Abstract
A new paradigm has emerged for osteogenesis imperfecta (OI) as a collagen-related disorder. The more prevalent autosomal dominant forms of OI are caused by primary defects in type I collagen, while autosomal recessive forms are caused by deficiency of proteins which interact with type I procollagen for post-translational modification and/or folding. Factors contributing to the mechanism of dominant OI include intracellular stress, disruption of interactions between collagen and non-collagenous proteins, compromised matrix structure, abnormal cell-cell and cell-matrix interactions and tissue mineralization. Recessive OI is caused by deficiency of any of the three components of the collagen prolyl 3-hydroxylation complex; absence of 3-hydroxylation is associated with increased modification of the collagen helix, supporting delayed collagen folding. Other causes of recessive OI include deficiency of collagen chaperones, FKBP65 or HSP47. Murine models are crucial to uncovering the common pathways in dominant and recessive OI bone dysplasia. Clinical management of OI is multidiscipinary, encompassing substantial progress in physical rehabilitation and surgical procedures, managment of hearing, dental and pulmonary abnormalities, as well as drugs such as bisphosphonates and rGH. Novel treatments using cell therapy or new drug regimens hold promise for the future.
INTRODUCTION
Osteogenesis Imperfecta (OI), or “brittle bone disease”, is a clinically heterogeneous heritable connective tissue disorder in which the causative defect is directly related to type I collagen, including abnormalities of collagen primary structure, insufficient quantity, abnormal post-translational modification, folding, intracellular transport or matrix incorporation. The clinical features of OI commonly include low bone mass and reduced bone material strength, resulting in bone fragility and easy susceptibility to fracture, bone deformity and growth deficiency. In its various types it occurs in approximately 1/15–20,000 births1. Most OI cases have autosomal dominant inheritance. Over 1500 dominant mutations in either COL1A1 or COL1A2, encoding the α-chains (α1(I) and α2(I)) of type I collagen, have been identified2 (See also the Osteogenesis Imperfecta Variant Database, at https://oi.gene.le.ac.uk). These mutations alter the structure or quantity of type I collagen and cause a skeletal phenotype ranging from subclinical to lethal.
Exciting developments have generated a new paradigm for OI as a collagen-related disorder. Recessive OI with lethal to moderate phenotypes is caused by defects in genes whose products interact with type I collagen. Most recessive cases have null mutations, causing absence of proteins involved in collagen prolyl 3-hydroxylation (CRTAP, LEPRE1 and PPIB)3–8, or helical folding (FKBP10 and SERPINH1)9, 10. Human cases and OI murine models are providing insight into common pathways in dominant and recessive OI, leading to re-evaluation of OI definition, classification and therapeutic approaches.
CLASSICAL OI (AUTOSOMAL DOMINANT INHERITANCE)
Defects in COL1A1 or COL1A2 cause autosomal dominant OI. OI features include bone fragility and deformity, as well as short stature, dentinogenosis imperfecta (DI) and hearing loss. Blue/grey sclerae and wormian bones are frequent, but not uniform, findings. In addition, there is overlap between the features of individuals with different Sillence types.
The Sillence classification included 4 types based on clinical, radiographic, and genetic criteria11, 12. Although proposed before collagen defects were identified in OI, it remains useful in an updated form which accounts for new gene defects or distinctive histomorphometry (Table 1).
Table 1.
OI Nosology
| OI Type |
Inheritance | Phenotype | Gene Defect | |
|---|---|---|---|---|
| Classical Sillence Types |
I | AD | Mild | Null COL1A1 allele |
| II | AD | Lethal | COL71A1/COL1A2 | |
| III | AD | Progressive Deforming | COL1A1/COL1A2 | |
| IV | AD | Moderate | COL1A1/COL1A2 | |
| Unknown Etiology |
V | AD | Distinctive Histology | Unknown |
| VI | AR? | Mineralization Defect | Unknown | |
| 3-Hydroxylation Defects |
VII | AR | Severe (Hypomorphic) Lethal (Null) |
CRTAP |
| VIII | AR | Severe to Lethal | LEPRE1 (P3H1) | |
| IX | AR | Moderate to Severe | PPIB (CyPB) | |
| Chaperone Defects |
X | AR | Severe to Lethal |
SERPINH1 (HSP47) |
| XI | AR | Progressive Deforming Bruck Syndrome? |
FKBP10 (FKBP65) | |
| Unclassified OI-like or Collagen-based Disorders | ||||
| Bruck Syndrome type 2 |
AR | Joint Contractures | PLOD2 | |
| Caffey Disease | AD | Cortical Hyperostosis | COL1A1 | |
| Osteoblast Maturation Defects |
AR | Moderate | SP7 (Osterix) | |
The classification shown in this review and elsewhere13 designates the original 4 Sillence types entirely for mutations in COL1A1 or COL1A2. It separates the novel OI types based on the gene in which the mutation occurs and the general function of that gene (collagen prolyl 3-hydroxylation, collagen chaperone, etc). This classification succinctly communicates the genetic defect and general phenotypic severity, while also generating homogenous groupings for therapeutic approaches and basic investigations of disease mechanism. Several versions of an alternative classification have been proposed14, 15, in which the recessive types are folded into the original Sillence numeration based on clinical phenotype; these classifications vary in whether the histologically defined types V and VI are retained or also classified clinically. The alternative classification results in children with defects in the same gene (ie LEPRE1) being classified as different types because of clinical variability (ie type II for lethal cases, or III for severe survivors), which is likely to confuse genetic counselling.
OI Type I, the mildest form, has a triad of features: fractures, blue sclera, and hearing loss. Fractures often begin with ambulation and decrease after puberty. These individuals have minimal bone deformity, near normal stature and rarely have DI.
OI Type II is perinatal lethal. Affected infants have short, bowed long bones with crumpling from in utero fractures, blue/grey sclerae, and a large, soft cranium. Radiographs reveal undertubulated long bones. The most common cause of death is respiratory failure, associated with small thorax, rib fractures, pneumonia, and perhaps with intrinsic collagen-related abnormalities of lung tissue16, 17.
OI Type III (progressive deforming) is the most severe, non-lethal form. Affected individuals may sustain hundreds of fractures. Most have triangular facies, frontal bossing, blue/grey sclerae, DI, vertebral compressions and scoliosis. Many have platybasia or basilar invagination. They have extremely short stature; about half have “popcorn” formation (sclerotic lines seen on radiographs representing growth plate fragmentation) at femoral growth plates18.
OI Type IV (moderately severe) has a broad phenotypic range overlapping types I and III. Affected individuals incur dozens of long bone fractures but most achieve ambulation. Scleral hue, DI, basilar impression, hearing loss and final stature are variable.
Genotype-Phenotype relationship
Type I procollagen is a heterotrimer, composed of two pro!1(I) and one pro!2(I) chains, flanked by globular pro-domains at both the amino (N-) and carboxyl (C-) termini (Figure 1). Glycine is obligatory at every third helical residue of collagen because of spatial constraints inside the triple helix19. Procollagen is extensively hydroxylated and glycosylated post-translationally (Box 1)20.
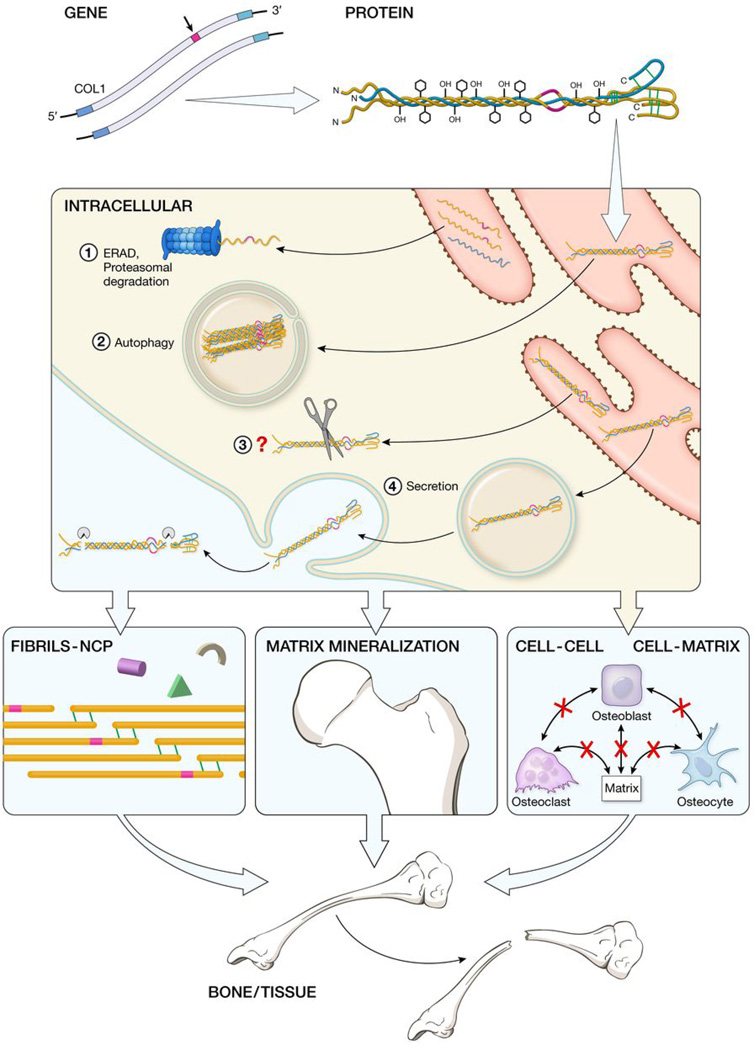
Figure 1. Mechanisms contributing to autosomal dominant OI bone dysplasia: from mutant type I collagen gene to bone defect.
Mutations in either COL1A1 or COL1A2 are translated into collagen α chains with abnormal structure, which delays folding of the heterotrimer and results in excess post-translational modification of the collagen helical region. Mutant procollagen chains unable to incorporate into heterotrimer are retrotranslocated into the cytosol and degraded by the ER-Associated Proteasomal (ERAD) pathway (1); fully misfolded heterotrimers with structural defects generate supramolecular aggregates that are eliminated by autophagy (2); mutant molecules with triple helical mutations are degraded through an unidentified pathway (3). Finally abnormal procollagen can be secreted, processed and incorporated in the extracellular matrix (4). The secreted mutant collagen affects fibril structure and interactions of NCPs with matrix, as well as matrix mineralization and osteoblast development and cell-cell and cell-matrix cross-talk. The overall result is bone deformity and fragility, although the relative importance of various contributions is under investigation.
Box 1.
COL1A1 and COL1A2 transcripts are translated in the ER into proα1(I) and proα2(I) chains, consisting of a central left handed triple helical domain (1014 aa) flanked by N- and C-terminal globular domains. The terminal C-noncollagenous (C-NC) domain of the proα chains contains interchain disulfide bonds and is important for the assembly and alignment of two α1(I) and one α2(I) chains210. Proper chain recognition and heterotrimer assembly through the C-NC alignment region is supported by interactions with ER-resident molecular chaperones such as immunoglobulin-heavy-chain binding protein (BiP), Serpinh1 (HSP47), peptidyl prolyl cis-trans isomerases and the recently identified prolyl 3-hydroxylation complex98, 211, 212. The assembled trimer then folds the helical domain from C- to N-termini. Proper folding of the triple helical domain requires the presence of a glycine residue at every third amino acid because of steric constrains in the interior aspect of the helix. The helical portions of the collagen chains are subject to a series of post-translational modifications during folding, until the chains become inaccessible in the tight helical configuration. These include prolyl 4-hydroxylation and lysyl hydroxylation of approximately half of proline and one-quarter of lysine residues along the length of the helical region of each chain, catalyzed by prolyl 4-hydroxylase (P4H) and lysyl hydroxylase, respectively, followed by hydroxylysyl glycosylation, catalyzed by glucosyl/galactosyl transferases. Specific proline residues, α1(I)Pro986 and α2(I)Pro707, are fully or partially 3-hydroxylated by the prolyl 3-hydroxylation complex (CRTAP/P3H1/CyPB). After procollagen is secreted into the pericellular space, the terminal propeptides are removed by specific N- and C-proteinases20. The mature triple helical collagen molecules participate in a higher order structure in the extracellular matrix, the heterotypic fibril. In the fibril, type I collagen is aligned in a quarter-staggered array, yielding D-period banding with overlap and gap regions. Collagen fibrils are stabilized by formation of covalent cross-links between the telopeptides and adjacent domains of collagen molecules, which are catalyzed by lysyl oxidase. Fibrils interact with non-collageneous proteins, bind soluble factors such as growth factors and cytokines, which regulate cell functions, and constitute the scaffold for mineral deposition24.
General principles have emerged for genotype-phenotype correlations in dominant OI. The molecular defect in type I OI is a null COL1A1 allele due to frameshifts or PTCs, causing reduced synthesis of structurally normal collagen. Also, splice site defects often lead to alternative splicing with subsequent PTCs. The phenotypes of null COL1A1 alleles and mild helical substitutions may overlap21, 22. We propose that type I OI should be limited to cases with type I collagen haploinsufficiency, including those individuals whose haploinsufficiency is associated with a moderate clinical outcome. The overwhelming majority of patients with a type I OI phenotype have a null COL1A1 allele. The occasional individual with a collagen structural mutation and a very mild phenotype will be designated type IV OI. In this approach, type I OI is a clinically and biochemically homogenous grouping, as well as the only dominant OI form in which structurally abnormal collagen is not present.
Types II – IV OI are caused by defects in type I collagen structure, most commonly glycine substitutions (80%) and splice site mutations (20%)2. Glycine substitutions delay helix folding, leading to post-translational overmodification (Box 1). The OI Mutation Consortium examined 832 mutations in Types II-IV OI, representing substitutions at ~ 44% of glycine residues2. In both α-chains, substitutions in the N-terminus are non-lethal (Figure 2). Overall, 36% of COL1A1 glycine substitutions had a lethal outcome, especially those with charged or branched side chains. Substitutions in two α1(I) Major Ligand Binding Regions (MLBR 2 and 3) are exclusively lethal, suggesting collagen-NCP interactions in matrix are essential to bone formation (Figure 2). Most COL1A2 glycine substitutions are non-lethal (81%). The α2(I) lethal substitutions occur in eight regularly spaced clusters, aligning with proteoglycan binding sites in the collagen fibril (Figure 2)2, 23, 24. The different patterns of lethality in α1(I) and α2(I) indicate each chain plays a different role in matrix organization. Also, substitutions at over 40 glycine residues result in both lethal and non-lethal forms of OI2, supporting the importance of modifying factors25, 26.
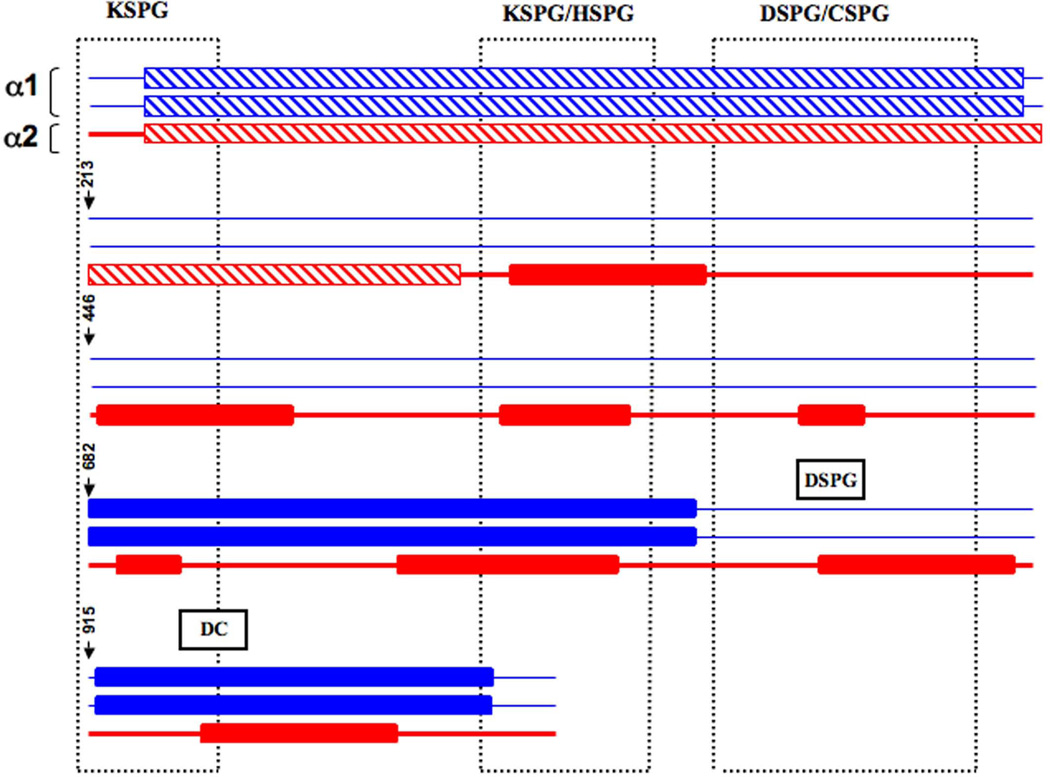
Figure 2. Distribution of lethal and non-lethal glycine substitutions causing OI along the type I collagen monomer and fibril.
The α1(I) chains are represented by blue coloration and the α2(I) chain by red coloration. Rectangles with hatched lines indicate the regions with predominantly non-lethal mutations located in the first quarter of both α chains. Filled rectangles symbolize the lethal regions in each chain. In the α1(I) monomer, stretches of exclusively lethal mutations were identified in the Major Ligand Binding Regions (MLBR2 and 3). In α2(I), lethal mutations were clustered in eight regions along the chain. The vertical boxes defined by dots represent the binding regions in the type I collagen fibril for keratin (KSPG), heparan (HSPG), dermatan (DSPG) and chondroitin sulphate (CSPG) proteoglycans. The monomer-binding sites for DSPG and decorin core protein overlap α2(I) lethal clusters. There is substantial alignment of the α2(I) lethal clusters and the proteoglycan binding sites on the fibril.
Detailed comparison of collagen quantitative and structural mutations with OI phenotype found higher lumbar spine areal BMD, greater cortical width and lower bone turnover parameters in type I OI27. Furthermore, BMD and histomorphometry of patients with non-lethal OI did not correlate with the α-chain containing the mutation, mutation location in the chain, or the substituting residue, suggesting other factors are crucial for outcome severity.
Rare mutations affecting procollagen processing sites or chain register lead to distinctive variants of OI. The procollagen N- and C-propeptides are cleaved by specific propeptidases in the pericellular space (Box 1). Glycine substitutions in the first 90 residues of the α1(I) helical region disrupt a stable N-anchor domain and prevent or delay N-propeptide removal. The pN-collagen is incorporated into matrix, decreases fibril size and causes a phenotype with characteristics of both OI and Ehlers-Danlos Syndrome (EDS)28, 29. Disruption of N-propeptide processing by helical defects in α2(I) also leads to OI/EDS30–34.
For C-propeptide processing, substitutions at the cleavage site Asp-Ala residues result in mild OI with increases in vertebral DXA z-scores and bone mineralization that are counterintuitive for OI, due to accelerated mineralization35. Substitutions in the proα1(I) or proα2(I) C-propeptide have broad phenotypic variability, causing types II-IV OI, though the majority are mild or lethal36–39. Since α-chains align at the C-terminal end, these mutations delay chain incorporation and helix formation. However, the C-propeptide is not normally incorporated into collagen fibrils, leaving the mechanism of these OI cases unclear.
Small triplet deletion or duplication mutations shift the register of α-chains in the helix. Although the Gly-X-Y sequence is maintained, salt bridges are disrupted by misalignment of X and Y residues between chains. These cases are severe or lethal, and have delayed collagen folding40, 41. The register shift can propagate to the end of the helix, and impact N-propeptide cleavage. Interestingly, substitutions for Y-position residues may also propagate a register shift nearly the full length of the collagen helix, interfering with N-propeptide processing and causing variable phenotypes including mild OI, hyperextensibility and Caffey Disease, a transient infantile cortical hyperostosis42–44. Several pedigrees with autosomal dominant Caffey Disease have been shown to have the same COL1A1 R836C (p.R1014C) Y-position change44–46, associated with self-resolving inflammation and subperiosteal new bone formation with reduced penetrance in infancy. The hyperostosis may be the consequence of the mutation disrupting binding of a ligand, such as IL-2, to collagen and causing increased susceptibility to periosteal injury during infancy44.
Understanding the Disease Mechanism: from gene to tissue
Almost all cases of dominant OI have low bone mass and increased skeletal fragility47. Histomorphometry of OI iliac crests revealed decreased trabecular and cancellous bone volume, increased osteoblast and osteoclast surface, and an overall increase in bone formation rate per bone surface. However, deposition of new bone at the single osteoblast level (MAR) is reduced, and is not compensated by the increased cell number48. Interestingly, FT-IR and qBEI both revealed elevated bone matrix mineralization. These data support the occurrence of a common defect in OI bone downstream from the collagen quantitative and qualitative mutations, altering bone cell function and the modelling/remodelling mechanisms which normally maintain bone homeostasis27, 48, 49.
A variety of murine models for OI are now available for investigation of OI mechanism and pilot treatment studies (Table 2). Mov13 mice have a null Col1a1 allele caused by a proviral insertion and model type I OI50, 51. The oim/oim mouse phenotypically resembles type III OI, although its recessive inheritance is atypical for collagen mutations. A spontaneous single nucleotide deletion in the oim Col1a2 C-propeptide prevents α2(I) incorporation into collagen52. However, the resulting α1(I) homotrimer does not account for the severe OI phenotype (see Gene and Protein Defects, below). More recent OI models were generated with knock-in technology or ENU mutagenesis. Knock-in Brtl53 and G610C OI (Amish)26 mice have classical glycine substitutions in α1(I) or α2(I) respectively, leading to phenotypes representative of type IV OI. Aga2 mice were generated by ENU mutagenesis; they have a proα1(I) C-propeptide mutation causing a type III OI phenotype54. Murine OI models provide direct access to intact long bone and tissues such as lung which are not available from patients; they provide large numbers of samples with the same mutation for studies. These models already play an important role in piloting therapy approaches. In Brtl and oim, cell transplantation has led to positive changes in mechanical properties despite low levels of cellular uptake into bone55–57. In the same mouse models treated with bisphosphonates, direct access to whole femora revealed both beneficial and potentially detrimental effects58, 59; RANKL inhibition has also been piloted in oim60, 61. Of equal importance, murine OI models have provided insight into basic mechanism, including elevated osteoclast function (Brtl and oim)62, 63, varability of expression (Brtl and Amish)25, 26, 64, ER Stress54, 65 and apoptosis (Aga2)54, which provides new targets for therapy.
Table 2.
OI Murine Models
| Mouse | Gene defect | Protein defect | Transmission | Bone phenotype | OI Type | Ref |
|---|---|---|---|---|---|---|
| Mov 13 +/− | M-MuLV insertion in endogenous Col1a1 intron 1 |
50% synthesis of normal proα1(I) |
AD | Reduced ductility and bone strength, increased tissue porosity and altered collagen organization |
Type I | 213 |
| Mov 13 −/− | Lack of proα1(I) | AR | Lethal in mid-stage gestation due to rupture of major blood vessels |
Type II | 51 | |
| Transgenic | Exogenous mutant murine Col1a1 cDNA |
Gly859Cys | AD | Deformed bones, poor mineralization and underdevelopment of the skeleton |
Type II | 214 |
| Transgenic | Human COL1A1 minigene | Human proα1(I) lacking 41 internal exons |
AD | Moderate to severe bone phenotype depending on transgene expression level. Multiple fractures, short femurs, reduced mineral and collagen content |
Type II–IV | 215 |
| Oim −/− | Naturally occurring Col1a2 c.3983delG |
Lack of proα2(I) in collagen I and synthesis of [α1(I)]3 homotrimers |
AR | Skeletal fractures, limb deformities, generalized osteopenia, small body size and reduced bone mineral density. Increased osteoclast activity. |
Type III-like | 52 |
| Brtl +/− | Knock-in Col1a1 c.1546G>T |
Gly349Cys | AD | 30% perinatal lethality, reduced body size, flared thorax, rib fractures, long bone deformity, bone fragility, and reduced bone mineral density. Increased bone turnover due to increased osteoclast precursors and reduced osteoblast activity. |
Type IV | 53 |
| Brtl −/− | AR | Absence of perinatal lethality, body size intermediate between Brtl+/− and WT, no rib fractures, no flared thorax, normal bone mineral density. |
Mild Type IV (near normal) |
76 | ||
| Aga2 +/− | ENU induced Col1a1 IVS50-2T>A c.4216-2T>A |
Frameshift of last 48 amino acids and addition of 90 amino acids beyond stop codon |
AD | Severe bone phenotype: early lethality, multiple fractures, and reduced bone mass. Disturbed osteoblast function. |
Type III | 54 |
| G610C OI (Amish) |
Knock-in Col1a2 c.2098G>T |
Gly610Cys | AD | Moderately severe phenotype affected by genetic background. Reduced body size, reduced bone mineral density and bone strength. |
Type IV | 26 |
| Crtap−/− | Knock-out of Crtap gene |
Absence of CRTAP protein |
AR | Moderate phenotype: growth delay, skeletal deformity, kyphosis, and reduced bone mineral density. |
Type VII | 3 |
| P3H1 null | Knock-out of Lepre1 gene |
Absence of P3H1 protein | AR | Moderate phenotype: reduced body size, and reduced bone mineral density. |
Type VIII | 105 |
| Ppib−/− | Knock-out of Ppib gene |
Absence of CyPB protein | AR | Severe phenotype: premature death, reduced body size, bone deformity, and reduced bone mineral density. |
Type IX | 110 |
| Osx −/− | Knock-out of Sp7 gene |
Absence of Osterix protein |
AR | Lethal phenotype: severe bone deformity, and absence of bone mineralization. |
OI-like | 127, 216 |
Factors contributing to the Mechanism of OI
The mechanisms of classical OI encompass the gene mutation, the collagen alteration, and dysfunction at the cellular, matrix (ECM) and tissue levels (Figure 1). The composition and organization of matrix influences the presence of growth factors and cytokines important for proliferation and differentiation of bone cells66, as well as matrix mineralization, which confers bone stiffness.
Gene and protein defects
The type I collagen biosynthetic pathway has been extensively reviewed67 and a brief description is provided in Box 1. The matrix insufficiency of type I OI results from a PTC in the COL1A1 transcript, which activates NMD, reducing mutant transcripts and leading to the synthesis of half the amount of normal collagen. Absence of α1(I) chains is not compatible with life, as demonstrated by embryonic lethality in the homozygous Mov13 mice50 (Table 2).
Homozygous null mutations in COL1A2 lead to a range of phenotypes. Those associated with NMD and loss-of-function lead to assembly of α1(I) homotrimer. Clinically, this causes mild EDS with hypermobility in childhood and cardiac valve disease in adulthood, rather than OI68. In contrast, both one patient with OI69, and the severe oim/oim mouse (Table 2) have a deletion in the α2(I) C-propeptide, which does not result in NMD. They produce normal levels of COL1A2 transcripts, which are translated into α2(I) chains that cannot incorporate into collagen. Since α1(I) homotrimer alone does not lead to OI, the intracellular accumulation of mutant α2(I) chains (see Intracellular Stress, below) may cause the skeletal dysplasia.
Glycine substitutions delay collagen folding and result in overmodified collagen, which may compromise secretion and/or processing2, 70. Substituting residues disrupt non-covalent bonds, causing local unwinding. Certain substituting residues have greater lethality, as do substitutions in clusters along α2(I), and in α1(I) MLBRs (Figure 2)2. Although the overmodification gradient does not correlate with clinical severity in α1(I), empirical rules correctly assign most lethal or non-lethal outcomes71. In addition, the overmodification of structurally normal collagen in recessive OI (Recessive OI, below) raises the possibility that excess hydroxylation and glycosylation have a direct detrimental role in matrix.
Intracellular Stress
Misfolded collagen chains in the ER activate the Unfolded Protein Response (UPR), triggering synthesis of chaperones to assist collagen folding or, alternatively, increasing mutant protein degradation72. Cellular response varies depending on the type of collagen mutation (Figure 1). Collagen with triple helical mutations is removed by autophagy, as are collagen aggregates in cells lacking HSP4773. In Aga2 cells, ER-retention of mutant collagen increases expression of chaperones BiP and HSP47, apoptosis-inducing transcription factor Gadd153/CHOP and activation of caspase-3 dependent apoptosis54. In calvaria of Brtl+/− perinatal lethal pups, relative intracellular retention of helices with one mutant chain65 is associated with increased expression of Gadd153/CHOP, but normal BiP expression, suggesting collagen misfolding activates the UPR through a BiP-independent response25. Finally, C-propeptide mutations that impair trimer assembly result in increased BiP expression, retrotranslocation of the misfolded proα chains into the cytosol and degradation via the proteasomal ER-associated degradation (ERAD) pathway74, 75.
Compromised ECM Structure and Mineralization
In OI types II-IV, the mixture of normal and mutant α-chains results in matrix heterogeneity and may contribute to the generally greater severity of α1(I) defects, since heterozygous α1(I) defects yield helices with two, one or no mutant chains, while α2(I) defects result in two helix compositions. In Brtl mice, homozygosity for the mutant allele leads to matrix homogeneity and, unexpectedly, to a less severe phenotype, suggesting this feature impacts bone properties76.
The association of lethal OI with MLBRs in α1(I) monomers or proteoglycan binding sites on fibrils for α2(I)2, 23, 24 most likely reflects compromised interactions of NCPs with fibrils (Figure 2). The NCP composition of matrix is altered secondarily in OI, which is also likely to impact bone properties. Cultured OI osteoblasts synthesize reduced amounts of osteonectin and proteoglycans, and increased amounts of fibronectin, thrombospondin and hyaluronan77, 78. Thrombospondin and decorin bind growth factors, while decorin and fibronectin are important for fibrillogenesis.
The normal D-periodic spacing of fibrils generates gap and overlap regions, which are important for mineral nucleation and collagen cross-links and NCP interactions, respectively24. In Brtl+/− bone matrix, the collagen fibril D-period has significantly greater variability in spacing than in wild-type littermates79. The abnormal structure of heterotypic fibrils could affect the type and amount of mineral deposited by increasing the density of nucleation sites80, 81; OI matrix also contains abnormal levels of NCPs82 known to regulate crystal deposition and growth83. Elevated mineral content has been demonstrated by FT-IR and BMDD in OI bone with collagen quantitative and structural defects, and is also found in murine models81, 84. The elevated mineral content and loss of mineralization heterogeneity contribute to the fragility of OI bone85, 86, possibly through loss of ductility.
Cell-Cell, Cell-Matrix Interactions
Cellular interactions with abnormal matrix and compromised osteoblast development influence signalling between osteoblasts and osteoclasts, increasing bone remodelling and exacerbating the bone weakness caused by the primary collagen change (Figure 1). Osteoblasts sense osteocyte apoptosis via gap junctions, and receive negative feedback from osteocytes through sclerostin87,88. Osteoblasts then trigger osteoclast maturation and recruitment87. Ultrastructural examination of OI bone revealed increased numbers of osteocytes and multiple osteocytes in some lacunae89. In the Brtl mouse, osteoclast numbers are elevated in femora, uncoupled from osteoblast numbers. Brtl osteoclast precursors from marrow are larger, more numerous and more intensely TRAP stained than in wild-type62. The RANKL/OPG ratio is normal in Brtl bone, so other soluble factors triggered by the abnormal matrix may increase osteoclast development. In the oim/oim mouse, elevation of the RANKL/OPG ratio and higher expression of TNF-α were detected in sorted immature osteoblasts, supporting cell-cell signalling as a key aspect of elevated bone turnover in OI63.
Cross-links in collagen fibrils are important for preosteoblast maturation90. In OI, collagen located at the surface of fibrils had fewer cross-links than in the fibril interior.91 Contact with cross-link deficient matrix by OI bone cell populations could contribute to impaired osteoblast maturation and increased osteoclast recruitment. Also, the effects of collagen heterogeneity in dominant OI could be mediated in part by abnormal cross-linking.
RECESSIVE OI
Beginning with the genes encoding the components of the collagen 3-hydroxylation complex, mutations in five genes have now been identified as causing recessive OI (Table 1). Collectively, they account for 2–5% of OI cases detected in North America and Europe4, 71. Other genes remain to be identified, including the genes causing types V and VI OI (See Discussion of type VI OI and FKBP10 mutations). The pattern emerging for OI is of a collagen-related condition, affecting the structure, synthesis, folding, secretion and matrix organization of type I collagen.
Defects in components of the collagen 3-hydroxylation complex
Prolyl 3-hydroxylase 1 (P3H1), cartilage-associated protein (CRTAP) and cyclophilin B (CyPB) assemble into a 1:1:1 complex within the ER that post-translationally modifies specific proline residues in unfolded collagen α-chains92. This includes nearly complete 3-hydroxylation of Pro986 residues of α1(I), α1(II) and α2(V) collagen chains, plus several partially modified sites in α2(I) (80% Pro707), α1(II), α1(V) and α2(V) chains93. The complex also has a chaperone function; furthermore, each complex component is a multifunctional protein, with independent extracellular functions94–96.
The importance of the complex and α1(I) Pro986 hydroxylation to bone development became apparent when the recessive bone defect in the Crtap−/− mouse3 converged with the chromosomal location of type VII OI (3p22–24.1)97 and with a biochemical approach to recessive OI based on overmodification of structurally normal collagen (Figure 3)98.
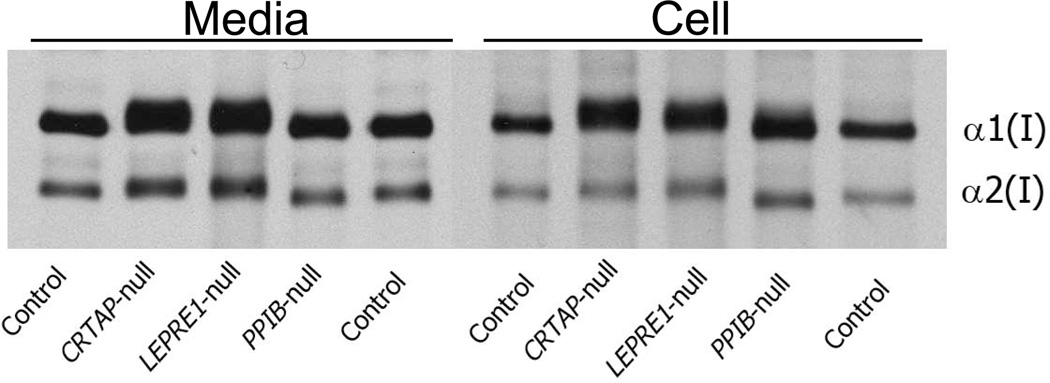
Figure 3. Electrophoretic analysis of type I collagen synthesized by dermal fibroblasts with mutations in genes coding for collagen 3-hydroxylation complex components.
Most mutations in CRTAP, LEPRE1 or PPIB cause decreased or absent protein due to nonsense mediated decay. Both [3H]-proline-labelled collagen alpha chains are fully overmodified in media and cell layer from primary fibroblast cultures of OI patients with CRTAP or LEPRE1 null mutations, indicating delayed folding of the collagen helix. However, in the fibroblasts of siblings with a mutated PPIB start codon, the secreted α chains have normal electrophoretic migration and collagen in the cell layer fraction has minimal backstreaking of α1(I).
CRTAP, the helper protein of the complex, is highly homologous to the amino end of P3H1, but lacks the C-terminal catalytic domain95, 98, 99. CRTAP is expressed in the skeletal system by chondrocytes, osteoblasts and osteoclasts. Although it is mainly localized in the ER, it is also secreted and may have a matrix function3, 95, 100. The Crtap−/− mouse has a moderately severe connective tissue disorder characterized by rhizomelia, kyphosis, growth deficiency, and osteopenia3, 101 (Table 2). In humans, CRTAP deficiency (type VII OI) presents as a moderate to lethal recessive osteochondrodystrophy, with growth deficiency and rhizomelia as in the mouse, and also with white sclerae, severe osteoporosis with neonatal fractures, and broad undertubulated long bones3, 4. Almost all reported CRTAP mutations cause frameshifts, resulting in NMD and absence of CRTAP protein, with loss of α1(I) 3-hydroxylation100. We had hypothesized that severe OI with collagen overmodification, but without a collagen structural defect, would be caused by defects in one or more proteins that interacted with collagen. However, the OI field had not anticipated that Pro986 3-hydroxylation or defects in members of the 3-hydroxylation complex, such as CRTAP, would delay type I collagen folding (Figure 3).
P3H1, the enzymatic component of the complex, is encoded by LEPRE1. P3H1 is the only complex component containing a KDEL sequence for ER retrieval; this KDEL-containing isoform is crucial for collagen modification102. A P3H1 isoform is also secreted as the chondroitin sulfate proteoglycan leprecan96. P3H1 expression is localized to tissues rich in fibrillar collagens, which are abundant during development103. Molecular defects in LEPRE1 (Type VIII OI), most of which led to reduced transcripts, were identified shortly after CRTAP mutations5, 8, 102 and found to outnumber type VII OI cases. Type VIII OI patients present with severe to lethal OI, recessive inheritance, white sclerae, rhizomelia, and undertubulation of long bones. Those who survive into childhood have extremely low BMD, severe growth deficiency and bulbous metaphyses. A LEPRE1 founder mutation (c.1080+1G>T), found in West Africans and African Americans, occurs in almost half of type VIII OI cases reported104. In Type VIII as in Type VII OI, there is absence of α1(I)Pro986 3-hydroxylation and overmodification of the collagen helical region, supporting delayed folding (Figure 3). The P3H1 null mouse has a milder phenotype than type VIII OI, although they share growth deficiency, rhizomelia, reduced BMD, abnormal hypertrophic chondrocytes, and delayed secretion of overmodified collagen from cultured cells105 (Table 2).
The third component of the collagen 3-hydroxylation complex, CyPB (encoded by PPIB), is a peptidyl-prolyl cis-trans isomerase. Isomerization of the naturally-occurring cis proline to the trans conformation is rate-limiting for collagen folding106. CyPB was thought to be the unique collagen isomerase107. Several mutations in PPIB (Type IX OI)7, 108 which lead to PTCs or misfolded protein result in severe to lethal OI. Their phenotype and overmodified collagen biochemistry are similar to OI types VII and VIII, except without rhizomelia, consistent with a dysfunctional 3-hydroxylation complex. In contrast, we identified a PPIB mutation (c.26T>C; referred to as c2T>G in ref. 6) in siblings with moderate OI and white sclerae, but without rhizomelia6. This mutation occurs at position M9 of the protein sequence predicted by GenBank (NM_000942.4). However, it was unproven (www.uniprot.org/uniprot/P23284), whether M1 or M9 was the initiation codon, and M1 is not fully conserved evolutionarily. Our data supported M9 as the start codon since PPIB transcripts were reduced and there was complete absence of CyPB, as expected for an initiator mutation109. CyPB was undetectable with multiple antibodies, in guanidinium extracts or after proteasomal inhibition109. P3H1 and CRTAP levels are one-third and two-thirds normal in the PPIB-null cells, indicating CyPB enhances complex stability. However, activity of the complex in the absence of CyPB is sufficient to normally 3-hydroxylate α1(I) Pro9866. Furthermore, normal helical modification in the absence of CyPB (Figure 3), indicating normal folding, argues for redundancy of collagen cis-trans isomerases in human cells.
A Ppib−/− mouse with an out-of-frame exon 2/4 junction has non-lethal recessive OI with reduced bone volume, kyphosis and growth deficiency110 (Table 2). Ppib transcripts are reduced and CyPB is undetectable, but murine collagen biochemistry differs from both human PTC and start codon mutations. Collagen gel electrophoretic migration had a delayed baseline, rather than a broadened band as in the human PTC cases; helical modification was not quantitated in the mouse. Pro986 3-hydroxylation was absent in murine collagen, although partial persistence of complex components in murine tissue is suggested by detection of reduced levels of P3H1. Functionally, procollagen remained in the ER and was not properly transported into the Golgi. The differences in collagen modification between the KO mouse and human start codon mutation may reflect alternative binding partners supporting P3H1/CRTAP stability in humans, or redundancy for PPIase.
Mechanisms of Recessive OI in Collagen 3-Hydroxylation Defects
Both P3H1 and CRTAP are absent or severely reduced in LEPRE1- or CRTAP-null cells, although transcript levels of the normal gene are somewhat increased. Transfection of null cells with constructs encoding the absent transcript restored both proteins111. These data indicate that CRTAP and P3H1 are mutually protective in the 3-hydroxylation complex, and explain the overlapping phenotypes of types VII and VIII OI. LEPRE1- or CRTAP-null cells lack the complex chaperone function, as well as collagen 3-hydroxylation. Absence of the P3H1/CRTAP complex also abrogates the collagen chaperone and PPIase functions of CyPB110, although cellular levels of CyPB are unaffected111. Similarly, binding of misfolded CyPB with P3H1/CRTAP appears to interfere with complex function, while P3H1/CRTAP can bind and modify collagen in the total absence of CyPB6, 7.
Crucial questions remain concerning the enzymatic and chaperone functions of the complex. Bachinger showed that the complex has collagen chaperone as well as PPIase activity94. Disregulation of the complex may eliminate 3-hydroxylation, but loss of complex collagen chaperone/PPIase functions may dominate disease mechanism. CyPB loses its ability to bind gelatin without CRTAP/P3H1, and knock-down of P3H1 severely reduces the ability of CyPB to bind nascent collagen chains in vitro110. The OI type IX case in which the P3H1/CRTAP complex was able to normally 3-hydroxylate collagen in the total absence of CyPB resulted in normal collagen folding and a moderate phenotype6.
It is unclear how deficiency of the 3-hydroxylation complex leads to collagen overmodification. Pro986 3-hydroxylation minimally affects collagen stability112. Although collagen folding has not been measured directly in these cases, overmodification is an established consequence of slow folding70. However, the extent of Pro986 hydroxylation does not correlate with overmodification or phenotype; patients with 0–5% Pro986 hydroxylation have the same helical overmodification and lethal outcome as those with 25% Pro986 hydroxylation100.
Another possible contribution to OI mechanism is a proposed role in matrix for the 3-hydroxylation modification. Eyre and co-workers suggested that 3Hyp sites function in fibril assembly through intermolecular hydrogen bonding93. Deficiency of type V collagen 3-hydroxylation could also affect formation of heterotypic fibrils. Alternatively, the 3-hydroxylation modification may not be crucial per se, but could serve as a binding epitope for NCPs involved in mineralization93.
Furthermore, absence of 3-hydroxylation complex components has a metabolic aspect, with intracellular effects beyond collagen hydroxylation. Mutant fibroblasts appear stellate and foamy in culture (unpublished data). The absence of complex chaperone activity would be expected to cause ER stress and ultimately apoptosis. We found a surprising increase in collagen secretion in LEPRE1-null fibroblasts that points to a possible role for P3H1 in regulation of proline metabolism or collagen synthesis5.
Collagen Chaperone Defects
Recently, absence or dysfunction of collagen chaperones HSP47 and FKBP65 have been reported to cause recessive OI9, 10. HSP47, encoded by SERPINH1, is an ER-resident collagen-specific chaperone that binds to and accompanies the assembled procollagen molecule together with CyPB into the Golgi113. Hsp47−/− mice are embryonic lethal, demonstrating HSP47 is required for normal development 114. Hsp47 defects caused intracellular aggregation and delayed secretion of collagen, with abnormal fibrils; type IV collagen misfolding disrupted basement membranes73, 115, 116. Severe bone dysplasia in canine and human cases (type X OI) is associated with SERPINH1 missense mutations10, 117. Dachshunds with a missense mutation (c.977C>T, p.L326P) causing OI with DI survived postnatally, probably due to residual HSP47117. The only child reported with HSP47 deficiency was homozygous for the missense mutation c.233T>C, p.Leu78Pro. The proband had a severe OI phenotype, including blue sclerae and DI. Atypical features included transient skin bullae, pyloric stenosis and renal stones requiring nephrectomy10. The mutant transcript was stable, but proteasomal degradation led to minimal HSP47 protein. Although collagen secretion was somewhat delayed, total collagen secretion was normal in culture and the secreted collagen had normal post-translational modification, indicating independence of the HSP47 chaperone function and 3-hydroxylation. However, proband types I and III collagen had increased sensitivity to protease digestion in vitro, suggesting HSP47 monitors triple helix folding and stabilizes the collagen helix during transit through the secretory pathway.
FKBP65, encoded by FKBP10, is ER-localized with chaperone activity for collagen118. Turkish and Mexican patients carrying FKBP10 frameshift mutations were first reported with recessive OI (Type XI OI)9. All probands have deforming OI including long bone fractures, ligamentous laxity, platyspondyly and scoliosis, although sclerae and teeth are normal. Biochemically, normal collagen 3-hydroxylation, without evidence of increased helical modification, was reported. FKBP10 mutations were associated with delayed collagen secretion and dilated ER. Consistent with a defect in the FKBP chaperone activity, intracellular aggregates of collagen were demonstrated. In one case, bone histology with an abnormal lamellar pattern resembling the fish scales of type VI OI was reported, as was elevated alkaline phosphatase in two individuals with the Turkish frameshift mutation9. Currently no mutation analysis has associated type VI OI with FKBP10 mutations; the similarity in bone histology may indicate a common pathway in separate gene defects.
Emerging data does indicate that the phenotypic spectrum of mutations in FKBP10 exons 5, 6 and 8 overlaps with Bruck syndrome (BRKS), an autosomal recessive condition characterized by osteoporosis, joint contractures at birth, fragile bones and short stature, and often thought of as “OI with congenital joint contractures”119. Siblings from Saudi Arabia have symptoms of Bruck syndrome caused by an FKBP10 exon 6 frameshift mutation that also alters the third PPIase domain120; these authors suggested calling FKBP10 mutations BRKS3, since FKBP10 does not map to either of the reported loci for BRKS1 or BRKS2. However, an exon 5 frameshift mutation, (c.831_832insC), predicted to lead to a PTC downstream of the third PPIase domain, has been detected in 5 pedigrees reflecting 4 ethnic groups121, including the Mexican family in the original report9, which was not noted to have contractures. Three of these 5 pedigrees had findings of Bruck syndrome, including a sibship with one child diagnosed as type III OI and the second with Bruck syndrome. Kelley et al proposed that FKBP10 mutations were the cause of BRKS1, although the map position of FKBP10 (17q21.2) does not coincide with the reported chromosomal position of BRKS1 based on a single 2-generation Kurdish family (17p12)121, 122. This issue remains unsettled: mapping results can be misleading and the enzyme (bone specific telopeptide TLH, encoded by PLOD2) whose deficiency was postulated to be involved in BRKS1 has subsequently been mapped to 3q23–24123. Bank and co-workers also reported that the original Kurdish family had no defects in PLOD2 leaving open the possibility that resequencing of the original Kurdish family might reveal an FKBP10 mutation122.
Unclassified OI-like and type I collagen based disorders
There are several OI-like or type I collagen based disorders that do not rise to the level of an OI type because of incomplete information. BRKS2 is a recessive condition caused by mutations in PLOD2 (3q23–24), which encodes bone-specific collagen telopeptide lysyl hydroxylase (TLH)124. Affected individuals with BRKS2 are reported to be clinically indistinguishable from BRKS1, “OI with joint contractures”. TLH deficiency results in underhydroxylation of the lysines of the collagen telopeptide, but not the triple helix, leading to abnormal collagen crosslinking.
Caffey Disease is also a distinctive syndrome, some cases of which are caused by a COL1A1 R836C (p.R1014C) substitution (see Dominant OI)44. The collagen matrix defect causes OI/EDS symptoms, while the partially penetrant cortical hyperostosis is limited to infancy.
Third, is a homozygous genetic defect in osterix (encoded by SP7), a member of the SP/KLF family of zinc-finger transcription factors125, 126. Osterix mutations might be expected to cause devastating defects of osteoblast differentiation; Osx-null mice are perinatal lethal with loss of both endochondral and intramembraneous bone formation, and reduced expression of osteoblast-specific markers Col1a1, BSP, osteonectin and osteopontin127. An Egyptian child, with a frameshift mutation in SP7 that leads to loss of the zinc-finger domain important for DNA binding, has a moderately severe OI-like phenotype with decreased vertebral DXA125. Classification of this defect is premature in the absence of biochemical, cellular or bone data, since SP7 (Osterix) does not have a selective direct effect on type I collagen.
CLINICAL ASPECTS OF OI
Secondary features of OI – Hearing, Dental, Neurological, Growth
Hearing loss is a common secondary feature of OI, affecting persons with all Sillence types128. It is generally progressive, often with mixed conductive and sensorineural deficiency, mostly bilateral and beginning in the second to fourth decades of life, although about 5% of OI children have been reported to have 20 dB hearing loss129. By age 50 years, about half of patients have subjective hearing loss in a Scottish survey130, while over 60% of Finnish OI adults had hearing loss on audiometry in a population study131. All OI types had hearing loss (approximately 60%, 80% and 40% of OI types I, III and IV, respectively). Further analysis of the Finnish OI population showed no correlation of hearing loss with collagen mutation type (null allele, glycine substitution or splicing defect) or mutated collagen gene, as well as a lack of penetrance in some family members132. About half of Finnish OI adults also have vestibular dysfunction, with vertigo generally secondary to inner ear pathology133.
The hearing loss in OI is clinically otosclerosis-like in that both result in footplate fixation, although the two conditions are distinct. When amplification is not adequate, surgical options may be indicated. Several large series of stapedectomies reported hearing gains of more than 20 dB in over 80% of operated ears, as well as improved bone conduction thresholds134–136. More recently, a second Dutch series137 reported success in type I OI but loss of hearing in a type III OI case, while a Swedish series138 encompassing multiple surgeons in different hospital settings reported more cautious gains, with worsening of hearing loss in 21% operated ears. For carefully selected OI patients with profound sensorineural hearing loss, cochlear implantation is an option139. The procedure is more challenging due to hypervascularity of the middle ear mucosa, but results similar to other sensorineural causes were obtained in most of the 10 cases reported139.
Dental abnormalities (opalescent teeth, obliterated pulp cavities, and constricted coronal-radicular junctions) were proposed as distinguishing genetic features of OI even before the Sillence classification, with almost complete penetrance in those pedigrees in which it occurred, and subsequently described as Sillence subtypes (A & B, with and without DI, respectively)140. Dental examinations with panoramic radiographs revealed 40–80% of children with types III and IV OI had DI in primary dentition141–143; microscopy suggests very mild DI may be missed radiographically144. Children with yellowish-brown discoloration had more enamel fractures and attrition than those with opalescent grey discoloration, and were more likely to need full-mouth restoration with crowns141, but discoloration was not related to OI type143. DI always improved in permanent teeth. A high incidence of malocclusion, impaction, and both delayed and accelerated tooth eruption were also noted. Interestingly, the majority of patients with quantitative collagen defects did not have DI145, while DI was not associated with any particular molecular abnormalities in patients with altered collagen structure145, 146. Expression of Col1a1 in homozygous mov13 odontoblasts suggests regulation of collagen expression may differ in teeth147, which could underlie DI inconsistencies. Histology reveals structurally abnormal dentin, with collagen hyperfibers and vesicles148. Reduced number and size variation of dentinal tubules were found on scanning EM of affected teeth, with an abnormally smooth dentin-enamel junction149. Recent microscopic and ultrastructural studies found occluded tubules, some with retro-curved processes and occlusion of the pulp chamber, consistent with odontoblast dysfunction150.
Various neurological features are associated with OI, including macrocephaly, hydrocephalus, syringomyelia and basilar invagination (BI) (an infolding of the skull base leading to brainstem distortion)151. Relative and absolute macrocephaly is common in OI caused by collagen structural defects. BI which progresses to brain stem impingement is relatively rare but its consequences are potentially devastating; progression should be followed with MRI152. Early intervention with occipitocervical bracing can delay progression in most cases153. Reducible BI (40%) is treated with posterior fossa decompression and occipitocervical fusion, while irreducible BI (60%) is treated with transoral-transpalatopharyngeal decompression. Despite successful decompression, 80% of BI progresses within 6 years post-surgery152.
Short stature is one of the cardinal features of OI. Endocrine evaluation of the growth axis was normal in most patients with collagen defects, however about half of children had a blunted response to the IGF-I stimulation test154. About half of type IV OI children treated with rGH double their baseline growth rate in the first rGH treatment year155. Given the chondro-osseus manifestations of recessive OI, it is reasonable to speculate that the short stature of dominant OI may be related to abnormalities at the transition from cartilage to bone.
Morbidity and Mortality in OI – Pulmonary and cardiovascular features
Extraskeletal manifestations of OI in the respiratory and cardiovascular systems are the most common causes of OI morbidity and mortality16, 156. Recurrent pneumonia is well-known in children with severe OI, as is right sided heart failure (cor pulmonale) in severe adults1. These effects have been considered secondary to skeletal changes16, 156, such as scoliosis, rib fractures, or thoracic cage deformity16, 47, 157. Individuals with OI and scoliosis have striking decline of pulmonary function after 60° curvature157. The presence of severe restrictive lung disease with minimal scoliosis raised the possibility that bone-independent pulmonary pathology also contributes substantially to morbidity in Types III-IV OI157. Two case studies of OI with lung hypoplasia add to the possible direct role of mutant collagen in lung pathology17, 158. Cor pulmonale is considered a late effect of pulmonary dysfunction in OI16. Pulmonary function data in OI patients with structurally abnormal collagen but without scoliosis, as well as data from both dominant and recessive murine models is needed to delineate the mechanism of pulmonary pathology.
Cardiovascular findings, including valvular insufficiency, aortic root dilatation, atrial septal defects and septal and posterior left ventricular wall thickening have been reported in OI159. In OI adults, aortic root dilatation is the most frequent valvular manifestation159, 160. When aortic regurgitation occurs, it is more commonly due to abnormal valvular structure than dilatation of the root160. Examination of asymptomatic OI adults by 12-lead EKG and 2D echocardiography revealed valvular regurgitation in 95%, with tricuspid and mitral regurgitation with or without aortic regurgitation accounting for 60% of cases, and impaired diastolic function161. These changes may reflect greater stiffness of myocardial tissue which may be primary effects of the mutant collagen.
Management of OI – Rehabilitation and physical therapy
The goal of physical rehabilitation in OI is to maximize the patient’s gross motor function and daily life competencies. This is especially important in childhood, when the fundamentals of life functioning are established, and for older individuals, who will experience combined OI and aging effects. Upper arm function, mobility, and annual fracture and surgery rates, but not pain, were reported to correlate with DXA z-scores in 20 patients with types I, III, IV162. The authors make the reverse inference that improving DXA scores will improve function, but this is not true especially for gross motor skills and is likely to be a simple correlation of baseline DXA and OI severity163, 164. Scoliosis and chest wall deformity also correlate with overall measures of physical health157. Further, earlier onset of scoliosis correlates with lower mean DXA z-scores and later age of achieving motor milestones165. However, children with mild bone disease and hyperlaxity of paraspinal ligaments may also develop early aggressive scoliosis, despite moderate DXA z-scores and earlier gross motor milestones42.
Physical rehabilitation of children at major OI clinical centers is individualized and actively promotes increased strength and mobility. Functional tests such as the BAMF166, the GMFM (originally designed for cerebral palsey)167 and Bleck score168 have been validated for OI; muscle strength is scored using consistent criteria individual to each center. The results of rehabilitation have been best documented for the Dutch pediatric OI population. A 4- year follow-up of 5–19 year olds showed that joint range of motion decreased significantly over time in type I OI, especially in the lower extremities, whereas types III and IV had severe motion limitations that did not change with time169. Children with all types of OI increased self-care and social function over time, but mobility level plateaued in types III and IV with muscle strength as the best predictor of ambulation. The type I OI children had no pulmonary or cardiac defects at rest170, while reduced exercise tolerance and muscle strength in types III and IV contributed to fatigue during activities of daily living. Children with types I and IV OI who participated in a low-resistance physical training program had improved peak oxygen consumption, maximal working capacity and muscle strength after 3 months, but the improvements diminished 6 months after program completion171, suggesting that regular exercise of the correct intensity is important to improving OI fitness. For immobilized children, a pilot study of whole body vibration using a tilt table in 4 type III or IV OI children was reported to achieve upright sitting in 2 children, and walking with minimal support in 2 others, that had not been achieved with several years of bisphosphonate172.
Management of OI – Orthopaedic Surgery
Orthopaedic surgery remains a mainstay of lifelong OI management, in a complementary function with physical rehabilitation. Osteotomies of long bones with placement of intramedullary rods are undertaken to correct deformity that impedes function and manage fracture recurrence. Corrective surgery is often crucial to ambulation. Currently, surgeons have at their disposal two types of telescoping rods (Fassier-Duval and Baily-Dubow/Sheffield) and non-elongating Rush pins for the immobilization of long bones after osteotomy procedures. Rod migration is a commonly reported complication in OI173, 174, occurring more frequently in non-telescoping than telescoping rods175. The Fassier-Duval rods have the advantage of percutaneous placement, minimizing trauma and allowing multiple bones to be treated in one session, followed by early rehabilitation176. Flexible nails unload less weight from the bone and are successfully utilized in single or double nail techniques177. Non-union occurs in about 15% of osteotomy in OI176; the increase in non-union after pamidronate may be related to thermal damage from use of an electric saw178.
The course of the scoliosis that is common in OI is minimally affected by bracing179. In Europe, successive use of halo traction followed by spine stabilization can be successful to stabilize (and sometimes reduce) the curve, improve respiratory function and pain180, 181. In contrast to common progression of scoliosis, basilar invagination uncommonly progresses to clinically significant compression151. To prevent hindbrain herniation and CSF obstruction, patients may require shunt placement or decompression with occipito-cervical fusion152, to be performed by experienced surgeons in specialized centers.
Management of OI – Benefits and limitations of Pharmacological therapy
Bisphosphonates are anti-resorptive compounds widely administered to children with OI, with the rationale that bones with increased volume of OI-quality matrix will be more fracture resistant182, 183. Positive effects on bone histology are obtained from treatment of OI children with bisphosphonates, including increased trabecular number and cortical thickness; vertebral DXA z-scores increase184, 185. Studies in OI children have shown that these gains are maximized in 2–4 years163, 186. While controlled trials concur that bisphosphonates improve vertebral geometry187, 188, the decrease in long bone fractures is equivocal, even in trials with over 125 children185, 189, 190. The claims of improved strength, motor function and decreased pain initially reported in observational trials are unsupported by controlled trials163. Human and murine studies have raised concerns about high cumulative doses impairing bone modelling and healing, decreasing bone material quality and mineralization heterogeneity, and impairing bone cells58, 86,191, but osteonecrosis of the jaw has not been seen in OI192. Because bisphosphonates have a decade-plus half-life in bone, it is crucial to determine the lowest effective cumulative dose for improved vertebral geometry and whether children should be treated until epiphyseal closure to avoid fractures at the junction of treated and untreated bone193. Junctional fractures have not been seen on the NIH bisphosphonate regimen; our view is that long-term suppression of bone remodelling is likely to be a greater detriment than junctional fracture.
Two short-acting compounds being investigated in OI murine models may be applicable to OI in the future. Denosumab is a fully humanized antibody to RANKL which has anti-resorptive action194. Inhibition of RANKL will shift the RANKL/OPG ratio, and decrease osteoblast signalling that normally stimulates osteoclast development. Animal studies showed OPG treatment inhibits dental eruption195; however, it remains to be determined whether denosumab will have a greater clinical impact on tooth eruption than bisphosphonates, which have been associated with a 1.67 year delay in tooth eruption in 6–14 year old children and may be longer in those treated since infancy196. Anti-sclerostin antibody works by an anabolic pathway to stimulate osteoblast production of matrix197 and may provide benefit without anti-resorptive side effects.
Growth hormone has been administered to both type I OI and type III/IV children in clinical trials155, 198. Although the GH axis is generally normal in OI children154, treatment with standard doses of rGH can produce significant increases in linear growth. In a study of children with types III/IV OI, about half of the type IV children doubled their baseline growth rate and maintained increased linear growth over multiple treatment years; they also experienced significant improvement in bone histology (BV/TV, TbN, BFR/BS) and in vertebral DXA. rGH responders had higher baseline type I collagen carboxyterminal propeptide, a marker for increased matrix production. Similarly, treatment of type I OI children with documented COL1A1 quantitative mutations resulted in positive changes in bone histology, muscle mass and strength, as well as improved linear growth198. The combination of bisphosphonate and rGH is being tested by several groups, and encouraging results are beginning to emerge199.
Novel molecular approaches to treatment
Potential molecular therapies for OI are modelled on mechanisms of milder disease. Mosaic carrier parents have barely detectable phenotypes, even with high burdens of mutant osteoblasts200. Cellular therapy recapitulates the mosaic situation by infusing normal mesenchymal stem cells (MSCs), with osteoblast differentiation potential, into affected individuals. Feasibility studies for MSC therapy were conducted in both humans and mice. Experiments in Brtl and oim showed improved bone properties, despite limited bone engraftment. Transplantation into developing animals55–57, 201, 202 yielded the highest donor cell engraftment (< 5%), and amelioration of bone structure and integrity. In utero transplantation of whole bone marrow into Brtl+/− mice showed that engrafted cells may synthesize a greater amount of normal matrix than the endogenous osteoblasts56. Also, MSCs could be used for correction of specific genetic mutations by homologous recombination prior to transplantation203, 204.
Another approach is allele-specific silencing, which models the null COL1A1 allele. Agents for targeting mutant transcripts include antisense oligonucleotides, ribozymes and siRNA205. Ribozymes, in particular, have specificity for single nucleotide changes, and have shown positive results in vitro and in culture206. Clinical implementation would require the development of appropriate delivery systems. Further, the importance of ER stress in OI pathology suggests strategies addressing protein folding, perhaps by chemical chaperones207–209, may be beneficial.
Common features of dominant and recessive OI
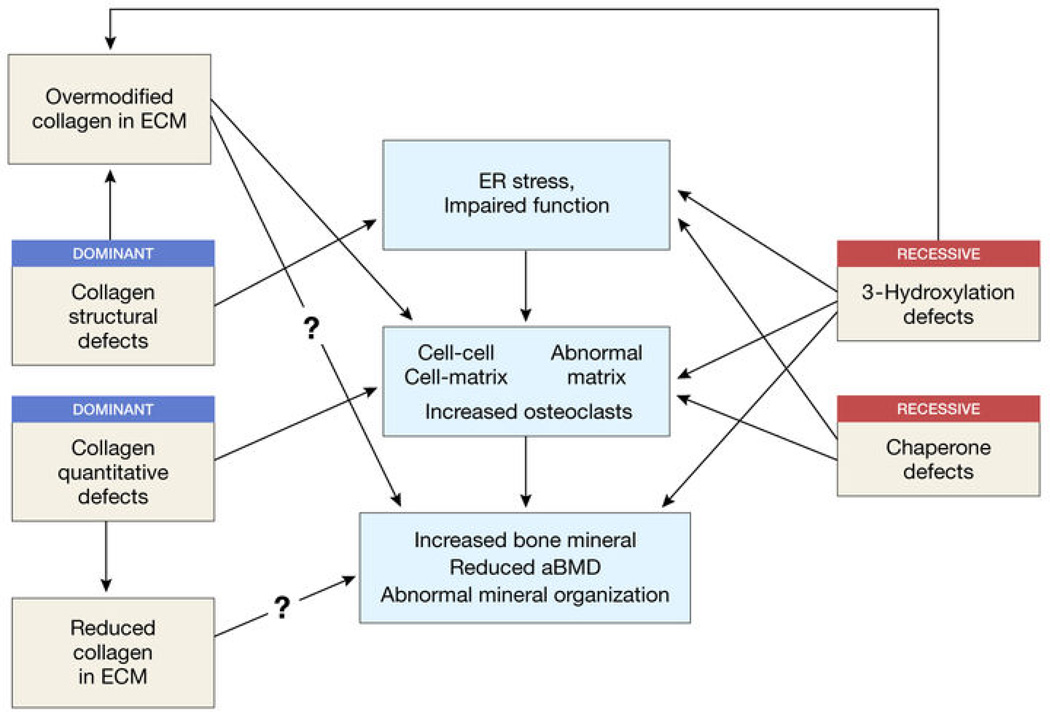
The overlapping features of dominant and recessive OI are likely to hold the key to a more complete understanding of mechanism and more targeted therapeutic approaches (Figure 4).
-
Genetic defects relate to collagen structure, modification, folding or processing
Many collagen structural defects and most 3-hydroxylation defects lead to significant overmodification, apparently due to delayed folding. FKBP10 and SERPINH1 defects do not cause overmodification, and are not associated with deficient 3-hydroxylation.
-
Osteochondrodystrophy
Because CRTAP was first identified in cartilage, it was clear that types VII, VIII, and IX OI were osteochondrodystrophies. Patients with dominant OI have significant growth deficiency, which may represent a defect in transition from cartilage to bone. Patients with FKBP10 defects have short stature, indicating a cartilaginous component.
-
ER stress and possible metabolic component
Collagen structural, modification and folding defects share swollen ER. Increased stress-related proteins in OI osteoblasts may lead to apoptosis. Dominant and recessive OI may share impaired osteoblast differentiation, altered production of non-collagenous components of matrix and abnormal cell-cell signalling in bone.
-
Collagen-collagen and collagen-NCP binding
For collagen structural defects, alignment of lethal regions on alpha chains with MLBR and proteoglycan binding sites points to important protein-protein interactions in ECM. The Pro986 3-hydroxylation site may be a binding epitope for NCP involved in mineralization. Altered collagen folding from chaperone deficiency could impact protein-protein interactions.
-
Cell-matrix effects
Osteoblasts in contact with abnormal OI matrix may have increased stress and altered cell-cell signalling. Matrix abnormalities including heterogeneity of collagen forms, altered modification and fibril organization may play a role.
-
Cell-cell signalling and histomorphometry
Dominant OI and recessive types VII and VIII OI have high bone turnover, with elevated osteoblast and osteoclast surface. Studies from murine models point to increased osteoblast-osteoclast signalling, perhaps because immature osteoblasts strongly support osteoclast development from marrow precursors.
-
Hypermineralization
OI bone has decreased aBMD, but elevated matrix mineral content. This is a common feature of bone with either quantitative or structural collagen defects causing dominant OI, as well as Crtap−/− mice and patients with hypomorphic CRTAP defects. The altered matrix content of non-collagenous proteins may disrupt mineralization kinetics.
Figure 4. Relationship between dominant and recessive forms of OI.
Boxes in the left and right columns identify features of dominant and recessive OI, respectively. The central column of boxes list mechanisms which may be shared by both sets of mutations.
Abbreviations
- BAMF
Brief Assessment of Motor Function
- qBEI
quantitative Backscattered Electron Imaging
- BMDD
Bone Mineral Density Distribution
- CSF
Cerebrospinal Fluid
- EM
Electron Microscopy
- FT-IR
Fourier Transform Infrared Spectroscopy
- GMFM
Gross Motor Function Measure
- rGH
recombinant Growth Hormone
- IGF-1
Insulin-like Growth Factor 1
- MAR
Mineral Apposition Rate
- NCP
Non-Collagenous Proteins
- NMD
Nonsense Mediated Decay
- PPIase
Peptidyl Prolyl cis-trans Isomerase
- PTC
Premature Termination Codon
Reference
- 1.Marini JC. In: Nelson Textbook of Pediatrics. Behrman RE, Kliegman RM, Jensen RM, editors. Philadelphia: Saunders; 2004. pp. 2336–2338. [Google Scholar]
- 2.Marini JC, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morello R, et al. CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127:291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Barnes AM, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabral WA, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes AM, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N Engl J Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk FS, et al. PPIB mutations cause severe osteogenesis imperfecta. Am J Hum Genet. 2009;85:521–527. doi: 10.1016/j.ajhg.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldridge D, et al. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat. 2008;29:1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alanay Y, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:551–559. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christiansen HE, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am J Hum Genet. 2010;86:389–398. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sillence DO, Rimoin DL. Classification of osteogenesis imperfecta. Lancet. 1978;1:1041–1042. doi: 10.1016/s0140-6736(78)90763-8. [DOI] [PubMed] [Google Scholar]
- 12.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop N. Characterising and treating osteogenesis imperfecta. Early Hum Dev. 2010;86:743–746. doi: 10.1016/j.earlhumdev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Van Dijk FS, Pals G, Van Rijn RR, Nikkels PG, Cobben JM. Classification of Osteogenesis Imperfecta revisited. Eur J Med Genet. 2010;53:1–5. doi: 10.1016/j.ejmg.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Glorieux FH. Osteogenesis imperfecta. Best Pract Res Clin Rheumatol. 2008;22:85–100. doi: 10.1016/j.berh.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 16.McAllion SJ, Paterson CR. Causes of death in osteogenesis imperfecta. J Clin Pathol. 1996;49:627–630. doi: 10.1136/jcp.49.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thibeault DW, Pettett G, Mabry SM, Rezaiekhaligh MM. Osteogenesis imperfecta Type IIA and pulmonary hypoplasia with normal alveolar development. Pediatr Pulmonol. 1995;20:301–306. doi: 10.1002/ppul.1950200508. [DOI] [PubMed] [Google Scholar]
- 18.Obafemi AA, Bulas DI, Troendle J, Marini JC. Popcorn calcification in osteogenesis imperfecta: incidence, progression, and molecular correlation. Am J Med Genet A. 2008;146A:2725–2732. doi: 10.1002/ajmg.a.32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 20.Myllyharju J, Kivirikko KI. Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet. 2004;20:33–43. doi: 10.1016/j.tig.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Willing MC, et al. Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. Am J Hum Genet. 1994;55:638–647. [PMC free article] [PubMed] [Google Scholar]
- 22.Willing MC, Deschenes SP, Slayton RL, Roberts EJ. Premature chain termination is a unifying mechanism for COL1A1 null alleles in osteogenesis imperfecta type I cell strains. Am J Hum Genet. 1996;59:799–809. [PMC free article] [PubMed] [Google Scholar]
- 23.Di Lullo GA, Sweeney SM, Korkko J, Ala-Kokko L, San Antonio JD. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277:4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 24.Sweeney SM, et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J Biol Chem. 2008;283:21187–21197. doi: 10.1074/jbc.M709319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forlino A, et al. Differential expression of both extracellular and intracellular proteins is involved in the lethal or nonlethal phenotypic variation of BrtlIV, a murine model for osteogenesis imperfecta. Proteomics. 2007;7:1877–1891. doi: 10.1002/pmic.200600919. [DOI] [PubMed] [Google Scholar]
- 26.Daley E, et al. Variable bone fragility associated with an Amish COL1A2 variant and a knock-in mouse model. J Bone Miner Res. 2010;25:247–261. doi: 10.1359/jbmr.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauch F, Lalic L, Roughley P, Glorieux FH. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J Bone Miner Res. 2010;25:1367–1374. doi: 10.1359/jbmr.091109. [DOI] [PubMed] [Google Scholar]
- 28.Cabral WA, et al. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J Biol Chem. 2005;280:19259–19269. doi: 10.1074/jbc.M414698200. [DOI] [PubMed] [Google Scholar]
- 29.Makareeva E, Cabral WA, Marini JC, Leikin S. Molecular mechanism of alpha 1(I)-osteogenesis imperfecta/Ehlers-Danlos syndrome: unfolding of an N-anchor domain at the N-terminal end of the type I collagen triple helix. J Biol Chem. 2006;281:6463–6470. doi: 10.1074/jbc.M511830200. [DOI] [PubMed] [Google Scholar]
- 30.Dombrowski KE, Vogel BE, Prockop DJ. Mutations that alter the primary structure of type I procollagen have long-range effects on its cleavage by procollagen N-proteinase. Biochemistry. 1989;28:7107–7112. doi: 10.1021/bi00443a048. [DOI] [PubMed] [Google Scholar]
- 31.Sippola M, Kaffe S, Prockop DJ. A heterozygous defect for structurally altered proalpha 2 chain of type I procollagen in a mild variant of osteogenesis imperfecta. The altered structure decreases the thermal stability of procollagen and makes it resistant to procollagen N-proteinase. J Biol Chem. 1984;259:14094–14100. [PubMed] [Google Scholar]
- 32.Feshchenko S, et al. Identification of a new heterozygous point mutation in the COL1A2 gene leading to skipping of exon 9 in a patient with joint laxity, hyperextensibility of skin and blue sclerae. Mutations in brief no. 166. Online. Hum Mutat. 1998;12:138. doi: 10.1002/(SICI)1098-1004(1998)12:2<138::AID-HUMU17>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 33.Nicholls AC, Oliver J, Renouf DV, Heath DA, Pope FM. The molecular defect in a family with mild atypical osteogenesis imperfecta and extreme joint hypermobility: exon skipping caused by an 11-bp deletion from an intron in one COL1A2 allele. Hum Genet. 1992;88:627–633. doi: 10.1007/BF02265286. [DOI] [PubMed] [Google Scholar]
- 34.Raff ML, Craigen WJ, Smith LT, Keene DR, Byers PH. Partial COL1A2 gene duplication produces features of osteogenesis imperfecta and Ehlers-Danlos syndrome type VII. Hum Genet. 2000;106:19–28. doi: 10.1007/s004390051004. [DOI] [PubMed] [Google Scholar]
- 35.Barnes AM, et al. COL1 C-propeptide cleavage site mutations cause OI with increased bone mineralization. J Bone Miner Res. 2009;24(Suppl 1) Available at http://www.asbmr.org/Meetings/AnnualMeeting/AbstractDetail.aspx?aid=ac38dee4-05df-47d7-a0e6-09ce416c5052, [Google Scholar]
- 36.Cole WG, Campbell PE, Rogers JG, Bateman JF. The clinical features of osteogenesis imperfecta resulting from a non-functional carboxy terminal pro alpha 1(I) propeptide of type I procollagen and a severe deficiency of normal type I collagen in tissues. J Med Genet. 1990;27:545–551. doi: 10.1136/jmg.27.9.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chessler SD, Wallis GA, Byers PH. Mutations in the carboxyl-terminal propeptide of the pro alpha 1(I) chain of type I collagen result in defective chain association and produce lethal osteogenesis imperfecta. J Biol Chem. 1993;268:18218–18225. [PubMed] [Google Scholar]
- 38.Pace JM, et al. Defective C-propeptides of the proalpha2(I) chain of type I procollagen impede molecular assembly and result in osteogenesis imperfecta. J Biol Chem. 2008;283:16061–16067. doi: 10.1074/jbc.M801982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willing MC, Cohn DH, Byers PH. Frameshift mutation near the 3' end of the COL1A1 gene of type I collagen predicts an elongated Pro alpha 1(I) chain and results in osteogenesis imperfecta type I. J Clin Invest. 1990;85:282–290. doi: 10.1172/JCI114424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pace JM, Atkinson M, Willing MC, Wallis G, Byers PH. Deletions and duplications of Gly-Xaa-Yaa triplet repeats in the triple helical domains of type I collagen chains disrupt helix formation and result in several types of osteogenesis imperfecta. Hum Mutat. 2001;18:319–326. doi: 10.1002/humu.1193. [DOI] [PubMed] [Google Scholar]
- 41.Cabral WA, et al. Type I collagen triplet duplication mutation in lethal osteogenesis imperfecta shifts register of alpha chains throughout the helix and disrupts incorporation of mutant helices into fibrils and extracellular matrix. J Biol Chem. 2003;278:10006–10012. doi: 10.1074/jbc.M212523200. [DOI] [PubMed] [Google Scholar]
- 42.Cabral WA, et al. Y-position cysteine substitution in type I collagen (alpha1(I) R888C/p.R1066C) is associated with osteogenesis imperfecta/Ehlers-Danlos syndrome phenotype. Hum Mutat. 2007;28:396–405. doi: 10.1002/humu.20456. [DOI] [PubMed] [Google Scholar]
- 43.Malfait F, et al. Three arginine to cysteine substitutions in the pro-alpha (I)-collagen chain cause Ehlers-Danlos syndrome with a propensity to arterial rupture in early adulthood. Hum Mutat. 2007;28:387–395. doi: 10.1002/humu.20455. [DOI] [PubMed] [Google Scholar]
- 44.Gensure RC, et al. A novel COL1A1 mutation in infantile cortical hyperostosis (Caffey disease) expands the spectrum of collagen-related disorders. J Clin Invest. 2005;115:1250–1257. doi: 10.1172/JCI22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suphapeetiporn K, Tongkobpetch S, Mahayosnond A, Shotelersuk V. Expanding the phenotypic spectrum of Caffey disease. Clin Genet. 2007;71:280–284. doi: 10.1111/j.1399-0004.2007.00768.x. [DOI] [PubMed] [Google Scholar]
- 46.Cho TJ, et al. The c.3040C >T mutation in COL1A1 is recurrent in Korean patients with infantile cortical hyperostosis (Caffey disease) J Hum Genet. 2008;53:947–949. doi: 10.1007/s10038-008-0328-5. [DOI] [PubMed] [Google Scholar]
- 47.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 48.Rauch F, Travers R, Parfitt AM, Glorieux FH. Static and dynamic bone histomorphometry in children with osteogenesis imperfecta. Bone. 2000;26:581–589. doi: 10.1016/s8756-3282(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 49.Lund AM, Molgaard C, Muller J, Skovby F. Bone mineral content and collagen defects in osteogenesis imperfecta. Acta Paediatr. 1999;88:1083–1088. doi: 10.1080/08035259950168135. [DOI] [PubMed] [Google Scholar]
- 50.Schnieke A, Harbers K, Jaenisch R. Embryonic lethal mutation in mice induced by retrovirus insertion into the alpha 1(I) collagen gene. Nature. 1983;304:315–320. doi: 10.1038/304315a0. [DOI] [PubMed] [Google Scholar]
- 51.Harbers K, Kuehn M, Delius H, Jaenisch R. Insertion of retrovirus into the first intron of alpha 1(I) collagen gene to embryonic lethal mutation in mice. Proc Natl Acad Sci U S A. 1984;81:1504–1508. doi: 10.1073/pnas.81.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chipman SD, et al. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: a model of human osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1993;90:1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forlino A, Porter FD, Lee EJ, Westphal H, Marini JC. Use of the Cre/lox recombination system to develop a non-lethal knock-in murine model for osteogenesis imperfecta with an alpha1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J Biol Chem. 1999;274:37923–37931. doi: 10.1074/jbc.274.53.37923. [DOI] [PubMed] [Google Scholar]
- 54.Lisse TS, et al. ER stress-mediated apoptosis in a new mouse model of osteogenesis imperfecta. PLoS Genet. 2008;4:e7. doi: 10.1371/journal.pgen.0040007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehrotra M, Rosol M, Ogawa M, Larue AC. Amelioration of a mouse model of osteogenesis imperfecta with hematopoietic stem cell transplantation: microcomputed tomography studies. Exp Hematol. 2010;38:593–602. doi: 10.1016/j.exphem.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panaroni C, et al. In utero transplantation of adult bone marrow decreases perinatal lethality and rescues the bone phenotype in the knockin murine model for classical, dominant osteogenesis imperfecta. Blood. 2009;114:459–468. doi: 10.1182/blood-2008-12-195859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Li F, Niyibizi C. Progenitors systemically transplanted into neonatal mice localize to areas of active bone formation in vivo: implications of cell therapy for skeletal diseases. Stem Cells. 2006;24:1869–1878. doi: 10.1634/stemcells.2005-0430. [DOI] [PubMed] [Google Scholar]
- 58.Uveges TE, et al. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res. 2009;24:849–859. doi: 10.1359/JBMR.081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rao SH, Evans KD, Oberbauer AM, Martin RB. Bisphosphonate treatment in the oim mouse model alters bone modeling during growth. J Biomech. 2008;41:3371–3376. doi: 10.1016/j.jbiomech.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bargman R, Huang A, Boskey AL, Raggio C, Pleshko N. RANKL inhibition improves bone properties in a mouse model of osteogenesis imperfecta. Connect Tissue Res. 2010;51:123–131. doi: 10.3109/03008200903108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delos D, et al. The effects of RANKL inhibition on fracture healing and bone strength in a mouse model of osteogenesis imperfecta. J Orthop Res. 2008;26:153–164. doi: 10.1002/jor.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uveges TE, et al. Cellular mechanism of decreased bone in Brtl mouse model of OI: imbalance of decreased osteoblast function and increased osteoclasts and their precursors. J Bone Miner Res. 2008;23:1983–1994. doi: 10.1359/JBMR.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H, et al. Immature osteoblast lineage cells increase osteoclastogenesis in osteogenesis imperfecta murine. Am J Pathol. 2010;176:2405–2413. doi: 10.2353/ajpath.2010.090704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Carleton SM, et al. Role of genetic background in determining phenotypic severity throughout postnatal development and at peak bone mass in Col1a2 deficient mice (oim) Bone. 2008;42:681–694. doi: 10.1016/j.bone.2007.12.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Forlino A, Kuznetsova NV, Marini JC, Leikin S. Selective retention and degradation of molecules with a single mutant alpha1(I) chain in the Brtl IV mouse model of OI. Matrix Biol. 2007;26:604–614. doi: 10.1016/j.matbio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Chavassieux P, Seeman E, Delmas PD. Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev. 2007;28:151–164. doi: 10.1210/er.2006-0029. [DOI] [PubMed] [Google Scholar]
- 67.Canty EG, Kadler KE. Procollagen trafficking, processing and fibrillogenesis. J Cell Sci. 2005;118:1341–1353. doi: 10.1242/jcs.01731. [DOI] [PubMed] [Google Scholar]
- 68.Malfait F, et al. Total absence of the alpha2(I) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems. J Med Genet. 2006;43:e36. doi: 10.1136/jmg.2005.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nicholls AC, et al. The clinical features of homozygous alpha 2(I) collagen deficient osteogenesis imperfecta. J Med Genet. 1984;21:257–262. doi: 10.1136/jmg.21.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raghunath M, Bruckner P, Steinmann B. Delayed triple helix formation of mutant collagen from patients with osteogenesis imperfecta. J Mol Biol. 1994;236:940–949. doi: 10.1006/jmbi.1994.1199. [DOI] [PubMed] [Google Scholar]
- 71.Bodian DL, et al. Mutation and polymorphism spectrum in osteogenesis imperfecta type II: implications for genotype-phenotype relationships. Hum Mol Genet. 2009;18:463–471. doi: 10.1093/hmg/ddn374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boot-Handford RP, Briggs MD. The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res. 2009;339:197–211. doi: 10.1007/s00441-009-0877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishida Y, et al. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell. 2009;20:2744–2754. doi: 10.1091/mbc.E08-11-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lamande SR, et al. Endoplasmic reticulum-mediated quality control of type I collagen production by cells from osteogenesis imperfecta patients with mutations in the pro alpha 1 (I) chain carboxyl-terminal propeptide which impair subunit assembly. J Biol Chem. 1995;270:8642–8649. doi: 10.1074/jbc.270.15.8642. [DOI] [PubMed] [Google Scholar]
- 75.Fitzgerald J, Lamande SR, Bateman JF. Proteasomal degradation of unassembled mutant type I collagen pro-alpha1(I) chains. J Biol Chem. 1999;274:27392–27398. doi: 10.1074/jbc.274.39.27392. [DOI] [PubMed] [Google Scholar]
- 76.Forlino A, et al. 9th International Meeting on Osteogenesis Imperfecta; Annapolis, MD, USA. 2005. [Google Scholar]
- 77.Fedarko NS, Robey PG, Vetter UK. Extracellular matrix stoichiometry in osteoblasts from patients with osteogenesis imperfecta. J Bone Miner Res. 1995;10:1122–1129. doi: 10.1002/jbmr.5650100718. [DOI] [PubMed] [Google Scholar]
- 78.Fedarko NS, Sponseller PD, Shapiro JR. Long-term extracellular matrix metabolism by cultured human osteogenesis imperfecta osteoblasts. J Bone Miner Res. 1996;11:800–805. doi: 10.1002/jbmr.5650110611. [DOI] [PubMed] [Google Scholar]
- 79.Wallace JM, Orr BG, Marini JC, Holl MM. Nanoscale morphology of Type I collagen is altered in the Brtl mouse model of Osteogenesis Imperfecta. J Struct Biol. 2010 doi: 10.1016/j.jsb.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fratzl-Zelman N, et al. CRTAP deficiency leads to abnormally high bone matrix mineralization in a murine model and in children with osteogenesis imperfecta type VII. Bone. 2009;46:820–826. doi: 10.1016/j.bone.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roschger P, et al. Evidence that abnormal high bone mineralization in growing children with osteogenesis imperfecta is not associated with specific collagen mutations. Calcif Tissue Int. 2008;82:263–270. doi: 10.1007/s00223-008-9113-x. [DOI] [PubMed] [Google Scholar]
- 82.Vetter U, et al. Osteogenesis imperfecta: changes in noncollagenous proteins in bone. J Bone Miner Res. 1991;6:501–505. doi: 10.1002/jbmr.5650060512. [DOI] [PubMed] [Google Scholar]
- 83.Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- 84.Camacho NP, Landis WJ, Boskey AL. Mineral changes in a mouse model of osteogenesis imperfecta detected by Fourier transform infrared microscopy. Connect Tissue Res. 1996;35:259–265. doi: 10.3109/03008209609029199. [DOI] [PubMed] [Google Scholar]
- 85.Renders GA, Mulder L, Langenbach GE, van Ruijven LJ, van Eijden TM. Biomechanical effect of mineral heterogeneity in trabecular bone. J Biomech. 2008;41:2793–2798. doi: 10.1016/j.jbiomech.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 86.Gourion-Arsiquaud S, et al. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010;46:666–672. doi: 10.1016/j.bone.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakahama KI. Cellular communications in bone homeostasis and repair. Cell Mol Life Sci. 2010 doi: 10.1007/s00018-010-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poole KE, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb J. 2005;19:1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 89.Sarathchandra P, Pope FM, Kayser MV, Ali SY. A light and electron microscopic study of osteogenesis imperfecta bone samples, with reference to collagen chemistry and clinical phenotype. J Pathol. 2000;192:385–395. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH704>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 90.Fernandes H, et al. The role of collagen crosslinking in differentiation of human mesenchymal stem cells and MC3T3-E1 cells. Tissue Eng Part A. 2009;15:3857–3867. doi: 10.1089/ten.tea.2009.0011. [DOI] [PubMed] [Google Scholar]
- 91.Bank RA, et al. Pyridinium cross-links in bone of patients with osteogenesis imperfecta: evidence of a normal intrafibrillar collagen packing. J Bone Miner Res. 2000;15:1330–1336. doi: 10.1359/jbmr.2000.15.7.1330. [DOI] [PubMed] [Google Scholar]
- 92.Vranka JA, Sakai LY, Bachinger HP. Prolyl 3-hydroxylase 1, enzyme characterization and identification of a novel family of enzymes. J Biol Chem. 2004;279:23615–23621. doi: 10.1074/jbc.M312807200. [DOI] [PubMed] [Google Scholar]
- 93.Weis MA, et al. Location of 3-hydroxyproline residues in collagen types I, II, III and V/XI implies a role in fibril supramolecular assembly. J Biol Chem. 2009;285:2580–2590. doi: 10.1074/jbc.M109.068726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ishikawa Y, Wirz J, Vranka JA, Nagata K, Bachinger HP. Biochemical characterization of the prolyl 3-hydroxylase 1.cartilage-associated protein.cyclophilin B complex. J Biol Chem. 2009;284:17641–17647. doi: 10.1074/jbc.M109.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Castagnola P, et al. Cartilage associated protein (CASP) is a novel developmentally regulated chick embryo protein. J Cell Sci. 1997;110(Pt 12):1351–1359. doi: 10.1242/jcs.110.12.1351. [DOI] [PubMed] [Google Scholar]
- 96.Wassenhove-McCarthy DJ, McCarthy KJ. Molecular characterization of a novel basement membrane-associated proteoglycan, leprecan. J Biol Chem. 1999;274:25004–25017. doi: 10.1074/jbc.274.35.25004. [DOI] [PubMed] [Google Scholar]
- 97.Labuda M, et al. Osteogenesis imperfecta type VII maps to the short arm of chromosome 3. Bone. 2002;31:19–25. doi: 10.1016/s8756-3282(02)00808-6. [DOI] [PubMed] [Google Scholar]
- 98.Marini JC, Cabral WA, Barnes AM, Chang W. Components of the collagen prolyl 3-hydroxylation complex are crucial for normal bone development. Cell Cycle. 2007;6:1675–1681. doi: 10.4161/cc.6.14.4474. [DOI] [PubMed] [Google Scholar]
- 99.Morello R, et al. cDNA cloning, characterization and chromosome mapping of Crtap encoding the mouse cartilage associated protein. Matrix Biol. 1999;18:319–324. doi: 10.1016/s0945-053x(99)00002-5. [DOI] [PubMed] [Google Scholar]
- 100.Marini JC, Cabral WA, Barnes AM. Null mutations in LEPRE1 and CRTAP cause severe recessive osteogenesis imperfecta. Cell Tissue Res. 2009;339:59–70. doi: 10.1007/s00441-009-0872-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baldridge D, et al. Generalized connective tissue disease in Crtap-/- mouse. PLoS One. 2010;5:e10560. doi: 10.1371/journal.pone.0010560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Willaert A, et al. Recessive osteogenesis imperfecta caused by LEPRE1 mutations: clinical documentation and identification of the splice form responsible for prolyl 3-hydroxylation. J Med Genet. 2009;46:233–241. doi: 10.1136/jmg.2008.062729. [DOI] [PubMed] [Google Scholar]
- 103.Vranka J, Stadler HS, Bachinger HP. Expression of prolyl 3-hydroxylase genes in embryonic and adult mouse tissues. Cell Struct Funct. 2009;34:97–104. doi: 10.1247/csf.09002. [DOI] [PubMed] [Google Scholar]
- 104.Cabral WA, et al. European Society of Human Genetics Annual Conference; Gothenburg, Sweeden. 2010. [Google Scholar]
- 105.Vranka JA, et al. Prolyl 3-hydroxylase 1 null mice display abnormalities in fibrillar collagen-rich tissues such as tendons, skin, and bones. J Biol Chem. 2010;285:17253–17262. doi: 10.1074/jbc.M110.102228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bachinger HP. The influence of peptidyl-prolyl cis-trans isomerase on the in vitro folding of type III collagen. J Biol Chem. 1987;262:17144–17148. [PubMed] [Google Scholar]
- 107.Steinmann B, Bruckner P, Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem. 1991;266:1299–1303. [PubMed] [Google Scholar]
- 108.Schwarze U, et al. 59th Annual Meeting of the American Society of Human Genetics; October 20–24, 2009; Honolulu. Available at http://www.ashg.org/2009meeting/pdf/platforms_4up.pdf. [Google Scholar]
- 109.Van Dijk FS, Cobben JM, Pals G. Osteogenesis Imperfecta, normal collagen folding, and lack of cyclophilin B. N Engl J Med. 2010;362:1941–1942. doi: 10.1056/NEJMc1002797. [DOI] [PubMed] [Google Scholar]
- 110.Choi JW, et al. Severe osteogenesis imperfecta in cyclophilin B-deficient mice. PLoS Genet. 2009;5:e1000750. doi: 10.1371/journal.pgen.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang W, Barnes AM, Cabral WA, Bodurtha JN, Marini JC. Prolyl 3-hydroxylase 1 and CRTAP are mutually stabilizing in the endoplasmic reticulum collagen prolyl 3-hydroxylation complex. Hum Mol Genet. 2009;19:223–234. doi: 10.1093/hmg/ddp481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mizuno K, Peyton DH, Hayashi T, Engel J, Bachinger HP. Effect of the -Gly-3(S)-hydroxyprolyl-4(R)-hydroxyprolyl- tripeptide unit on the stability of collagen model peptides. Febs J. 2008;275:5830–5840. doi: 10.1111/j.1742-4658.2008.06704.x. [DOI] [PubMed] [Google Scholar]
- 113.Smith T, Ferreira LR, Hebert C, Norris K, Sauk JJ. Hsp47 and cyclophilin B traverse the endoplasmic reticulum with procollagen into pre-Golgi intermediate vesicles. A role for Hsp47 and cyclophilin B in the export of procollagen from the endoplasmic reticulum. J Biol Chem. 1995;270:18323–18328. doi: 10.1074/jbc.270.31.18323. [DOI] [PubMed] [Google Scholar]
- 114.Nagai N, et al. Embryonic lethality of molecular chaperone hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol. 2000;150:1499–1506. doi: 10.1083/jcb.150.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ishida Y, et al. Type I collagen in Hsp47-null cells is aggregated in endoplasmic reticulum and deficient in N-propeptide processing and fibrillogenesis. Mol Biol Cell. 2006;17:2346–2355. doi: 10.1091/mbc.E05-11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ishida Y, Nagata K. Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy. 2009;5:1217–1219. doi: 10.4161/auto.5.8.10168. [DOI] [PubMed] [Google Scholar]
- 117.Drogemuller C, et al. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet. 2009;5:e1000579. doi: 10.1371/journal.pgen.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ishikawa Y, Vranka J, Wirz J, Nagata K, Bachinger HP. The rough endoplasmic reticulum-resident FK506-binding protein FKBP65 is a molecular chaperone that interacts with collagens. J Biol Chem. 2008;283:31584–31590. doi: 10.1074/jbc.M802535200. [DOI] [PubMed] [Google Scholar]
- 119.Breslau-Siderius EJ, Engelbert RH, Pals G, van der Sluijs JA. Bruck syndrome: a rare combination of bone fragility and multiple congenital joint contractures. J Pediatr Orthop B. 1998;7:35–38. [PubMed] [Google Scholar]
- 120.Shaheen R, Al-Owain M, Sakati N, Alzayed ZS, Alkuraya FS. FKBP10 and Bruck Syndrome: Phenotypic Heterogeneity or Call for Reclassification? Am J Hum Genet. 2010;87:571. doi: 10.1016/j.ajhg.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kelley BP, et al. Mutations in FKBP10 cause recessive osteogenesis imperfecta and type 1 bruck syndrome. J Bone Miner Res. 2010;2010:13. doi: 10.1002/jbmr.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bank RA, et al. Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc Natl Acad Sci U S A. 1999;96:1054–1058. doi: 10.1073/pnas.96.3.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Szpirer C, et al. Localization of the gene encoding a novel isoform of lysyl hydroxylase. Mamm Genome. 1997;8:707–708. doi: 10.1007/s003359900549. [DOI] [PubMed] [Google Scholar]
- 124.van der Slot AJ, et al. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J Biol Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 125.Lapunzina P, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am J Hum Genet. 2010;87:110–114. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 127.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 128.Pedersen U. Hearing loss in patients with osteogenesis imperfecta. A clinical and audiological study of 201 patients. Scand Audiol. 1984;13:67–74. doi: 10.3109/01050398409043042. [DOI] [PubMed] [Google Scholar]
- 129.Kuurila K, Grenman R, Johansson R, Kaitila I. Hearing loss in children with osteogenesis imperfecta. Eur J Pediatr. 2000;159:515–519. doi: 10.1007/s004310051322. [DOI] [PubMed] [Google Scholar]
- 130.Paterson CR, Monk EA, McAllion SJ. How common is hearing impairment in osteogenesis imperfecta? J Laryngol Otol. 2001;115:280–282. doi: 10.1258/0022215011907442. [DOI] [PubMed] [Google Scholar]
- 131.Kuurila K, Kaitila I, Johansson R, Grenman R. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. Ann Otol Rhinol Laryngol. 2002;111:939–946. doi: 10.1177/000348940211101014. [DOI] [PubMed] [Google Scholar]
- 132.Hartikka H, et al. Lack of correlation between the type of COL1A1 or COL1A2 mutation and hearing loss in osteogenesis imperfecta patients. Hum Mutat. 2004;24:147–154. doi: 10.1002/humu.20071. [DOI] [PubMed] [Google Scholar]
- 133.Kuurila K, et al. Vestibular dysfunction in adult patients with osteogenesis imperfecta. Am J Med Genet A. 2003;120A:350–388. doi: 10.1002/ajmg.a.20088. [DOI] [PubMed] [Google Scholar]
- 134.Pedersen U, Elbrond O. Stapedectomy in osteogenesis imperfecta. ORL J Otorhinolaryngol Relat Spec. 1983;45:330–337. doi: 10.1159/000275663. [DOI] [PubMed] [Google Scholar]
- 135.Garretsen AJ, Cremers CW, Huygen PL. Hearing loss (in nonoperated ears) in relation to age in osteogenesis imperfecta type I. Ann Otol Rhinol Laryngol. 1997;106:575–582. doi: 10.1177/000348949710600709. [DOI] [PubMed] [Google Scholar]
- 136.Shea JJ, Postma DS. Findings and long-term surgical results in the hearing loss of osteogenesis imperfecta. Arch Otolaryngol. 1982;108:467–470. doi: 10.1001/archotol.1982.00790560005002. [DOI] [PubMed] [Google Scholar]
- 137.van der Rijt AJ, Cremers CW. Stapes surgery in osteogenesis imperfecta: results of a new series. Otol Neurotol. 2003;24:717–722. doi: 10.1097/00129492-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 138.Kuurila K, Pynnonen S, Grenman R. Stapes surgery in osteogenesis imperfecta in Finland. Ann Otol Rhinol Laryngol. 2004;113:187–193. doi: 10.1177/000348940411300303. [DOI] [PubMed] [Google Scholar]
- 139.Rotteveel LJ, et al. Cochlear implantation in 3 patients with osteogenesis imperfecta: imaging, surgery and programming issues. Audiol Neurootol. 2008;13:73–85. doi: 10.1159/000111779. [DOI] [PubMed] [Google Scholar]
- 140.Levin LS, Salinas CF, Jorgenson RJ. Classification of osteogenesis imperfecta by dental characteristics. Lancet. 1978;1:332–333. doi: 10.1016/s0140-6736(78)90108-3. [DOI] [PubMed] [Google Scholar]
- 141.O'Connell AC, Marini JC. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:189–196. doi: 10.1016/s1079-2104(99)70272-6. [DOI] [PubMed] [Google Scholar]
- 142.Malmgren B, Norgren S. Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol Scand. 2002;60:65–71. doi: 10.1080/000163502753509446. [DOI] [PubMed] [Google Scholar]
- 143.Majorana A, et al. Dentinogenesis imperfecta in children with osteogenesis imperfecta: a clinical and ultrastructural study. Int J Paediatr Dent. 2010;20:112–118. doi: 10.1111/j.1365-263X.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- 144.Waltimo J, Ojanotko-Harri A, Lukinmaa PL. Mild forms of dentinogenesis imperfecta in association with osteogenesis imperfecta as characterized by light and transmission electron microscopy. J Oral Pathol Med. 1996;25:256–264. doi: 10.1111/j.1600-0714.1996.tb01381.x. [DOI] [PubMed] [Google Scholar]
- 145.Lund AM, Jensen BL, Nielsen LA, Skovby F. Dental manifestations of osteogenesis imperfecta and abnormalities of collagen I metabolism. J Craniofac Genet Dev Biol. 1998;18:30–37. [PubMed] [Google Scholar]
- 146.Rauch F, Lalic L, Roughley P, Glorieux FH. Genotype-phenotype correlations in nonlethal osteogenesis imperfecta caused by mutations in the helical domain of collagen type I. Eur J Hum Genet. 2010;18:642–647. doi: 10.1038/ejhg.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schwarz M, Harbers K, Kratochwil K. Transcription of a mutant collagen I gene is a cell type and stage-specific marker for odontoblast and osteoblast differentiation. Development. 1990;108:717–726. doi: 10.1242/dev.108.4.717. [DOI] [PubMed] [Google Scholar]
- 148.Waltimo J. Hyperfibers and vesicles in dentin matrix in dentinogenesis imperfecta (DI) associated with osteogenesis imperfecta (OI) J Oral Pathol Med. 1994;23:389–393. doi: 10.1111/j.1600-0714.1994.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 149.Lygidakis NA, Smith R, Oulis CJ. Scanning electron microscopy of teeth in osteogenesis imperfecta type I. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81:567–572. doi: 10.1016/s1079-2104(96)80048-5. [DOI] [PubMed] [Google Scholar]
- 150.Hall RK, Maniere MC, Palamara J, Hemmerle J. Odontoblast dysfunction in osteogenesis imperfecta: an LM, SEM, and ultrastructural study. Connect Tissue Res. 2002;43:401–405. doi: 10.1080/03008200290001005. [DOI] [PubMed] [Google Scholar]
- 151.Charnas LR, Marini JC. Communicating hydrocephalus, basilar invagination, and other neurologic features in osteogenesis imperfecta. Neurology. 1993;43:2603–2608. doi: 10.1212/wnl.43.12.2603. [DOI] [PubMed] [Google Scholar]
- 152.Sawin PD, Menezes AH. Basilar invagination in osteogenesis imperfecta and related osteochondrodysplasias: medical and surgical management. J Neurosurg. 1997;86:950–960. doi: 10.3171/jns.1997.86.6.0950. [DOI] [PubMed] [Google Scholar]
- 153.Menezes AH. Specific entities affecting the craniocervical region: osteogenesis imperfecta and related osteochondrodysplasias: medical and surgical management of basilar impression. Childs Nerv Syst. 2008;24:1169–1172. doi: 10.1007/s00381-008-0602-z. [DOI] [PubMed] [Google Scholar]
- 154.Marini JC, Bordenick S, Heavner G, Rose S, Chrousos GP. Evaluation of growth hormone axis and responsiveness to growth stimulation of short children with osteogenesis imperfecta. Am J Med Genet. 1993;45:261–264. doi: 10.1002/ajmg.1320450223. [DOI] [PubMed] [Google Scholar]
- 155.Marini JC, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res. 2003;18:237–243. doi: 10.1359/jbmr.2003.18.2.237. [DOI] [PubMed] [Google Scholar]
- 156.Singer RB, Ogston SA, Paterson CR. Mortality in various types of osteogenesis imperfecta. J Insur Med. 2001;33:216–220. [PubMed] [Google Scholar]
- 157.Widmann RF, et al. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine (Phila Pa 1976) 1999;24:1673–1678. doi: 10.1097/00007632-199908150-00008. [DOI] [PubMed] [Google Scholar]
- 158.Shapiro JR, et al. Pulmonary hypoplasia and osteogenesis imperfecta type II with defective synthesis of alpha I(1) procollagen. Bone. 1989;10:165–171. doi: 10.1016/8756-3282(89)90049-5. [DOI] [PubMed] [Google Scholar]
- 159.Hortop J, Tsipouras P, Hanley JA, Maron BJ, Shapiro JR. Cardiovascular involvement in osteogenesis imperfecta. Circulation. 1986;73:54–61. doi: 10.1161/01.cir.73.1.54. [DOI] [PubMed] [Google Scholar]
- 160.Bonita RE, Cohen IS, Berko BA. Valvular heart disease in osteogenesis imperfecta: presentation of a case and review of the literature. Echocardiography. 2010;27:69–73. doi: 10.1111/j.1540-8175.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 161.Migliaccio S, et al. Impairment of diastolic function in adult patients affected by osteogenesis imperfecta clinically asymptomatic for cardiac disease: casuality or causality? Int J Cardiol. 2009;131:200–203. doi: 10.1016/j.ijcard.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 162.Huang RP, Ambrose CG, Sullivan E, Haynes RJ. Functional significance of bone density measurements in children with osteogenesis imperfecta. J Bone Joint Surg Am. 2006;88:1324–1330. doi: 10.2106/JBJS.E.00333. [DOI] [PubMed] [Google Scholar]
- 163.Letocha AD, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005;20:977–986. doi: 10.1359/JBMR.050109. [DOI] [PubMed] [Google Scholar]
- 164.Sakkers R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet. 2004;363:1427–1431. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 165.Engelbert RH, et al. Scoliosis in children with osteogenesis imperfecta: influence of severity of disease and age of reaching motor milestones. Eur Spine J. 2003;12:130–134. doi: 10.1007/s00586-002-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cintas HL, Siegel KL, Furst GP, Gerber LH. Brief assessment of motor function: reliability and concurrent validity of the Gross Motor Scale. Am J Phys Med Rehabil. 2003;82:33–41. doi: 10.1097/00002060-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 167.Ruck-Gibis J, Plotkin H, Hanley J, Wood-Dauphinee S. Reliability of the gross motor function measure for children with osteogenesis imperfecta. Pediatr Phys Ther. 2001;13:10–17. [PubMed] [Google Scholar]
- 168.Bleck EE. Nonoperative treatment of osteogenesis imperfecta: orthotic and mobility management. Clin Orthop Relat Res. 1981:111–122. [PubMed] [Google Scholar]
- 169.Engelbert RH, et al. Osteogenesis imperfecta in childhood: impairment and disability. A prospective study with 4-year follow-up. Arch Phys Med Rehabil. 2004;85:772–778. doi: 10.1016/j.apmr.2003.08.085. [DOI] [PubMed] [Google Scholar]
- 170.Takken T, et al. Cardiopulmonary fitness and muscle strength in patients with osteogenesis imperfecta type I. J Pediatr. 2004;145:813–818. doi: 10.1016/j.jpeds.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 171.Van Brussel M, et al. Physical training in children with osteogenesis imperfecta. J Pediatr. 2008;152:111–116. e1. doi: 10.1016/j.jpeds.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 172.Semler O, Fricke O, Vezyroglou K, Stark C, Schoenau E. Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. J Musculoskelet Neuronal Interact. 2007;7:77–81. [PubMed] [Google Scholar]
- 173.Luhmann SJ, Sheridan JJ, Capelli AM, Schoenecker PL. Management of lowerextremity deformities in osteogenesis imperfecta with extensible intramedullary rod technique: a 20-year experience. J Pediatr Orthop. 1998;18:88–94. [PubMed] [Google Scholar]
- 174.Zionts LE, Ebramzadeh E, Stott NS. Complications in the use of the Bailey-Dubow extensible nail. Clin Orthop Relat Res. 1998:186–195. [PubMed] [Google Scholar]
- 175.El-Adl G, Khalil MA, Enan A, Mostafa MF, El-Lakkany MR. Telescoping versus non-telescoping rods in the treatment of osteogenesis imperfecta. Acta Orthop Belg. 2009;75:200–208. [PubMed] [Google Scholar]
- 176.Esposito P, Plotkin H. Surgical treatment of osteogenesis imperfecta: current concepts. Curr Opin Pediatr. 2008;20:52–57. doi: 10.1097/MOP.0b013e3282f35f03. [DOI] [PubMed] [Google Scholar]
- 177.Boutaud B, Laville JM. [Elastic sliding central medullary nailing with osteogenesis imperfecta. Fourteen cases at eight years follow-up] Rev Chir Orthop Reparatrice Appar Mot. 2004;90:304–311. doi: 10.1016/s0035-1040(04)70125-7. [DOI] [PubMed] [Google Scholar]
- 178.Munns CF, Rauch F, Zeitlin L, Fassier F, Glorieux FH. Delayed osteotomy but not fracture healing in pediatric osteogenesis imperfecta patients receiving pamidronate. J Bone Miner Res. 2004;19:1779–1786. doi: 10.1359/JBMR.040814. [DOI] [PubMed] [Google Scholar]
- 179.Hanscom DA, Bloom BA. The spine in osteogenesis imperfecta. Orthop Clin North Am. 1988;19:449–458. [PubMed] [Google Scholar]
- 180.Topouchian V, Finidori G, Glorion C, Padovani JP, Pouliquen JC. [Posterior spinal fusion for kypho-scoliosis associated with osteogenesis imperfecta: long-term results] Rev Chir Orthop Reparatrice Appar Mot. 2004;90:525–532. doi: 10.1016/s0035-1040(04)70426-2. [DOI] [PubMed] [Google Scholar]
- 181.Janus GJ, Finidori G, Engelbert RH, Pouliquen M, Pruijs JE. Operative treatment of severe scoliosis in osteogenesis imperfecta: results of 20 patients after halo traction and posterior spondylodesis with instrumentation. Eur Spine J. 2000;9:486–491. doi: 10.1007/s005860000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rauch F, Glorieux FH. Treatment of children with osteogenesis imperfecta. Curr Osteoporos Rep. 2006;4:159–164. doi: 10.1007/s11914-996-0025-2. [DOI] [PubMed] [Google Scholar]
- 183.Cheung MS, Glorieux FH. Osteogenesis Imperfecta: update on presentation and management. Rev Endocr Metab Disord. 2008;9:153–160. doi: 10.1007/s11154-008-9074-4. [DOI] [PubMed] [Google Scholar]
- 184.Bachrach LK, Ward LM. Clinical review 1: Bisphosphonate use in childhood osteoporosis. J Clin Endocrinol Metab. 2009;94:400–409. doi: 10.1210/jc.2008-1531. [DOI] [PubMed] [Google Scholar]
- 185.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005088.pub2. CD005088. [DOI] [PubMed] [Google Scholar]
- 186.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110:1293–1299. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Marini JC. Should children with osteogenesis imperfecta be treated with bisphosphonates? Nat Clin Pract Endocrinol Metab. 2006;2:14–15. doi: 10.1038/ncpendmet0075. [DOI] [PubMed] [Google Scholar]
- 188.Land C, Rauch F, Munns CF, Sahebjam S, Glorieux FH. Vertebral morphometry in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate treatment. Bone. 2006;39:901–906. doi: 10.1016/j.bone.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 189.Castillo H, Samson-Fang L. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51:17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 190.Ward LM, et al. Alendronate for the Treatment of Pediatric Osteogenesis Imperfecta: A Randomized Placebo-Controlled Study. J Clin Endocrinol Metab. 2010 doi: 10.1210/jc.2010-0636. [DOI] [PubMed] [Google Scholar]
- 191.Marini JC. Bone: Use of bisphosphonates in children-proceed with caution. Nat Rev Endocrinol. 2009;5:241–243. doi: 10.1038/nrendo.2009.58. [DOI] [PubMed] [Google Scholar]
- 192.Malmgren B, Astrom E, Soderhall S. No osteonecrosis in jaws of young patients with osteogenesis imperfecta treated with bisphosphonates. J Oral Pathol Med. 2008;37:196–200. doi: 10.1111/j.1600-0714.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 193.Ward KA, Adams JE, Freemont TJ, Mughal MZ. Can bisphosphonate treatment be stopped in a growing child with skeletal fragility? Osteoporos Int. 2007;18:1137–1140. doi: 10.1007/s00198-007-0330-3. [DOI] [PubMed] [Google Scholar]
- 194.Hussar DA, Stevenson T. New drugs: Denosumab, dienogest/estradiol valerate, and polidocanol. J Am Pharm Assoc. 2003;50:658–662. doi: 10.1331/JAPhA.2010.10536. 2003. [DOI] [PubMed] [Google Scholar]
- 195.Dunn MD, Park CH, Kostenuik PJ, Kapila S, Giannobile WV. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone. 2007;41:446–455. doi: 10.1016/j.bone.2007.04.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kamoun-Goldrat A, Ginisty D, Le Merrer M. Effects of bisphosphonates on tooth eruption in children with osteogenesis imperfecta. Eur J Oral Sci. 2008;116:195–198. doi: 10.1111/j.1600-0722.2008.00529.x. [DOI] [PubMed] [Google Scholar]
- 197.Paszty C, Turner CH, Robinson MK. Sclerostin: a gem from the genome leads to bone-building antibodies. J Bone Miner Res. 2010;25:1897–1904. doi: 10.1002/jbmr.161. [DOI] [PubMed] [Google Scholar]
- 198.Antoniazzi F, et al. Growth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesis. J Pediatr. 1996;129:432–439. doi: 10.1016/s0022-3476(96)70077-x. [DOI] [PubMed] [Google Scholar]
- 199.Antoniazzi F, et al. GH in combination with bisphosphonate treatment in osteogenesis imperfecta. Eur J Endocrinol. 2010;163:479–487. doi: 10.1530/EJE-10-0208. [DOI] [PubMed] [Google Scholar]
- 200.Cabral WA, Marini JC. High proportion of mutant osteoblasts is compatible with normal skeletal function in mosaic carriers of osteogenesis imperfecta. Am J Hum Genet. 2004;74:752–760. doi: 10.1086/383252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li F, Wang X, Niyibizi C. Distribution of single-cell expanded marrow derived progenitors in a developing mouse model of osteogenesis imperfecta following systemic transplantation. Stem Cells. 2007;25:3183–3193. doi: 10.1634/stemcells.2007-0466. [DOI] [PubMed] [Google Scholar]
- 202.Guillot PV, et al. Intrauterine transplantation of human fetal mesenchymal stem cells from first-trimester blood repairs bone and reduces fractures in osteogenesis imperfecta mice. Blood. 2008;111:1717–1725. doi: 10.1182/blood-2007-08-105809. [DOI] [PubMed] [Google Scholar]
- 203.Chamberlain JR, et al. Gene targeting in stem cells from individuals with osteogenesis imperfecta. Science. 2004;303:1198–1201. doi: 10.1126/science.1088757. [DOI] [PubMed] [Google Scholar]
- 204.Chamberlain JR, et al. Gene targeting of mutant COL1A2 alleles in mesenchymal stem cells from individuals with osteogenesis imperfecta. Mol Ther. 2008;16:187–193. doi: 10.1038/sj.mt.6300339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Millington-Ward S, McMahon HP, Farrar GJ. Emerging therapeutic approaches for osteogenesis imperfecta. Trends Mol Med. 2005;11:299–305. doi: 10.1016/j.molmed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 206.Dawson PA, Marini JC. Hammerhead ribozymes selectively suppress mutant type I collagen mRNA in osteogenesis imperfecta fibroblasts. Nucleic Acids Res. 2000;28:4013–4020. doi: 10.1093/nar/28.20.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Navon A, Ciechanover A. The 26 S proteasome: from basic mechanisms to drug targeting. J Biol Chem. 2009;284:33713–33718. doi: 10.1074/jbc.R109.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sloan LA, Fillmore MC, Churcher I. Small-molecule modulation of cellular chaperones to treat protein misfolding disorders. Curr Opin Drug Discov Devel. 2009;12:666–681. [PubMed] [Google Scholar]
- 209.Rochet JC. Novel therapeutic strategies for the treatment of protein-misfolding diseases. Expert Rev Mol Med. 2007;9:1–34. doi: 10.1017/S1462399407000385. [DOI] [PubMed] [Google Scholar]
- 210.Khoshnoodi J, Cartailler JP, Alvares K, Veis A, Hudson BG. Molecular recognition in the assembly of collagens: terminal noncollagenous domains are key recognition modules in the formation of triple helical protomers. J Biol Chem. 2006;281:38117–38121. doi: 10.1074/jbc.R600025200. [DOI] [PubMed] [Google Scholar]
- 211.Nishikawa Y, et al. A structure-activity relationship study elucidating the mechanism of sequence-specific collagen recognition by the chaperone HSP47. Bioorg Med Chem. 2010;18:3767–3775. doi: 10.1016/j.bmc.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 212.Chessler SD, Byers PH. BiP binds type I procollagen pro alpha chains with mutations in the carboxyl-terminal propeptide synthesized by cells from patients with osteogenesis imperfecta. J Biol Chem. 1993;268:18226–18233. [PubMed] [Google Scholar]
- 213.Bonadio J, et al. A murine skeletal adaptation that significantly increases cortical bone mechanical properties. Implications for human skeletal fragility. J Clin Invest. 1993;92:1697–1705. doi: 10.1172/JCI116756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Stacey A, et al. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988;332:131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- 215.Khillan JS, Olsen AS, Kontusaari S, Sokolov B, Prockop DJ. Transgenic mice that express a mini-gene version of the human gene for type I procollagen (COL1A1) develop a phenotype resembling a lethal form of osteogenesis imperfecta. J Biol Chem. 1991;266:23373–23379. [PubMed] [Google Scholar]
- 216.Baek WY, et al. Positive regulation of adult bone formation by osteoblast-specific transcription factor osterix. J Bone Miner Res. 2009;24:1055–1065. doi: 10.1359/jbmr.081248. [DOI] [PMC free article] [PubMed] [Google Scholar]