T. F., Lee H. C. (1993) Synthesis and characterization of antagonists of cyclic ADP-ribose-induced Ca2+ release. Biochim. Biophys. Acta 1178, 235–242 [DOI] [PubMed] [Google Scholar]
- 52. Naylor E., Arredouani A., Vasudevan S. R., Lewis A. M., Parkesh R., Mizote A., Rosen D., Thomas J. M., Izumi M., Ganesan A., Galione A., Churchill G. C. (2009) Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 5, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Walseth T. F., Aarhus R., Zeleznikar R. J., Jr., Lee H. C. (1991) Determination of endogenous levels of cyclic ADP-ribose in rat tissues. Biochim. Biophys. Acta 1094, 113–120 [DOI] [PubMed] [Google Scholar]
- 54. Graeff R., Lee H. C. (2002) A novel cycling assay for cellular cADP-ribose with nanomolar sensitivity. Biochem. J. 361, 379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kuroda R., Kontani K., Kanda Y., Katada T., Nakano T., Satoh Y., Suzuki N., Kuroda H. (2001) Increase of cGMP, cADP-ribose, and inositol 1,4,5-trisphosphate preceding Ca2+ transients in fertilization of sea urchin eggs. Development 128, 4405–4414 [DOI] [PubMed] [Google Scholar]
- 56. Billington R. A., Ho A., Genazzani A. A. (2002) Nicotinic acid adenine dinucleotide phosphate (NAADP) is present at micromolar concentrations in sea urchin spermatozoa. J. Physiol. 544, 107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Graeff R., Lee H. C. (2002) A novel cycling assay for nicotinic acid-adenine dinucleotide phosphate with nanomolar sensitivity. Biochem. J. 367, 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim B. J., Park K. H., Yim C. Y., Takasawa S., Okamoto H., Im M. J., Kim U. H. (2008) Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes 57, 868–878 [DOI] [PubMed] [Google Scholar]
- 59. Mushtaq M., Nam T. S., Kim U. H. (2011) Critical role for CD38-mediated Ca2+ signaling in thrombin-induced procoagulant activity of mouse platelets and hemostasis. J. Biol. Chem. 286, 12952–12958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shawl A. I., Park K. H., Kim U. H. (2009) Insulin receptor signaling for the proliferation of pancreatic β-cells: involvement of Ca2+ second messengers, IP3, NAADP, and cADPR. Islets 1, 216–223 [DOI] [PubMed] [Google Scholar]
- 61. Lee H. C., Aarhus R. (1991) ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 2, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. States D. J., Walseth T. F., Lee H. C. (1992) Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem. Sci. 17, 495. [DOI] [PubMed] [Google Scholar]
- 63. Howard M., Grimaldi J. C., Bazan J. F., Lund F. E., Santos-Argumedo L., Parkhouse R. M., Walseth T. F., Lee H. C. (1993) Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science 262, 1056–1059 [DOI] [PubMed] [Google Scholar]
- 64. Takasawa S., Tohgo A., Noguchi N., Koguma T., Nata K., Sugimoto T., Yonekura H., Okamoto H. (1993) Synthesis and hydrolysis of cyclic ADP-ribose by human leukocyte antigen CD38 and inhibition of the hydrolysis by ATP. J. Biol. Chem. 268, 26052–26054 [PubMed] [Google Scholar]
- 65. Kim H., Jacobson E. L., Jacobson M. K. (1993) Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science 261, 1330–1333 [DOI] [PubMed] [Google Scholar]
- 66. Jackson D. G., Bell J. I. (1990) Isolation of a cDNA encoding the human CD38 (T10) molecule, a cell surface glycoprotein with an unusual discontinuous pattern of expression during lymphocyte differentiation. J. Immunol. 144, 2811–2815 [PubMed] [Google Scholar]
- 67. Liu Q., Kriksunov I. A., Graeff R., Munshi C., Lee H. C., Hao Q. (2005) Crystal structure of human CD38 extracellular domain. Structure 13, 1331–1339 [DOI] [PubMed] [Google Scholar]
- 68. Prasad G. S., McRee D. E., Stura E. A., Levitt D. G., Lee H. C., Stout C. D. (1996) Crystal structure of Aplysia ADP ribosyl cyclase, a homolog of the bifunctional ectozyme CD38. Nat. Struct. Biol. 3, 957–964 [DOI] [PubMed] [Google Scholar]
- 69. Munshi C., Thiel D. J., Mathews I. I., Aarhus R., Walseth T. F., Lee H. C. (1999) Characterization of the active site of ADP-ribosyl cyclase. J. Biol. Chem. 274, 30770–30777 [DOI] [PubMed] [Google Scholar]
- 70. Munshi C., Aarhus R., Graeff R., Walseth T. F., Levitt D., Lee H. C. (2000) Identification of the enzymatic active site of CD38 by site-directed mutagenesis. J. Biol. Chem. 275, 21566–21571 [DOI] [PubMed] [Google Scholar]
- 71. Graeff R., Liu Q., Kriksunov I. A., Kotaka M., Oppenheimer N., Hao Q., Lee H. C. (2009) Mechanism of cyclizing NAD to cyclic ADP-ribose by ADP-ribosyl cyclase and CD38. J. Biol. Chem. 284, 27629–27636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lee H. C. (2000) Enzymatic functions and structures of CD38 and homologs. Chem. Immunol. 75, 39–59 [DOI] [PubMed] [Google Scholar]
- 73. Graeff R., Liu Q., Kriksunov I. A., Hao Q., Lee H. C. (2006) Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J. Biol. Chem. 281, 28951–28957 [DOI] [PubMed] [Google Scholar]
- 74. Liu Q., Graeff R., Kriksunov I. A., Jiang H., Zhang B., Oppenheimer N., Lin H., Potter B. V., Lee H. C., Hao Q. (2009) Structural basis for enzymatic evolution from a dedicated ADP-ribosyl cyclase to a multifunctional NAD hydrolase. J. Biol. Chem. 284, 27637–27645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Liu Q., Kriksunov I. A., Graeff R., Lee H. C., Hao Q. (2007) Structural basis for formation and hydrolysis of the calcium messenger cyclic ADP-ribose by human CD38. J. Biol. Chem. 282, 5853–5861 [DOI] [PubMed] [Google Scholar]
- 76. Liu Q., Kriksunov I. A., Graeff R., Munshi C., Lee H. C., Hao Q. (2006) Structural basis for the mechanistic understanding of human CD38-controlled multiple catalysis. J. Biol. Chem. 281, 32861–32869 [DOI] [PubMed] [Google Scholar]
- 77. Liu Q., Kriksunov I. A., Jiang H., Graeff R., Lin H., Lee H. C., Hao Q. (2008) Covalent and noncovalent intermediates of an NAD utilizing enzyme, human CD38. Chem. Biol. 15, 1068–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu Q., Kriksunov I. A., Moreau C., Graeff R., Potter B. V., Lee H. C., Hao Q. (2007) Catalysis-associated conformational changes revealed by human CD38 complexed with a non-hydrolyzable substrate analog. J. Biol. Chem. 282, 24825–24832 [DOI] [PubMed] [Google Scholar]
- 79. Kotaka M., Graeff R., Chen Z., Zhang L. H., Lee H. C., Hao Q. (2012) Structural studies of intermediates along the cyclization pathway of Aplysia ADP-ribosyl cyclase. J. Mol. Biol. 415, 514–526 [DOI] [PubMed] [Google Scholar]
- 80. Zhang H., Graeff R., Chen Z., Zhang L., Zhang L., Lee H., Hao Q. (2011) Dynamic conformations of the CD38-mediated NAD cyclization captured in a single crystal. J. Mol. Biol. 405, 1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rah S. Y., Mushtaq M., Nam T. S., Kim S. H., Kim U. H. (2010) Generation of cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate by CD38 for Ca2+ signaling in interleukin-8-treated lymphokine-activated killer cells. J. Biol. Chem. 285, 21877–21887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kim S. Y., Cho B. H., Kim U. H. (2010) CD38-mediated Ca2+ signaling contributes to angiotensin II-induced activation of hepatic stellate cells. Attenuation of hepatic fibrosis by CD38 ablation. J. Biol. Chem. 285, 576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cosker F., Cheviron N., Yamasaki M., Menteyne A., Lund F. E., Moutin M. J., Galione A., Cancela J. M. (2010) The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J. Biol. Chem. 285, 38251–38259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Partida-Sánchez S., Cockayne D. A., Monard S., Jacobson E. L., Oppenheimer N., Garvy B., Kusser K., Goodrich S., Howard M., Harmsen A., Randall T. D., Lund F. E. (2001) Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx, and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 7, 1209–1216 [DOI] [PubMed] [Google Scholar]
- 85. Kato I., Yamamoto Y., Fujimura M., Noguchi N., Takasawa S., Okamoto H. (1999) CD38 disruption impairs glucose-induced increases in cyclic ADP-ribose, [Ca2+]i, and insulin secretion. J. Biol. Chem. 274, 1869–1872 [DOI] [PubMed] [Google Scholar]
- 86. Malavasi F., Deaglio S., Funaro A., Ferrero E., Horenstein A. L., Ortolan E., Vaisitti T., Aydin S. (2008) Evolution and function of the ADP-ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol. Rev. 88, 841–886 [DOI] [PubMed] [Google Scholar]
- 87. Savarino A., Bensi T., Chiocchetti A., Bottarel F., Mesturini R., Ferrero E., Calosso L., Deaglio S., Ortolan E., Buttò S., Cafaro A., Katada T., Ensoli B., Malavasi F., Dianzani U. (2003) Human CD38 interferes with HIV-1 fusion through a sequence homologous to the V3 loop of the viral envelope glycoprotein gp120. FASEB J. 17, 461–463 [DOI] [PubMed] [Google Scholar]
- 88. Galione A., White A., Willmott N., Turner M., Potter B. V., Watson S. P. (1993) cGMP mobilizes intracellular Ca2+ in sea urchin eggs by stimulating cyclic ADP-ribose synthesis. Nature 365, 456–459 [DOI] [PubMed] [Google Scholar]
- 89. Reyes-Harde M., Potter B. V., Galione A., Stanton P. K. (1999) Induction of hippocampal LTD requires nitric oxide-stimulated PKG activity and Ca2+ release from cyclic ADP-ribose-sensitive stores. J. Neurophysiol. 82, 1569–1576 [DOI] [PubMed] [Google Scholar]
- 90. Bruzzone S., Moreschi I., Usai C., Guida L., Damonte G., Salis A., Scarfì S., Millo E., De Flora A., Zocchi E. (2007) Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc. Natl. Acad. Sci. U.S.A. 104, 5759–5764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. De Flora A., Zocchi E., Guida L., Franco L., Bruzzone S. (2004) Autocrine and paracrine calcium signaling by the CD38/NAD+/cyclic ADP-ribose system. Ann. N.Y. Acad. Sci. 1028, 176–191 [DOI] [PubMed] [Google Scholar]
- 92. Bruzzone S., Guida L., Zocchi E., Franco L., De Flora A. (2001) Connexin-43 hemichannels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 15, 10–12 [DOI] [PubMed] [Google Scholar]
- 93. Guida L., Bruzzone S., Sturla L., Franco L., Zocchi E., De Flora A. (2002) Equilibrative and concentrative nucleoside transporters mediate influx of extracellular cyclic ADP-ribose into 3T3 murine fibroblasts. J. Biol. Chem. 277, 47097–47105 [DOI] [PubMed] [Google Scholar]
- 94. Bruzzone S., Franco L., Guida L., Zocchi E., Contini P., Bisso A., Usai C., De Flora A. (2001) A self-restricted CD38-connexin-43 cross-talk affects NAD+ and cyclic ADP-ribose metabolism and regulates intracellular calcium in 3T3 fibroblasts. J. Biol. Chem. 276, 48300–48308 [DOI] [PubMed] [Google Scholar]
- 95. Song E. K., Rah S. Y., Lee Y. R., Yoo C. H., Kim Y. R., Yeom J. H., Park K. H., Kim J. S., Kim U. H., Han M. K. (2011) Connexin-43 hemichannels mediate cyclic ADP-ribose generation and its Ca2+-mobilizing activity by NAD+/cyclic ADP-ribose transport. J. Biol. Chem. 286, 44480–44490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stewart R. S., Harris D. A. (2003) Mutational analysis of topological determinants in prion protein (PrP) and measurement of transmembrane and cytosolic PrP during prion infection. J. Biol. Chem. 278, 45960–45968 [DOI] [PubMed] [Google Scholar]
- 97. Seppälä S., Slusky J. S., Lloris-Garcerá P., Rapp M., von Heijne G. (2010) Control of membrane protein topology by a single C-terminal residue. Science 328, 1698–1700 [DOI] [PubMed] [Google Scholar]
- 98. Ohta Y., Kitanaka A., Mihara K., Imataki O., Ohnishi H., Tanaka T., Taminato T., Kubota Y. (2011) Expression of CD38 with intracellular enzymatic activity: a possible explanation for the insulin release induced by intracellular cADPR. Mol. Cell. Biochem. 352, 293–299 [DOI] [PubMed] [Google Scholar]
- 99. Zhao Y. J., Zhang H. M., Lam C. M., Hao Q., Lee H. C. (2011) Cytosolic CD38 protein forms intact disulfides and is active in elevating intracellular cyclic ADP-ribose. J. Biol. Chem. 286, 22170–22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Yue J., Wei W., Lam C. M., Zhao Y. J., Dong M., Zhang L. R., Zhang L. H., Lee H. C. (2009) CD38/cADPR/Ca2+ pathway promotes cell proliferation and delays nerve growth factor-induced differentiation in PC12 cells. J. Biol. Chem. 284, 29335–29342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Morgan A. J., Platt F. M., Lloyd-Evans E., Galione A. (2011) Molecular mechanisms of endolysosomal Ca2+ signaling in health and disease. Biochem. J. 439, 349–374 [DOI] [PubMed] [Google Scholar]