Abstract
Acinetobacter baumannii is an opportunistic bacterial pathogen primarily associated with hospital-acquired infections. The recent increase in incidence, largely associated with infected combat troops returning from conflict zones, coupled with a dramatic increase in the incidence of multidrug-resistant (MDR) strains, has significantly raised the profile of this emerging opportunistic pathogen. Herein, we provide an overview of the pathogen, discuss some of the major factors that have led to its clinical prominence and outline some of the novel therapeutic strategies currently in development.
Keywords: Acinetobacter baumannii, antibiotic, infection, pathogen, stress, virulence
Introduction
Acinetobacter baumannii is a Gram-negative bacillus that is aerobic, pleomorphic and non-motile. An opportunistic pathogen, A. baumannii has a high incidence among immunocompromised individuals, particularly those who have experienced a prolonged (> 90 d) hospital stay.1 Commonly associated with aquatic environments,2 it has been shown to colonize the skin as well as being isolated in high numbers from the respiratory and oropharynx secretions of infected individuals.3 In recent years, it has been designated as a “red alert” human pathogen, generating alarm among the medical fraternity, arising largely from its extensive antibiotic resistance spectrum.4
This phenomenon of multidrug-resistant (MDR) pathogens has increasingly become a cause for serious concern with regard to both nosocomial and community-acquired infections.5 Indeed, the World Health Organization (WHO) has recently identified antimicrobial resistance as one of the three most important problems facing human health.6 The most common and serious MDR pathogens have been encompassed within the acronym “ESKAPE,” standing for Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.7
While in the 1970s A. baumannii is thought to have been sensitive to most antibiotics, today the pathogen appears to exhibit extensive resistance to most first-line antibiotics.8 More recently, A. baumannii has become a major cause for concern in conflict zones, and has gained particular notoriety in the resent desert conflicts in Iraq, earning it the moniker “Iraqibacter.” In particular, high incidences of MDR bacteremia (bloodstream infections) have been noted among US Army service members following Operation Iraqi Freedom (OIF).9 Interest from the scientific community over the past 15 years has led to significant advances of our understanding of this organism.10
Genus Acinetobacter
The Dutch microbiologist Beijerinck first isolated the organism in 1911 from soil using minimal media enriched with calcium acetate.11 Originally described as Micrococcus calco-aceticus, the genus Acinetobacter (coming from the Greek “akinetos,” meaning non-motile) was proposed some 43 years later by Brisou and Prevot12 to differentiate it from the motile organisms within the genus Achromobacter. The genus Acinetobacter was widely accepted by 1968 after Baumann et al.13 published a comprehensive study of organisms such as Micrococcus calco-aceticus, Alcaligenes hemolysans, Mima polymorpha, Moraxella lwoffi, Herellea vaginicola and Bacterium anitratum, which concluded that they belonged to a single genus and could not be further sub-classified into different species based on phenotypical characteristics.13 In 1971, the sub-committee on the Taxonomy of Moraxella and Allied Bacteria officially acknowledged the genus Acinetobacter based on the results of Baumann’s 1968 publication.14
The genus Acinetobacter, as currently defined, comprises Gram-negative, strictly aerobic, non-fermenting, non-fastidious, non-motile, catalase-positive, oxidase-negative bacteria with a DNA G + C content of 39% to 47%.5 Following DNA-DNA hybridization studies performed by Bouvet and Grimnot in 1986, the Acinetobacter genus now consists of 26 named species and nine genomic species.15 Four species of Acinetobacters (A. calcoaceticus, A. baumannii, Acinetobacter genomic species 3 and Acinetobacter genomic species 13TU) have such phenotypic similarities that they are difficult to differentiate, and as such are often referred to as the A. calcoaceticus-complex.16 This nomenclature can be misleading as the environmental species A. calcoaceticus has not been implicated in clinical disease, while the other three species in the A. calcoaceticus-complex are perhaps the most clinically significant species, being implicated in both community-acquired and nosocomial infections.5
Species
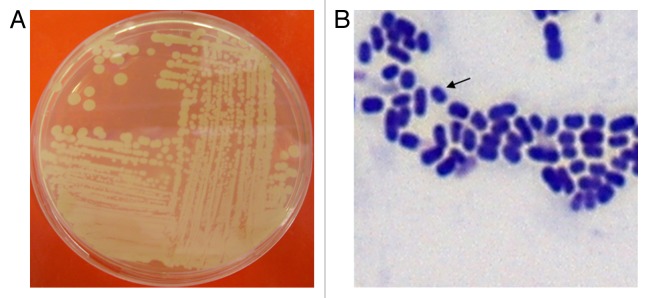
Acinetobacters may be identified presumptively to the genus level as Gram-negative, catalase-positive, oxidase-negative, non-motile, non-fermenting coccobacilli. However, the organisms are often difficult to de-stain and, as such, are often incorrectly identified as Gram-positive (see Fig. 1). There is no definitive metabolic test that can distinguish Acinetobacters from other non-fermenting Gram-negative bacteria.5 A method which is often used to identify to the genus level relies on the ability of the mutant A. baylyi strain BD413 trpE27 to be transformed by crude DNA of any Acinetobacter species to a wild-type phenotype (i.e., the transformation assay of Juni17). While for species level identification, the 28 available phenotypic tests have proven to be 95.6% effective in identifying human skin-derived Acinetobacters.18 However, phenotypic tests alone have proven to be ineffective in identifying more recently discovered genomic strains of Acinetobacters.5
Figure 1. (A) Complex streak of Acinetobacter baumannii following overnight growth on Luria-Bertani agar at 37°C. (B) Gram-stain of log phase A. baumannii cells grown in Luria-Bertani broth. Arrow indicates an individual A. baumannii cell.
More advanced molecular diagnostic methods have been developed for identification of Acinetobacters to the species level, these include:
• Amplified 16S rRNA gene restriction analysis (ARDRA)19
• High-resolution fingerprint analysis by amplified fragment length polymorphism (AFLP)20
• Ribotyping21
• tRNA spacer fingerprinting22
• Restriction analysis of the 16S–23S rRNA intergenic spacer sequences23
• Sequence analysis of the 16S–23S rRNA gene spacer region24
• Sequencing of the rpoB (RNA polymerase β-subunit) gene and its flanking spacers25
Natural Habitat
Organisms belonging to the genus Acinetobacter are often considered to be ubiquitous in nature given that they can be recovered from almost all soil and surface water samples.13 This understanding has contributed to the common misconception that A. baumannii is also ubiquitous.26 While not all Acinetobacters find their habitat in the natural environment, a thorough and systematic study to investigate the natural occurrence of the various Acinetobacter species in the environment has yet to be performed.5
As a pathogen, A. baumannii specifically targets moist tissues such as mucous membranes or areas of the skin that are exposed, either through accident or injury. Skin and soft tissues infected with A. baumannii initially present with a “peau d’orange” appearance (similar to the skin of an orange) followed by a sandpaper-like presentation which eventually gives way to clear vesicles on the skin.3 In areas of skin disruption hemorrhagic bullae can be seen, with a visible necrotizing process followed by bacteremia.3 If left untreated, this infection can lead to septicemia and death. Although it is likely that A. baumannii is responsible for these recognizable features, copathogens, such as Klebsiella pneumoniae, Candida albicans and Enterococcus faecalis, are thought to be a contributing factor. These co-pathogens may cause necrotizing infection and may create a nidus of entry into the bloodstream for A. baumannii.3
Despite its association with skin infections, A. baumannii is found only rarely as part of the normal skin microflora, with one study estimating that only 3% (at most) of the population are colonized by the bacterium.18 Interestingly, Acinetobacter was recovered from 22% of body lice sampled from homeless people, suggesting another potentially important reservoir for the pathogen.25
A key risk group for A. baumannii infection is members of the armed forces who have been deployed to conflict zones, particularly Iraq, earning A. baumannii the notorious moniker of ‘Iraqibacter.’ The dry, sandy conditions associated with these desert campaigns provide an ideal environment for the physiologically robust A. baumannii, making it the main source of infection among injured soldiers.9 A 2003 study on board the US Navy hospital ship USNS Comfort (T-AH-20) providing emergency on-site care for injured US combat forces situated on the Persian Gulf, revealed that 4.1% of all skin and soft-tissue infections (SSTIs) encountered were A. baumannii related, with the axilla, groin and toe webs being the areas of highest colonization.9
Furthermore, although originating in isolated conflict zones, the incidence of A. baumannii infections is on the increase, particularly in the UK and the US, as the coalition troops exposed to the bacterium in field hospitals return home to convalesce,27 making it a formidable emerging pathogen.28 Once A. baumannii is isolated in a hospital environment, this poses a significant risk, particularly in ICU wards where patients are chronically ill. As most of these patients are immunocompromised and spend a prolonged period of time in hospital, they represent a high risk group for A. baumannii infection.1 Patients that acquire artificial devices such as catheters, sutures, ventilators and those who have undergone dialysis or antimicrobial therapy within the past 90 days are also at risk of developing A. baumannii infections.1 The respiratory tract, blood, pleural fluid, urinary tract, surgical wounds, CNS, skin and eyes may be sites for infection or colonization.29,30 Pneumonia may pose a threat to those patients who require mechanical ventilation as A. baumannii has the ability to form biofilms on the surface of the endotracheal tube, which may account for the relatively high levels of colonization in the lower part of the respiratory tract.31
Pathogenesis-Virulence Potential
Despite extensive research into the virulence potential of this emerging pathogen, little is still known about its true pathogenic potential or virulence repertoire. While it is believed that several factors may contribute to the virulence potential of A. baumannii, one factor in particular, OmpA, a member of the Outer membrane proteins (OMPs), has been determined to contribute significantly to the disease causing potential of the pathogen.32 A. baumannii OmpA bind to the host epithelia and mitochondria, once bound to the mitochondria, OmpA induces mitochondrial dysfunction and causes the mitochondria to swell. This is followed by the release of cytochrome c, a heme protein, which leads to the formation of apoptosome. These reactions all contribute to apoptosis of the cell.32 OmpA, being the most abundant surface protein on the pathogen, is also involved in resistance to complement and the formation of biofilms33,34—two key stress survival strategies and potentially important virulence associated factors that help to promote bacterial survival both inside and outside the host. The ability of A. baumannii to form biofilms allows it to grow persistently in unfavorable conditions and environments. Indeed, A. baumannii has been shown to form biofilms on abiotic surfaces, which can include glass and equipment used in intensive care units, and/or on biotic surfaces such as epithelial cells.33 The most common factors that control biofilm formation can include nutrient availability, the presence of pili and outer membrane proteins and macromolecular secretions. Pili assembly and production of biofilm-associated protein (BAP) both contribute to the initiation of biofilm production and maturation after A. baumannii attach to particular surfaces.33 When pili attach to abiotic surfaces, they initiate the formation of microcolonies, followed by the full development of biofilm structures. BAP are present on the surface of bacterial cells and they contribute to biofilm development and maturation by stabilizing the mature biofilm on abiotic or biotic surfaces. Environmental signals, such as metal cations, also play a role in controlling the formation of biofilms, increasing the ability of A. baumannii to adhere to particular surfaces.33
Other key proteins that have been shown to contribute to A. baumannii virulence include phospholipase D and C. While phospholipase D is important for resistance to human serum, epithelial cell evasion and pathogenesis,35 phospholipase C enhances toxicity to epithelial cells.36 Along with OmpA, fimbria, also expressed on the surface of the bacterial cell, contribute to the adhesion of the pathogen to host epithelia.
Antibiotic Resistance
The rapid emergence of multi- and pandrug-resistant strains of Acinetobacter highlights the organism’s ability to quickly acclimatize to selective changes in environmental pressures. The upregulation of the organism’s innate resistance mechanisms coupled with the acquisition of foreign determinants have played a crucial role in the express route the organism has taken to becoming a multidrug-resistant pathogen.5
A 2006 study undertaken by Fournier et al.8 compared the genome of AYE and SDF Acinetobacter using whole shotgun genome sequences. In France, the epidemic AYE strain had a 26% mortality rate in infected patients,37 while the SDF strain came from the same geographical region, but was associated with human body lice, and was fully susceptible to antimicrobial agents. The genomic comparisons revealed that the genome of the virulent AYE strain contained an 86 kb region called a resistance “island” that contained a cluster of 45 resistance genes. The homologous location in the susceptible strain exhibited a 20 kb genomic island that is devoid of these resistance markers. This ability to “switch” its genomic structure goes some way to explaining the speed with which Acinetobacter captures resistance markers when under antibacterial pressure, such as may occur in a high risk environment, such as in hospital intensive care units5 (where broad spectrum antimicrobials are commonly used). Sequence similarity and phylogenetic analyses confirmed that most of the resistance genes found in the Acinetobacter strain AYE had been recently acquired from bacteria of the genera Pseudomonas, Salmonella or Escherichia.8
All genomic variants of A. baumannii contain a non-inducible chromosomal AmpC cephalosporinase, also known as Acinetobacter-derived cephalosporinases (ADCs).38 The presence of an upstream IS element known as ISAba1 determines the regulation of the AmpC gene. Overexpression of AmpC cephalosporinase and resistance to extended spectrum cephalosporin is intrinsically linked to the presence of ISAba1.39 Cefepime and carbapenems, however, appear to be stable in response to these enzymes.38
A. baumannii also possess an intrinsic class D oxacillinase belonging to the OXA-51-like group of enzymes that constitutes over 40 sequence variants.40 The ubiquitous nature of OXA-51-like genes in A. baumannii has led to this gene becoming an important genetic marker in identification of the organism to the species level.2 OXA-51-like enzymes are able to hydrolyze penicillins (benzylpenicillin, ampicillin, ticarcillin and piperacillin) and carbapenems (imipenem and meropenem) but do so only very weakly.5 A significant contribution to lactam resistance by OXA-51-like enzymes therefore requires the presence of an insertion element ISAba1 upstream of the gene, which acts as a strong transcriptional promoter.2 The most common enzymatic mode of carbapenem resistance is the production of oxacillinases encoded by genes of the blaOXA-23, blaOXA-40 and blaOXA-58-like lineage.
In Europe, the spread of multidrug-resistant Acinetobacter is not confined to hospitals within a city but also occurs on a national scale, mostly through inter-hospital patient transfers, for example the spread of the so-called Southeast clone and the Oxa-23 clones 1 and 2 in southeast England.41 International transfer of colonized patients has led to the introduction and subsequent epidemic spread of multidrug-resistant A. baumannii strains from southern into northern European countries, such as Belgium and Germany.42
In an industry-supported surveillance report (MYSTIC) from 48 European hospitals for the period 2002–2004, just 73.1% of A. baumannii isolates were susceptible to meropenem and 69.8% were susceptible to imipenem. Susceptibility to other antibiotics was also very low, with 32.4%, 34.0% and 47.6% being susceptible to ceftazidime, ciprofloxacin and gentamicin, respectively.43
There is a long history of multidrug-resistant A. baumannii infections occurring in the United States. In 1991 and 1992, outbreaks of carbapenem-resistant A. baumannii were observed in a hospital in New York City.44 This followed an outbreak of infections due to ESBL-producing Klebsiella pneumoniae during which use of imipenem increased substantially.45 In a more recent industry-supported surveillance study including isolates of Acinetobacter collected between 2004 and 2005 from 76 centers throughout the United States, only 60.2% were susceptible to imipenem.46
Numerous outbreaks of pandrug-resistant A. baumannii have been documented in Asian and Middle Eastern hospitals. Rates of non-susceptibility in SENTRY (Anti-microbial Surveillance Program) isolates (2001–2004) exceeded 25% for imipenem and meropenem, 40% for cefepime and ceftazidime, 40% for ampicillin-sulbactam, 35% for amikacin, and 45% for ciprofloxacin.47 It is worth noting that resistance to tigecycline and polymyxin B (drugs relied on heavily to treat infection with A. baumannii) both already exist in this region.48
Clinical Symptoms
A. baumannii infections are implicated across a wide range of anatomical regions and with varying severity and patient outcomes.49 There is considerable debate relating to the actual clinical impact of infection and its relationship to patient mortality. While a number of studies have concluded that infection with Acinetobacter has a detrimental effect on patient outcome,50,51 other similar studies implied little or no effect on patient outcome as a result of infection.52,53
The lack of consensus is most likely due to the difference in the approaches of the various studies; some being prospective while others have been of retrospective samples.49 The results generated by some studies have also only identified the organism to genus level but not to species level, with many referring to infection with Acinetobacter calcoaceticus-baumannii complex which could conceivably indicate colonization with the environmental species Acinetobacter calcoaceticus coupled with a polymicrobial infection, rather than a monomicrobial infection with a virulent Acinetobacter species such as MDR Acinetobacter.54
Hospital-acquired pneumonia
Ventilator associated pneumonia (VAP) is commonly linked to infection.55 Longer periods of hospitalization, longer time on mechanical ventilation and prior use of antibiotics are the recognized factors increasing the risk of VAP due to Acinetobacter. Nosocomial outbreaks have also been described due to health care professionals with colonized hands and poor personal hygiene;5 such individuals may act as opportunist carriers of an epidemic stain. Contaminated ventilators or respiratory care equipment as well as intra-hospital transmission may also contribute to the beginning of an outbreak.56
Community-acquired pneumonia
Pneumonia acquired outside of the hospital setting and caused by Acinetobacter has been noted in Australia and Asia.57 The source of infection may be throat carriage, which occurs in up to 10% of community residents with excessive alcohol consumption.57 It is characterized by a severe and sudden onset coupled with secondary bloodstream infection and has a mortality rate of between 40% and 60%.58
Bloodstream infections
In a seven year review (1995–2002) of nosocomial bloodstream infections in the United States, Acinetobacter accounted for 1.3% of all monomicrobial bloodstream infections.59 Acinetobacter was a more common cause of ICU-acquired bloodstream infection than of non-ICU-ward infection (1.6% vs. 0.9% of bloodstream infections, respectively, in those locations). Crude mortality figures overall from Acinetobacter bloodstream infection was 34.0% to 43.4% in the ICU and 16.3% outside the ICU.59 Acinetobacter bloodstream infection had the third highest crude mortality rate in the ICU, exceeded only by P. aeruginosa and Candida spp infections.59 It is notable that 102 patients had bloodstream infections at sites treating US military personnel injured in Iraq or Afghanistan from January 1, 2002 and August 31, 2004.9
Battlefield trauma and other wounds
Acinetobacter is a well-documented pathogen of burns units and is difficult to treat in patients with severe burns.60 However, infection of the skin and soft tissue outside of a military environment is uncommon.61 A retrospective review of 57 patients with SSTI revealed that eight cases were infected with Acinetobacter.3 In this instance all patients were male, ranging in age from 13 to 55 and of both American and Iraqi nationality. The median time from trauma to diagnosis with Acinetobacter infection was 15 d. All eight patients had a similar clinical presentation of SSTI; characteristic cellulitis with “peau d’orange” appearance, severe infection resulted in formation of bullae on the skin surface. The mortality rate in this instance was 12.5% (i.e., one of the eight died; however given that the patient was admitted with a gunshot wound to the groin, mortality cannot be solely assigned to infection).
Meningitis
Nosocomial, post-neurosurgical Acinetobacter meningitis is becoming increasingly more common with many other Gram-negative bacteria also becoming problematic in post-operative care.62 Installation of an external ventricular drain becomes a site for opportunistic infection. The mortality rate may be as high as 70%; however it is not possible to discern the definitive cause of mortality.63
Therapeutic Strategies
Existing antimicrobials
As mentioned previously, one of the distinguishing features of A. baumannii is its impressive array of acquired antibiotic resistance mechanisms, which although beyond the scope of this review, includes degradation enzymes against β-lactams, modification enzymes against aminoglycosides, altered binding sites for quinolones, and a variety of efflux mechanisms and changes in outer membrane proteins (see Peleg et al.5 for a detailed overview). Any and all of these elements can be combined to result in a highly drug-resistant pathogen; making selection of an appropriate empirical antimicrobial agent extremely difficult. Indeed, given the probability that A. baumannii would be most likely resistant to one of the common first line antibiotics, treatment of the infection should be performed following sound consideration of antimicrobial susceptibility testing. Nevertheless, as a delay in accessing correct treatment may have adverse consequences for a patient’s health, carbapenems such as Imipenem are often given as a drug of preference for serious and suspected Acinetobacter infections.64 However, despite its utility short-term, this prescription method jeopardizes future efficacy of such drugs as effective antimicrobial agents.
Future therapies
Given the rapid and extensive development of antibiotic resistance, several attempts have been made to develop alternative control strategies for dealing with A. baumannii including, but not limited to the following:
Bacteriophage
Recently renewed interest in the area of antibacterial phage therapy has gained some traction.67 Due to the high specificity of phage and their ability to work quickly, bacteriophage therapy is being re-examined as an alternative treatment to help counteract the phenomenon of antibiotic resistance.68 Indeed, a recent study by Yang et al.69 has resulted in the isolation and characterization of the virulent AB1 bacteriophage which has been shown to be effective against A. baumannii and as such represents a novel therapeutic of some potential.
Bactericidal gene transfer therapy
The design and delivery of vectors containing bactericidal genes that can be introduced into recipient pathogenic organisms by conjugation using attenuated donor cells is referred to as bactericidal gene transfer therapy. While the therapeutic potential of this approach is limited by the requirement for donor cells to be in contact with the pathogen (to facilitate vector transfer), positive effects have nonetheless been observed using murine burn infection models. Using this approach, Shankar et al.65 have shown that mice treated with a single dose of 1010 CFU of donor cells containing bactericidal genes had lower levels of A. baumannii in burn wounds compared with untreated mice.
Cathelicidins
Marsupials give birth to immunologically naïve, altricial young that reside in the maternal pouch for 9–10 mo while being supported by a sophisticated lactation system. In the pouch, cathelicidins interact with and destroy Gram-positive and Gram-negative bacteria, protozoa and fungi via electrostatic interactions between their positively charged peptides and the negatively charged molecules found in the cell membranes of their targets.70 The best studied cathelicidin is human LL-37; the only human cathelicidin, it exhibits both anti-tumor and anti-HIV activity.71 The Tammar Wallaby cathelicidin WAM1 has been shown to be effective against Acinetobacter, and is 3–80 times more potent than LL-37 against a host of bacterial pathogens. WAM1 was not hemolytic against human red blood cells indicating potential for parenteral use in humans.70 Indeed, WAM1’s anti-microbial activity and tolerance to salt concentrations similar to those found in the human body make it seem a likely candidate for further in vivo studies.72
Radioimmunotherapy
Although not yet not exploited as a therapeutic antimicrobial strategy in the clinic, radioimmunotherapy can target microorganisms as quickly and efficiently as cancer cells.66 This approach takes advantage of the specificity of antigen-antibody interactions to deliver radionuclides that emanate lethal doses of cytotoxic radiation directly to the target cell. Producing only transient hematological toxicity in experimental animals, radioimmunotherapy has been successfully adapted for the treatment of bacterial,73 fungal74 and viral75 infections. Given that previous studies have already described the development of antibodies against A. baumannii,76,77 the application of radioimmunotherapy as a novel therapeutic strategy for A. baumannii is a definite possibility.
Photodynamic therapy
Involves the combination of nontoxic photosensitizers (PSs) with oxygen and visible to produce reactive oxygen species that oxidize biomolecules thereby killing cells.78 The use of photodynamic therapy (PDT) to treat localized bacterial infections generally involves the topical application of a PS into the infected tissue, followed by illumination with red (or near-infrared) which is capable of penetrating the infected tissue.79 Using a murine burn wound model, this technique has previously been shown to be effective against A. baumannii while having no obvious effects on wound healing.80 Recently, Tsai et al.81 investigated the effect of chitosan, a polycationic biopolymer, on increasing the efficacy of PDT against a number of pathogens including A. baumannii. Under conditions in which hematoporphyrin-PDT exhibited a bacteriocidal effect on a 2- to 4-log scale, subsequent treatment with chitosan (0.025%) for a further 30 min completely eradicated the bacteria (at a starting inoculum of 108 CFU/ml). Chitosan alone did not exert significant antimicrobial activity, without prior PDT, suggesting that the potentiated effect of chitosan worked only after the bacterial damage induced by PDT. Furthermore, the potentiated PDT effect of chitosan appears to be related to the level of PDT damage and the deacetylation level of the chitosan.
Nanoparticle technology
Nitric oxide (NO) has been shown to exhibit potent antimicrobial activity as well as playing an important role in modulating immunity82 and regulating wound healing.83 Using nanotechnology based on a silane hydrogel, Friedman et al.84 have designed a stable nitric oxide (NO)-releasing nanoparticle (NO-np) platform. With the potential to serve as a novel, inexpensive and easily applied topical class of antimicrobials, this technology has been shown to be effective for the treatment of complex cutaneous infections such as those caused by A. baumannii. Indeed, Mihu et al.85 recently demonstrated the effect of NO-np against A. baumannii using murine wound and soft tissue models. Compared with control animals, NO-np-treated mice exhibited significant reductions in bacterial burden, enhanced wound healing rates and less collagen degradation by bacterial collagenases.
Conclusions
In conclusion, A. baumannii is an important opportunistic and emerging pathogen that can lead to serious nosocomial infections. Its pathogenic potential includes the ability to adhere to surfaces, form biofilms, display antimicrobial resistance and acquire genetic material from unrelated genera, making it a versatile and difficult adversary to control and eliminate.57 The optimal treatment for A. baumannii, especially nosocomial infections resulting from multiple resistant strains, remains to be established. It is thus a clinical imperative that well-designed procedures are put in place to help guide clinicians on decisions regarding the current best therapeutic practice.86 Furthermore, new experimental approaches are warranted to develop and evaluate novel therapeutic strategies for dealing with A. baumannii infections.
Acknowledgments
R.D.S. is an ECSMID Research Fellow and the recipient of a Society for Applied Microbiology, New Lecturer Research Grant 2011. A.F. is funded by an IRCSET EMBARK Postgraduate Scholarship RS/2010/2300. The authors would like to acknowledge the technical assistance of Mr John Murphy, Department of Biological Sciences, CIT, in obtaining the Gram-stain image.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/19700
References
- 1.Montefour K, Frieden J, Hurst S, Helmich C, Headley D, Martin M, et al. Acinetobacter baumannii: an emerging multidrug-resistant pathogen in critical care. Crit Care Nurse. 2008;28:15–25, quiz 26. [PubMed] [Google Scholar]
- 2.Turton JF, Kaufmann ME, Gill MJ, Pike R, Scott PT, Fishbain J, et al. Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict. J Clin Microbiol. 2006;44:2630–4. doi: 10.1128/JCM.00547-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebeny PJ, Riddle MS, Petersen K. Acinetobacter baumannii skin and soft-tissue infection associated with war trauma. Clin Infect Dis. 2008;47:444–9. doi: 10.1086/590568. [DOI] [PubMed] [Google Scholar]
- 4.Cerqueira GM, Peleg AY. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–60. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- 5.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21:538–82. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassetti M, Ginocchio F, Mikulska M. New treatment options against gram-negative organisms. Crit Care. 2011;15:215. doi: 10.1186/cc9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis. 2008;197:1079–81. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 8.Fournier PE, Vallenet D, Barbe V, Audic S, Ogata H, Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006;2:e7. doi: 10.1371/journal.pgen.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC Acinetobacter baumannii infections among patients at military medical facilities treating injured U.S. service members, 2002-2004 (Reprinted from MMWR, vol 53, pg 1063-1066, 2004) JAMA. 2004;292:2964–6. doi: 10.1001/jama.292.24.2964. [DOI] [PubMed] [Google Scholar]
- 10.Bergogne-Bérézin E, Towner KJ. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin Microbiol Rev. 1996;9:148–65. doi: 10.1128/cmr.9.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beijerinck MW. Über Pigmentbildung bei Essigbakterien. Centr Bakteriol Parasitenk Abt 1911:167-76. [Google Scholar]
- 12.Brisou J, Prevot AR. [Studies on bacterial taxonomy. X. The revision of species under Acromobacter group] Ann Inst Pasteur (Paris) 1954;86:722–8. [PubMed] [Google Scholar]
- 13.Baumann P, Doudoroff M, Stanier RY. A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter) J Bacteriol. 1968;95:1520–41. doi: 10.1128/jb.95.5.1520-1541.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lessel EF. International Committee on Nomenclature of Bacteria Subcommittee on the Taxonomy of Moraxella and Allied Bacteria: Minutes of the Meeting, 11 August 1970. Room Constitution C, Maria-Isabel Hotel, Mexico City, Mexico. Int J Syst Bacteriol. 1971;21:213–4. doi: 10.1099/00207713-21-2-213. [DOI] [Google Scholar]
- 15.Di Nocera PP, Rocco F, Giannouli M, Triassi M, Zarrilli R. Genome organization of epidemic Acinetobacter baumannii strains. BMC Microbiol. 2011;11:224. doi: 10.1186/1471-2180-11-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerner-Smidt P, Tjernberg I, Ursing J. Reliability of phenotypic tests for identification of Acinetobacter species. J Clin Microbiol. 1991;29:277–82. doi: 10.1128/jcm.29.2.277-282.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972;112:917–31. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seifert H, Dijkshoorn L, Gerner-Smidt P, Pelzer N, Tjernberg I, Vaneechoutte M. Distribution of Acinetobacter species on human skin: comparison of phenotypic and genotypic identification methods. J Clin Microbiol. 1997;35:2819–25. doi: 10.1128/jcm.35.11.2819-2825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaneechoutte M, Dijkshoorn L, Tjernberg I, Elaichouni A, de Vos P, Claeys G, et al. Identification of Acinetobacter genomic species by amplified ribosomal DNA restriction analysis. J Clin Microbiol. 1995;33:11–5. doi: 10.1128/jcm.33.1.11-15.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen P, Maquelin K, Coopman R, Tjernberg I, Bouvet P, Kersters K, et al. Discrimination of Acinetobacter genomic species by AFLP fingerprinting. Int J Syst Bacteriol. 1997;47:1179–87. doi: 10.1099/00207713-47-4-1179. [DOI] [PubMed] [Google Scholar]
- 21.Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol. 1992;30:2680–5. doi: 10.1128/jcm.30.10.2680-2685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrenstein B, Bernards AT, Dijkshoorn L, Gerner-Smidt P, Towner KJ, Bouvet PJ, et al. Acinetobacter species identification by using tRNA spacer fingerprinting. J Clin Microbiol. 1996;34:2414–20. doi: 10.1128/jcm.34.10.2414-2420.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolzani L, Tonin E, Lagatolla C, Prandin L, Monti-Bragadin C. Identification of Acinetobacter isolates in the A. calcoaceticus-A. baumannii complex by restriction analysis of the 16S-23S rRNA intergenic-spacer sequences. J Clin Microbiol. 1995;33:1108–13. doi: 10.1128/jcm.33.5.1108-1113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang HC, Wei YF, Dijkshoorn L, Vaneechoutte M, Tang CT, Chang TC. Species-level identification of isolates of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex by sequence analysis of the 16S-23S rRNA gene spacer region. J Clin Microbiol. 2005;43:1632–9. doi: 10.1128/JCM.43.4.1632-1639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.La Scola B, Raoult D. Acinetobacter baumannii in human body louse. Emerg Infect Dis. 2004;10:1671–3. doi: 10.3201/eid1009.040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42:692–9. doi: 10.1086/500202. [DOI] [PubMed] [Google Scholar]
- 27.Turton JF, Kaufmann ME, Gill MJ, Pike R, Scott PT, Fishbain J, et al. Comparison of Acinetobacter baumannii isolates from the United Kingdom and the United States that were associated with repatriated casualties of the Iraq conflict. J Clin Microbiol. 2006;44:2630–4. doi: 10.1128/JCM.00547-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gootz TD, Marra A. Acinetobacter baumannii: an emerging multidrug-resistant threat. Expert Rev Anti Infect Ther. 2008;6:309–25. doi: 10.1586/14787210.6.3.309. [DOI] [PubMed] [Google Scholar]
- 29.Bayuga S, Zeana C, Sahni J, Della-Latta P, el-Sadr W, Larson E. Prevalence and antimicrobial patterns of Acinetobacter baumannii on hands and nares of hospital personnel and patients: the iceberg phenomenon again. Heart Lung. 2002;31:382–90. doi: 10.1067/mhl.2002.126103. [DOI] [PubMed] [Google Scholar]
- 30.Gusten WM, Hansen EA, Cunha BA. Acinetobacter baumannii pseudomeningitis. Heart Lung. 2002;31:76–8. doi: 10.1067/mhl.2002.120258. [DOI] [PubMed] [Google Scholar]
- 31.Lorente C, Del Castillo Y, Rello J. Prevention of infection in the intensive care unit: current advances and opportunities for the future. Curr Opin Crit Care. 2002;8:461–4. doi: 10.1097/00075198-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol. 2005;7:1127–38. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 33.Gaddy JA, Actis LA. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009;4:273–8. doi: 10.2217/fmb.09.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, et al. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett. 2009;301:224–31. doi: 10.1111/j.1574-6968.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs AC, Hood I, Boyd KL, Olson PD, Morrison JM, Carson S, et al. Inactivation of phospholipase D diminishes Acinetobacter baumannii pathogenesis. Infect Immun. 2010;78:1952–62. doi: 10.1128/IAI.00889-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camarena L, Bruno V, Euskirchen G, Poggio S, Snyder M. Molecular mechanisms of ethanol-induced pathogenesis revealed by RNA-sequencing. PLoS Pathog. 2010;6:e1000834. doi: 10.1371/journal.ppat.1000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poirel L, Menuteau O, Agoli N, Cattoen C, Nordmann P. Outbreak of extended-spectrum beta-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J Clin Microbiol. 2003;41:3542–7. doi: 10.1128/JCM.41.8.3542-3547.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hujer KM, Hamza NS, Hujer AM, Perez F, Helfand MS, Bethel CR, et al. Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 beta-lactamase: defining a unique family of class C enzymes. Antimicrob Agents Chemother. 2005;49:2941–8. doi: 10.1128/AAC.49.7.2941-2948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corvec S, Caroff N, Espaze E, Giraudeau C, Drugeon H, Reynaud A. AmpC cephalosporinase hyperproduction in Acinetobacter baumannii clinical strains. J Antimicrob Chemother. 2003;52:629–35. doi: 10.1093/jac/dkg407. [DOI] [PubMed] [Google Scholar]
- 40.Alsultan AA, Hamouda A, Evans BA, Amyes SG. Acinetobacter baumannii: emergence of four strains with novel bla(OXA-51-like) genes in patients with diabetes mellitus. J Chemother. 2009;21:290–5. doi: 10.1179/joc.2009.21.3.290. [DOI] [PubMed] [Google Scholar]
- 41.Turton JF, Kaufmann ME, Warner M, Coelho J, Dijkshoorn L, van der Reijden T, et al. A prevalent, multiresistant clone of Acinetobacter baumannii in Southeast England. J Hosp Infect. 2004;58:170–9. doi: 10.1016/j.jhin.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 42.Schulte B, Goerke C, Weyrich P, Gröbner S, Bahrs C, Wolz C, et al. Clonal spread of meropenem-resistant Acinetobacter baumannii strains in hospitals in the Mediterranean region and transmission to South-west Germany. J Hosp Infect. 2005;61:356–7. doi: 10.1016/j.jhin.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Unal S, Garcia-Rodriguez JA. Activity of meropenem and comparators against Pseudomonas aeruginosa and Acinetobacter spp. isolated in the MYSTIC Program, 2002-2004. Diagn Microbiol Infect Dis. 2005;53:265–71. doi: 10.1016/j.diagmicrobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Go ES, Urban C, Burns J, Kreiswirth B, Eisner W, Mariano N, et al. Clinical and molecular epidemiology of acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet. 1994;344:1329–32. doi: 10.1016/S0140-6736(94)90694-7. [DOI] [PubMed] [Google Scholar]
- 45.Rahal JJUC, Urban C, Horn D, Freeman K, Segal-Maurer S, Maurer J, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA. 1998;280:1233–7. doi: 10.1001/jama.280.14.1233. [DOI] [PubMed] [Google Scholar]
- 46.Halstead DC, Abid J, Dowzicky MJ. Antimicrobial susceptibility among Acinetobacter calcoaceticus-baumannii complex and Enterobacteriaceae collected as part of the Tigecycline Evaluation and Surveillance Trial. J Infect. 2007;55:49–57. doi: 10.1016/j.jinf.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Gales AC, Jones RN, Sader HS. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: report from the SENTRY antimicrobial surveillance programme (2001-2004) Clin Microbiol Infect. 2006;12:315–21. doi: 10.1111/j.1469-0691.2005.01351.x. [DOI] [PubMed] [Google Scholar]
- 48.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, et al. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60:1163–7. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 49.Gordon NC, Wareham DW. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int J Antimicrob Agents. 2010;35:219–26. doi: 10.1016/j.ijantimicag.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 50.Grupper M, Sprecher H, Mashiach T, Finkelstein R. Attributable mortality of nosocomial Acinetobacter bacteremia. Infect Control Hosp Epidemiol. 2007;28:293–8. doi: 10.1086/512629. [DOI] [PubMed] [Google Scholar]
- 51.Lee NY, Lee HC, Ko NY, Chang CM, Shih HI, Wu CJ, et al. Clinical and economic impact of multidrug resistance in nosocomial Acinetobacter baumannii bacteremia. Infect Control Hosp Epidemiol. 2007;28:713–9. doi: 10.1086/517954. [DOI] [PubMed] [Google Scholar]
- 52.Sunenshine RH, Wright MO, Maragakis LL, Harris AD, Song X, Hebden J, et al. Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg Infect Dis. 2007;13:97–103. doi: 10.3201/eid1301.060716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect. 2009;73:143–50. doi: 10.1016/j.jhin.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 54.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–84. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 55.Garnacho-Montero J, Ortiz-Leyba C, Ferńndez-Hinojosa E, Aldabó-Pallás T, Cayuela A, Marquez-Vácaro JA, et al. Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med. 2005;31:649–55. doi: 10.1007/s00134-005-2598-0. [DOI] [PubMed] [Google Scholar]
- 56.Luna CM, Aruj PK. Nosocomial Acinetobacter pneumonia. Respirology. 2007;12:787–91. doi: 10.1111/j.1440-1843.2007.01147.x. [DOI] [PubMed] [Google Scholar]
- 57.Anstey NM, Currie BJ, Hassell M, Palmer D, Dwyer B, Seifert H. Community-acquired bacteremic Acinetobacter pneumonia in tropical Australia is caused by diverse strains of Acinetobacter baumannii, with carriage in the throat in at-risk groups. J Clin Microbiol. 2002;40:685–6. doi: 10.1128/JCM.40.2.685-686.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leung WS, Chu CM, Tsang KY, Lo FH, Lo KF, Ho PL. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest. 2006;129:102–9. doi: 10.1378/chest.129.1.102. [DOI] [PubMed] [Google Scholar]
- 59.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–17. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 60.Trottier V, Segura PG, Namias N, King D, Pizano LR, Schulman CI. Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J Burn Care Res. 2007;28:248–54. doi: 10.1097/BCR.0B013E318031A20F. [DOI] [PubMed] [Google Scholar]
- 61.Gaynes R, Edwards JR, National Nosocomial Infections Surveillance System Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41:848–54. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 62.Briggs S, Ellis-Pegler R, Raymond N, Thomas M, Wilkinson L. Gram-negative bacillary meningitis after cranial surgery or trauma in adults. Scand J Infect Dis. 2004;36:165–73. doi: 10.1080/00365540410027193. [DOI] [PubMed] [Google Scholar]
- 63.Metan G, Alp E, Aygen B, Sumerkan B. Acinetobacter baumannii meningitis in post-neurosurgical patients: clinical outcome and impact of carbapenem resistance. J Antimicrob Chemother. 2007;60:197–9. doi: 10.1093/jac/dkm181. [DOI] [PubMed] [Google Scholar]
- 64.Kwon KT, Oh WS, Song JH, Chang HH, Jung SI, Kim SW, et al. Impact of imipenem resistance on mortality in patients with Acinetobacter bacteraemia. J Antimicrob Chemother. 2007;59:525–30. doi: 10.1093/jac/dkl499. [DOI] [PubMed] [Google Scholar]
- 65.Shankar R, He LK, Szilagyi A, Muthu K, Gamelli RL, Filutowicz M, et al. A novel antibacterial gene transfer treatment for multidrug-resistant Acinetobacter baumannii-induced burn sepsis. J Burn Care Res. 2007;28:6–12. doi: 10.1097/BCR.0b013e31802c8861. [DOI] [PubMed] [Google Scholar]
- 66.Mihu MR, Martinez LR. Novel therapies for treatment of multi-drug resistant Acinetobacter baumannii skin infections. Virulence. 2011;2:97–102. doi: 10.4161/viru.2.2.15061. [DOI] [PubMed] [Google Scholar]
- 67.Coates AR, Hu Y. Novel approaches to developing new antibiotics for bacterial infections. Br J Pharmacol. 2007;152:1147–54. doi: 10.1038/sj.bjp.0707432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poole K. Overcoming multidrug resistance in gram-negative bacteria. Curr Opin Investig Drugs. 2003;4:128–39. [PubMed] [Google Scholar]
- 69.Yang H, Liang L, Lin S, Jia S. Isolation and characterization of a virulent bacteriophage AB1 of Acinetobacter baumannii. BMC Microbiol. 2010;10:131. doi: 10.1186/1471-2180-10-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Wong ES, Whitley JC, Li J, Stringer JM, Short KR, et al. Ancient antimicrobial peptides kill antibiotic-resistant pathogens: Australian mammals provide new options. PLoS One. 2011;6:e24030. doi: 10.1371/journal.pone.0024030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 72.Lisanby MW, Swiecki MK, Dizon BL, Pflughoeft KJ, Koehler TM, Kearney JF. Cathelicidin administration protects mice from Bacillus anthracis spore challenge. J Immunol. 2008;181:4989–5000. doi: 10.4049/jimmunol.181.7.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dadachova E, Burns T, Bryan RA, Apostolidis C, Brechbiel MW, Nosanchuk JD, et al. Feasibility of radioimmunotherapy of experimental pneumococcal infection. Antimicrob Agents Chemother. 2004;48:1624–9. doi: 10.1128/AAC.48.5.1624-1629.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dadachova E, Nakouzi A, Bryan RA, Casadevall A. Ionizing radiation delivered by specific antibody is therapeutic against a fungal infection. Proc Natl Acad Sci U S A. 2003;100:10942–7. doi: 10.1073/pnas.1731272100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dadachova E, Patel MC, Toussi S, Apostolidis C, Morgenstern A, Brechbiel MW, et al. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Med. 2006;3:e427. doi: 10.1371/journal.pmed.0030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McConnell MJ, Domínguez-Herrera J, Smani Y, López-Rojas R, Docobo-Pérez F, Pachón J. Vaccination with outer membrane complexes elicits rapid protective immunity to multidrug-resistant Acinetobacter baumannii. Infect Immun. 2011;79:518–26. doi: 10.1128/IAI.00741-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Islam AH, Singh KK, Ismail A. Demonstration of an outer membrane protein that is antigenically specific for Acinetobacter baumannii. Diagn Microbiol Infect Dis. 2011;69:38–44. doi: 10.1016/j.diagmicrobio.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 78.Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci U S A. 2008;105:5495–500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demidova TN, Hamblin MR. Photodynamic therapy targeted to pathogens. Int J Immunopathol Pharmacol. 2004;17:245–54. doi: 10.1177/039463200401700304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–34. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tsai T, Chien HF, Wang TH, Huang CT, Ker YB, Chen CT. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2011;55:1883–90. doi: 10.1128/AAC.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mowbray M, Tan XJ, Wheatley PS, Rossi AG, Morris RE, Weller RB. Topically applied nitric oxide induces T-lymphocyte infiltration in human skin, but minimal inflammation. J Invest Dermatol. 2008;128:352–60. doi: 10.1038/sj.jid.5701096. [DOI] [PubMed] [Google Scholar]
- 83.Soneja A, Drews M, Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep. 2005;57(Suppl):108–19. [PubMed] [Google Scholar]
- 84.Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, et al. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide. 2008;19:12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR. The use of nitric oxide releasing nanoparticles as a treatment against Acinetobacter baumannii in wound infections. Virulence. 2010;1:62–7. doi: 10.4161/viru.1.2.10038. [DOI] [PubMed] [Google Scholar]
- 86.Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis. 2010;51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]