Abstract
Genetic approaches to analyzing neuronal circuits and learning would benefit from a technology to first deliver a specific gene into presynaptic neurons, and then deliver a different gene into an identified subset of their postsynaptic neurons, connected by a specific synapse type. Here, we describe targeted gene transfer across a neocortical glutamatergic synapse, using as the model the projection from rat postrhinal to perirhinal cortex. The first gene transfer, into the presynaptic neurons in postrhinal cortex, used a virus vector and standard gene transfer procedures. The vector expresses an artificial peptide neurotransmitter containing a dense core vesicle targeting domain, a NMDA NR1 subunit binding domain (from a monoclonal antibody), and the His tag. Upon release, this peptide neurotransmitter binds to NMDA receptors on the postsynaptic neurons. Antibody-mediated targeted gene transfer to these postsynaptic neurons in perirhinal cortex used a His tag antibody, as the peptide neurotransmitter contains the His tag. Confocal microscopy showed that with untargeted gene transfer, ~3 % of the transduced presynaptic axons were proximal to a transduced postsynaptic dendrite. In contrast, with targeted gene transfer, ≥20 % of the presynaptic axons were proximal to a transduced postsynaptic dendrite. Targeting across other types of synapses might be obtained by modifying the artificial peptide neurotransmitter to contain a binding domain for a different neurotransmitter receptor. This technology may benefit elucidating how specific neurons and subcircuits contribute to circuit physiology, behavior, and learning.
Keywords: glutamatergic synapse, vesicular glutamate transporter 1 promoter, peptide neurotransmitter, antibody-mediated targeted gene transfer, herpes simplex virus vector
1. Introduction
Understanding how specific neurons and subcircuits support circuit physiology, behavior, and learning is one of the fundamental challenges in modern neuroscience. Forebrain areas contain many neuron types, and each type forms precise connnections with specific neuron types, to support circuit physiology (Dudai, 1989). In neocortex, a column contains tens to hundreds of diferent neuron types, with precise connections both within the column and to distant areas, each supporting distinct physiological functions (Peters and Jones, 1984; Sugino et al., 2006). Thus, elucidating the function of a specific subcircuit has proven to be a challenging task. Analyses using classical neuroantomical, cellular and molecular techniques, and electrophysiology are only beginning to elucidate the physiology of specific subcircuits, and how they contribute to circuit physiology and behavior. Genetic approaches to activate specific neuron types, using optogenetic tools or activation of specific signaling or transcriptional pathways, typically affect the physiology of an entire circuit (Dymecki and Kim, 2007; Fenno et al., 2011; Luo et al., 2008; Zhang et al., 2005). Analyses of specific subcircuits would benefit from a technology to first deliver a specific gene into a particular type of presynaptic neuron, and then deliver a different gene into an identified subset of their postsynaptic neurons, connected by a specific type of synapse. This powerful technology will enable studies on specific subcircuits: Following activation of presynaptic neurons, essential subcircuits and postsynaptic neurons might be identified by blocking the activity of these neurons, using the Drosophila allatostatin receptor or other genes (Lechner et al., 2002); and the function of these postsynaptic neurons might be studied by expressing critical physiological genes, such as specific glutamate receptors, postsynaptic density components, or transcription factors, or specific sensors, such as calcium or transcriptional sensors (Dymecki and Kim, 2007; Luo et al., 2008).
In particular, delivering different genes to monosynaptically connected neurons may benefit analyzing circuits that support cognitive learning. Of note, an identified circuit in rat postrhinal (POR) cortex can encode some essential information for specific visual object discriminations (Zhang et al., 2005; Zhang et al., 2010a). Genetic activation of protein kinase C (PKC) pathways in several hundred spatially-grouped glutamatergic and GABAergic neurons in POR cortex increased activation-dependent neurotransmitter release, and improved both the learning rate and accuracy for new visual discriminations (Zhang et al., 2005). Some of the essential information for performance is encoded in the genetically-modified circuit (Zhang et al., 2010a): After gene transfer and learning, creation of small neurochemical lesions, proximal to the gene transfer site, selectively reduced performance for only discriminations learned after gene transfer. Interestingly, POR cortex projects to more than ten neocortical areas (Agster and Burwell, 2009; Burwell and Amaral, 1998a; Burwell and Amaral, 1998b; Burwell, 2000), including areas important in this learning, such as perirhinal (PER) cortex (Murray et al., 2007; Winters et al., 2004), and many of the transduced neurons in POR cortex project to PER cortex (Zhang et al., 2010b), but the role of this subcircuit in this learning remains to be determined. Thus, we used this subcircuit as the model for developing targeted gene transfer to specific presynaptic neurons and a subset of their postsynaptic neurons.
Genetic technologies for mapping circuits or visualizing synapses are highly valuable, but exhibit distinct capabilities from the technology developed here. Specific viruses; including Rabies Virus, Vesicular Stomatitis Virus, Sindbis Virus, Herpes Simplex Virus (HSV-1), and Pseudorabies Virus; have been developed to map anteriograde or retrograde projections, across one, or multiple, synapses (reviewed in (Lo and Anderson, 2011)). These technologies rely on the spread of a single virus across one or more synapses, and deliver the same genes into the presynaptic and postsynaptic neurons. Synapses between specific neurons have been visualized using GRASP or BLINC (Feinberg et al., 2008; Thyagarajan and Ting, 2010); these technologies visualize specific synapses after untargeted gene transfer, and do not selectively deliver genes into neurons that are connected.
Here, we report a technology for delivering different genes into specific presynaptic neurons and an identified subset of their postsynaptic neurons that are connected by a glutamatergic synapse. As a model system, we chose the large projection from POR to PER cortex. First, gene transfer into the presynaptic neurons in POR cortex used a HSV-1 vector that expresses an artificial peptide neurotransmitter, containing i) a dense core vesicle (DCV) targeting domain (Dikeakos and Reudelhuber, 2007), ii) a NMDA receptor NR1 subunit binding domain (Moskal et al., 2001; Moskal et al., 2005), and iii) the His tag. Upon release, this peptide neurotransmitter binds to NMDA receptors on postsynaptic neurons. The second gene transfer selectively targeted the postsynaptic neurons in PER cortex, using antibody-mediated targeted gene transfer (Cao et al., 2010; Cao et al., 2011) and a His tag antibody, as the peptide neurotransmitter contains the His tag. With untargeted gene transfer, ~3 % of the transduced presynaptic axons were proximal to a transduced postsynaptic dendrite. In contrast, with targeted gene transfer, ≥20 % of the presynaptic axons were proximal to a transduced postsynaptic dendrite. Targeting across other synapse types of might be obtained by modifying the artificial peptide neurotransmitter to contain a binding domain for a different neurotransmitter receptor. This technology may benefit elucidating the function of specific subcircuits, particularly their roles in circuit physiology, behavior, and learning.
2. Results
2.1. The targeting strategy, two artificial peptide neurotransmitters, and the presynaptic and postsynaptic vectors
The strategy for targeting gene transfer across a glutamatergic synapse, and the model system, is shown in Fig. 1. For the first artificial peptide neurotransmitter (Fig. 2), we fused i) a DCV targeting domain from human Secretogranin II (Courel et al., 2008; Gerdes et al., 1989), to ii) a 10 aa peptide that binds to the NMDA NR1 subunit, derived from a monoclonal anti-NR1 antibody (Moskal et al., 2001), to iii) the His tag. For the second artificial peptide neurotransmitter, we fused i) a DCV targeting domain from mouse pro-opiomelanocortin (POMC) (Cool and Loh, 1994; Cool et al., 1995), to ii) the variable region of the anti-NR1 antibody (Moskal et al., 2005) to iii) the His tag.
Fig. 1.
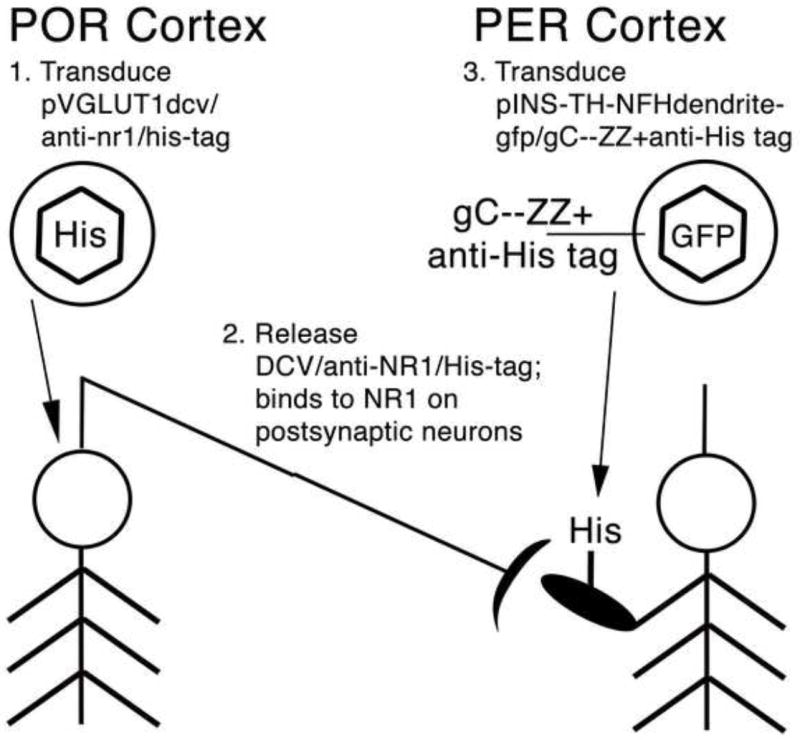
The targeting strategy and the model system. POR cortex has a large projection to PER cortex (Agster and Burwell, 2009), shown as a neuron in POR cortex with an axon projecting to PER cortex. First, gene transfer into the presynaptic neurons, in POR cortex, uses standard procedures. This vector uses a glutamatergic-specific promoter, the VGLUT1 promoter, to express an artificial peptide neurotransmitter, containing i) a DCV targeting domain, ii) a NMDA NR1 binding domain, and iii) the His tag. Second, upon release, the peptide neurotransmitter (solid elipse with His) binds to NMDA receptors on the postsynaptic neurons in PER cortex. Third, targeted gene transfer to the postsynaptic neurons uses antibody-mediated targeting and anti-His tag antibodies (gC--ZZ+anti-His tag). The postsynaptic vector uses a neuron-specfic promoter (INS-TH-NFH) to express a dendrite-targeted GFP.
Fig. 2.
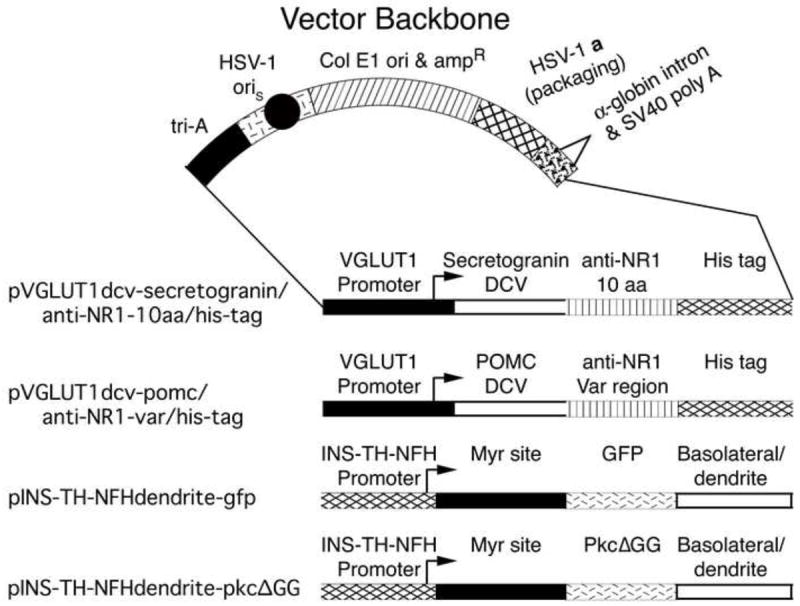
Vectors for targeted gene transfer to presynaptic and postsynaptic neurons. Top: The vector backbone. The expressed gene is followed by the mouse α-globin second intron (triangle) and the SV40 polyadenylation site (brick segment). A cassette of three polyadenylation sites (tri-A, black segment) was placed 5’ to the promoter to reduce any effects on expression from the HSV-1 immediate early 4/5 promoter (short line segment). An HSV-1 origin of DNA replication (oriS, black circle) and the HSV-1 a sequence (cross hatched segment), which contains the packaging site, support replication and packaging of the vector. Sequences from pBR322 (diagonal line segment) support propagation in E. coli. Bottom: The four vectors. The two presynaptic vectors use a glutamatergic-specific promoter, the VGLUT1 promoter, to express an artificial peptide neurotransmitter containing a DCV targeting domain from either Secretogranin II or POMC, an NMDA receptor NR1 subunit binding domain from a monoclonal anti-NR1 antibody, and the His tag. The two postsynaptic vectors use a neuron-specific promoter, the INS-TH-NFH promoter, to express a dendrite-targeted protein (Kameda et al., 2008); these constructs contain a myristoylation/palmitoylation (Myr) site from Fyn, a marker protein, either GFP or an enzymatically inactive PKC (PkcΔGG), and a basolateral/dendrite membrane-sorting domain from the low density lipoprotein receptor.
The two presynaptic vectors (Fig. 2) used the vesicular glutamate transporter-1 (VGLUT1) promoter (Rasmussen et al., 2007; Zhang and Geller, 2010) to express each artificial peptide neurotransmitter in VGLUT1-containing glutamatergic neurons, the predominant type of neocortical glutamatergic neuron. Helper virus-free HSV-1 vector packaging was performed using standard procedures (Fraefel et al., 1996). Each vector was injected into POR cortex, the rats were sacrificed 8 days later, and immunofluorescent analyses revealed His-tag-immunoreactive (IR) cell bodies in POR cortex (Fig. 3A and B). These vectors also supported expression in axon terminals in PER cortex, as detailed below.
Fig. 3.
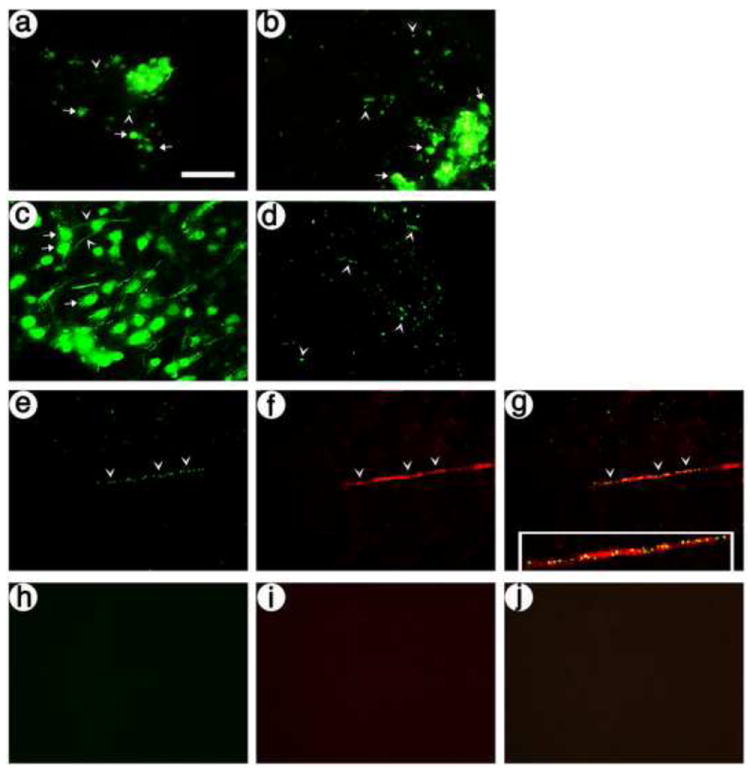
The presynaptic vectors support expression of the artificial peptide neurotransmitters, and these gene transfer conditions support expression in neurons in POR cortex with axons that innervate PER cortex. Rats were sacrified at 8 days after injection of vectors into POR cortex. (A and B) Each presynaptic vector supports expression in cell bodies and proximal processes in POR cortex, visualized by His-tag-IR; (A) pVGLUT1dcv-secretogranin/anti-NR1-10aa/his-tag, and (B) pVGLUT1ldcv-pomc/anti-NR1-var/his-tag. Arrows, cell bodies; arrowheads, axons. (C and D) pVGLUT1gaplac supports expression in cell bodies and proximal processes in POR cortex and in axons in PER cortex, visualized by ß-gal-IR; (C) POR cortex, and (D) PER cortex. (E-G) pVGLUT1gaplac supports expression in axons in PER cortex, identified by costaining with an axon marker, tau; (E) ß-gal-IR, (F) tau-IR, and (G) merge. (H-J) Omitting the primary antibodies from the assay resulted in background levels of fluorescence; (H) fluorescein-conjugated secondary antibody-IR, (I) Texas red-conjugated secondary antibody-IR (I), and (J) merge. Scale bar: 50 μm.
To locate the site of the projection in PER cortex, for subsequent use as the site for injecting the postsynaptic vector, we used a previously reported vector that expresses an axon-targeted ß-galactosidase (ß-gal) from the VGLUT1 promoter (pVGLUT1gap-lac) (Zhang et al., 2010b). This vector was injected into POR cortex, the rats were sacrificed 8 days later, and immunofluorescent analyses revealed ß-gal-IR cell bodies and processes in POR cortex (Fig. 3C), and ß-gal-IR processes in PER cortex (Fig. 3D). To confirm that the processes in PER cortex were axons, we performed costaining for ß-gal-IR and an axon marker, tau-IR, and found that most of the transduced processes in PER cortex contained tau-IR (Fig. 3E-G). As a control, omission of the primary antibodies resulted in no IR (Fig. 3H-J).
To label dendrites in the postsynaptic neurons, the vector (Fig. 2) used a neuron-specific promoter to express a dendrite-targeted green fluorescent protein (GFP) (Kameda et al., 2008). The promoter contains an insulator (INS), an upstream enhancer from the tyrosine hydroxylase (TH) promoter, and a neurofilament heavy gene (NFH) promoter; the INS-TH-NFH promoter supports ≥90 % neuron-specific expression (Zhang et al., 2000). The dendrite-targeted GFP fuses both a myristoylation/palmitoylation site and a basolateral (dendrite) membrane-sorting domain to GFP (Kameda et al., 2008). To establish expression in dendrites, this vector was packaged using standard conditions, and injected into PER cortex. The rats were sacrificed at 8 days after gene transfer, and immunofluorescent analyses showed GFP-IR in processes with dendritic morphology (Fig. 4A). The dendritic identity of these processes was confirmed by costaining for GFP-IR and a dendrite marker, MAP2-IR (Huber and Matus, 1984), and most of the transduced processes contained MAP2-IR (Fig. 4B-D). Further, high power views under the confocal microscope also showed that these GFP-IR processes contained MAP2-IR (Fig. 4E-G). In contrast, after injecting one of the presynaptic vectors into POR cortex, in PER cortex, we observed His-tag-IR in axon terminals that lacked MAP2-IR (Fig. 4H-J). As a control, omission of the primary antibodies resulted in no IR under the confocal microscope (Fig. 4K-M). In summary, the results show that the vector expressing dendrite-targeted GFP supports labeling of dendrites. For use in a comparison, untargeted postsynaptic vector, we constructed a dendrite-targeted, enzymatically inactive PKC (Fig. 2; dendrite-targeted-PkcΔGG); the GFP in dendrite-targeted GFP was replaced with PkcΔGG, which contains a point mutation that blocks PKC activity (Song et al., 1998).
Fig. 4.
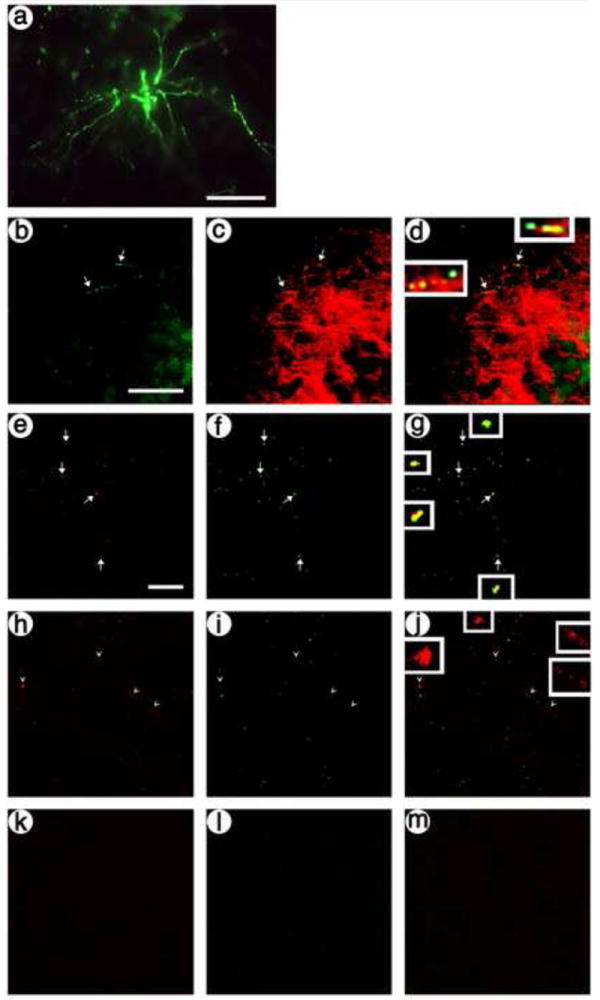
The postsynaptic, dendrite-targeted GFP vector supports expression of GFP in cell bodies and dendrites in PER cortex. Rats were sacrified at 8 days after gene transfer. (A) pINS-TH-NFHdendrite-gfp supports expression in cell bodies and dendrites in PER cortex, GFP-IR. (B-D) pINS-TH-NFHdendrite-gfp supports expression in dendrites in PER cortex, identified by costaining with an dendrite marker, MAP2; (B) GFP-IR, (C) MAP2-IR, and (D) merge. Arrows, dendrites; arrowheads, axons. (E-G) Confocal microscopy confirms that pINS-TH-NFHdendrite-gfp supports expression in dendrites in PER cortex; (E) GFP-IR, (F) MAP2-IR, and (G) merge. (H-J) Confocal microscopy shows that a presynaptic vector, pVGLUT1dcv-secretogranin/anti-NR1-10aa/his-tag, supports expression in axons in PER cortex that lack the dendrite marker, MAP2; (H) His tag-IR, (I) MAP2-IR, and (J) merge. (K-M) Under the confocal microscope, omitting the primary antibodies from the assay resulted in background levels of fluorescence; (K) Texas red-conjugated secondary antibody-IR, (L) fluorescein-conjugated secondary antibody-IR, and (M) merge. Scale bars: (A) 50 μm; (B-D) 50 μm; (E-M) 40 μm.
2.2. Targeted gene transfer to presynaptic and postsynaptic neurons that form a glutamatergic synapse
Each presynaptic vector, expressing an artificial peptide neurotransmitter, was injected into POR cortex. The targeted postsynaptic vector, expressing dendrite-targeted GFP, was packaged for antibody-mediated targeting, and complexed with anti-His tag antibodies. For antibody-mediated targeting, we previously (Cao et al., 2010) added the Staphylococcus A ZZ domain, which binds antibodies, to a protein on the surface of an HSV-1 vector particle, glycoprotein C (gC). After packaging, the modified vector particles (containing gC--ZZ) are complexed with an antibody for a specific protein on the surface of specific neuron types; these vector particle/antibody complexes bind to a specific neuron type, and entry occurs by the mechanisms used by wt HSV-1 (Spear and Longnecker, 2003). The comparison postsynaptic vector, expressing dendrite-targeted PkcΔGG, was packaged using standard conditions. Eight days after the presynaptic gene transfer, a 1:1 mixture of the targeted and control postsynaptic vectors was injected into PER cortex; and 8 days later, the rats were sacrificed. Targeting was analyzed by costaining alternating sections for the presynaptic vector (His-tag-IR) and either the targeted postsynaptic vector (GFP-IR) or the control, untargeted postsynaptic vector (flag-IR, PkcΔGG contains the flag tag).
The results showed that each artificial peptide neurotransmitter supports targeted gene transfer to postsynaptic neurons that form synapses with the transduced presynaptic neurons. We first examined the presynaptic vector containing the Secretogranin DCV domain peptide neurotransmitter and the targeting postsynaptic vector: Fluoresence microscopy showed that in PER cortex, transduced presynaptic axons were closely associated with transduced postsynaptic neurons/dendrites (Fig. 5). Further, confocal images showed transduced axons made contacts with dendrites transduced by the postsynaptic targeting vector (Fig. 6A-C), but not with dendrites transduced by the control, untargeted postsynaptic vector (Fig. 6D-F). Additionally, using the presynaptic vector containing the POMC DCV domain peptide neurotransmitter, confocal images showed transduced axons made contacts with dendrites transduced by the postsynaptic targeting vector (Fig. 7A-C), but not with dendrites transduced by the control, untargeted postsynaptic vector (Fig. 7D-F).
Fig. 5.
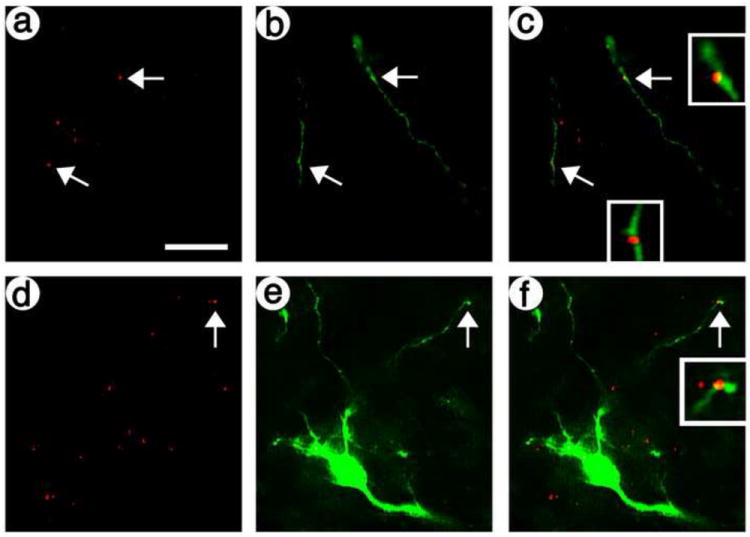
The artificial peptide neurotransmitter containing the Secretogranin DCV domain supports targeted gene transfer to dendrites closely associated with transduced axons. The presynaptic vector containing the Secretogranin DCV domain was injected into POR cortex; 8 days later, the targeting postsynaptic vector was injected into PER cortex; and 8 days later, the rats were sacrificed. PER cortex was costained for transduced presynaptic axons and transduced postsynaptic dendrites: (A and D) transduced axons, His-tag-IR; (B and E) transduced dendrites, GFP-IR; and (C and F) merge. Arrows, costaining of a transduced axon proximal to a transduced dendrite. Scale bar: 25 μm.
Fig. 6.
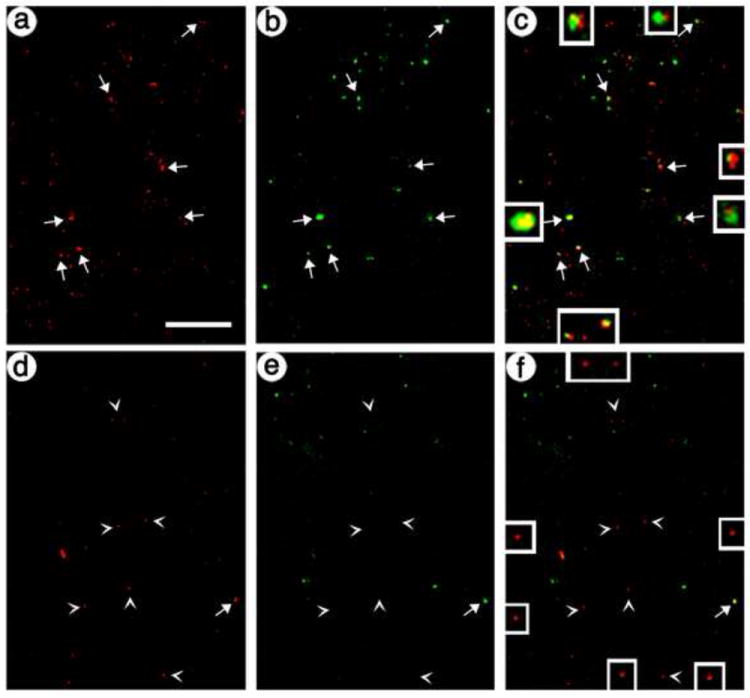
Confocal images show targeted gene transfer across synapses is supported by the artificial peptide neurotransmitter containing the Secretogranin DCV domain. The presynaptic vector containing the Secretogranin DCV domain was injected into POR cortex; 8 days later, a 1:1 mixture of the targeting and control postsynaptic vectors was injected into PER cortex; and 8 days later, the rats were sacrificed. PER cortex was costained for transduced presynaptic axons and transduced postsynaptic dendrites. (A-C) The targeting postsynaptic vector: (A) axons, His-tag-IR; (B) dendrites, GFP-IR; and (C) merge. Arrows, costaining. (D-F) The control, untargeted postsynaptic vector: (D) axons, His-tag-IR; (E) dendrites, flag-IR; and (F) merge. Arrowheads, His-tag-IR only. Scale bar: 20 μm.
Fig. 7.

Confocal images show targeted gene transfer across synapses is supported by the artificial peptide neurotransmitter containing the POMC DCV targeting construct. The presynaptic vector containing the POMC DCV domain was injected into POR cortex; 8 days later, a 1:1 mixture of the targeting and control postsynaptic vectors was injected into PER cortex; and 8 days later, the rats were sacrificed. PER cortex was costained for transduced presynaptic axons and transduced postsynaptic dendrites. (A-C) The targeting postsynaptic vector: (A) axons, His-tag-IR; (B) dendrites, GFP-IR; and (C) merge. Arrows, costaining. (D-F) The control, untargeted postsynaptic vector: (D) axons, His-tag-IR; (E) dendrites, flag-IR; and (F) merge. Arrowheads, His-tag-IR only. Scale bar: 20 μm.
Counts of the confocal images showed that targeted gene transfer supported a statistically significant increase in transduction of postsynaptic neurons that form synapses with transduced presynaptic neurons. Using either presynaptic vector, and targeted gene transfer for the postsynaptic vector, ≥20 % of the transduced presynaptic axons were associated with a transduced postsynaptic dendrite (Table 1). In contrast, for control, untargeted gene transfer for the postsynaptic vector, only 3 % of the transduced presynaptic axons were associated with a transduced postsynaptic dendrite (Secretogranin DCV domain peptide neurotransmitter, F(1,4)=176 p<0.0002; POMC DCV domain peptide neurotransmitter, F(1,4)=62.6 p<0.002).
Table 1.
The efficiency of targeted gene transfer across glutamatergic synapses; the % transduced presynaptic axons connected to a transduced postsynaptic dendrite
| Postsynaptic vector | ||||
|---|---|---|---|---|
| Presynaptic Vector, DCV domain | Targeting vector | Control vector | ||
| His-tag-IR axons | % GFP-IR costaining | His-tag-IR axons | % Flag-IR costaining | |
| Secretogranin | 260±38 | 20±1 | 276±12 | 3±0 |
| POMC | 188+14 | 21+2 | 333+79 | 3+1 |
In each section that was examined, all the transduced presynaptic axons were scored for being adjacent to, or distant from, a transduced postsynaptic dendrite. Three rats per condition; mean±s.e.m. are shown.
3. Discussion
We have developed a powerful new technology to analyze how specific neurons and subcircuits contribute to circuit physiology, behavior, and learning; we delivered a gene into a particular type of presynaptic neuron, and then delivered a different gene into an identified subset of their postsynaptic neurons, connected by a glutamatergic synapse. To support targeted gene transfer across a glutamatergic synapse, the presynaptic vector expresses an artificial peptide neurotransmitter containing a DCV targeting domain (Dikeakos and Reudelhuber, 2007), a NMDA NR1subunit binding domain (Moskal et al., 2001; Moskal et al., 2005), and the His tag. Upon release, this peptide neurotransmitter binds to NMDA receptors on postsynaptic neurons. The second gene transfer targets the postsynaptic neurons, using antibody-mediated targeted gene transfer (Cao et al., 2010) and a His tag antibody. For the model system, we studied the projection from POR to PER cortex (Agster and Burwell, 2009; Burwell and Amaral, 1998a; Burwell and Amaral, 1998b; Burwell, 2000), and showed that with targeted gene transfer, ≥20 % of the transduced presynaptic axons were associated with a transduced postsynaptic dendrite; but with untargeted gene transfer, only ~3 % of transduced presynaptic axons were associated with a transduced postsynaptic dendrite. Of note, this technology delivers a gene into an identified subset of the postsynaptic neurons; neurons in POR cortex project to multiple neocortical areas (Agster and Burwell, 2009), but postsynaptic neurons in PER cortex were selectively transduced. This technology can be adapted to other virus vectors, as most virus vectors are capable of expressing the artificial peptide neurotransmitter, and Staphylococcus A protein ZZ domain-mediated targeted gene transfer to peripheral cell types has been established with classical retrovirus, lentivirus, AAV, adenovirus, and Sindbis virus vectors (reviewed in (Cao et al., 2010)).
Additional specificity is already available for the presynaptic neuron type, postsynaptic neuron type, or synapse type. In this study, the presynaptic vector was delivered by standard, untargeted gene transfer, and this vector used the VGLUT1 promoter to restrict expression to VGLUT1-containing glutamatergic neurons (Rasmussen et al., 2007; Zhang and Geller, 2010). The presynaptic vector could be delivered into specific neuron types using targeted gene transfer; we established antibody-mediated targeted gene transfer to NR1-, NR2A-, or NR2B-containing neurons (Cao et al., 2010; Cao et al., 2011), and ligand-mediated targeted gene transfer to neurons containing specific neurotrophic factor receptors (Cao et al., 2008; Wang et al., 2005). Further, expression from the presynaptic vector could be restricted to specific glutamatergic neuron subtypes by using specific VGLUT1 promoter fragments (Zhang et al., 2011). Here, the postsynaptic vector used a neuron-specific promoter to support expression in most neuron types (Zhang et al., 2000), but expression could be restricted to specific neuron types by using promoters that support enkephalinergic-, catecholaminergic-, GABAergic-, glutamatergic-, or glutamatergic subtype-specific expression (Jin et al., 1996; Kaplitt et al., 1994; Rasmussen et al., 2007; Song et al., 1997; Zhang and Geller, 2010; Zhang et al., 2011). Here, targeting across most glutamatergic synapses was obtained by using artificial peptide neurotransmitters that can bind to NMDA NR1 subunits. Of note, targeting across other synapse types could be obtained by replacing the NR1 binding domain in the artificial peptide neurotransmitter with binding domains for different neurotransmitter receptors. Importantly, targeting was supported by artificial peptide neurotransmitters containing either an anti-NR1 variable region or a 10 aa fragment containing NR1 binding activity (Moskal et al., 2001; Moskal et al., 2005), establishing that either an entire variable region or a small peptide can support targeting.
The technology developed here is novel. Synapses between specific neurons have been visualized using GRASP or BLINC (Feinberg et al., 2008; Thyagarajan and Ting, 2010); these technologies use untargeted gene transfer, and do not selectively deliver genes into connected neurons. Specific viruses for mapping anteriograde or retrograde projections across one, or multiple, synapses exploit the spread of a single virus across synapses, thereby delivering the same gene into the presynaptic and postsynaptic neurons. A Rabies Virus-based technology can first deliver a gene into postsynaptic neurons, and then deliver a different gene into all the presynaptic neurons (Osakada et al., 2011). This rabies-based technology supports retrograde monosynaptic gene transfer; in contrast, the technology reported here supports anterograde monosynaptic gene transfer; thus, these two technologies will support different physiological applications. Further, this rabies-based technology requires a cre-expressing mouse line, lacks targeting specificity for either synapse type or subsets of the presynaptic neurons, and physiologically useful expression is limited to 5-11 days after gene transfer (Osakada et al., 2011).
This powerful new capability to deliver a specific gene into a particular type of presynaptic neuron, and then deliver a different gene into an identified subset of their postsynaptic neurons, connected by a specific synapse type, will help elucidate how specific neurons and subcircuits support circuit physiology, behavior, and learning. Importantly, this technology may support identification of specific subcircuits that are required for specific behaviors or learning: Following activation of presynaptic neurons (Fenno et al., 2011), essential subcircuits and postsynaptic neurons might be identified by blocking the activity of these neurons, using the Drosophila allatostatin receptor or other genes (Dymecki and Kim, 2007; Lechner et al., 2002; Luo et al., 2008). In particular, the POR to PER subcircuit studied here may be essential for visual learning, and this hypothesis can be tested using this new technology. Activation of PKC pathways in the presynaptic POR cortex neurons supports enhanced visual discrimination learning and encoding of some essential information in the genetically-modified circuit in POR cortex (Zhang et al., 2005; Zhang et al., 2010a); further, the transduced neurons have a large projection to PER cortex (Zhang et al., 2010b). Thus, the role of the postsynaptic PER cortex neurons in this learning can now be studied by first activating PKC pathways in the presynaptic POR cortex neurons, and then using this new technology to selectively block activity in the postsynaptic PER cortex neurons. More generally, the function of specific subcircuits might be studied by delivering different physiological genes into the presynaptic or postsynaptic neurons. For example, after optogenetic activation of the presynaptic neurons, the function of specific postsynaptic neurons could be studied by expressing critical physiological genes; such as specific glutamate receptors, postsynaptic density components or transcription factors; or specific sensors, such as calcium or transcriptional sensors (Dymecki and Kim, 2007; Luo et al., 2008).
4. Materials and Methods
4.1. Materials
OptiMEM, penicillin/streptomycin, Dulbecco’s modified minimal essential medium (DMEM), and fetal bovine serum (FBS) were obtained from Invitrogen; G418 was obtained from RPI; and X-gal was from Sigma. The primary antibodies were rabbit anti-β-gal (Chemicon), mouse anti-His tag (for targeting, Qiagen), rabbit anti-His tag (for immunohistochemistry, Cell Signaling), rabbit anti-GFP (Invitrogen), rabbit anti-flag (Sigma), mouse anti-tau (Millipore), and mouse anti-MAP2 (Sigma). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (IgG), FITC-conjugated goat anti-rabbit IgG, and Texas red-conjugated horse anti-mouse IgG were from Vector Labs.
4.2. Vectors
All the vectors used in this study contain our standard vector backbone (Fig. 2; (Song et al., 1997; Zhang et al., 2000)).
pVGLUT1lac contains the mouse VGLUT1 promoter, consisting of an upstream promoter fragment (7 kb) and the first intron (4.6 kb) (Rasmussen et al., 2007). To construct pVGLUT1linker, pVGLUT1lac was digested with Asc I and BsiW I; these sites are located at the 3’ end of the VGLUT1 first intron or near the 3’ end of the Lac Z gene, respectively; and the following two oligonucleotides were inserted: Sense 5’ CGCGCCGTTTAAACCAGTTCTACGTATTAATTAAC 3’ and antisense 5’ GTACGTTAATTAATACGTAGAACTGGTTTAAACGG 3’. The artificial peptide neurotransmitter containing the Secretogranin domain fused i) a DCV targeting domain from human Secretogranin II (signal peptide and amino acids (aa) 1-41 (Courel et al., 2008; Gerdes et al., 1989)), to ii) a 10 aa peptide that binds the NMDA NR-1 subunit, derived from an anti-NR-1 monoclonal antibody (CQQHYSTPPC (Moskal et al., 2001; Moskal et al., 2005)), to iii) a 20 aa spacer, to iv) the His tag; this construct was inserted into pUC57 (pUC57dcv-secretogranin/anti-NR1-10aa/his-tag, synthesized by Genscript; DNA sequence in Sup. Fig. S1). The artificial peptide neurotransmitter containing the POMC domain fused i) a DCV targeting domain from mouse POMC (the signal peptide and the DCV targeting domain, aa -26 to 101 (Cool and Loh, 1994; Cool et al., 1995)), to ii) the variable region of the anti-NR-1 antibody (107 aa (Moskal et al., 2001; Moskal et al., 2005)), to iii) the His tag; this construct was inserted into pUC57 (pUC57dcv-pomc/anti-NR1-var/his-tag, DNA sequence in Sup. Fig. S2). The DNA sequences encoding the 10 aa peptide that binds NR-1, the 20 aa spacer, the his tag, and the monoclonal antibody variable region were chosen with reference to human codon biases (Lavner and Kotlar, 2005), and a Kozak consensus translation initiation sequence was used in each construct (Kozak, 1986; Kozak, 1987; Nakagawa et al., 2008). To isolate HSV-1 vectors expressing each artificial peptide neurotransmitter, pVGLUT1linker, pUC57dcv-secretogranin/anti-NR1-10aa/his-tag, and pUC57dcv-pomc/anti-NR1-var/his-tag were digested with Asc I and Pac I; and each peptide neurotransmitter was inserted into the vector backbone to yield pVGLUT1dcv-secretogranin/anti-NR1-10aa/his-tag or pVGLUT1dcv-pomc/anti-NR1-var/his-tag (Fig. 2). pVGLUT1gap-lac has been described (Zhang et al., 2010b).
Dendrite-targeted GFP (Kameda et al., 2008) fused both a myristoylation/palmitoylation site (from Fyn) and a basolateral (dendrite) membrane-sorting domain (from the low density lipoprotein receptor) to GFP. pINS-TH-NFHdendrite-gfp (Fig. 2) has been described (Zhang et al., 2012). Dendrite-targeted PkcΔGG, which replaced the GFP gene in dendrite-targeted GFP with PkcΔGG (Song et al., 1998), was synthesized and inserted into pUC57 (pUC57dendrite-kkcΔGG; DNA sequence in Sup. Fig. S3). pINS-TH-NFHdendrite-gfp and pUC57dendrite-pkcΔGG were each digested with Asc I and Pac I; and the dendrite-PkcΔGG fragment was inserted into vector-backbone-INS-TH-NFH fragment to yield pINS-TH-NFHdendrite-pkcΔGG (Fig. 2).
4.3. Cells and vector packaging
BHK21 and 2-2 cells were maintained in DMEM supplemented with 10 % FBS, 4 mM glutamine and penicillin/streptomycin. They were grown in an incubator at 37 °C, 5 % CO2, and 100 % humidity. 2-2 cells were used for HSV-1 vector packaging; G418 (0.5 mg/ml), present during the growth of 2-2 cells, was removed before plating cells for packaging; 2-2 cells contain the HSV-1 immediate early 2 (IE 2) gene and were maintained under previously characterized selective conditions (Smith et al., 1992). Late-log phase, confluent cultures of BHK21 cells were used for titering the resulting vector stocks.
Vectors were packaged into HSV-1 particles using a modified form of the helper-virus free packaging protocol, described previously (Fraefel et al., 1996; Sun et al., 1999). For targeted gene transfer, pINS-TH-NFHdendrite-gfp was packaged in the presence of gC--ZZ and gBpK- (lacks the glycosaminoglycan binding domain that supports binding to many cell types), and complexed with anti-His tag antibodies (1:1 mixture of two mouse anti-His tag antibodies, Qiagen 34650 and 34660; 5 μg/ml total antibody concentration in the vector binding procedure), as described (Cao et al., 2010).
Purified vectors were titered on BHK cells, by either performing immunocytochemistry or X-gal staining at 24 hours after transduction. We previously quantified the titer of vector genomes (VG/ml), and the packaging efficiency (VG/ml / IVP/ml), for pVGLUT1lac, pINS-TH-NFHlac, and specific vector stocks for antibody-mediated targeted gene transfer, using a PCR assay; the VG/ml titer, and the packaging efficiencies, for these stocks were similar to a number of other vectors we have studied (Cao et al., 2010; Cao et al., 2011; Gao et al., 2007; Rasmussen et al., 2007; Yang et al., 2001; Zhang et al., 2000). We did not repeat the VG/ml assay here because the vectors used here are similar to previously studied vectors. The titers of the vector stocks used in this study were: pVGLUT1dcv-secretogranin/anti-NR1-10aa/his-tag 1 × 106 IVP/ml, pVGLUT1dcv-pomc/anti-NR1-var/his-tag 1 × 106 IVP/ml, pVGLUT1gap-lac 2 × 106 IVP/ml, pINS-TH-NFHdendrite-gfp/gC--wt 2 × 106 IVP/ml (for vector testing, Fig. 4), pINS-TH-NFHdendrite-gfp/gC--ZZ+anti-His tag 2 × 106 IVP/ml, and pINS-TH-NFHdendrite-pkcΔGG 2 × 106 IVP/ml. Wild-type HSV-1 was not detected (<10 plaque forming units/ml) in any of the vector stocks studied here.
4.4. Stereotactic injections of vectors into rat neocortex
The VA Boston Healthcare System IACUC approved all the animal procedures. Adult male Long-Evans rats (150-200 g) were anesthetized by ip injection of a Ketamine (20 mg/ml) Xylazine (2 mg/ml) mixture with a final dose of 60 mg/kg and 6 mg/kg, respectively. Additional anesthesia was administered as needed. For the presynaptic gene transfer, each rat received a single injection of a specific vector into the left POR cortex: The injection coordinates were anterior-posterior (AP) -8.0, medial-lateral (ML) -6.0, dorsal-ventral (DV) -5.2 (Paxinos and Watson, 1986). For the postsynaptic gene transfer, each rat each rat received a single injection of a specific vector mixture into the left PER cortex: The injection coordinates were AP -5.0, ML -6.6, DV -7.0. AP is measured relative to bregma, ML is relative to the sagittal suture, and DV is relative to the bregma-lambda plane. A micropump (model 100, KD Scientific) was used for the injections; 3 μl of vector stock was injected over 5 minutes, and after 5 additional minutes, the needle was slowly retracted.
4.5. The targeting strategy
The first gene transfer (Fig. 1), to the presynaptic neurons in POR cortex, used standard procedures, and the injection coordinates used to study visual learning (Zhang et al., 2005; Zhang et al., 2010a). With these conditions, the vast majority of the gene transfer is to neurons in POR cortex; specific neocortical areas with large projections to POR cortex, including PER cortex, contain ~1 % of the number of transduced neurons as POR cortex. A previously reported VGLUT1 promoter restricts expression to VGLUT1-containing neurons; after gene transfer into POR cortex, >90 % of the transduced neurons are glutamatergic, VGLUT1-containing neurons (Rasmussen et al., 2007; Zhang and Geller, 2010). The presynaptic vectors use the VGLUT1 promoter to express an artificial peptide neurotransmitter. Upon release, this peptide neurotransmitter binds to NMDA receptors on the postsynaptic neurons.
The second gene transfer targets the postsynaptic neurons, using antibody-mediated targeted gene transfer and an anti-His tag antibody, as the peptide neurotransmitter contains the His tag (Fig. 1). We previously developed antibody-mediated targeted gene transfer to specific types of neurons with HSV-1 vectors (Cao et al., 2010; Cao et al., 2011). The Staphylococcus A ZZ domain, which binds antibodies, was added to a protein on the surface of an HSV-1 vector particle, glycoprotein C (gC), to yield gC--ZZ. After vector packaging, the modified vector particles, which contain gC--ZZ, are complexed with an antibody that recognizes a specific protein on the surface of specific neuron types. Thus, these vector particle/antibody complexes bind to a specific neuron type, and entry occurs by the mechanisms used by wild type (wt) HSV-1; gD binds to a specific receptor and the HSV-1 envelope fuses to the cell membrane (Spear and Longnecker, 2003). Antibody-mediated targeted gene transfer was established to neurons that contain NMDA NR1, NR2A, or NR2B subunits; this technology supports targeting to rare neuron types with high specificity, as shown by targeting to NR2A-containing neurons in POR cortex (Cao et al., 2010; Cao et al., 2011).
The postsynaptic targeting vector (Fig. 1) is packaged using gC--ZZ (Cao et al., 2010), for targeting with anti-His antibodies. The postsynaptic vectors used the INS-TH-NFH promoter (Zhang et al., 2000). To label dendrites in the postsynaptic neurons, we used a previously reported dendrite-targeted GFP (Kameda et al., 2008).
4.6. Immunohistochemistry
Brains were perfused as described (Zhang et al., 2000), and 25 μm coronal sections were prepared using a freezing microtome. Immunohistochemistry was performed on free-floating sections as described (Zhang et al., 2000). The primary antibodies were rabbit anti-β-gal (1:400 dilution), rabbit anti-His tag (1:100 dilution), rabbit anti-GFP (1:400 dilution), rabbit anti-flag (1:400 dilution), mouse anti-tau (1:50 dilution), and mouse anti-MAP2 (1:200 dilution). This anti-MAP2 antibody is a dendrite-specific marker (Huber and Matus, 1984). Primary antibodies were visualized with FITC-conjugated goat anti-mouse IgG or with FITC-conjugated goat anti-rabbit IgG and Texas red-conjugated horse anti-mouse IgG (1:200 dilutions).
4.7. Axon and dendrite counts
Digital images were taken at 60X magnification under the confocal microscope. In the fields that were examined, all the His tag-IR axons were scored for being proximal to, or distant from, a GFP-IR or flag-IR dendrite. All counts were done at least two separate times, and results differed by <10 %.
Supplementary Material
Acknowledgments
We thank Dr. R. Cook for motivating this study by emphasizing the importance of this technique. We gratefully thank Drs. N. Brose and C. Rosenmund for the VGLUT1 promoter, Dr. G. Felsenfeld for the ß-globin insulator, Dr. K. O’Malley for the TH promoter, Dr. W. Schlaepfer for the NFH promoter, and Dr. A. Davison for HSV-1 cosmid set C. This work was supported by NIH Grants AG025894 (G.Z.), NS045855 and NS057558 (A.I.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agster KL, Burwell RD. Cortical efferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. Hippocampus. 2009;19:1159–86. doi: 10.1002/hipo.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Perirhinal and postrhinal cortices of the rat: interconnectivity and connections with the entorhinal cortex. J Comp Neurol. 1998a;391:293–321. doi: 10.1002/(sici)1096-9861(19980216)391:3<293::aid-cne2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. J Comp Neurol. 1998b;398:179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Ann NY Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Cao H, Zhang GR, Wang X, Kong L, Geller AI. Enhanced nigrostriatal neuron-specific, long-term expression by using neural-specific promoters in combination with targeted gene transfer by modified helper virus-free HSV-1 vector particles. BMC Neurosci. 2008;9:37. doi: 10.1186/1471-2202-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang GR, Geller AI. Antibody-mediated targeted gene transfer to NMDA NR1-containing neurons in rat neocortex by helper virus-free HSV-1 vector particles containing a chimeric HSV-1 glycoprotein C--Staphylococcus A protein. Brain Res. 2010;1351:1–12. doi: 10.1016/j.brainres.2010.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Zhang GR, Geller AI. Antibody-mediated targeted gene transfer to NMDA NR2A- or NR2B-containing neurons in rat neocortex by helper virus-free HSV-1 vector particles. Brain Research. 2011;1415:127–135. doi: 10.1016/j.brainres.2010.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Loh YP. Identification of a sorting signal for the regulated secretory pathway at the N-terminus of pro-opiomelanocortin. Biochimie. 1994;76:265–70. doi: 10.1016/0300-9084(94)90156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool DR, Fenger M, Snell CR, Loh YP. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J Biol Chem. 1995;270:8723–9. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- Courel M, Vasquez MS, Hook VY, Mahata SK, Taupenot L. Sorting of the neuroendocrine secretory protein Secretogranin II into the regulated secretory pathway: role of N- and C-terminal alpha-helical domains. J Biol Chem. 2008;283:11807–22. doi: 10.1074/jbc.M709832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikeakos JD, Reudelhuber TL. Sending proteins to dense core secretory granules: still a lot to sort out. J Cell Biol. 2007;177:191–6. doi: 10.1083/jcb.200701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y. The Neurobiology of Memory. Oxford Univ. Press; Oxford, England: 1989. [Google Scholar]
- Dymecki SM, Kim JC. Molecular neuroanatomy’s “Three Gs”: a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) Defines Cell Contacts and Synapses in Living Nervous Systems. Neuron. 2008;57:353–63. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraefel C, Song S, Lim F, Lang P, Yu L, Wang Y, Wild P, Geller AI. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J Virol. 1996;70:7190–7. doi: 10.1128/jvi.70.10.7190-7197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Sun M, Wang X, Geller AI. Isolation of an enhancer from the rat tyrosine hydroxylase promoter that supports long-term, neuronal-specific expression from a neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 2007;1130:1–16. doi: 10.1016/j.brainres.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes HH, Rosa P, Phillips E, Baeuerle PA, Frank R, Argos P, Huttner WB. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989;264:12009–15. [PubMed] [Google Scholar]
- Huber G, Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984;4:151–60. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin BK, Belloni M, Conti B, Federoff HJ, Starr R, Son JH, Baker H, Joh TH. Prolonged in vivo gene expression driven by a tyrosine hydroxylase promoter in a defective herpes simplex virus amplicon vector. Hum Gene Ther. 1996;7:2015–24. doi: 10.1089/hum.1996.7.16-2015. [DOI] [PubMed] [Google Scholar]
- Kameda H, Furuta T, Matsuda W, Ohira K, Nakamura K, Hioki H, Kaneko T. Targeting green fluorescent protein to dendritic membrane in central neurons. Neurosci Res. 2008;61:79–91. doi: 10.1016/j.neures.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Kaplitt MG, Kwong AD, Kleopoulos SP, Mobbs CV, Rabkin SD, Pfaff DW. Preproenkephalin promoter yields region-specific and long-term expression in adult brain after direct in vivo gene transfer via a defective herpes simplex viral vector. Proc Natl Acad Sci U S A. 1994;91:8979–83. doi: 10.1073/pnas.91.19.8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–92. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5’-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–48. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavner Y, Kotlar D. Codon bias as a factor in regulating expression via translation rate in the human genome. Gene. 2005;345:127–38. doi: 10.1016/j.gene.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Lechner HA, Lein ES, Callaway EM. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–90. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo L, Anderson DJ. A cre-dependent, anterograde transsynaptic viral tracer for mapping output pathways of genetically marked neurons. Neuron. 2011;72:938–50. doi: 10.1016/j.neuron.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–60. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal JR, Yamamoto H, Colley PA. The use of antibody engineering to create novel drugs that target N-methyl-D-aspartate receptors. Curr Drug Targets. 2001;2:331–45. doi: 10.2174/1389450013348399. [DOI] [PubMed] [Google Scholar]
- Moskal JR, Kuo AG, Weiss C, Wood PL, O’Connor Hanson A, Kelso S, Harris RB, Disterhoft JF. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacology. 2005;49:1077–87. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual Perception and Memory: A New View of Medial Temporal Lobe Function in Primates and Rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Niimura Y, Gojobori T, Tanaka H, Miura K. Diversity of preferred nucleotide sequences around the translation initiation codon in eukaryote genomes. Nucleic Acids Res. 2008;36:861–71. doi: 10.1093/nar/gkm1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Mori T, Cetin AH, Marshel JH, Virgen B, Callaway EM. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron. 2011;71:617–31. doi: 10.1016/j.neuron.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; Sidney: 1986. [DOI] [PubMed] [Google Scholar]
- Peters A, Jones E, editors. Cellular components of the cerebral cortex. Plenum Press; New York: 1984. [Google Scholar]
- Rasmussen M, Kong L, Zhang G, Liu M, Wang X, Szabo G, Curthoys NP, Geller AI. Glutamatergic or GABAergic neuron-specific, long-term expression in neocortical neurons from helper virus-free HSV-1 vectors containing the phosphate-activated glutaminase, vesicular glutamate transporter-1, or glutamic acid decarboxylase promoter. Brain Res. 2007;1144:19–32. doi: 10.1016/j.brainres.2007.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IL, Hardwicke MA, Sandri-Goldin RM. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology. 1992;186:74–86. doi: 10.1016/0042-6822(92)90062-t. [DOI] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak SY, Lang P, Ullrey D, Neve RL, O’Malley KL, Geller AI. An HSV-1 vector containing the rat tyrosine hydroxylase promoter enhances both long-term and cell type-specific expression in the midbrain. J Neurochem. 1997;68:1792–803. doi: 10.1046/j.1471-4159.1997.68051792.x. [DOI] [PubMed] [Google Scholar]
- Song S, Wang Y, Bak SY, During MJ, Bryan J, Ashe O, Ullrey DB, Trask LE, Grant FD, O’Malley KL, Riedel H, Goldstein DS, Neve KA, LaHoste GJ, Marshall JF, Haycock JW, Neve RL, Geller AI. Modulation of rat rotational behavior by direct gene transfer of constitutively active protein kinase C into nigrostriatal neurons. J Neurosci. 1998;18:4119–32. doi: 10.1523/JNEUROSCI.18-11-04119.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77:10179–85. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, Huang ZJ, Nelson SB. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhang GR, Yang T, Yu L, Geller AI. Improved titers for helper virus-free herpes simplex virus type 1 plasmid vectors by optimization of the packaging protocol and addition of noninfectious herpes simplex virus-related particles (previral DNA replication enveloped particles) to the packaging procedure. Hum Gene Ther. 1999;10:2005–11. doi: 10.1089/10430349950017365. [DOI] [PubMed] [Google Scholar]
- Thyagarajan A, Ting AY. Imaging activity-dependent regulation of neurexin-neuroligin interactions using trans-synaptic enzymatic biotinylation. Cell. 2010;143:456–69. doi: 10.1016/j.cell.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang X, Kong L, Zhang G, Sun M, Geller AI. Targeted gene transfer to nigrostriatal neurons in the rat brain by helper virus-free HSV-1 vector particles that contain either a chimeric HSV-1 glycoprotein C--GDNF or a gC--BDNF protein. Molec Brain Res. 2005;139:88–102. doi: 10.1016/j.molbrainres.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Zhang G, Zhang W, Sun M, Wang X, Geller AI. Enhanced reporter gene expression in the rat brain from helper virus-free HSV-1 vectors packaged in the presence of specific mutated HSV-1 proteins that affect the virion. Molec Brain Res. 2001;90:1–16. doi: 10.1016/s0169-328x(01)00059-6. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Yang T, Sun M, Zhang W, Wang Y, Geller AI. A tyrosine hydroxylase--neurofilament chimeric promoter enhances long-term expression in rat forebrain neurons from helper virus-free HSV-1 vectors. Molec Brain Res. 2000;84:17–31. doi: 10.1016/s0169-328x(00)00197-2. [DOI] [PubMed] [Google Scholar]
- Zhang G, Wang X, Kong L, Lu X, Lee B, Liu M, Sun M, Franklin C, Cook RG, Geller AI. Genetic enhancement of visual learning by activation of protein kinase C pathways in small groups of rat cortical neurons. J Neurosci. 2005;25:8468–81. doi: 10.1523/JNEUROSCI.2271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cao H, Kong L, O’Brien J, Baughns A, Jan M, Zhao H, Wang X, Lu X, Cook RG, Geller AI. Identified circuit in rat postrhinal cortex encodes essential information for performing specific visual shape discriminations. Proc Natl Acad Sci U S A. 2010a;107:14478–14483. doi: 10.1073/pnas.0912950107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Cao H, Li X, Zhao H, Geller AI. Genetic labeling of both the axons of transduced, glutamatergic neurons in rat postrhinal cortex and their postsynaptic neurons in other neocortical areas by Herpes Simplex Virus vectors that coexpress an axon-targeted ß-galactosidase and wheat germ agglutinin from a vesicular glutamate transporter-1 promoter. Brain Res. 2010b;1361:1–11. doi: 10.1016/j.brainres.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Geller AI. An HSV-1 vector containing the VGLUT1 promoter is expressed only in VGLUT1-, and not VGLUT2-, containing glutamatergic neurons. Brain Res. 2010;1331:12–19. doi: 10.1016/j.brainres.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li X, Cao H, Zhao H, Geller AI. The vesicular glutamate transporter-1 upstream promoter and first intron each support glutamatergic-specific expression in rat postrhinal cortex. Brain Research. 2011;1377:1–12. doi: 10.1016/j.brainres.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Zhao H, Cao H, Geller AI. Overexpression of either lysine-specific demethylase-1 or CLOCK, but not Co-Rest, improves long-term expression from a modified neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 2012;1436:157–67. doi: 10.1016/j.brainres.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


