Abstract
Punishment can help maintain cooperation by deterring free-riding and cheating. Of particular importance in large-scale human societies is third-party punishment in which individuals punish a transgressor or norm violator even when they themselves are not affected. Nonhuman primates and other animals aggress against conspecifics with some regularity, but it is unclear whether this is ever aimed at punishing others for noncooperation, and whether third-party punishment occurs at all. Here we report an experimental study in which one of humans' closest living relatives, chimpanzees (Pan troglodytes), could punish an individual who stole food. Dominants retaliated when their own food was stolen, but they did not punish when the food of third-parties was stolen, even when the victim was related to them. Third-party punishment as a means of enforcing cooperation, as humans do, might therefore be a derived trait in the human lineage.
Keywords: social evolution, human evolution, negative reciprocity, norm enforcement, great apes
Humans cooperate with unrelated individuals on a much larger scale and in a wider variety of ways than do any other species (1), and how “ultra-sociality” (2, 3) could have evolved is a matter of debate. A cooperative tendency is only a partial solution to the problem of cooperation. The problem comes from free-riding; individuals who do not pay the cost of cooperation, or who exploit the efforts of others, have an advantage over cooperators. Punishment is an important mechanism for stabilizing cooperation against the degrading influences of selfish individuals because it makes cheating costly (4). Second-party (direct) punishment is important for the maintenance of cooperation in humans and other species (4–7), such as eusocial insects (8), fish (9), birds (10), and mammals (11), including nonhuman primates (12). A difficulty with second-party punishment is that it is not possible to distinguish between a coercive strategy employed by dominant individuals with cooperation as a byproduct, and a behavior that is intended to produce cooperative behavior.
Third-party punishment is a particularly important form of punishment for the maintenance of human cooperation (13). Individuals punish a norm violator for acts not directly affecting them. Unlike second-party punishment, third-party punishers do not stand to gain material or fitness benefits directly, yet suffer a cost to the benefit of others. The motivation of the punisher is therefore less likely to be self-serving, and benefits from reforming the behaviors of free-riders can extend to others and not just the punisher. People in many societies will engage in third-party punishment against violations of cooperative and other social norms, even when it is costly to do so (14). Impersonal enforcement of violations is especially critical to the maintenance of cooperation in large-scale human societies in which individuals rarely, if ever, encounter each other directly (15, 16).
Very little is known about how human third-party punishment evolved. Prototypically, human third-party punishment involves responses by an unaffected individual to violations of social norms, such as socially agreed upon rules for cooperation (13). Processes resembling third-party punishment have been described in other animals (17–22), including in nonhuman primates, namely, chimpanzees (Pan troglodytes) (23, 24) and pigtailed macaques (Macaca nemestrina) in which powerful individuals successfully intervene in fights (25) and whose absence results in increased conflicts among the remaining group members (26). However, the removal of a dominant is likely to create a power vacuum, which results in increased conflict as the other animals jockey for status, and any cooperativeness caused by the dominant’s presence might arise as a byproduct. To date, there have been no direct tests of third-party punishment of violations of cooperation in nonhuman animals.
Chimpanzees are one of humans’ two closest living relatives (along with bonobos). They live in small social groups (typically of a few dozen individuals, but up to 150 individuals) (27). Chimpanzees cooperate in group defense, intragroup coalitions, and hunting (28), and do so with kin as well as nonkin (29). They also retaliate when personally harmed by conspecifics (30) and dominants sometimes intervene to break up fights among third parties (23, 24, 31). It has also been suggested that chimpanzees share with humans another important mechanism for sociality, sensitivity to fairness (32). It would thus seem plausible that the conditions for third-party punishment would be ripe in chimpanzees. However, chimpanzees do not cooperate in the same manner nor to the same degree as humans (33), their social networks within and between groups are much smaller (34), and it has been called into question whether they are averse to inequity (35, 36). The question then arises whether the antecedents of human third-party punishment for enforcing cooperation would be exhibited by chimpanzees.
To determine whether human third-party punishment and norm enforcement have their evolutionary roots in other great apes, we conducted a third-party punishment study with chimpanzees. Because chimpanzees retaliate against personally harmful behavior (30), we asked whether they would also punish harmful behavior directed at conspecifics (third-party). Based on past reports of “policing” by dominants in other species (5, 21–26), we expected that dominant individuals would be more likely to engage in third-party punishment than individuals subordinate to the thief. In addition, because kin-selection accounts for much of the cooperative behavior in primate societies, we anticipated that there would be more third-party punishment when the victim was kin (37). Like third-party punishment experiments in humans (13), an actor witnessed a violation between two conspecifics. In this case, the violation of interest was theft in which one individual (thief) pulled food away from another (victim).
Results and Discussion
We presented 13 captive chimpanzees with an opportunity to punish third-party violations. Based on previous work that showed that chimpanzees will retaliate against conspecifics who steal their food (30), chimpanzees in this study could react toward third-party violations in addition to personal (second-party) violations. Punishment was defined as collapsing a platform, causing food on the platform to be knocked away from any individual in the thief’s position (Fig. 1). The expectation was that if chimpanzees are sensitive to third-party violations, they would react most strongly to third-party theft (3P theft) in which one chimpanzee (thief) took food away from another (victim). To control for alternative motivations that might account for the punishment behavior, there were three other conditions with the subject (actor) in the actor's position: third-party unfair (3P unfair), in which the experimenter moved the victim’s food to a conspecific in the thief’s position; third-party loss (3P loss), in which the experimenter took the food from the victim and put it in front of an empty cage; and third-party no-victim (3P no victim), in which the “thief” could pull food but no other chimpanzee was affected. Importantly, to validate the method and to demonstrate that chimpanzees understood the mechanics of the task, we presented them with opportunities to retaliate against personal loss because of theft (2P theft) and personal loss that did not benefit a conspecific (2P loss). See Fig. 2 for diagrams of the conditions.
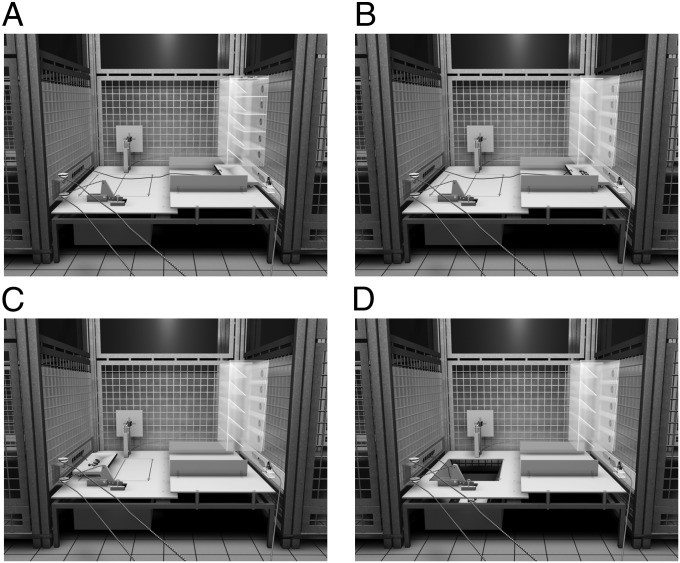
Fig. 1.
Illustration of the testing environment. The three cages are shown with the thief’s cage on the left, the actor’s in the center, and the victim’s to the right. The apparatus was in a booth that allowed limited access by the chimpanzees. (A) In the starting position, the food is on the top tray of the victim’s food box. (B) By manipulating five sliders (not shown), the food drops from the top shelf to the bottom, where it falls onto a food tray. Once the food is on the food tray, the experimenter (outside the room) pulls a rope, allowing the victim to slide a panel to access the food tray. (C) The food is then moved to the thief’s position, either by the thief (after the experimenter allows access to the rope via a rope-and-pulley), or manually by the experimenter. (D) Once the food tray is in front of the thief’s cage (whether a chimpanzee is there or not), the trapdoor could be collapsed by the actor, either by pulling a rope or pressing a large button (the experimenter first would cause the trapdoor to engage with the releasing mechanisms to prevent premature collapsing).
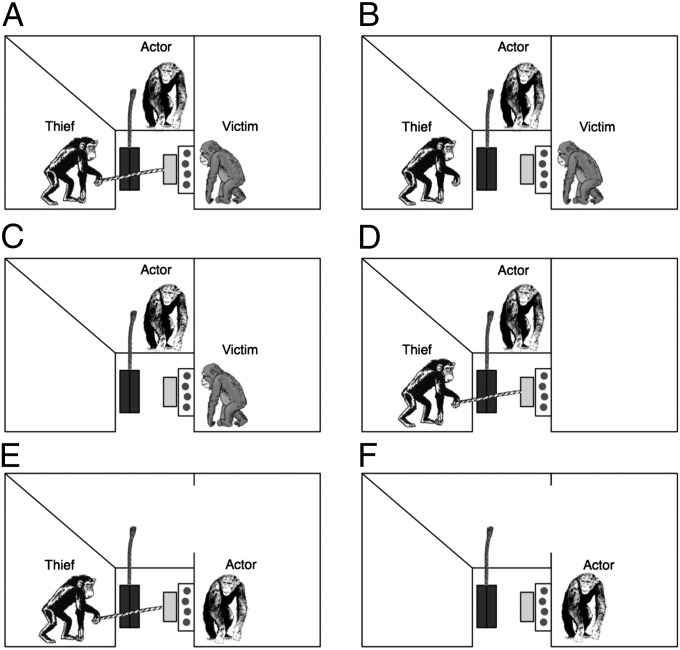
Fig. 2.
Experimental setup. Chimpanzees could occupy three cages designated as “victim,” “thief,” or “actor.” In all conditions, food (circles) had to be manipulated to drop out of a clear box onto a sliding tray (light gray). A rope, if available, could be used to pull the tray from the victim’s cage toward the thief’s cage. Another rope, as well as a large button (not shown) could be used to release a trapdoor (dark gray) underneath the tray, causing the tray and the food to fall into a box, out of the chimpanzees’ reach and sight. The four third-party conditions in which a punisher had no access to food were (A) third-party theft (thief could pull food tray away from victim), (B) third-party unfair (experimenter moved food tray away from victim and toward thief), (C) third-party loss (experimenter moved food tray from victim to empty cage), and (D) third-party no-victim (thief could pull food tray away from empty victim’s cage). The second-party conditions in which the victim could enter the actor’s cage were (E) second-party theft (thief could pull food tray away from the actor) and (F) second-party loss (experimenter moved food tray away from actor to the empty thief’s cage).
We investigated whether dominant individuals were more likely than subordinates to use coercive (punitive) strategies (5, 30) and whether kin-based nepotism would lead to more retaliation. Because there were 1,716 possible triads with a group of 13, we selected 60 triads corresponding to the following four test groups: (i) actor dominant to the thief and related to the victim, (ii) actor dominant to the thief and unrelated to the victim, (iii) actor subordinate to the thief and related to the victim, and (iv) actor subordinate to the thief and unrelated to the victim.
The key findings were that: (i) whereas chimpanzees punish those who steal from them directly, they do not punish those who steal from third parties; and (ii) dominants were more likely than subordinates to retaliate when they were stolen from directly (Fig. 3).
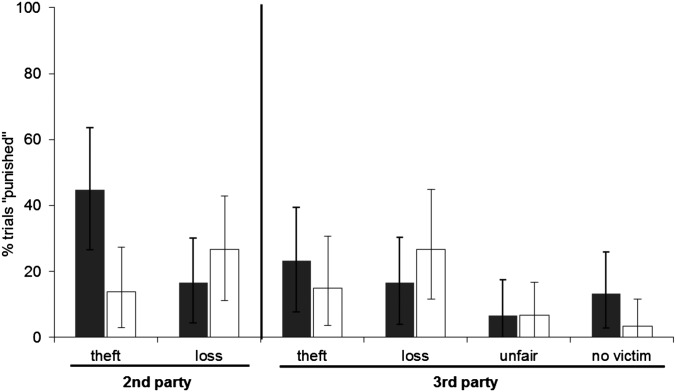
Fig. 3.
Relative frequency of actors collapsing the trapdoor in second-party and third-party conditions. Results for actors (third-party) and victims/actors (second-party) who were dominant to the thief are shown as solid bars. Individuals subordinate to the thief are shown by open bars. Note that when there was no thief (3P loss and 2P loss), the dominance status is based on the assigned pairings in the matched conditions. Values are expressed as means and error bars as bootstrapped 95% confidence intervals.
Overall, “actor” was the only random-effects factor that significantly explained variance in the response variable, namely collapsing the food platform (χ21 = 12.584, P < 0.001). In other words, individuals varied in their behavior of collapsing the platform. Neither the identity of the thief nor the victim influenced the actor’s response. With one exception, control variables (order of conditions within each session, as well as actors’ sex, age, and rearing history) had no explanatory value (Table S1). The exception was “session order” (χ21 = 11.549, P < 0.001): actors were less likely to respond in later sessions than earlier ones (Z = −3.254, P = 0.001), likely because of habituation to food loss. A comparison of the full model, including all experimental test factors and control variables against a model without any of the experimental test factors was significant (χ27 = 26.927, P < 0.001), indicating that the experimental factors as a whole, namely “dominance,” “kinship,” and “condition,” contributed to the collapsing of the platform. For the experimental test factors, “condition” was a significant predictor for actors collapsing the trapdoor (χ25 = 19.508, P = 0.002). Furthermore, actors’ responses were influenced by their dominance relative to the thieves (χ21 = 8.6729, P = 0.003); that is, dominant individuals were more likely to collapse the trapdoor than subordinates (Z = 2.884, P = 0.004). However, kin relation with the victim was not a significant predictor (χ21 = 0.1476, P = 0.701). We investigated the potential interaction effects of condition, dominance, and kinship using only the 3P unfair and 3P theft conditions because these were the only conditions in which both thieves and victims were present. Neither the interactions between condition*dominance nor dominance*kinship were significant (Table S2). In summary, actors’ responses were predicted by the random effects factor “actor” and the fixed effects factors “condition,” “dominance,” and “session order” (Table S3); that is, punishment rates could be explained by individual variation, dominance relationships between thief and actor, and order of sessions. Kinship with the victim had no effect on the actors’ behavior.
Next, we analyzed the data separately for dominant and subordinate actors because dominants, but not subordinates, take on a policing role (5, 21–26, 31), a result that was confirmed in chimpanzees faced with 2P theft (30). Both the experimental test variable “condition” and the control variable “session order” were significant predictors (condition: χ25 = 17.252, P = 0.004; session order: χ21 = 7.8767, P = 0.005). Dominants responded less in later sessions than in earlier ones (Z = −2.665, P = 0.007). In general, dominants collapsed the trapdoor significantly more often when the subordinate thief had stolen from them (2P theft) than in any other experimental condition (Table S4 for all pairwise comparisons). Most importantly, and consistent with ref. 30, dominant individuals were more likely to collapse the trapdoor in response to second-party theft than to nonsocial food loss (2P loss) when the experimenter moved their food to an empty cage (Z = −2.494, P = 0.0126). Dominants collapsed the trapdoor significantly more often when they were personally affected as opposed to when they observed theft (2P theft vs. 3P theft; Z = 1.995, P = 0.0460).
In contrast, dominant individuals did not engage in third-party punishment. Aside from a trend toward collapsing the trapdoor more often in 3P theft than 3P unfair (Z = −1.754, P = 0.0793), there was no difference in rate of collapsing the trapdoor in 3P theft than in the other 3P conditions. Although overall rates were low, dominants showed more anger (threats and displays) toward thieves in the 2P theft than in the 3P theft condition [Friedman’s χ25 test = 12.735, P = 0.02; Wilcoxon T+ test = 33, n = 12 (four ties), P = 0.039], with no difference across the other conditions in which there were partners in the thief’s position.
Similar to dominant actors, both the factors “condition” (χ25 = 16.223, P = 0.006) and “session order” (χ21 = 3.9659, P = 0.046) significantly explained variance in the subordinate actors’ response. Additionally, response rates tended to decrease across sessions (Z = −1.906, P = 0.0567). Subordinate actors, like dominants, did not punish third-party theft. Unlike dominants, however, subordinate actors did not retaliate against second-party theft more frequently than they collapsed the trapdoor in any other condition (Table S5 for all pairwise comparisons). Subordinates did not differ in their displays of anger toward thieves across conditions and in 2P theft relative to 3P theft [Friedman’s χ25 test = 7.250, P = 0.181; Wilcoxon T+ test = 3, n = 11 (nine ties), P = 0.500], as was seen in dominants.
Because it takes few punishers to maintain cooperation in a group (38), we examined individual responses. As indicated by the significant random-effects factor “actor,” there was variance between individuals on collapsing the trapdoor across conditions. However, a visual inspection of the data suggests that no individual consistently punished third-party theft (Table S6).
The results suggest that chimpanzees do not punish third-party violations of cooperative behavior. Chimpanzees were as unlikely to collapse a table, causing food to be lost, in response to a thief stealing food from a victim (3P theft) as they were when the experimenter took food away from a victim (3P unfair) when there was no thief (3P loss) and when there was no victim (3P no victim). Contrary to what would be expected if nonhuman primates engage in third-party interventions (23–26, 31), dominant individuals did not intervene by punishing. Nor, contrary to predictions from kin selection, did relatedness with the victim induce third-party punishment. The only situation in which chimpanzees react in a manner that resembles punishment was when dominant individuals had their own food taken away (2P theft) compared with reacting out of frustration to food loss in which no one benefits (2P loss). Subordinate individuals, on the other hand, showed a tendency to react more often by collapsing the table when there was no thief, likely reflecting frustration tempered by fear of the dominant thieves. The results from the second-party conditions validate our experiment by demonstrating that chimpanzees are able to “punish” others, but do not do so when not personally affected, or when not protected by a power asymmetry. Overall, chimpanzee punishment appears confined to retaliation against personal harm when the punisher is in a position of dominance: chimpanzee punishment is of the “might makes right” variety.
These findings are consistent with observations that nonhuman primates and other animals use aggression as a coercive strategy (5, 39). Second-party punishment in nonhuman primates may be used to target uncooperative behavior (e.g., ref. 12), but it is quite probable that these displays are used to assert dominance, to subjugate or coerce another individual, rather that to modify behavior (6, 7, 40). The difficulty with second-party punishment is that the punisher stands to gain directly—in the form of dominance—securing a resource and sexual coercion (5, 39), as well as reputation, at least in humans (41, 42). Second-party punishment may be moralistic aggression (43) and it might result in altruistic punishment (44), such that others benefit, but it is difficult to ascertain the motives (goals) and motivations (emotional drives) of the punisher in dyadic encounters.
Third-party punishment can reveal a sensitivity of the punisher to norms of behavior applicable to all. In the third-party punishment game with humans, an observer can pay a cost to punish a violation of a distribution norm in a dictator game played between other players (13). Third-party punishment has been demonstrated in a variety of cultures (14, 45), although there is some question about how third-party punishment could have evolved in humans. Even children, it least in one Western society, will protest and intervene against violations of norms (46, 47). People punish others even if doing so does not provide any material or reputation benefit to the punisher, likely because of concern for fairness norms or other other-regarding concerns [although some authors wonder whether, even in humans, third-party punishment is not some sort of “big mistake” (48, 49)].
Our main conclusion is thus that, in contrast to humans, chimpanzees do not engage in third-party punishment. Unlike studies in experimental economics, there was no anonymity among subjects in this study but, if anything, the lack of anonymity should have increased third-party punishment for reputation benefits. Although it is possible that there might be contexts in which chimpanzees would punish others for violations against third parties, our experimental context—theft of food—is ecologically valid and effective at eliciting second-party, but not third-party, punishment. Punishment on behalf of third-parties—a critical component of large-scale human cooperation—would not seem to be an ancestral feature of the last common ancestor to humans and chimpanzees.
Materials and Methods
Subjects.
Animal husbandry and research complied with the EAZA Minimum Standards for the Accommodation and Care of Animals in Zoos and Aquaria and the WAZA Ethical Guidelines for the Conduct of Research on Animals by Zoos and Aquariums. Additionally, this study was conducted according to the laws of Germany and approved by the joint ethical committee of the MPI-EVA and the Zoo Leipzig. Subjects were 13 chimpanzees (Pan troglodytes, nine females, four males) from a single social group at the Wolfgang Köhler Primate Research Center in Leipzig, Germany. During the day, the chimpanzees live in a 430-m2 indoor and 4,000-m2 outdoor enclosure, both observable by the public. Enclosures provide natural vegetation, various enrichment items, ropes and trees for vertical climbing. Overnight, the group stays together in a 47-m2 sleeping room that consists of five interconnected cages. This sleeping room, which is familiar with the chimpanzees, served as the testing room for this study. Details on the 13 chimpanzees included in this study can be found in Table S7.
All chimpanzees had prior testing experience in various studies on social and physical cognition. The chimpanzees were not food deprived, and they had ad libitum access to water throughout the study.
Apparatus and Setup.
For testing, we used two adjacent cages (6.75 m2 and 8.3 m2) of the sleeping room. The cages were separated by mesh panels, allowing the chimpanzees to see and hear each other while separating them from direct physical contact. This separation was done to allow individuals to engage in the study without interference from the others. The larger cage was subdivided (5.45 m2 and 2.85 m2) by removable mesh panels to create a third cage. Viewed from the experimenter’s perspective, as shown in the four panels of Fig. 1, the leftmost cage (5.45 m2) was designated as the “thief’s” cage, the middle one (2.85 m2) was the “actor’s” cage, and the one to the right (6.75 m2) was the “victim’s” cage. The cages were arranged around a 1-m2 space (booth) where the test apparatus sat. The chimpanzees could manipulate the apparatus through the mesh.
The apparatus was designed to be intuitive for the chimpanzees to use with minimal familiarization. Despite appearing complex, the apparatus worked in a straightforward manner, which we describe in the sequential manner in which it was used. The sequences of steps are working for the food, accessing the food, losing the food, and collapsing the trapdoor.
Working for the food.
A clear Plexiglas box, with five hinged shelves inside, held highly desirable food items (10 grapes and 10 dry food pellets: OWM chunks banana, Mazuri Zoo Foods). This food was similar to “enrichment boxes” permanently installed in the chimpanzees’ enclosures. The subjects, therefore, had prior experience with working on a box to gain food. However, the mechanism was novel to them in that they had to use their fingers to reach through the mesh panel to shift Plexiglas sliders. Doing so caused the hinged shelf it was supporting to collapse. If the food was on that shelf, it would also drop. The chimpanzees had to manipulate all five sliders to get the food to fall from the top shelf to the bottom one (Fig. 1A). The purpose of this setup was to get them to invest effort into getting the food, and therefore to increase the likelihood of a sense of possession (50). Chimpanzees in the victim position could only begin to move the sliders in the food box after the experimenter had left the room: access to the food box was blocked by a thin, transparent Lexan panel until it was raised by the experimenter using a pulley mechanism from outside the testing room. The reason for this setup was to ensure that no one was in the room during any part of the test, to minimize the likelihood that subjects were somehow interacting with the experimenter rather than their conspecifics.
Accessing the food.
After the last slider of the food box had been shifted successfully, food dropped onto a food tray on the table in the booth (Fig. 1B). To prevent chimpanzees in the victim’s position from accidentally pushing the food tray away before it had been baited, a small Plexiglas panel blocked the access to the food tray until it was released by the experimenter from outside the testing room. This release was done once the chimpanzee had successfully dropped the food from the food box onto the tray. The last action the chimpanzee had to perform to access the food on the tray was to slide this Plexiglas panel away.
Losing the food.
The food tray had rollers underneath, allowing it to slide along the table in the booth. A rope attached at the food tray led across the table to the cage mesh opposite the one with the food box (thief’s position). By pulling the tray rope, another chimpanzee (thief) could move the food tray across the table toward himself (Fig. 1C). Alternatively, the experimenter could move the tray by reentering the room and sliding it from the victim’s position to the thief’s position. (This process was not done remotely from outside the room because it was important that all subjects be able to see that the food loss was created by a human, and not by another chimpanzee. A “ghost” pull would likely have been ambiguous.) A Plexiglas panel prevented the thief from attempting to pull the rope until food had dropped onto the tray; once food was on the tray, the experimenter would release the panel from outside of the testing room by means of a rope and pulley system. The victim could not pull the tray back.
Collapsing the trapdoor.
Once the food tray had been moved from the victim’s position to the thief’s, it stood on a trapdoor. When the trapdoor was opened, the food tray and its contents fell into an opaque box under the table. The food in the box was not visible to the chimpanzees, thereby eliminating any possible enticement that might lead the actors to open the trapdoor under the false expectation that they could get the food for themselves. Furthermore, because the experimenter did not remove the food between trials, there was a reduced association between the experimenter and the lost food. The trapdoor could be opened by the subject either by pulling a rope or by pressing a large plastic button (Fig. 1D). To prevent subjects from opening the trapdoor prematurely, the releasing mechanisms were disengaged from the trapdoor. Once the food tray was in front of the thief’s position, the experimenter (from outside the room) pulled a rope, causing the trapdoor to become engaged with the rope and button that could collapse it (this was achieved by a combination of magnets and springs). If the actor did nothing after 2 min, the experimenter entered the room and removed the food tray.
All test sessions were filmed with four video cameras. The overview camera faced the booth centering on the actor; the mesh panels of the other cages were also visible from this angle. Two other cameras were positioned to film the thief and the victim. The fourth camera was positioned underneath the apparatus so that the experimenter could determine how the trapdoor was collapsed. The overview camera was connected to a Sony DV-Walkman, allowing the experimenters to observe the test from outside of the testing room.
Procedure.
Apparatus familiarization.
All subjects received training in which they were introduced to the three different roles. For all subjects, familiarization started with the victim’s role, followed by the thief’s role, and finally by the actor’s role. All familiarization was nonsocial, in that individuals did not interact with other individuals during familiarization. Doors between the cages were closed to restrict the subjects to the position to which they were being familiarized.
Starting with the victim’s role, the subjects were given a variable number of sessions (mean 5.8, range 3–9 sessions) to shape them to get food out of the food box. Food-box familiarization sessions consisted of six trials, and subjects passed when they successfully moved the sliders, causing the food (two grapes and two pellets) to drop onto the food tray from which they could freely eat. The shelves could be released in any sequence. To shape the subjects, the number of sliders that had to be released to achieve this outcome was increased until they were successfully able to manipulate all five to get the food. The criterion for passing the food box familiarization was sliding the five sliders in at least three consecutive trials within a session for two successive sessions.
The second phase of familiarization introduced all subjects to the thief’s role. All but one individual received three sessions each with three trials. One individual, Fraukje, received four sessions because of a lack of motivation in the second of these sessions. In the first session of this phase, the doors between the cages were open, and individuals again had to manipulate the food box. However, instead of eating directly from the food tray, subjects had to go over to the thief’s cage and pull the rope of the food tray. In the other two sessions, individuals were in the thief’s cage from the beginning and only had to pull the food tray, which was baited with two grapes and two pellets across the table. Criterion was reached once the subject successfully moved the food tray across the table in all three trials of one session.
The final phase of familiarization was for the actor’s role. From the actor’s cage, subjects learned how to open the trapdoor. Subjects were not rewarded for collapsing the trapdoor and knocking the food tray down. Most chimpanzees spontaneously collapsed the trapdoor by exploring the button or rope. Those who did not were shown the consequences by calling them by name and pointing to the button and the rope. As a last option, the experimenter put the food tray, baited with food, in front of them, called the subject’s name, and pointed toward rope and button. Once enticed, the chimpanzees would then push the button or pull the rope, only to see the food fall through the trapdoor. Criterion was reached when chimpanzees released the trapdoor three times in two to four sessions (mean 2.6, range 2–4 sessions), each consisting of three trials.
Testing: Experimental and control conditions.
All actors participated in the following six test conditions. There were four third-party test conditions (3P conditions) in which actors were in the middle cage and two second-party test conditions (2P conditions) in which the door between the victim’s position and the actor’s position was open, allowing the actor manipulating the food box in the victim position and to collapse the trapdoor (see Fig. 2). Sixty triads were selected from a potential pool of 1,716 combinations of unique triads among the 13 subjects. Dominance relationships were determined from routinely conducted focal animal samples as well as data on dyadic feeding tolerance; kinship was based on known family history data. These data were used to compose test groups on the basis of a 2 × 2 design with the factors dominance (actor’s dominance relationship to the thief) and kinship (actor’s maternal or full-sibling relatedness to the victim) (for details, see Table S8). There were, therefore, four test categories: dominant/kin, dominant/nonkin, subordinate/kin, and subordinate/nonkin. We did not test for kin relatedness between subject and thief, as this would have decreased the likelihood of punishment as a result of nepotism. Subjects were related (full-sib) to the thief in only two of the triads. Each subject was the actor in four or five triads, and it was in as many of the four test categories as possible (Table S9). With few exceptions, subjects never encountered their particular triads more than once.
The main test condition was third-party theft (3P theft) (Fig. 2A). Once the victim had caused food to drop on the food tray, the thief could pull the tray away and the actor could respond by collapsing the trapdoor. In other words, the actor could “punish” the thief for stealing food from the victim, but could not get any food for herself. If the actor did nothing within 2 min of the food tray being pulled to the thief’s position, the trial ended. To help specify what the chimpanzees might be reacting to, we also ran several control conditions in which the target/thief did not do anything harmful at all, and so was undeserving of punishment. In one such control condition, the experimenter herself created an unfair outcome by moving food from the victim to the thief as the actor watched (3P unfair) (Fig. 2B). There was also a loss condition (3P loss) (Fig. 2C) in which the experimenter moved the food from the victim to the empty thief’s position. As an additional control for food tray movement, which might induce pulling by the actor because of response facilitation, there was a third-party no-victim condition (3P no-victim) (Fig. 2D), in which the subject in the thief’s position would pull the food tray, but there was no one in the victim’s position. In addition, to ensure that chimpanzees were sensitive to personally harmful outcomes as had been previously demonstrated experimentally (30), we had two second-party conditions, one in which the actor had its food stolen by a thief (2P theft) (Fig. 2E) and a control condition with nonsocial food loss (2P loss) (Fig. 2F). In both of these conditions, the door between the victim’s position and the actor’s position was open, allowing the victim to collapse the trapdoor. A filler trial was interspersed at random with each test session. In filler trials, the victims were able to eat the food from the tray for 2 min after manipulating the food box. This setup was done to maintain the motivation of the victims who would typically lose food after working for it. The thief and the actor could not interact with the apparatus at this time.
Each actor received all six conditions over a period of three sessions (days). The 2P and 3P conditions were counter balanced in a blocked design so that subjects either had 2P or 3P conditions on a given day, and order of the conditions was counterbalanced across subjects and across and within sessions.
Coding and Analyses.
All data were live-coded, and videotapes were coded for actor’s choice and arousal. We coded whether or not actors collapsed the trapdoors within 2 min from the moment the food tray moved to the thief’s position. Arousal, which is an indication of anger, was measured by the intensity of tantrums and displays including behavioral elements of rocking, foot stamps, and hand clapping combined with hair erection (51). For purposes of interobserver reliability, 20% of all trials were coded by a second observer blind to the study’s design (choice: Cohen’s κ = 1.0; anger: Cohen’s κ = 0.95).
We analyzed the data by using a generalized linear mixed model (GLMM) (52) and nonparametric statistics. The GLMM provides additional details on factors influencing the response variable that cannot be revealed by nonparametric tests. The GLMM was carried out with the function “lmer” of the statistics package R (v2.9.1, R Development Core Team 2009) using the package “lme4” (53). All reported P values are two-tailed. The testing structure using GLMM can be described as follows: for the whole sample of 60 test groups, there was an initial full model consisting of four types of variables. The response variable was “collapsing the trapdoor.” Three random-effects factors that could have explained variance in the response were the subject roles, namely actor, thief, and victim. Test variables that defined the experimental design were condition, dominance, and kinship. “Condition” described the different study conditions, namely 2P theft, 2P loss, 3P theft, 3P loss, 3P unfair, 3P no-victim. We predicted that the actor should collapse the trapdoor more often in the theft conditions (2P theft, 3P theft) than in other test conditions (2P loss, 3P loss, 3P unfair, 3P no-victim), and that dominant actors would do so more frequently than subordinates (5, 21–25, 29). “Dominance” referred to the rank of the actor relative to the thief and “kinship” indicated the degree of genetic relatedness between actor and victim (either mother-offspring or full-siblings, Wright coefficient = 0.5). Other factors not included in the experimental design that could have influenced the results and were included as control variables in the model were actor’s age, sex, rearing history (nursery vs. mother-reared), session order, and trial order per session. Hence, the initial full model including all potential predictors was:
response ∼ condition + dominance + kinship + age + sex + rearing + session order + trial order within session + actor + thief + victim.
Factor examination always followed the same logic. The coefficients were estimated by using maximum likelihood, and we used a binominal error structure and logit link function. A likelihood ratio test (using the ANOVA function in R) was used to measure the change of the fit between the full model and a reduced model (not comprising the factor of interest). A significant finding indicated that the factor in question contributed to the variance of the response variable “collapsing the trapdoor.” First, we examined which of the random effects factors influenced the response variable by taking out each random-effects factor one by one and comparing each reduced model to the initial full model. In the same way, we examined the importance of control variables on the response, namely by taking out each control variable and comparing the reduced model against the full one. Before testing for individual effects of the experimental test factors, we examined whether the whole set of experimental test variables significantly explained variance on the response variable by testing the full model against the null model (which did not comprise any of the test factors but the controls) to prevent false-positives (54).
Supplementary Material
Acknowledgments
We thank the keepers of the Leipzig zoo, notably Stefan Leideritz, Daniel Geissler, Nico Schenk, and ‘Mozart’ Herrmann for their care for and work with the chimpanzees; Raik Pieszek and Ulrich Manfred for their help in building the apparatuses; research assistant Angela Loose and student assistants Christian Nawroth, Ulrike Arnold, Georg Keller, Monique Junge, Diana Paschenda, Marthe Krüger, and Anita Akmentina for assisting with data collection and reliability coding; Marlen Sureck for illustrations; and Roger Mundry for statistical advice.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203179109/-/DCSupplemental.
References
- 1.Richerson PJ, Boyd R. Not by Genes Alone: How Culture Transformed Human Evolution. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 2.Richerson PJ, Boyd R. The evolution of human ultra-sociality. In: Eibl-Eibisfeldt I, Salter F, editors. Ideology, Warfare, and Indoctrinability; Evolutionary Perspectives. London, UK: Berghahn Books; 1998. pp. 71–95. [Google Scholar]
- 3.Hill K, Barton M, Hurtado AM. The emergence of human uniqueness: Characters underlying behavioral modernity. Evol Anthropol. 2009;18:187–200. [Google Scholar]
- 4.Boyd R, Richerson PJ. Punishment allows the evolution of cooperation (or anything else) in sizable groups. Ethol Sociobiol. 1992;13:171–195. [Google Scholar]
- 5.Clutton-Brock TH, Parker GA. Punishment in animal societies. Nature. 1995;373:209–216. doi: 10.1038/373209a0. [DOI] [PubMed] [Google Scholar]
- 6.Jensen K. Punishment and spite, the dark side of cooperation. Philos Trans R Soc Lond B Biol Sci. 2010;365:2635–2650. doi: 10.1098/rstb.2010.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen K, Tomasello M. Punishment. In: Breed MD, Moore J, editors. Encyclopedia of Animal Behavior. Oxford: Academic; 2010. pp. 800–805. [Google Scholar]
- 8.Wenseleers T, Ratnieks FLW. Enforced altruism in insect societies. Nature. 2006;444:50. doi: 10.1038/444050a. [DOI] [PubMed] [Google Scholar]
- 9.Bshary R, Grutter AS. Punishment and partner switching cause cooperative behaviour in a cleaning mutualism. Biol Lett. 2005;1:396–399. doi: 10.1098/rsbl.2005.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulder RA, Langmore NE. Dominant males punish helpers for temporary defection in superb fairy-wrens. Anim Behav. 1993;45:830–833. [Google Scholar]
- 11.Clutton-Brock TH, Russell AF, Sharpe LL, Jordan NR. 'False feeding' and aggression in meerkat societies. Anim Behav. 2005;69:1273–1284. [Google Scholar]
- 12.Hauser MD. Costs of deception: Cheaters are punished in rhesus monkeys (Macaca mulatta) Proc Natl Acad Sci USA. 1992;89:12137–12139. doi: 10.1073/pnas.89.24.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehr E, Fischbacher U. Third-party punishment and social norms. Evol Hum Behav. 2004;25:63–87. [Google Scholar]
- 14.Henrich J, et al. Markets, religion, community size, and the evolution of fairness and punishment. Science. 2010;327:1480–1484. doi: 10.1126/science.1182238. [DOI] [PubMed] [Google Scholar]
- 15.Boyd R, Richerson PJ. Culture and the evolution of the human social instincts. In: Enfield NJ, Levinson SC, editors. Roots of Human Sociality: Culture, Cognition, and Interaction. Oxford: Berg; 2006. pp. 453–477. [Google Scholar]
- 16.Mathew S, Boyd R. Punishment sustains large-scale cooperation in prestate warfare. Proc Natl Acad Sci USA. 2011;108:11375–11380. doi: 10.1073/pnas.1105604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratnieks FLW, Wenseleers T. Evolution. Policing insect societies. Science. 2005;307:54–56. doi: 10.1126/science.1106934. [DOI] [PubMed] [Google Scholar]
- 18.Monnin T, Ratnieks FLW, Jones GR, Beard R. Pretender punishment induced by chemical signalling in a queenless ant. Nature. 2002;419:61–65. doi: 10.1038/nature00932. [DOI] [PubMed] [Google Scholar]
- 19.Smith AA, Hölldober B, Liebig J. Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol. 2009;19:78–81. doi: 10.1016/j.cub.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 20.Ratnieks FLW, Wenseleers T. Altruism in insect societies and beyond: Voluntary or enforced? Trends Ecol Evol. 2008;23:45–52. doi: 10.1016/j.tree.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 21.Raihani NJ, Grutter AS, Bshary R. Punishers benefit from third-party punishment in fish. Science. 2010;327:171. doi: 10.1126/science.1183068. [DOI] [PubMed] [Google Scholar]
- 22.Raihani NJ, Pinto AI, Grutter AS, Wismer S, Bshary R. Male cleaner wrasses adjust punishment of female partners according to the stakes. Proc Biol Sci. 2012;279:365–370. doi: 10.1098/rspb.2011.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Waal FBM. Chimpanzee Politics: Power and Sex Among Apes. Baltimore: Johns Hopkins Univ Press; 1989. [Google Scholar]
- 24.von Rohr CR, et al. Impartial third-party interventions in captive chimpanzees: A reflection of community concern. PLoS ONE. 2012;7:e32494. doi: 10.1371/journal.pone.0032494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flack JC, de Waal FBM, Krakauer DC. Social structure, robustness, and policing cost in a cognitively sophisticated species. Am Nat. 2005;165:E126–E139. doi: 10.1086/429277. [DOI] [PubMed] [Google Scholar]
- 26.Flack JC, Girvan M, de Waal FBM, Krakauer DC. Policing stabilizes construction of social niches in primates. Nature. 2006;439:426–429. doi: 10.1038/nature04326. [DOI] [PubMed] [Google Scholar]
- 27.Mitani JC. Demographic influences on the behavior of chimpanzees. Primates. 2006;47:6–13. doi: 10.1007/s10329-005-0139-7. [DOI] [PubMed] [Google Scholar]
- 28.Muller MN, Mitani JC. Conflict and cooperation in wild chimpanzees. Adv Stud Behav. 2005;35:275–331. [Google Scholar]
- 29.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci USA. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen K, Call J, Tomasello M. Chimpanzees are vengeful but not spiteful. Proc Natl Acad Sci USA. 2007;104:13046–13050. doi: 10.1073/pnas.0705555104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Waal FBM. Good Natured. Cambridge, MA: Harvard Univ Press; 1996. [Google Scholar]
- 32.Brosnan SF, Schiff HC, de Waal FBM. Tolerance for inequity may increase with social closeness in chimpanzees. Proc Biol Sci. 2005;272:253–258. doi: 10.1098/rspb.2004.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behav Brain Sci. 2005;28:675–691, discussion 691–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- 34.Chapais B. Primeval Kinship: How Pair-Bonding Gave Birth to Human Society. Cambridge, MA: Harvard Univ Press; 2008. [Google Scholar]
- 35.Bräuer J, Call J, Tomasello M. Are apes inequity averse? New data on the token-exchange paradigm. Am J Primatol. 2009;71:175–181. doi: 10.1002/ajp.20639. [DOI] [PubMed] [Google Scholar]
- 36.Jensen K, Call J, Tomasello M. Chimpanzees are rational maximizers in an ultimatum game. Science. 2007;318:107–109. doi: 10.1126/science.1145850. [DOI] [PubMed] [Google Scholar]
- 37.Aureli F, Cozzolino R, Cordischi C, Scucchi S. Kin-oriented redirection among Japanese macaques: An expression of a revenge system? Anim Behav. 1992;44:283–291. [Google Scholar]
- 38.O’Gorman R, Henrich J, Van Vugt M. Constraining free riding in public goods games: Designated solitary punishers can sustain human cooperation. Proc Biol Sci. 2009;276:323–329. doi: 10.1098/rspb.2008.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clutton-Brock TH, Parker GA. Sexual coercion in animal societies. Anim Behav. 1995;49:1345–1365. [Google Scholar]
- 40.Gros-Louis J. The function of food-associated calls in white-faced capuchin monkeys, Cebus capucinus, from the perspective of the signaller. Anim Behav. 2004;67:431–440. [Google Scholar]
- 41.Panchanathan K, Boyd R. Indirect reciprocity can stabilize cooperation without the second-order free rider problem. Nature. 2004;432:499–502. doi: 10.1038/nature02978. [DOI] [PubMed] [Google Scholar]
- 42.Barclay P. Reputational benefits for altruistic punishment. Evol Hum Behav. 2006;27:325–344. [Google Scholar]
- 43.Trivers RL. The evolution of reciprocal altruism. Q Rev Biol. 1971;46:35–57. [Google Scholar]
- 44.Fehr E, Gächter S. Altruistic punishment in humans. Nature. 2002;415:137–140. doi: 10.1038/415137a. [DOI] [PubMed] [Google Scholar]
- 45.Henrich J, et al. Costly punishment across human societies. Science. 2006;312:1767–1770. doi: 10.1126/science.1127333. [DOI] [PubMed] [Google Scholar]
- 46.Vaish A, Missana M, Tomasello M. Three-year-old children intervene in third-party moral transgressions. Br J Dev Psychol. 2011;29:124–130. doi: 10.1348/026151010X532888. [DOI] [PubMed] [Google Scholar]
- 47.Rakoczy H, Warneken F, Tomasello M. The sources of normativity: Young children’s awareness of the normative structure of games. Dev Psychol. 2008;44:875–881. doi: 10.1037/0012-1649.44.3.875. [DOI] [PubMed] [Google Scholar]
- 48.Baumard N. Has punishment played a role in the evolution of cooperation? A critical review. Mind and Society. 2010;9:171–192. [Google Scholar]
- 49.Boyd R, Richerson PJ. Solving the puzzle of human cooperation. In: Levinson SC, editor. Evolution and Culture. Cambridge, MA: MIT Press; 2005. pp. 105–132. [Google Scholar]
- 50.Kummer H, Cords M. Cues of ownership in long-tailed macaques, Macaca fascicularis. Anim Behav. 1991;42:529–549. [Google Scholar]
- 51.Nishida T, Kano T, Goodall J, McGrew WC, Nakamura M. Ethogram and ethnography of Mahale chimpanzees. Anthropol Sci. 1999;107:141–188. [Google Scholar]
- 52.Baayen RH. Analyzing Linguistic Data. A Practical Introduction to Statistics Using R. Cambridge, UK: Cambridge Univ Press; 2008. p. 368. [Google Scholar]
- 53.Bates D, Maechler M. 2009. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-31. Available at http://cran.r-project.org/
- 54.Forstmeier W, Schielzeth H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol. 2011;65:47–55. doi: 10.1007/s00265-010-1038-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.