Abstract
SR Ca2+ ATPase 2a (SERCA2a) is a critical ATPase responsible for Ca2+ re-uptake during excitation-contraction coupling. Impaired SR Ca2+ uptake resulting from decreased expression and reduced activity of SERCA2a is a hallmark of heart failure (HF)1. Accordingly, restoration of SERCA2a expression by gene transfer has proven to be effective in improving cardiac function in HF patients2 as well as in animal models3. The small ubiquitin-related modifier (SUMO) can be conjugated to lysine residues of target proteins4, which is involved in most cellular process5. Here, we show that SERCA2a is SUMOylated at lysine 480 and 585 and that this SUMOylation is essential for preserving SERCA2a ATPase activity and stability. The levels of SUMO1 and SUMOylation of SERCA2a itself were greatly reduced in failing hearts. SUMO1 restitution by adeno-associated virus-mediated gene delivery maintained protein abundance of SERCA2a and significantly improved cardiac function in HF mice. This effect was comparable to SERCA2a gene delivery. Moreover, SUMO1 overexpression in isolated cardiomyocytes augmented contractility and accelerated Ca2+ decay. Transgene-mediated SUMO1 overexpression rescued pressure overload-induced cardiac dysfunction concomitantly with increased SERCA2a function. By contrast, down-regulation of SUMO1 using shRNA accelerated pressure overload-induced deterioration of cardiac function and was accompanied by decreased SERCA2a function. However, knockdown of SERCA2a resulted in severe contractile dysfunction both in vitro and in vivo, which was not rescued by overexpression of SUMO1. Taken together, our data show that SUMOylation is a critical post-translational modification that regulates SERCA2a function and provides a platform for the design of novel therapeutic strategies for HF.
It was previously reported that SERCA2a activity could be modulated by post-translational modifications (PTMs) such as glutathiolation6 and nitration7. We identified SUMO1 as a SERCA2a interactor in a proteomic screen (Supplementary Fig. 1a, b). We confirmed that SERCA2a indeed binds to SUMO1 and Ubc9, a SUMO conjugating enzyme in vivo (Supplementary Fig. 1c-e).
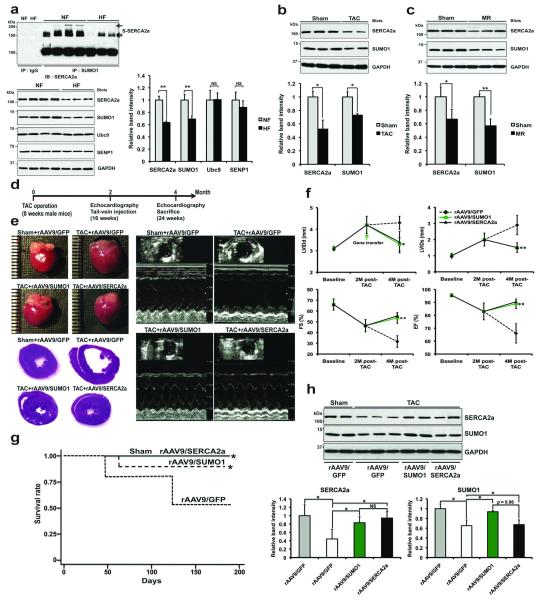
We then examined whether SERCA2a is indeed SUMOylated in human hearts. The SERCA2a band was present along with other slowly migrating SERCA2a bands (Fig. 1a, top), which represent SUMOylated SERCA2a. The level of SUMOylated SERCA2a was significantly reduced in failing hearts compared to normal hearts. SUMOylation of SERCA2a was specific for SUMO1 (but not for SUMO2/3) (Supplementary Fig. 1f). Along with the reduction in SERCA2a, which is consistent with previous reports, the level of SUMO1 was also significantly reduced in failing hearts. However, the levels of Ubc9 and SENP1, critical SUMOylating and de-SUMOylating enzymes, were unaltered in failing hearts (Fig. 1a, bottom). These data indicate that the reduction in SERCA2a SUMOylation might be primarily due to the reduced level of SUMO1 and not to alterations in the level or activity of SUMOylating or de-SUMOylating factors. In additional experiments, we observed that the levels of both SUMO1 and SERCA2a were significantly reduced in a murine model of HF induced by pressure-overload (Fig. 1b) and in a porcine model of HF induced by volume-overload (Fig. 1c).
Figure 1. Endogenous SUMO1 protein levels are decreased in both experimental animal and human heart failure.
(a) SERCA2a SUMOylation in human cardiac tissue (n=4 per HF and n=3 per NF). Representative immunoblots and protein quantification results are shown (n=5 per each group). S-SERCA2a: SUMOylated SERCA2a. NF: non-failing control; HF: heart failure.
(b-c) Representative immunoblots for SUMO1 and SERCA2a and quantification of protein levels in a TAC-induced mouse HF model (b) and mitral valve regurgitation (MR) induced porcine HF model (c). (b) TAC: TAC-induced failing hearts (n=5); Sham: sham-operated control hearts (n=8). (c) MR: HF porcine hearts (n=3); Sham: Sham-operated porcine hearts (n=5).
(d) Protocol for SUMO1 gene therapy in mouse model of HF.
(e) Representative gross images of whole hearts (top left panel) and hematoxylin and eosin staining results (bottom left panel), and two-dimensional guided M-mode images of the LV (right panel) from rAAV9/SUMO1 injecting mice or rAAV9/SERCA2a or rAAV9/GFP negative control subjected to TAC.
(f) Effect of rAAV9/SUMO1 treatment on echocardiography indices of LV function measured by fractional shortening (FS) and ejection fraction (EF) and LV end-diastolic diameter measured by LV internal diameters in end-diastole (LVIDd) and end-systole (LVIDs). Sham: rAAV9/GFP, n=12; TAC: rAAV9/GFP, n=10; rAAV9/SUMO1, n=14; rAAV9/SERCA2a, n=12.
(g) Survival of animals following rAAV9-mediated SUMO1 restoration. The Kaplan-Meier method was used to analyze animal lifespan following infection with different kinds of virus. rAAV9/GFP, n=12 per group; rAAV9/SUMO1, or rAAV9/SERCA2a (5 × 1010 vg/mice, n=24 per each group). Of 50 TAC-operated animals, 34 mice survived and were further divided into groups that received rAAV9/GFP (n=9) or rAAV9/SUMO1 (n=13) or rAAV9/SERCA2a (n=12).
(h) Immunoblot results from a representative experiment and protein quantification (n=5 per each group).
All data represent the mean ± the SD. * p < 0.05 vs. the respective control as determined by Student’s t-test.
To characterize the potential role of SUMO1 during HF, we restored SUMO1 expression in a murine model of transverse aortic constriction (TAC)-induced HF. We delivered the cardiotropic recombinant adeno-associated virus serotype 9 (rAAV9) when mice developed HF (Fig. 1d). rAAV9/SUMO1 gene transfer resulted in a dose-dependent increase in SUMO1 in the myocardium one month post-gene transfer (Supplementary Fig. 2). After induction of HF and prior to gene delivery (at 2 month post-TAC), the cardiac function was significantly decreased in the mice. The TAC mice received tail-vein injection of either rAAV9/GFP (which was used as a negative control), rAAV9/SERCA2a (positive control) or rAAV9/SUMO1 and were followed for 2 more months. Echocardiograhically assessed cardiac function revealed significant improvements following rAAV9/SUMO1 gene delivery (at 4 month post-TAC in total), despite constant pressure-overload, compared with severe deterioration in the rAAV9/GFP control (Fig. 1e, f and Supplementary Table 1). The mice injected with rAAV9/SUMO1 recovered cardiac function to the same degree as the mice injected with rAAV9/SERCA2a. Furthermore, hemodynamic analyses showed substantially improved LV function in the SUMO1 treated mice and stabilization of heart weight to body weight ratio (Supplementary Fig. 3 and Supplementary Table 2). Furthermore, the survival rate in the rAAV9/SUMO1-injected mice was significantly extended compared with rAAV9/GFP-injected HF mice (Fig. 1g). Consistent with the improvement in cardiac performance in rAAV9/SUMO1-injected mice, the protein levels of SERCA2a were significantly increased in rAAV9/SUMO1-injected mice compared with rAAV9/GFP-injected HF animals, suggesting that SUMO1 restoration maintained SERCA2a level during HF (Fig. 1h). Taken together, we provide the first evidence that cardiac restoration of SUMO1 by rAAV9 gene transfer enables long-term improvement of cardiac function. These findings provide further evidence that manipulating PTM of SERCA2a may result in a functional benefit.
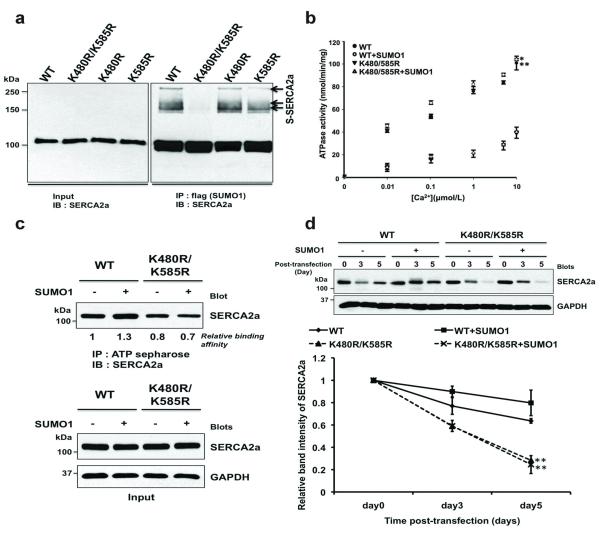
SUMOylation usually occurs within a highly conserved recognition motif8. We found two putative SUMO conjugation sites in human SERCA2a, lysines 480 and 585. These residues are perfectly conserved in mouse, rat, pig, and human SERCA2a (Supplementary Fig. 4). We generated three SERCA2a variants. While K480R and K585R were SUMOylated at levels indistinguishable from that of wild type (WT) SERCA2a, K480R/K585R was completely un-SUMOylated (Fig. 2a). Since the SUMOylated lysine residues, K480 and K585, reside in the nucleotide-binding domain that binds ATP, we predicted that SUMOylation might affect the ATPase activity of SERCA2a. As expected, K480R/K585R demonstrated a significantly decreased ATPase activity compared to WT. SUMO1 co-expression significantly increased the ATPase activity of WT but did not affect the ATPase activity of K480R/K585R (Fig. 2b). We further tested whether SUMOylation affects the ATP-binding affinity of SERCA2a. Co-expression of SUMO1 significantly increased the ATP-binding affinity of WT. By contrast, K480R/K585R had significantly less ATP-binding affinity, which was not affected by SUMO1 co-expression (Fig. 2c). These data suggest that SUMOylation increases the ATPase activity of SERCA2a at least partially by enhancing its ATP-binding affinity.
Figure 2. SUMO1 is conjugated to lysines 480 and 585 of SERCA2a and is required for SERCA2a function.
(a) in vivo SUMOylation of SERCA2a. HEK293 cells were co-transfected with plasmids expressing flag-tagged SUMO1 and myc-tagged Ubc9 and WT or mutant SERCA2a. SUMOylated forms of SERCA2a were detected by immunoblot analysis using anti-SERCA2a antibody.
(b) Ca2+-dependent ATPase activity of WT SERCA2a and the K480R/K585R mutant in the presence and absence of additional SUMO1. The data represent three independent experiments, and each experiment was performed in duplicate.
(c) The ATP binding capacity of WT and K480R/K585R SERCA2a. Lysates from HEK293 cells transfected with the indicated plasmids were affinity-precipitated with ATP-sepharose and subsequently subjected to immunoblot analysis with an anti-SERCA2a antibody.
(d) Effects of SUMO1 overexpression on the stability of WT and K480R/K585R SERCA2a mutant protein in HEK293 cells. HEK293 cells were transfected with WT or K480R/K585R SERCA2a expression plasmids together with empty or SUMO1 expression plasmids. Forty-eight hours after transfection, the cells were treated with cycloheximide, a protein synthesis inhibitor. Immunoblot analysis was performed with the indicated antibodies at different time points. The quantification data represent the ratio relative to day 0 (n=3).
All data represent the mean ± the SD. * p < 0.05; ** p < 0.001 vs. the respective control as determined using Student’s t-test.
Since we observed that both SERCA2a and SUMO1 levels were reduced in HF, we studied whether SUMOylation affects SERCA2a stability. The estimated half-life of WT was 4.9 days, which increased to 5.9 days when SUMO1 was co-expressed. The estimated half-life of K480R/K585R was significantly reduced compared to WT (Fig. 2d). In the ER-associated protein degradation, misfolded proteins are usually degraded by 26S proteasomes after poly-Ubiquitination9. To determine whether the instability of K480R/K585R mutant involves Ubiquitin dependent degradation, we performed ubiquitination assays of SERCA2a in HEK293 cells. SUMO1 overexpression decreased the poly-Ubiquitin-conjugated form of WT SERCA2a whereas it did not alter poly-Ubiquitination of the K480R/K585R SERCA2a mutant (Supplementary Fig. 5). These data support our hypothesis that SUMOylation may compete with Ubiquitination on SERCA2a and suggest that SUMOylation increases the stability of SERCA2a by preventing Ubiquitin/26S proteasomal degradation of SERCA2a.
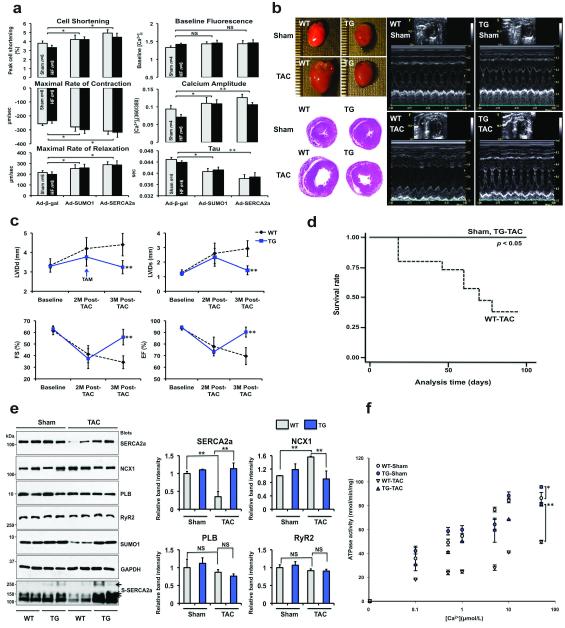
To examine the physiological function of SUMO1, we isolated mouse adult cardiomyocytes from sham-operated or TAC-induced HF and then infected the cells with adenovirus expressing either β-gal (Ad-β-gal) or SUMO1 (Ad-SUMO1). When infected with Ad-SUMO1, sham cardiomyocytes showed significantly enhanced contractility compared to Ad-β-gal-infected cardiomyocytes. A more prominent enhancement in contractility was observed when failing cardiomyocytes were infected with Ad-SUMO1. Ad-SUMO1-infected cardiomyocytes showed increased Ca2+ amplitude and faster Ca2+ decay compared to the Ad-β-gal-infected cardiomyocytes. The overall inotropic effect of SUMO1 overexpression was comparable with that induced by SERCA2a overexpression (Fig. 3a). It is possible that SUMO1 overexpression enhanced cardiomyocyte contractility at least in part by increasing the enzymatic activity and stability of SERCA2a.
Figure 3. SUMO1 overexpression restores TAC-induced cardiac dysfunction.
(a) The bar graphs show the average of the contractility and Ca2+ transient parameters. Isolated mouse cardiomyocytes were infected with indicated adenoviruses and their mechanical properties were measured. Sham contractility, n=143 cells from 6 mice; HF contractility, n=181 cells from 8 mice; Sham Ca2+ transient, n=65 cells from 4 mice; HF Ca2+ transient, n=131 cells from 6 mice.
(b) Representative gross images of whole hearts (top left panel) and hematoxylin and eosin staining results (bottom left panel), and two-dimensional guided M-mode images of the left ventricle (right panel) from SUMO1 transgenic mice or WT control subjected to Sham or TAC.
(c) Echocardiographic measurements of the LV internal diameters in end-diastole (LVIDd), end-systole (LVIDs), fractional shortening (FS) and ejection fraction (EF). Sham: WT, n=12; TG, n=10; TAC: WT, n=14; TG, n=12.
(d) Kaplan-Meier survival curves of WT mice (dotted line, n=15) and SUMO1 transgenic mice following TAC operations (closed line, n=14).
(e) Representative immunoblot analysis is shown that indicate alternations in the levels of cardiac proteins in SUMO1 transgenic mice (left panel), and their quantification (right panel; n=4 for each group).
(f) Ca2+-dependent ATPase activity of SERCA2a in preparations from sham-operated WT ( ), sham-operated SUMO1 transgenic (
), sham-operated SUMO1 transgenic ( ), TAC-operated WT (
), TAC-operated WT ( ), and TAC-operated SUMO1 transgenic (
), and TAC-operated SUMO1 transgenic ( ) mouse hearts (n=3 per each group).
) mouse hearts (n=3 per each group).
All data represent the mean ± the SD. * p < 0.05; ** p < 0.001 vs. the respective control as determined using Student’s t-test. NS: non-significant.
Given our positive in vitro and gene delivery results, we then proceeded to define the physiological consequences of SUMO1 overexpression in vivo. For this purpose, we generated cardiac specific Cre/loxP conditional SUMO1 transgenic (TG) mice (Supplementary Fig. 6a, b). Along with increased SUMO1 levels, the level of both SERCA2a itself and its SUMOylation were significantly induced by tamoxifen administration (Supplementary Fig. 6c, d). No apparent cardiac dysfunction was present in these transgenic animals (Supplementary Table 3). WT and TG mice were subjected to TAC operations. HF developed in 2 months, characterized by a decrease of less than 50% in fractional shortening (FS). One month after tamoxifen was administered (3 months post-TAC in total), cardiac function was examined. At 3 months post-TAC, WT mice exhibited severe failing phenotypes with significant left ventricular (LV) dilation and a low ejection fraction (EF). However, tamoxifen-induced SUMO1 overexpression dramatically reversed these failing phenotypes and resulted in less LV dilation and improved FS and EF (Fig. 3b, c and Supplementary Table 4). Hemodynamic analyses also showed improved LV function in the TG mice along with stabilization of heart weight to body weight ratio (Supplementary Fig. 7 and Supplementary Table 5). The recovery of cardiac dysfunction induced by SUMO1 overexpression was also manifested by the increased survival rate of TG animals under prolonged pressure-overload (Fig. 3d). All TG animals survived 100 days after tamoxifen administration, whereas at this time point only seven out of 15 WT animals survived. These findings are consistent with the results from the rAAV9 gene delivery study.
Next, we monitored the expression levels of key regulatory proteins involved in Ca2+ homeostasis. Following TAC, a notable reduction in the levels of SERCA2a was observed while NCX1 levels increased compared to sham (Fig. 3e). A decrease in SERCA2a function is coupled with an increase in NCX function in failing hearts10. The TAC-induced changes in the levels of SERCA2a and NCX1 were normalized in TG animals.
In WT animals, TAC resulted in a significant reduction of SERCA2a ATPase activity. However, this TAC-induced reduction in ATPase activity was significantly ameliorated in the TG animals (Fig. 3f). Taken together, these data suggest that SUMO1 overexpression restores cardiac dysfunction induced by pressure overload.
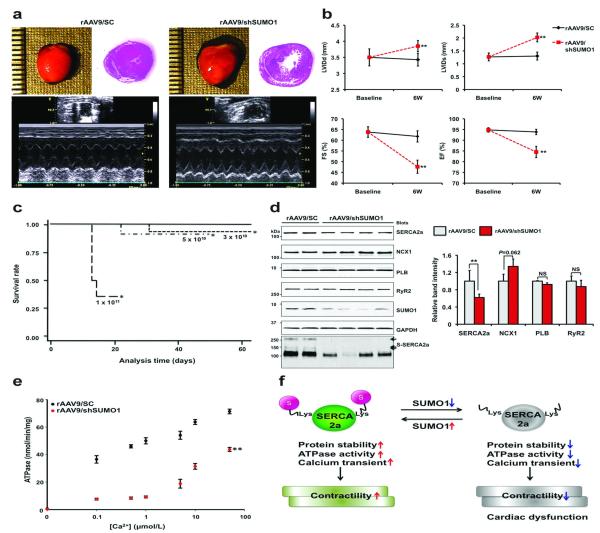
To evaluate the effects of down-regulation of SUMO1 in hearts, we generated rAAV9 that express SUMO1-directed shRNA (rAAV9/shSUMO1) or a scrambled sequence (rAAV9/SC). We confirmed the efficiency and specificity of the shSUMO1 in mice (Supplementary Fig. 8a, b). Six weeks after tail-vein injection, cardiac function was evaluated. Hearts from rAAV9/shSUMO1-injected mice showed LV dilation and functional deterioration compared to hearts from rAAV9/SC-injected mice (Fig. 4a, b and Supplementary Tables 6, 7). Injection of an increased dose of rAAV9/shSUMO1 resulted in more severe cardiac dysfunction (Supplementary Fig. 8c, d). rAAV9/shSUMO1-induced cardiac dysfunction manifested as sudden death of rAAV9/shSUMO1-injected mice. All mice receiving 1 × 1011 vg of rAAV9/shSUMO1 died within 3 weeks. The death rates of mice that received 3 × 1010 vg and 5 × 1010 vg of rAAV9/shSUMO1 were slightly higher than the control mice that received rAAV9/SC which had no death (Fig. 4c). SERCA2a protein levels were significantly decreased by approximately 40% in rAAV9/shSUMO1-injected hearts, while NCX levels were slightly elevated, although not statistically significant. As expected, SUMOylation of SERCA2a was also significantly blunted (Fig. 4d and Supplementary Fig. 8e). These results support the premise that SUMO1 is important for SERCA2a’ stability and function. The down-regulation of SUMO1 also significantly suppressed the ATPase activity of SERCA2a (Fig. 4e). The ATPase activity of SERCA2a was further impaired when a higher dose of rAAV9/shSUMO1 was injected (Supplementary Fig. 8f). Taken together, these data show that SUMO1 is an essential regulator of SERCA2a function in the heart.
Figure 4. Reduction of SUMO1 levels accelerates cardiac dysfunction.
(a) Heart morphology, cardiac function, and heart dimensions were examined in mice injected with 5 × 1010 vg of rAAVs at 6 weeks after injection. Representative gross images of the hearts (top left panel), hematoxylin and eosin staining results (top right panel), and M-mode imaging data (bottom panel) are shown.
(b) Quantifications of the LV internal diameters in end-diastole (LVIDd) and end-systole (LVIDs) and the fractional shortening (FS) and ejection fraction (EF) by echocardiography in rAAV9/shSC or rAAV9/shSUMO1 injected mice (5 × 1010 vg/mice per each group, n=14 per each group).
(c) Survival of animals following rAAV9-mediated cardiac knockdown of SUMO1.The Kaplan-Meier curves are shown for different doses of rAAV9/ shSUMO1 (n=14 per each group) or rAAV9/shSC (5 × 1010 vg/mice, n=24).
(d) Representative immunoblot analysis for the indicated cardiac proteins are shown from samples collected 6 weeks after tail-vein injection with rAAV9/shSC (5 × 1010 vg/mice, n=4) or rAAV9/shSUMO1 (5 × 1010 vg/mice, n=7) (left panel). Quantification of GAPDH corrected cardiac protein immunoblot signals (right panel).
(e) Ca2+-dependence of SERCA2a’s ATPase activity. The ATPase activity was examined in preparations from scramble (●) injected and SUMO1 shRNA ( ) injected hearts (5 × 1010 vg/mice per each group, n=3 per each group).
) injected hearts (5 × 1010 vg/mice per each group, n=3 per each group).
All data represent the mean ± the SD. * p < 0.05; ** p < 0.001 vs. the respective control as determined using Student’s t-test. NS: non-significant.
(f) A working model for the regulation of SERCA2a function by SUMOylation. Under basal conditions, SUMOylation enhances SERCA2a’s protein stability and its Ca2+ pump function to regulate cardiac contractility. However, increased levels of unSUMOylated SERCA2a due to low SUMO1 protein pools triggers impaired SERCA2a activity and induces cardiac dysfunction under pathophysiological conditions.
We then tested the SUMOylation status of some important cardiac transcriptional factors such as GATA411 and SRF12 involved in cardiac pathogenesis which are regulated SUMOylation in our gene therapy model (Supplementary Fig. 9). In these experiments, SUMOylation status and protein expression level of GATA4 were not altered by SUMO1 gene delivery whereas SUMOylated SRF was increased. This indicates that increased SRF SUMOylation, following SUMO1 restoration, may contribute to the activation of certain genes such as SERCA2a and contractile proteins during HF13. However, there are several other transcriptional factors such as SP1, SP3 and calcium regulatory signaling pathways are associated with SERCA2a gene expression14,15. One interesting finding is that the cleaved form of SRF was detected in failing hearts and this was decreased by SUMO1 and SERCA2a gene delivery. Recent studies have shown that caspase-3 mediated SRF cleavage occurs in HF16 and SUMO1 overexpression blocks both TNF and FAS-induced cell death signaling17. Our findings and recent studies suggest that SUMO1 restoration increases SUMOylated SRF and may alter apoptotic signaling in HF.
To determine the critical link between SUMO1 and SERCA2a in the setting of cardiac dysfunction, we knocked down SERCA2a using a lentiviral system carrying a short hairpin directed towards SERCA2a (shSERCA2a). In rodent and murine isolated cardiomyocytes, shSERCA2a significantly decreased contractile parameters, which were not improved by overexpression of SUMO1 (Supplementary Figs 10a, b and 11a). In vivo, rat ventricular function was significantly decreased following gene transfer of shSERCA2a but no improvements were observed with co-infection with Ad-SUMO1 (Supplement Fig. 10c-f and Supplementary Table 8). In mice, in vivo antecedent overexpression of SUMO1 did not rescue ventricular function following lentiviral gene transfer of shSERCA2a (Supplement Fig. 11b-e and Supplementary Table 9). Taken together, these four different experiments show that SERCA2a is critical in modulating SUMO1’s beneficial effects in the setting of heart failure. However we cannot exclude that knockdown of SERCA2a may have effects on other proteins that would not allow SUMO1 to rescue contractile function.
In this study, we showed that SERCA2a is SUMOylated at two lysine residues. We found the levels of both SERCA2a itself and SERCA2a SUMOylation were significantly reduced in failing hearts. We provide compelling evidence suggesting that the reduction of SERCA2a SUMOylation is a direct result of reduced SUMO1 levels in failing hearts. This reduction in SUMOylation correlated with reduced ATPase activity and decreased SERCA2a stability. Moreover, restoration of SUMO1 reversed contractile dysfunction in failing hearts. Our findings are summarized in Fig. 4f.
It is possible that SUMOylation may induce a conformational change18 within SERCA2a or may provide an additional interface for ATP binding, leading to increase ATPase activity. It is also possible that SUMOylation may reciprocally and competitively affect other PTMs of SERCA2a, and in particular, may alter acetylation19. Interestingly, acetylation of SERCA2a was recently identified in a large-scale analysis of the human acetylome in different cancer cell lines20. We also found that SERCA2a is indeed acetylated and that this acetylation is more prominent in failing hearts and can be reversed by Sirt1 deacetylase (Kho et al., unpublished data). Further elucidation of the reciprocal or competitive relationship between SUMOylation and acetylation of SERCA2a is currently underway. There is precedence for this type of SUMOylation-mediated inhibition of protein degradation. For example, SUMOylation of Axin, a negative regulator of Wnt signaling, prevents ubiquitination and thus induces a prolonged Axin half-life21. Similarly, SUMOylation of the RNA helicases p68 and p72 increases their stability by reducing ubiquitin-proteasome-mediated protein degradation22. Detailed biochemical analyses are underway to identify the ubiquitination sites in SERCA2a.
Our data show that the amount of SUMO1 is significantly reduced in failing hearts from different species, suggesting that the level of cellular SUMO1 needs to be precisely maintained and controlled for proper cardiomyocyte function. Our findings that restoration of SUMO1 reversed TAC-induced HF indicate that a reduction in SUMO1 levels directly causes contractile dysfunction. Our study was based on strong background data that has clearly established impaired SERCA2a as a key molecular abnormality in HF. The therapeutic effects of SUMO1 with near-complete recovery of contractile function are quite remarkable considering we are altering the expression of a single gene. In contrast to the reduction of SUMO1 levels in failing hearts, the levels of the SUMOylating and de-SUMOylating enzymes, Ubc9 and SENP1, were unaltered either in failing hearts or when shSUMO1 was administered or when SUMO1 levels were restored (data not shown). Therefore, the specificity and capacity of SUMOylation itself is unlikely to be altered in failing hearts. Instead, what appears to have the largest impact on SUMOylation is the supply of SUMO1 itself. In this regard, it is intriguing to note that depletion of cellular ubiquitin is sufficient to cause neuronal dysfunction and death23.
In this study, we demonstrate a novel regulatory mechanism whereby SUMOylation affects SERCA2a activity and the overall contractile properties of the heart while highlighting the importance of post-translational mechanisms in experimental and human heart failure. In addition, the beneficial effects of SUMO1 on cardiac contractility and survival suggest a targeting SUMO1 may provide a novel therapeutic strategy for the treatment of heart failure.
METHODS SUMMARY
Animals
All mice and rats housed and treated in accordance with NIH and IACUC guidelines, and used protocols approved by the Mount Sinai School of Medicine Animal Care and Use Committees.
Human heart samples
Left ventricular samples were obtained from explanted human hearts obtained at the time of cardiac transplantation. Non-failing hearts (which were used as controls) were obtained from donors who died from neurological diseases or motor vehicle accidents and who had normal cardiac function by echocardiography. The five donors, three males and two females, had a median age of 62. The heart failure patients, three males and two females had a median age of 60 and their average ejection fraction was 20±3%.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Methods
In vivo SUMOylation assay
To analyze SUMOylation within cells, Lipofectamine 2000 was used to transfect HEK293 cells with plasmids encoding WT or SERCA2a SUMOylation site mutants, along with flag-tagged SUMO1 and myc-tagged Ubc9. The cells were lysed by sonication in ice-cold lysis buffer [50 mM Tris-Cl pH 8.0, 150 mM NaCl, 0.1% Triton X-100, 10 mM EDTA, complete protease inhibitor (one tablet per 10 ml, Roche), and a protein phosphatase inhibitor cocktail (Sigma)] containing 20 mM N-ethylmaleimide. Lysates were cleared by centrifugation at 30,000 g for 20 min. Cell lysates were then immunoprecipitated with a flag-specific affinity matrix gel (Sigma) overnight at 4°C, after which the immunoprecipitates were washed in cold lysis buffer. The immunocomplexes were then resolved by SDS-PAGE, and immunoblot analysis was performed with an sSERCA2a-specific antibody.
Fresh tissue extracts were prepared in lysis buffer for in vivo SUMOylation assays. Hearts from each experimental and control group were frozen in liquid nitrogen. The frozen tissues were crushed and homogenized in lysis buffer as described above using the MP homogenate system (FastPrep homogenizer). The insoluble portion was removed by centrifugation at 30,000 g for 20 min. The extracts were incubated with anti-SUMO1 agarose resin with agitation overnight. The SUMO conjugated forms were detected by immunoblot analysis with specific primary antibodies.
SERCA2a activity assay
SERCA2a activity was determined using pyruvate/NADH-coupled reactions based assay, as previously described24. The activity of the Ca2+-ATPase was calculated as follows: Δabsorbance/6.22 × protein × time (in nmol ATP/mg protein × min). All assays were performed in triplicate.
Generation of conditional SUMO1 transgenic mice
The αMHC-flox-mouse SUMO1 transgene was subcloned into the pML2G vector (a kind gift of Dr. Yibin Wang), which encodes EGFP cDNA between two loxP sites. The DNA construct was microinjected into fertilized eggs from B6C3 mice, and transgenic integration was confirmed by PCR (Dr. Kevin Kelly, Mount Sinai School of Medicine).
Antibodies
The following antibodies were used for Immunoblotting: SERCA2a (produced by 21st Century Biochemicals), GAPDH (Sigma), myc (Sigma), flag (Sigma), SUMO1 (Cell signaling), SUMO1 agarose resin (Santa Cruz Biotech), SUMO2/3 (MBL), Ubc9 (Boston Biochem), SENP1 (Pierce), PLN (Badrilla), RyR2 (Badrilla), NCX1 (Chemicon), GATA4 (Millipore), SRF (Millipore), anti-rabbit-HRP and anti-mouse-HRP secondary antibodies (Sigma).
Primers for mutagenesis
The following primer sets were used for mutagenesis: K480R_sense, TCA GTC ATT AAA CAG CTG ATG AGA AAG GAA TTC ACT CTA GAG; K480R_anti-sense, CTC TAG AGT GAA TTC CTT TCT CAT CAG CTG TTT AAT GAC TGA; K585R_sense, GAG GAC TCT GCC AAC TTT ATT AGA TAT GAG ACC AAT CTG ACC; K585R_anti-sense, GGT CAG ATT GGT CTC ATA TCT AAT AAA GTT GGC AGA GTC CTC
Proteomic analysis
Solubilized immuno-complexes were combined with a rehydration buffer (9 M urea, 2 M thiourea, 4 M CHAPS, 16 mM DTT, 2% [w/v] Pharmalyte 3-11, and trace amounts of bromophenol blue) to a final volume of 340 μl and were rehydrated for 16 hours. After rehydration, strips were focused at 60 kVh at 20°C (IPGphor III, GE Healthcare). Once IEF was complete the strips were equilibrated, separated on 12.5% SDS-PAGE gels and visualized by silver staining using the plus one silver staining kit (GE Healthcare) with minor modifications to ensure compatibility with subsequent mass spectrometry analysis. Stained two-dimensional gel images were scanned (ImageScanner II, GE Healthcare) and analyzed by PDquest 8.1 software system (Bio-Rad). Interesting spots were excised from the gels by spot picker and prepared for MS analysis as previously described25.
Mass spectrometric analysis
Identification of gel spots after was accomplished by ESI/LC/MS/MS system (Thermo-Scientific). Full scan spectra were recorded in positive mode over the mass range 350-2000 Da. MS/MS data were automatically acquired on the two most intense precursor ions in each full scan spectrum and interpreted using Xcalibur 2.0 and Bioworks 3.2 software.
Contractility measurement
The mechanical properties of isolated ventricular cardiomyocytes were assessed using a video-based edge detection system (IonOptix) as previously described26.
Adenoviruses
Adenoviruses encoding SERCA2a and SUMO1 were generated using the pAdEasy XL adenoviral Vector System (Stratagene) according to the manufacturer’s protocols.
rAAV vector production and purification
rAAV9/SERCA2a, rAAV9/shSUMO1 and rAAV9/scramble were produced using the two-plasmids protocol described by Zolotukhin et al27 with the following modifications: HEK293T cells (ATCC) were grown in triple flasks for 24 hours (DMEM, 10% FBS) prior to adding the calcium phosphate precipitate. After 72 hours, the virus was purified from benzonase-treated cell crude lysates over an iodixanol density gradient (Optiprep, Greiner Bio-One Inc), followed by heparin-agarose type I affinity chromatography (Sigma). Finally viruses were concentrated and formulated into lactated Ringer’s solution (Baxter Healthcare Corporation) using a Vivaspin 20 Centrifugal concentrators 50K MWCO (Vivascience Inc), and stored at − 80°C.
Lentivirus
The lentiviral plasmids encoding for the shRNA to knockdown SERCA2a were previously described by us28. SERCA2a shRNA constructs is the following sequence, where the segment in italic type indicates the loop: 5′-GATCCGACTTACTAGTTAGAATTTGGCTAAGAGCAAATTCTAACTAGTAAGTC TTTTTTGGAATTAAT-3′. We used third generation lentiviral systems to generate the vectors29.
Transverse aortic constriction
Mice underwent TAC using a supraclavicular construction model as previously described26. TAC or sham surgery was performed in 8 weeks old male mice (BW: 18 – 22 g). Mice were anesthetized with intraperitoneal ketamine and placed on ventilator. A 2- to 3-mm longitudinal cut was made in the proximal portion of the sternum, allowing visualization of the aortic arch. The transverse aortic arch was ligated between the innominate and left common carotid arteries with an overlaying 27-gauge needle. The needle was immediately removed leaving a discrete region of constriction. Animal sham group underwent a similar procedure without ligation.
in vivo Gene Transfer
Adult Sprague-Dawley rats (240-260 g) underwent gene transfer via aortic cross-clamping as previously described30. After dissection of the aorta and pulmonary artery lentiviral shSERCA2a with or without adenoviral SUMO1 were injected into the LV cavity through a 22G catheter while the aorta and pulmonary artery were cross-clamped for 50 seconds. In sham operated animals normal saline was injected into the LV cavity while the aorta and pulmonary artery were cross-clamped for 50 seconds. After cross-clamping was released the chest was closed.
Echocardiography
Transthoracic echocardiography was performed using a Vivid 7000 equipped (GE healthcare) with a H13L transducer (14 MHz). Two-dimensional and M-mode images were obtained in the short-axis view. The heart rate (HR), left ventricular end-diastolic internal diameter (LVIDd), left ventricular end-systolic internal diameter (LVIDs) were measured over the course of at least three repeated cardiac cycles. The ejection fraction (EF) and fractional shortening (FS) were then calculated.
Hemodynamics
Hemodynamic measurements are performed using a 1.2 Fr pressure-volume (PV) conductance catheter (Scisense). PV loop analysis was performed as previously described31. Mice were injected intraperitoneal with urethane (1 g/kg), etomidate (10 mg/kg), morphine (1 mg/kg) and mechanically ventilated with 7 μl/g stroke volume at 125 respirations/min. The chest was opened to expose the heart for an apical stab approach. In order to determine absolute ventricular volumes via admittance technology, myocardial and blood conductance were obtained prior to PV catheter placement in the left ventricle32. The inferior vena cava was transiently occluded to reduce ventricular preload to obtain load-independent pressure-volume relationships. Hemodynamic measurements were acquired and analyzed using IOX software (EMKAtech).
Histological analysis
Hearts was prepared in Tissue-Tek OCT compound (Sakura Finetechnical) and sectioned into 6 μm slices (Microm HM560 Cryo-star, Thermo Scientific). The sections were stained with hematoxylin-eosin.
Statistical analysis
Statistical analyses were performed using Student’s t-test, and significant differences are demonstrated by either a single asterisk (*), which indicates p < 0.05, or a double asterisk (**), which indicates p < 0.001. Data in the figures represents the mean ± the SD.
Supplementary Material
Acknowledgements
This work is supported by NIH RO1 HL083156, HL080498, HL093183, and P20HL100396 (R. J. H.). W. J. P. is funded by Global Research Laboratory Program (M6-0605-00-0001) of the Korean Ministry of Science and Technology.
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author contributions C. K., A. L., and R.J.H. conceived of the project and its design. C. K., A. L., D. J., J. G. O., and A. H. C. performed experiments and data analysis. E. K. aided in experimental design. C. K., A. L., W. J. P. and R. J. H. wrote this manuscript.
The authors declare no competing financial interests. Readers are welcome to comment on the online version of this article at www.nature.com/nature.
REFERENCES
- 1.Meyer M, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92(4):778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, et al. [accepted 27 June, 2011];Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca2+-ATPase in Patients With Advanced Heart Failure. Circulation. doi: 10.1161/CIRCULATIONAHA.111.022889. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawase Y, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008;51(11):1112–1119. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- 5.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8(12):947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 6.Adachi T, et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10(11):1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 7.Knyushko TV, Sharov VS, Williams TD, Schoneich C, Bigelow DJ. 3-Nitrotyrosine modification of SERCA2a in the aging heart: a distinct signature of the cellular redox environment. Biochemistry. 2005;44(39):13071–13081. doi: 10.1021/bi051226n. [DOI] [PubMed] [Google Scholar]
- 8.Sampson DA, Wang M, Matunis MJ. The small ubiquitin-like modifier-1 (SUMO-1) consensus sequence mediates Ubc9 binding and is essential for SUMO-1 modification. J Biol Chem. 2001;276(24):21664–21669. doi: 10.1074/jbc.M100006200. [DOI] [PubMed] [Google Scholar]
- 9.Plemper RK, Wolf DH. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24(7):266–270. doi: 10.1016/s0968-0004(99)01420-6. [DOI] [PubMed] [Google Scholar]
- 10.Schillinger W, Fiolet JW, Schlotthauer K, Hasenfuss G. Relevance of Na+-Ca2+ exchange in heart failure. Cardiovasc Res. 2003;57(4):921–933. doi: 10.1016/s0008-6363(02)00826-x. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Feng XH, Schwartz RJ. SUMO-1 modification activated GATA4-dependent cardiogenic gene activity. J Biol Chem. 2004;279(47):49091–49098. doi: 10.1074/jbc.M407494200. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki K, et al. Serum response factor is modulated by the SUMO-1 conjugation system. Biochem Biophys Res Commun. 2003;306(1):32–38. doi: 10.1016/s0006-291x(03)00910-0. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Schwartz RJ. Sumoylation and regulation of cardiac gene expression. Circ Res. 2010;107(1):19–29. doi: 10.1161/CIRCRESAHA.110.220491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brady M, et al. Sp1 and Sp3 transcription factors are required for trans-activation of the human SERCA2 promoter in cardiomyocytes. Cardiovasc Res. 2003;60(2):347–354. doi: 10.1016/s0008-6363(03)00529-7. [DOI] [PubMed] [Google Scholar]
- 15.Vlasblom R, et al. Contractile arrest reveals calcium-dependent stimulation of SERCA2a mRNA expression in cultured ventricular cardiomyocytes. Cardiovasc Res. 2004;63(3):537–544. doi: 10.1016/j.cardiores.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Drewett V, et al. Serum response factor cleavage by caspases 3 and 7 linked to apoptosis in human BJAB cells. J Biol Chem. 2001;276(36):33444–33451. doi: 10.1074/jbc.M103877200. [DOI] [PubMed] [Google Scholar]
- 17.Okura T, et al. Protection against Fas/APO-1- and tumor necrosis factor-mediated cell death by a novel protein, sentrin. J Immunol. 1996;157(10):4277–4281. [PubMed] [Google Scholar]
- 18.Baba D, et al. Crystal structure of thymine DNA glycosylase conjugated to SUMO-1. Nature. 2005;435(7044):979–982. doi: 10.1038/nature03634. [DOI] [PubMed] [Google Scholar]
- 19.Van Rechem C, et al. Differential regulation of HIC1 target genes by CtBP and NuRD, via an acetylation/SUMOylation switch, in quiescent versus proliferating cells. Mol Cell Biol. 2010;30(16):4045–4059. doi: 10.1128/MCB.00582-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choudhary C, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325(5942):834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Chia IV, Costantini F. SUMOylation target sites at the C terminus protect Axin from ubiquitination and confer protein stability. FASEB J. 2008;22(11):3785–3794. doi: 10.1096/fj.08-113910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mooney SM, Grande JP, Salisbury JL, Janknecht R. Sumoylation of p68 and p72 RNA helicases affects protein stability and transactivation potential. Biochemistry. 2010;49(1):1–10. doi: 10.1021/bi901263m. [DOI] [PubMed] [Google Scholar]
- 23.Ryu KY, Garza JC, Lu XY, Barsh GS, Kopito RR. Hypothalamic neurodegeneration and adult-onset obesity in mice lacking the Ubb polyubiquitin gene. Proc Natl Acad Sci U S A. 2008;105(10):4016–4021. doi: 10.1073/pnas.0800096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hajjar RJ, Schmidt U, Kang JX, Matsui T, Rosenzweig A. Adenoviral gene transfer of phospholamban in isolated rat cardiomyocytes. Rescue effects by concomitant gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circ Res. 1997;81(2):145–153. doi: 10.1161/01.res.81.2.145. [DOI] [PubMed] [Google Scholar]
- 25.Lee AY, et al. Identification of the degradome of Isp-1, a major intracellular serine protease of Bacillus subtilis, by two-dimensional gel electrophoresis and matrix- assisted laser desorption/ionization-time of flight analysis. Proteomics. 2004;4(11):3437–3445. doi: 10.1002/pmic.200400997. [DOI] [PubMed] [Google Scholar]
- 26.Jeong D, et al. PICOT inhibits cardiac hypertrophy and enhances ventricular function and cardiomyocyte contractility. Circ Res. 2006;99(3):307–314. doi: 10.1161/01.RES.0000234780.06115.2c. [DOI] [PubMed] [Google Scholar]
- 27.Zolotukhin S, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6(6):973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 28.Kizana E, Cingolani E, Marban E. Non-cell-autonomous effects of vector-expressed regulatory RNAs in mammalian heart cells. Gene Ther. 2009;16(9):1163–1168. doi: 10.1038/gt.2009.64. [DOI] [PubMed] [Google Scholar]
- 29.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003;100(4):1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajjar RJ, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998;95(9):5251–5256. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3(9):1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porterfield JE, et al. Dynamic correction for parallel conductance, GP, and gain factor, alpha, in invasive murine left ventricular volume measurements. J Appl Physiol. 2009;107(6):1693–1703. doi: 10.1152/japplphysiol.91322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.