Abstract
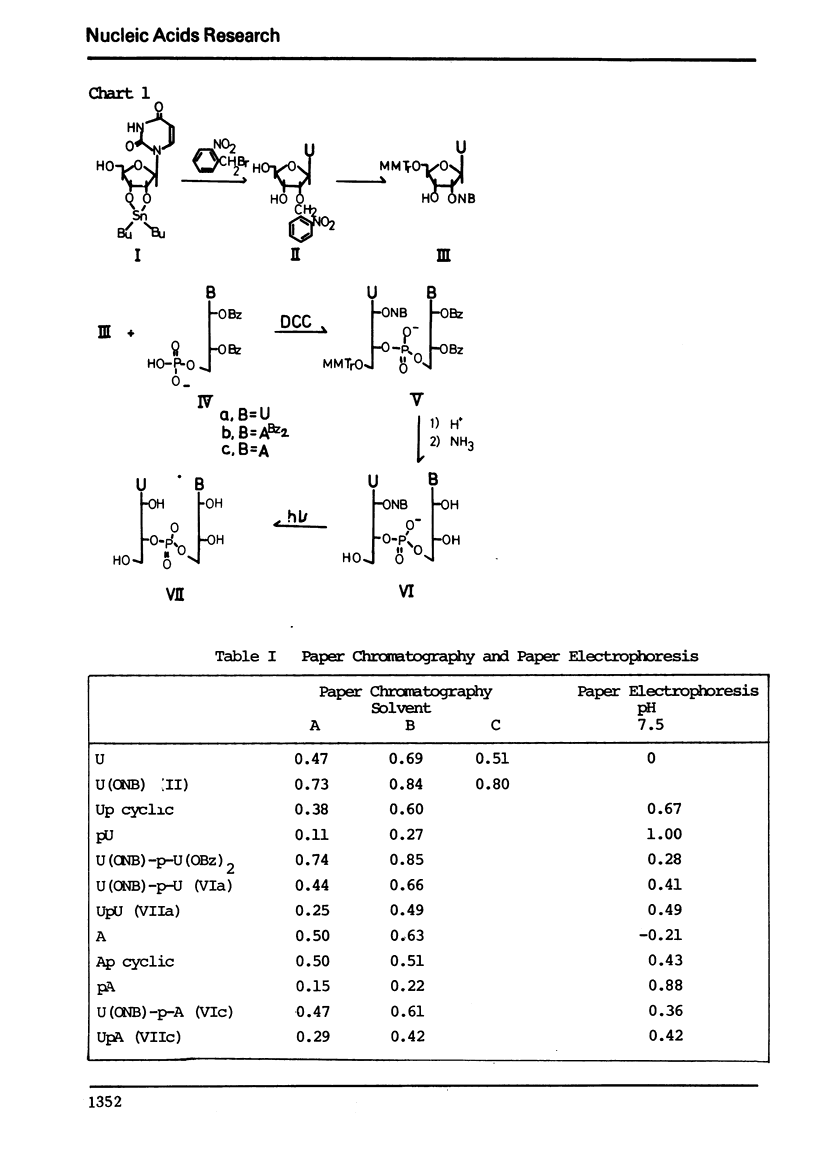
o-Nitrobenzyl group was introduced to the 2′-hydroxyl function of uridine via 2′,3′-O-(dibutylstannylene) uridine. The benzylated uridine was protected at the 5′-hydroxyl group with monomethoxytrityl chloride and condensed with 2′,3′-O-dibenzoyluridine 5′-phosphate or N,N′,2′,3′-O-tetrabenzoyladenosine 5′-phosphate using dicyclohexylcarbodiimide (DCC). o-Nitrobenzyl ether linkage of the dinucleotides was removed by UV irradiation with wavelength longer than 320 nm. Deprotected UpU and UpA thus obtained were characterized by RNase A digestion.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown I. H., Freeman K. B., Johns H. E. Photochemistry of uridylyl-(3'--5')-uridine. J Mol Biol. 1966 Feb;15(2):640–662. doi: 10.1016/s0022-2836(66)80133-x. [DOI] [PubMed] [Google Scholar]
- Christensen L. F., Broom A. D. Specific chemical synthesis of ribonucleoside O-benzyl ethers. J Org Chem. 1972 Nov 3;37(22):3398–3401. doi: 10.1021/jo00795a003. [DOI] [PubMed] [Google Scholar]
- Griffin B. E., Reese C. B., Stephenson G. F., Trentham D. R. Oligoribonucleotide synthesis from nucleoside 2'-O-benzyl ethers. Tetrahedron Lett. 1966 Sep;36:4349–4354. doi: 10.1016/s0040-4039(00)76063-1. [DOI] [PubMed] [Google Scholar]
- Kikugawa K., Sato F., Tsuruo T., Imura N., Ukita T. On the benzylation of nucleosides. II. A novel synthesis of 2'-o-benzyluridine. Chem Pharm Bull (Tokyo) 1968 Jun;16(6):1110–1115. doi: 10.1248/cpb.16.1110. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Murao K., Ubasawa M., Ikehara M. Studies on transfer ribonucleic acids and related compounds. I. Synthesis of ribooligonucleotides using aromatic phosphoramidates as a protecting group. J Am Chem Soc. 1970 Jun 3;92(11):3441–3445. doi: 10.1021/ja00714a036. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Ubasawa M., Ikehara M. Studies on transfer ribonucleic acids and related compounds. II. A method for synthesis of protected ribooligonucleotides using a ribonuclease. J Am Chem Soc. 1970 Jun 3;92(11):3445–3451. doi: 10.1021/ja00714a037. [DOI] [PubMed] [Google Scholar]
- Otsuka E., Honda A., Shigyo H., Morioka S., Sugiyama T. Studies on transfer ribonucleic acids and related compounds. 8(1). Further studies on aromatic phosphoramidates as a protecting group for phosphomonoesters. Nucleic Acids Res. 1974 Feb;1(2):223–234. doi: 10.1093/nar/1.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka E., Nakamura S., Yoneda M., Ikehara M. Polynucleotides. 23. A synthesis of ribodinucleoside monophosphates using nucleoside 5'-phosphates. Nucleic Acids Res. 1974 Feb;1(2):323–329. doi: 10.1093/nar/1.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka E., Ubasawa M., Morioka S., Ikehara M. Studies on transfer ribonucleic acids and related compounds. VI. Synthesis of yeast alanine transfer ribonucleic acid 3'-terminal nonanucleotides and 5'-terminal hexanucleotides. J Am Chem Soc. 1973 Jul 11;95(14):4725–4733. doi: 10.1021/ja00795a042. [DOI] [PubMed] [Google Scholar]
- Zehavi U., Patchornik A. Oligosaccharide synthesis on a light-sensitive solid support. I. The polymer and synthesis of isomaltose (6-O-alpha-D-glucopyranosyl-D-glucose). J Am Chem Soc. 1973 Aug 22;95(17):5673–5677. doi: 10.1021/ja00798a036. [DOI] [PubMed] [Google Scholar]


