Abstract
Glucose-regulated protein 94 is the HSP90-like protein in the lumen of the endoplasmic reticulum and therefore it chaperones secreted and membrane proteins. It has essential functions in development and physiology of multicellular organisms, at least in part because of this unique clientele. GRP94 shares many biochemical features with other HSP90 proteins, in particular its domain structure and ATPase activity, but also displays distinct activities, such as calcium binding, necessitates by the conditions in the endoplasmic reticulum. GRP94’s mode of action varies from the general HSP90 theme in the conformational changes induced by nucleotide binding, and in its interactions with co-chaperones, which are very different from known cytosolic co-chaperones. GRP94 is more selective than many of the ER chaperones and the basis for this selectivity remain obscure. Recent development of molecular tools and functional assays has expanded the spectrum of clients that rely on GRP94 activity, but it is still not clear how the chaperone binds them, or what aspect of folding it impacts. These mechanistic questions and the regulation of GRP94 activity by other proteins and by post-translational modification differences pose new questions and present future research avenues.
Keywords: glucose-regulated protein 94, GRP94, endoplasmic reticulum, chaperone, heat shock protein 90, HSP90, secretory proteins
1. Protein folding in the endoplasmic reticulum vs. the cytosol
While most of our knowledge about the HSP90 family of stress proteins is derived from studying the cytosolic HSP90s in unicellular and multicellular organisms, an important feature of this family is that it also has representatives in two organellar compartments, the endoplasmic reticulum (ER) and the mitochondrion. The HSP90 family member that resides in the ER is glucose-regulated protein 94 (GRP94), encoded by the HSP90B1 gene. It is also commonly known as gp96 or endoplasmin. The various family members are involved in different biological processes and distinct protein-protein interactions and therefore comparisons between them necessarily informs about the unique as well as the common activities of HSP90 proteins. Here we review the current knowledge about the functions, mode of action and regulation of GRP94, highlighting the aspects that are common to HSP90s and those unique to the ER member of the family,
Since the molecular environment in the ER is rather different from that of the cytosol (Table I), and since the ER is the compartment where membrane and secreted proteins fold and assemble, GRP94 had to evolve to deal with a distinct clientele than HSP90 under different conditions. Proteins that fold in the ER tend to have distinct folds, such as the immunoglobulin domain fold, which is found in a large fraction of secretory proteins but not in cytosolic proteins. Many of the clients of GRP94 have one or more such domains. More than 97% of all secretory and membrane proteins contain disulfide bonds and are dependent on proper oxidative folding to attain their biological function. The cysteines in these proteins tend to oxidize because the lumen of the ER is 30-fold more oxidizing than the cytosol [1] and there is a sophisticated protein-based mechanism that extracts electrons from, and oxidizes cysteines in proteins that fold in the ER via concerted action of one of the many protein disulfide isomerases in the ER (reviewed in [2, 3]). Although GRP94 itself is not an oxidoreductase, all its known bona fide substrates are disulfide-bonded proteins.
Table I.
Comparison of conditions that affect protein folding and disposal of misfolded proteins (parameter) and the response to changes in these conditions between the ER and the cytosol.
| Parameter | ER | Cytosol |
|---|---|---|
| Redox state | Oxidizing | Reducing |
| Calcium | From 0 to 1 mM free Ca++ Major protein-bound storage | <1 μM |
| Energy generating system | No | Yes |
| Glycosylation machinery | Yes | No |
| Proteolytic machinery | No | Many |
| HSP70 chaperones | BiP/GRP78, GRP170 | HSP70, HSC70 |
| HSP90 chaperones | GRP94 | HSP90 |
| Stress response | ER stress = unfolded protein response | Heat shock response and metabolic stress |
A post-translational modification that characterizes the vast majority of secretory and membrane proteins is Asn-linked glycosylation. This modification introduces important constraints on folding in the ER lumen, with chaperones and enzymes that interact directly with the carbohydrates and thereby affect GRP94 action, at least indirectly. Furthermore, the status of glycosylation is an important parameter that is monitored by the cell to gauge the quality of protein folding, and is used to determine which proteins should be targeted for degradation. The disposal relies of translocation of defective proteins to the cytosol for degradation by the proteasome, and therefore misfolded proteins in the lumen of the ER require triage, in which GRP94 participates. It is remarkable that many protein folding and other enzymatic reactions that take place in the ER require ATP, yet there is no local production of ATP in the lumen. The ER, unlike the cytosol, must rely completely on import and exchange of nucleotides from and to the cytosol (Table I). This has a direct effect on GRP94, as well as many other ER proteins, which use ATP binding and hydrolysis in their action cycle.
Another important constraint on GRP94 activity is calcium. The ER lumen is a major calcium storage compartment, where Ca++ is stored by binding to multiple proteins, and GRP94 is one of the major Ca++ binding proteins [4]. In addition, GRP94 has to contend during its action cycle with fluctuations in free Ca++ in the lumen, as protein-bound Ca++ is released and leaks through the ER membrane channels to the cytosol, based on the physiological demands of the cell. Since all the above parameters define quite a different homeostatic environment in the ER than in the cytosol, it also follows that changes in these parameters pose distinct stresses to these two compartments. Alteration of either ER Ca++ balance, the redox status or the glycosylation machinery each initiates a distinct signal that is processed by stress signal transducers located in the ER membrane, triggering a complicated signaling system that together is known as the ER stress response or the unfolded protein response (UPR). The purpose of this system, much like the corresponding stress signaling in the cytosol is to either restore homeostasis or target the cell to apoptosis. However, the two stress responses are initiated by distinct stress signals, use distinct signal transducers, rely on distinct transcription factors and target distinct gene sets. GRP94 and other ER chaperones are among the main target of the ER stress response and in addition play a role in mediating the response.
GRP94 has long been an enigma. While it was recognized long ago as a major indication of ER stress [5], a major calcium-binding [6] and a peptide-binding protein [7], it was not clear how it recognizes its substrate proteins, whether or how it uses ATP hydrolysis in its action cycle, whether it uses co-chaperones to regulate its activities, and perhaps most critically – how important it is for cellular functions. In the following sections we describe the considerable recent progress that provides answers to some of these questions.
2. Evolutionary relation
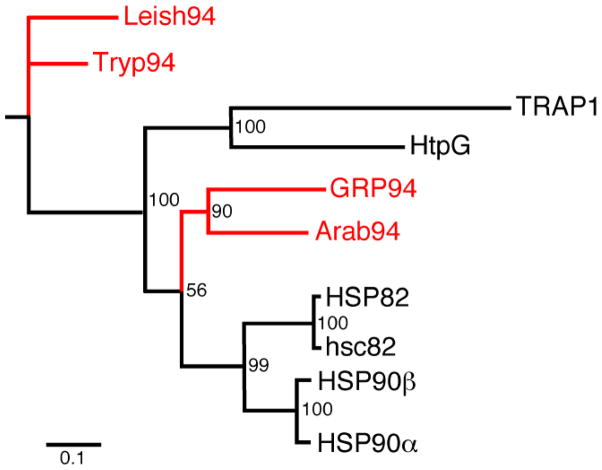
GRP94 is present as a single gene in all metazoa, whether from the animal or plant kingdom. Even though GRP94 is not found in bacteria, archea, yeast, most fungi and other unicellular organisms, it is present in the parasites Leishmania and Trypanosoma. Phylogenetic analysis indicates that organellar and cytosolic HSP90 family members evolved separately from a common ancestor, distinct from the ancestor of HtpG and TRAP (Fig. 1 and [8, 9]). Remarkably, the GRP94s in the unicellular parasitic organisms evolved separately from the plant or animal kingdom GRP94 (Fig. 1), perhaps fulfilling different functions. Apparently the distinction among the GRP94s occurred after the evolution of eukaryotic compartments. If so, unicellular eukaryotes like most yeasts, that have ER but do not have GRP94 orthologues, may have lost them during evolution. The absence of GRP94 from Saccharomyces deprived researchers of a powerful genetic approach to understand GRP94’s activity, no doubt diminishing the ability to place this chaperone in the context of ER functions.
Figure 1. Phylogenetic analysis of some HSP90 family members.
The analysis is based on sequence comparisons of human cytosolic HSP90α (AAI21063.1) and HSP90β (NP_031381.2), human GRP94 (AAH66656.1), Arabidopsis thaliana GRP94 (NP_974606.1) and TRAP-1 (NP_057376.2), yeast HSP82 (P02829.1) and HSC82 (AAA02813.1), E. coli HtpG (AP_001122.1) and Leishmania infantum GRP94 (ADP89477.1) and Trypanosoma cruzi GRP94 by the algorithm MUSCLE, using the web site T-Coffee. The GRP94s are highlighted in red. Numbers at the nodes are bootstrapping values to validate the analysis, performed using PhyML3.0. The X axis is indicating the expected amount of sequence change, which is a combination of time and inherent mutation rate. Inherent mutation rate can be different for each genome, complicating the comparisons. Bar, 0.1 units.
3. GRP94 serves an essential function
In metazoans GRP94 is an essential gene. RNAi knockdown GRP94 in Dictyostelium leads to delayed development and small fruiting bodies [10]. Loss of function of Arabidopsis GRP94 (the Shepherd mutant) causes defects in meristem development through loss of interaction with CLAVATA proteins [11]. GRP94 is also essential in C. elegans, since RNAi knockdown each causes a development arrest at an early larval stage, without maturation of adult worms (WormBase, release WS198). In Drosophila, the orthologous protein gp93 is also needed for larval development and mutant larvae have pronounced defects in their midgut epithelium [12]. Finally, mouse development is arrested without GRP94 in gestational day 7, before gastrulation and formation of mesodermal tissues [13]. Thus, in each organism, GRP94 is required during a specific, early developmental stage. The genetic requirement for GRP94 does not coincide with the onset of its expression (GRP94 is made throughout embryogenesis, including in the zygote) and there is likely maternal inheritance of GRP94, which sustains its functions in early embryos. This is supported by the data on zygote-specific knockout of GRP94, which reveals a requirement for it during the first mitosis [14]. Thus, it is likely that GRP94’s essential function is exposed when it is needed for particular protein-protein interactions. The required interactions likely differ from organism to organism, as the developmental schemes vary considerably. Nonetheless, whatever the molecular functions, they are essential primarily for metazoa, because GRP94 is not expressed in most unicellular organisms, and it is also not essential for growth of individual mammalian cells in culture. GRP94-deficient cells can grow normally [15], albeit more slowly [16], do not have global deficiency in surface receptors expression [15], and are still capable of differentiation [13]. Therefore, GRP94 is not needed to support traffic of secreted or plasma membrane proteins in general, but has more selective effects. This is surprising for a protein that is expressed in virtually every cell (including the zygote) and is among the most abundant ER constituents [17].
The functions of GRP94 in unicellular organisms may be different than in metazoa. Leishmania GRP94 (LPG3) is not needed for viability, but rather is involved in virulence of the parasite [18]. A point mutant in LPG3 affects the addition of galactosyl residues during the biosynthesis of lipophosphoglycan, a major surface protein of Leishmania and also has other defects in conjugation of glycans [18].
4. Subcellular Localization
High-resolution microscopy techniques show that GRP94 is confined to the lumen of the ER, or the related sarcoplasmic reticulum of muscle cells [19]. GRP94 is targeted to the ER by its 21 amino acid N-terminal signal sequence, which is cleaved upon entry to the lumen, exposing the DDEV terminus of the mature protein (Fig. 2). GRP94 maintains its residence in the ER by using its C-terminal sequence KDEL, which serves as an ER retrieval signal for the KDEL receptor [20]. The KDEL-mediated retention system is important for the localization of GRP94, because a human variant lacking this signal is secreted rather efficiently, leading to lower steady state levels of GRP94 in the cell (Marzec, Eletto and Argon, unpublished). Nonetheless, the ER retention is not absolute and GRP94 has been reported to escape retention in several cell types. A remarkable exception to the ER localization of GRP94 is its association with the bile salt-dependent lipase (BSDL), also known as cholesterol esterase. This enzyme is secreted by pancreatic acinar cells in a complex with GRP94 and the two remain associated in the pancreatic juice until it arrives at the intestinal lumen [21]. There, the complex is somehow internalized by enterocytes and both molecules are found in endosomes. BSDL, but not GRP94, is eventually deposited on the basolateral membrane of enterocytes. It is not clear whether GRP94 is needed for BSDL uptake or whether it simply protects BSDL from degradation in the duodenal lumen [21, 22]. Secretion of GRP94 (and other ER luminal proteins) by exocrine pancreatic cells [23] and by hepatocytes [24] has also been reported. In all these cases it would be very interesting to elucidate the mechanism that overcomes ER retention. Knowledge of the mechanism also has practical importance, given the potential power of deploying extracellular GRP94 as a peptide-binding vaccine (section 9.4).
Figure 2. Comparison of structural features between GRP94 and HSP90β.
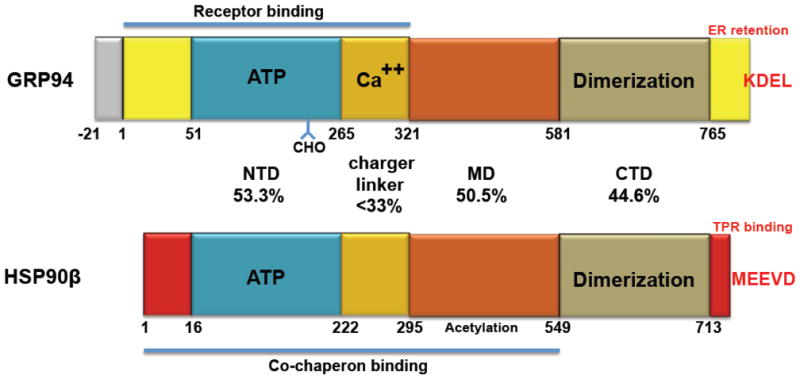
Schematic representation of the domain organization of human GRP94 and human HSP90β. Blue, N-terminal domain (NTD). Beige, charged linker domain. Orange, middle domain (MD). Brown, C-terminal domain (CTD). The similarity between them is indicated by the percent identity indicated for each of the 4 domains. Grey, the signal sequence of GRP94. Yellow and red, unique sequences that distinguish the two members of the family. KDEL, the C-terminal tetrapeptide of GRP94 that serves as the ER retention/retrival ligand for the KDEL receptor [20]. MEEVD, the C-terminal peptide of HSP90, which serves as the target for binding of various TPR containing proteins. ATP, the nucleotide binding site. CHO, the N-glycosylation site of GRP94. Ca++, the known calcium binding activity of the charged linker of GRP94 [92].
A second reported exceptional location is the cell surface. In tumor cell lines and in the myoblast line C2C12 GRP94 is detectable as a surface protein (e.g. [25, 26]), where its peptide binding ability could affect the interaction of tumor cells with the immune system. The physiological significance of surface GRP94 expression remains unclear in the absence of an in vivo tumor model. Another interesting case of surface GRP94 expression is presented by thymocyte development. At a specific, early developmental stage, immature thymocytes express 1–5% of their GRP94 and calnexin on the plasma membrane and as they mature, the ER proteins are again confined to their normal intracellular location ([27] and Argon, unpublished data). The escape from ER localization is perhaps due to masking of ER retention by association with other proteins; in the case of calnexin it was attributed to assembly of partial T cell receptor complexes [27] that perhaps masks ER retention signals. Another possibility is that there is an alternative membrane-spanning form of GRP94 [28], but this had not been confirmed by other studies and GRP94 is highly soluble in the absence of detergents.
A membrane-bound sub-population of GRP94 was proposed to also account for observed interactions between GRP94 and cytosolic proteins. For example, such physical association was proposed to regulate the level of expression of Fanconi Anemia Group C protein [29]. Similarly, the frequent observations of phosphorylation of GRP94 by the cytosolic enzyme casein kinase II [30, 31] could perhaps be due to a membrane- spanning fraction of GRP94.
Another mechanism that could account for the published association with cytosolic proteins is the presence of a distinct cytosolic sub-population of GRP94. Precedents for such a population are provided by observations that two other luminal chaperones, calreticulin and BiP, have minor cytosolic forms. Cytosolic calreticulin could be created because of its inherently inefficient signal peptide, which fails to target a fraction of the molecules into the ER [32]. Cytosolic BiP can be created by alternative splicing that causes alternative translation initiation [33]. Yet a third possible mechanism for cytosolic localization of luminal proteins is retro-translocation through the ER membrane. There is yet no compelling evidence for the existence of a cytosolic population of GRP94 molecules, via any of the abovementioned mechanisms. Such existence would be convincing if the subpopulation of GRP94 would acquire a new property or a new function.
5. Transcriptional regulation
Together with BiP/GRP78, GRP94 is probably best known as the hallmark of the UPR response. It was originally described as a glucose responsive protein whose transcription and translation are induced when the cellular supply of glucose diminishes below physiological levels [5, 34]. When glucose is low within physiological levels, GRP94 expression is actually downregulated [35]. GRP94 has since been shown to respond in the same manner to all types of stresses that affect the ER machinery – perturbations of calcium or redox homeostasis, inhibition of glycosylation, amino acid analogs that likely cause nascent chain misfolding, hypoxia, etc. [36, 37]. Unlike HSP90α and β, GRP94 is not induced by high temperature or other stresses that are unique to the cytosol [36]. Importantly, GRP94 and BiP are induced also by physiological ER stress, for example during differentiation of cells [38–43]. Consequently, the steady state level of GRP94 expression in liver, a professional secretory tissue, is 5 times higher than in skeletal muscle or other non-secretory cells (Argon et al., unpublished data). The main mechanism controlling the expression of GRP94 is transcriptional regulation [17, 36, 37]. Transcription of GRP94 is usually initiated by activation of the signal transduction pathways that inform on changes in physiological conditions in the ER lumen and cause the activation of transcription factors - the Ire-1/XBP-1 pathway, the PERK/eIF2a pathway and the ATF6 pathway (reviewed in [44]).
Sequence analysis shows that the promoter of GRP94 is highly similar to that of other ER stress inducible promoters characterized by the presence of a large number of CCAAT-like motifs flanked by GC-rich regions [45, 46]. These signature features were later characterized as ER stress elements (ERSE) which are cis-acting elements shown to mediate the responses to ER stress [47, 48]. ERSEs consist of an evolutionarily conserved tripartite structure CCAAT(N9)CCACG, where N9 is a GC-rich linker. The CCAAT motif is bound by the multimeric nuclear factor NF-Y, but while this binding is required for GRP promoter activity, it is not sufficient and specificity is conferred by the binding of the UPR transcription factor ATF6 to the 3′ CCACG motif of ERSEs. The 9 bp linker is also functionally important since mutations disrupting a GGC triplet in it lead to loss of stress inducibility [47].
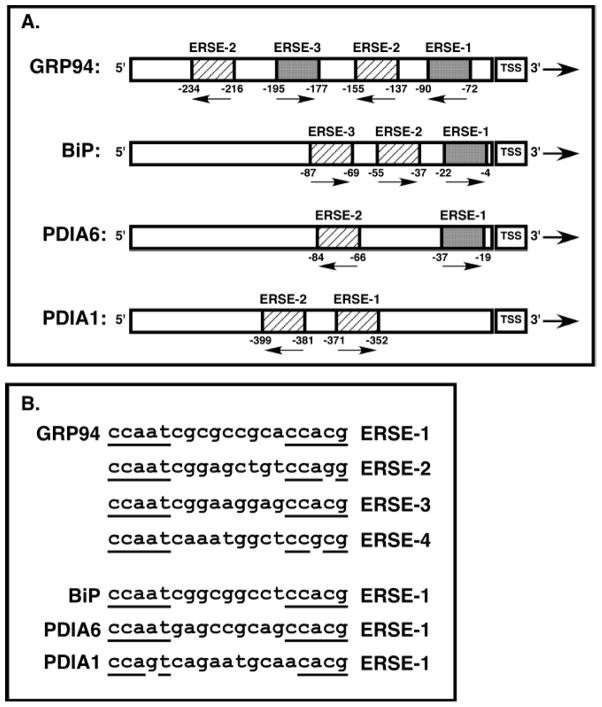
Human GRP94 promoter contains four ERSEs (Fig. 3). Using reporter assays, most of the transcriptional activation in response to tunicamycin was accounted for by ERSE-1 and -3 [47]. This may be due by the fact that binding of ATF6 is profoundly inhibited by mismatches in the CCACG motifs of ERSE-2 and -4 (Fig. 3B).
Figure 3. Comparison of the promoter of GRP94 with other ER chaperone genes.
A. Schematic representation of the promoter of human GRP94, compared to other ER chaperone genes. The ERSEs in each promoter are indicated by boxes and are numbered. Arrows indicate the orientation of the ERSEs whose function is orientation-independent. The numbers below the ERSEs define the bp position relative to the transcription start site (TSS). The dark grey boxes depict perfectly matched ERSEs, and the light grey boxes - imperfectly homologous ERSE, see below. GRP94 promoter has two canonical ERSEs, BiP and PDIA6 have one. In contrast, the PDIA1 promoter of a poorly inducible gene in mammals, has only two mismatched ERSEs. Note: the scheme is not to scale.
B. Sequence comparison of the ERSEs of the indicated genes. The CCACG 3′ motif of ERSE-2 and -4 of the GRP94 promoter have one base mismatch with the consensus sequence. The PDIA1 ERSE-1 contains two mismatches.
The GRP94 promoter can be activated not only by ATF6 but also by another transcription factor that characterizes the UPR, XBP1 [49]. It is not entirely clear what sequences XBP1 binds in mammalian promoters. The ER stress-responsive element UPRE [50], which contains the consensus sequence TGACGTGG/A, is the only element responsible for UPR in yeast, mediated by XBP1. The promoter of GRP94, as well as of many other ER chaperone genes, does not contain a canonical UPRE. However, XBP1 can also bind the CCACG motif in the ERSE (Fig. 3), at lower affinity than ATF6, [50].
The functionality of GRP94 promoter has only been tested in response to chemical inducers of ER stress and clearly, other elements that are yet to be discovered, mediate the transcriptional regulation in other conditions. For example, basal expression of GRP94 and BiP is dependent upon and regulated by a mitogenic pathway distinct from the UPR and probably controls the basal expression needed to assist protein biogenesis in the ER of normal, non-stressed cells [51]. Another example is hypoxia, where GRP94 is the most induced gene [52]. This response is mediated by hypoxia inducible factor 1 via a site that is also distinct from ERSE [52].
There is an interesting transcriptional parallelism between the ER compartment and the cytosol: when HSP90s are inhibited, a significant induction of cytosolic HSP70s, dependent on the transcription factor HSF1, is observed (reviewed in [53]). Similarly, in both worms and cell culture, inhibition or ablation of GRP94 induces the expression of BiP [54](Eletto et al., unpublished data). It should be informative to pursue the physiological significance of this transcriptional connection given that the respective HSP90/HSP70 chaperones do not have the same interactome, but often work in concerted fashion.
6. Posttranslational modifications
The function and action cycle of cytosolic HSP90 are regulated by posttranslational modifications: it is known to undergo phosphorylation on multiple tyrosine, serine and threonine residues, as well as acetylation on lysine residues, and these modifications provide fast and precise mechanisms to regulate its conformation, substrate specificity and activation [55, 56]. Being in the ER, separated from cytosolic kinases, phosphatases, acetylases etc., much less is known about the role of posttranslational modifications in GRP94 activity.
6.1. Glycosylation
The only certain post-translational modification on GRP94 is the attachment of one N-linked carbohydrate attached to Asn196. There are 4 other N-linked acceptor sites in the N-terminal domain (NTD) and the middle domain (MD), but none is apparently used. Likewise, no O-linked glycosylation of GRP94 has been reported. The N-linked glycosylation does not have any known impact on the biological activity of GRP94: even when non-glycosylated, GRP94 associates with Ig light chain [57] and with α1-anti-trypsin (Dersh and Argon, unpublished).
6.2. Phosphorylation
Ser/Thr phosphorylation of GRP94 has been described for the first time by Cala et al. in cardiac sarcoplasmic reticulum [19] and then by Trujillo et al. who ascribed the phosphorylation to casein kinase 2 (CK2) [58, 59]. Phosphorylated GRP94 and CK2 have also been co-purified from Golgi membranes [60–62]. The conceptual problem with these studies is that CK2 is considered to be a cytoplasmic protein with no known ER targeting signal or a transmembrane domain that would explain its association with ER or Golgi complex [62]. A distinct modification, tyrosine phosphorylation of GRP94 by the Src kinase Fyn was reported to occur during myogenic differentiation in C2C12 cells [26]. Evidence was provided, by confocal microscopy, immuno-electron microscopy and cell fractionation, that Fyn indeed co-localizes with GRP94 in the ER. As with CK2, there is no molecular explanation for the localization of a Src kinase in the lumen of the ER, so it is still unclear what is the physiological significance of this Tyr phosphorylation, although it correlates temporally with myocyte differentiation.
Two groups reported that GRP94 possesses autophosphorylating activity [30, 63], but Reed et al argued that it was due to insufficient purification of the protein and was not an authentic GRP94 activity [64].
As discussed in section 4, an alternative explanation is that phospho-GRP94 is a sub-population of the protein that is somehow located in the cytosol, where it could be phosphorylated by soluble kinases. A precedent for this explanation is the cytosolic pool of calreticulin (section 4). On the other hand, unlike calreticulin, GRP94 seems to be targeted to the lumen rather efficiently, is not subject to significant proteasomal degradation [65] and there is no evidence for alternative splicing of exons, all of which argues against the presence of a significant cytosolic population of GRP94.
It is worth noting that other luminal ER proteins were shown to be phosphorylated. GRP58, GRP75 and BiP are phosphorylated in vivo, on their luminal domains [66–68] and importantly, BiP phosphorylation has regulatory function towards its peptide binding capacity [69]. Nonetheless, those reports are difficult to interpret for the same reasons listed above regarding GRP94.
6.3. Acetylation
HSP90 is known to undergo acetylation [70] and this modification has functional significance [71–73]): Acetylation of Lys 294 in the middle domain regulates HSP90 client binding and association with co-chaperones [56]. This Lys is conserved in GRP94, and other Ac-Lys sites are predicted (PHOSIDA – Posttranslational Modification Database), but not demonstrated yet. Recently, the first acetylation of a luminal ER protein, BiP has been reported [74], and shown to be involved in its binding to PERK and subsequent regulation of the unfolded protein response [75]. Furthermore, work by Ko et al. described ER acetyl-CoA:lysine acetyltransferases, ATase1 and ATase2 [76], as well as a membrane transporter, AT-1, that translocates acetyl-CoA into the lumen of the ER [77]. Thus, the machinery for acetylation in the ER is in place and it seems prudent to ask whether acetylation affects GRP94 activity in analogous fashion to its effect on HSP90.
7. Structural Comparison between GRP94 and HSP90
The domain organization of all HSP90 family members is conserved in GRP94s: an N terminal domain (NTD) followed by a charged linker domain, then the middle domain (MD) which is divided to two sub-domains, and finally the C-terminal domain (CTD). Two obvious structural differences are in the N- and C-termini of GRP94 vs. HSP90 (Fig. 2). The first ~50 amino acids of mature GRP94 are distinct from the N terminus of HSP90 in both length and sequence. This implies that the function/conformational change performed by the first 24 residues of cytosolic HSP90, including participation in the dimerization of N-terminal domains after ATP binding and lid closure [78](see section 8.2) are likely distinct in GRP94.
7.1. The N-terminal domain
The distinct N-terminal sequences are followed by highly homologous sequences throughout the NTD. The identity includes all the residues that contact the nucleotide in the binding pocket and those that catalyze ATP hydrolysis. The homology between GRP94 and HSP90 is such that compounds that bind competitively to this site have similar affinity for GRP94 and for HSP90 [79–82]. The NTD belongs to the Bergerat fold of ATP-binding proteins [83, 84] and has the unusual property for an ATPase of similar affinity for either ADP or ATP [82, 85]. In addition, the nucleotide binding site of GRP94 accommodate geldanamycin, radicicol and their derivatives [79, 82, 85–87], albeit at slightly different docking orientations [88]. So far, all known compounds that bind HSP90 also bind GRP94. This is an important feature that is often under-emphasized while interpreting the effects of inhibitors on an HSP90-dependent process. The one exceptional inhibitor is the adenine analogue NECA, which binds specifically to GRP94 and not HSP90s, due to a modified lid over the binding site, formed by a 5 amino acids insertion between helix 4 and 5 that is specific for the GRP94s [82]. Binding of all the geldanamycin or radicicol derivatives to GRP94 has been shown to have important functional consequences. The activity of GRP94 towards clients and its importance during cellular stress are impaired by either compound [79, 89]. This inhibitory activity is mediated by conversion of the protein to a ‘closed’, more compact conformation [80, 90](see section 8 below).
The NTD contains one N-linked glycosylation site at Asn196 (four other similar sites do not appear to be used normally), but the glycosylation is not essential for the biological activity of GRP94 [57].
7.2. The Charged Linker Domain
The second domain of GRP94 is the charged linker, whose structure cannot be resolved by crystallography. This domain is essential for the activity of GRP94, because it mediates conformational changes that, among other things, are needed for ATP hydrolysis [79, 80]. More recently, similar functional importance for the charged linker domain of HSP90 has also been determined both in vitro and in vivo, and it affects interaction with co-chaperones and clients [91]. The charged linker domain is shorter in GRP94s than in HSP90s and is divided more cleanly to an E/D-rich sequence followed by a poly-K stretch (Fig. 4). The former sequence contains 1–2 calcium binding sites whose occupation induces a conformational change that impacts the NTD [92]. Presumably, the HSP90 linker domain does not share this Ca++-binding property because its negatively charged residues are interspersed frequently with lysines, creating sequences that are not conducive to coordination of metal ions.
Figure 4. Comparisons of the linker domains of human HSP90β (hum90β) and GRP94 (hum94).
*, identical residues. :, similar residues. Blue, acidic residues. Red, basic residues. Purple, Asn and Gln. Green, aromatic residues. Orange, aliphatic residues. Brown, Pro. Black, small side chain residues. Strands 8 and 9, which flank much of the charged stretches and bind the domain to the N-terminal domain are indicated, and the Trp zipper domain interaction motif within strand 9 is underlined. The heavily negative sequence in the middle of the GRP94 domain is likely to contain a Ca++ binding site [92]. A human GRP94 allele has Pro300Leu substitution (yellow highlight) (http://browser.1000genomes.org/index.html, rs116891695). This sequence comparison is instructive because a 3D structure for the charged linkers has proven elusive.
The charged linker domain also contains the epitope for an anti-GRP94 monocloncal antibody which is conformation-sensitive [80], indicating that this charged domain is important for the activity of the chaperone. Strands 8 and 9, which flank the charged linker, form an anti-parallel sheet in at least one crystal form [86], and the Trp zipper motif WDWE, in strand 9 was proposed as an interface that communicates with the NTD [93].
7.3. The Middle Domain
The high sequence and structure similarity with HSP90 continues throughout much of the MD. Its N-terminal subdomain forms the split ATPase structure in both GRP94s and HSP90s; it harbors the catalytic loop, which interact with the nucleotide-binding site in the NTD and is needed for ATP hydrolysis [84](Fig. 2) as well as a hydrophobic patch, which like in HSP90 is important for domain interactions. All the functionally important residues in the catalytic loop, the hydrophobic loop and other elements of the M domain in HSP90 are remarkably conserved in the ER paralogue. Other than the residues that interact with the NTD, no functions have yet been mapped to the MD despite the conservation of other residues, that in cytosolic HSP90s mediate contacts with co-chaperones [94].
The next region of diversion follows the MD helix 5. Plant GRP94s have a ~20 residues insertion rich in charged residues, which is missing from the other GRP94s and from the HSP90s. Perhaps this sub-domain mediates divergent functions or protein interactions in the plant and animal ER.
7.4. The C-terminal domain
The main known molecular function of the C-terminal domain (CTD) of GRP94 is to provide a homo-dimerization interface. This is accomplished, as proposed by Nemoto et al., through a pair of interdomain interactions between the MD and CTD [95]. The interface includes four helices - helix 9 from the C-terminus of the MD and helix 2, 4 and 5 of the CTD [95, 96]. The latter two helices correspond to the hydrophobic segment originally identified in [97]. This interface is used constitutively, accounting for the primary cellular form of GRP94 [97]. Detailed examination of the crystal structure reveals that CTD helix 6 from each GRP94 monomer contacts the loop region between helix 3 and strand 3 of the opposite CTD, an interaction that acts as a strap that enforces the dimerization. This is perhaps the reason for the apparent obligatory dimerization of GRP94.
A novobiocin binding site in the CTD of human HSP90α, first identified by Marcu et al. [98, 99] and mapped by Matts et al. [100], centers around the K559KQEEKK564 sequence. In the closed conformation of the CTD, the most frequently crystallized conformation [86], this site in not accessible, indicating that ligand binding to this site is coupled to the conformational equilibrium of the entire protein. Interestingly, this sequence is not conserved in the GRP94 sequences, and plant GRP94s even have a 4 amino acid deletion in this peptide. Despite such sequence divergence, the SPC597 sequence which is adjacent to the novobiocin binding site in the crystal structure [86] and was shown to be part of a conformational control point of HSP90 that impacts the entire dimer [101], is conserved in GRP94s. Because the allosteric regulation of the ATPase cycle and NTD and CTD conformations likely underlie the mode of action of GRP94s and HSP90s (section 7), possible differences between these orthologues should be investigated further.
The protein terminates with a ~ 55 residue acidic region, which is longer by about 20 residues than the corresponding CTD of HSP90, is more continually acidic in GPR94s, lacks the C-terminal RMEEVD sequence of HSP90 and instead encodes the KDEL terminus that specifies ER retention/retrieval. Consequently, GRP94 lacks the motif shared between HSP70 and HSP90 that is essential for their activity [102], because it binds TPR motifs that are present in a number of co-chaperones [103]. Instead, the KDEL peptide is the binding site for an intracellular receptor, which shuttles between the Golgi and the ER and retrieves luminal KDEL proteins that have escaped the ER.
7.5. Where is the client binding site?
The main enigma about GRP94’s structure/function relationship is the identity of the sites for binding other proteins, either clients or co-factors. The ample literature on peptide binding by GRP94 and its use as a peptide presentation device for T cell responses (for reviews see [104, 105]) apparently focuses on the activity of the peptide-binding site in the NTD [106]. However, peptide presentation does not seem to be a major in vivo function of GRP94 [107], and furthermore, while in many other chaperones their peptide binding capacity was a valid surrogate measure of client protein binding activity, the peptide binding activity of GRP94 appears to be distinct from the chaperoning activity [108]. Thus there must be an active site(s) elsewhere in the protein. GRP94 shares this enigma with HSP90s, whose mode of binding clients has also not yet been elucidated. The circumstantial evidence for all family members suggest that clients bind somewhere along the MD and CTD [109, 110]. While mapping of such a site in GRP94 is in progress, none has yet been defined.
8. How does GRP94 work?
8.1. Conformational Equilibrium
GRP94 had long been thought to function in unique manner when compared with its cytosolic homologs. Nonetheless, Agard et al. have shown that it cycles in vitro through an equilibrium of solution conformations that is similar to that seen in Hsp90α and Hsc82 [111]. One main population is in an extended, chair-like conformation with a rotated CTD (relative to the crystal structure). A second, minor population is in a less extended conformation whose CTD orientation is similar to that in the GRP94 crystal structure. Yet a third, low level population is even more closed, a conformation represented by the “twisted V” crystal structures of GRP94 with nucleotides [82, 85, 86, 112]. The extended conformation could allow for a large surface of interaction with client proteins and it is also a conformation that is receptive for nucleotide binding [81]. The addition of nucleotide, and probably of client proteins and co-chaperones, shifts the preexisting equilibrium toward the more closed states, as opposed to determine a specific conformation. The presumption is that GRP94 functions by a ligand-dependent conversion from an inactive to an active conformation [113], though which ligand is activating and what function is activated are questions that are yet to be resolved.
Though GRP94 exist almost exclusively as a dimer in solution, the importance of the dimeric nature is not fully understood, because individual monomers can bind ATP [113] and peptide [80]. It is not yet known if monomeric GRP94 can be an active ATPase or an active chaperone in the cell. Indirect evidence for the importance of the dimeric structure was provided when native subunit interactions in GRP94 were analyzed using chemical cross-linking and mass spectrometry sequencing [96]. In addition to the C-terminal dimerization domain, another intermolecular contact was identified -between the NTD and MD of opposing subunits. This N-to-M domain interaction is found in apo-GRP94 as well as the nucleotide- or geldanamycin-bound states and presumably reflects the more intertwined conformations [96]. Similar interactions were demonstrated to cooperatively stabilize the active site of each monomer in HSP90 [114], reinforcing the importance of the dimeric structure in the biological activity of the family members.
Since GRP94 is a high capacity Ca++ binder and the free Ca++ level in the lumen changes over three orders of magnitude, it is surprising that in vitro Ca++ does not affect the global conformational equilibrium of GRP94 [111] even though a local conformation change is transmitted from the charged linker domain to the NTD [92]. It is also notable that no role has yet been assigned to cysteines in the activity of GRP94, despite the unique redox state of the ER and the fact that all GRP94’s clients are disulfide-bonded proteins.
8.2 Nucleotide binding, hydrolysis and NTD dimerization
While the conformational equilibrium of GRP94 resembles that of the other family members, GRP94’s action cycle is regulated differently from that of HSP90s. One major difference is the response to nucleotide binding: GRP94 adopts the twisted V conformation upon binding either ADP or ATP [86]. The two nucleotides bind to GRP94 with similar affinities and ATP does not become kinetically trapped upon binding, as happens when it binds to the HSP90 NTD. This difference is rooted in the effect that binding of either nucleotide exerts on GRP94’s structure: the “lid” over the nucleotide binding pocket, formed by helices 1,4,5 and their connecting loops, opens upon nucleotide binding, rather than closing and trapping ATP, as in HSP90 [81, 85, 112].
A second aspect of regulation that distinguishes GRP94 from HSP90 is the dimerization of NTDs. In HSP90, the changes in the conformation of NTD elements during the nucleotide cycle couple the activity of the chaperone to transient clamp-like dimerization of the NTDs, primarily via helix N1 [78, 94, 115, 116]. GRP94 also requires NTD dimerization for nucleotide hydrolysis, because mutants of GRP94 lacking strand N1, or strand N1 and helix N1, were defective in ATP hydrolysis, like the corresponding mutants in HSP90 [86]. In addition, the crystallized isolated NTD in a nucleotide-bound state has a conformation that leads to N-domain dimerization [86]. However, in contrast to HSP90, the twisted conformation of GRP94 keeps the structural elements necessary for dimerization of the two NTDs apart, on opposite sides of the dimer [86], so to achieve a catalytically active conformation, GRP94 must rotate the two NTDs about the N-M and M-C junctions. Since the isolated NTDs do not change their conformation in response to ATP or ADP, perhaps the impetus for NTD dimerization in GRP94 is provided by binding of clients or co-chaperones, linking protein-protein interactions to ATP hydrolysis.
It is still not known how the opening of the lid in GRP94 and the rotations of the NTD regulate the action cycle of GRP94 mechanistically. They may be related to the ability of each monomer of GRP94 to cycle between the open and closed conformations independently of each other [113], which would provide a mechanism for sensing nucleotides’ level in the lumen.
8.3. ATPase activity
The hydrolytic activity of GRP94 had been controversial for years, but is now known to be as efficient as other HSP90s [81, 86]. In fact, there is a larger difference in the Km and Kcat of the respective ATPase activities between yeast and human HSP90 than between yeast HSP90 and mammalian GRP94 [81, 86]. The disparity in the inherent catalytic rates between the two paralogues is due to sequence differences in the NTDs, as shown by the kinetic analysis of the Hsp82/GRP94 chimeric enzymes [81, 86]. Importantly, ATP hydrolysis is essential for GRP94’s activity in cells [108], at least with respect to chaperoning insulin-like growth factors.
8.4. Co-chaperones
A third potential difference with cytosolic HSP90s is the interactions with co-chaperones. Many well-characterized molecules serve as specific HSP90 co-chaperones, including HOP, p23, AHA1, CDC37, FKBP-52 and UNC45, to facilitate client protein folding [109, 117, 118]. These helper proteins clearly affect the conformational equilibrium of Hsp90 and assist in the loading of client proteins and in the regulation of the chaperone cycle. Unlike its cytosolic homologs, GRP94 has no known co-chaperones. These negative data in combination with the alternative conformation seen in the crystal structure may suggest a unique conformational and functional cycle for GRP94. Alternatively, it is possible that interactions with co-chaperones have not yet been defined because techniques to uncover protein-protein interactions in the ER are not yet sophisticated enough.
Recently CNPY3 (PRAT4A) has been shown to be necessary for proper folding of Toll-like receptors [119, 120](section 9.1). CNPY3 has many characteristics of a GRP94 co-chaperone: it is needed during TLR loading on GRP94; it interacts with GRP94 in nucleotide-dependent manner; it is neither a chaperone for GRP94, nor a client. Remarkably, unlike the co-chaperones that impact the general action cycle of HSP90, CNPY3 is a client-specific co-chaperone, involved in TLR but not integrin biogenesis [119, 120]. This finding suggests that the possibility that the conformational state of GRP94 varies when it is engaged with different clients, a possibility that is also consistent with the structural data in [86]. It would be interesting to determine how CNPY3 impacts the action cycle of GRP94, how it affects the ATPase activity of GRP94 and how it modulates the client specificity or conformational state of the chaperone.
ASNA1 (TRC40/ARSA/GET3) is an ATPase required for the post-translational delivery of tail-anchored (TA) proteins to the ER [121]. At the same time, it was also discovered to be a necessary factor for insulin secretion [122]. Recently, it was proposed that activity of ASNA-1 is dependent on the presence of active GRP94 in the cell, since simple overexpression of ASNA-1 does not restore insulin secretion in GRP94 KO cells. In addition ASNA-1 has been shown to be able to associate with GRP94 by yeast two-hybrid and GST pull-down techniques (G. Kao, B. Natarajan, P. Naredi, Personal communication).
OS-9, a third co-factor of GRP94, is not client-specific, but rather dedicated to a biological process: targeting misfolded proteins for ER-associated degradation (section 9.3). Its expression does not depend on GRP94 activity and mutant GRP94 with or without ATPase activity still bind to OS-9, unlike client proteins (Dersh and Argon unpublished data). OS-9 has a mannose receptor homology domain which endows it with carbohydrate-sensing capability [123]. OS-9 itself can distinguish between folded and misfolded conformations of proteins, apparently using polypeptide-based cues to bind glycoproteins [124–126]. Thus, OS-9 may be a co-chaperone that directs the activity of GRP94 towards quality control.
8.5. Activity in cells
The individual biochemical activities of GRP94 (ATP hydrolysis, conformational changes, calcium and peptide binding) provide important molecular information, but to determine how they affect the global in vivo activity of the chaperone, a cellular assay that depends on chaperone activity of GRP94 toward IGF has been developed [16](section 9.1). As shown in Fig. 5, varying GRP94 mutants have been tested and the ability to discriminate between wild type, hypomorphic and inactive versions of the chaperone has been proven. Existence of mutants that seems to have modulatory impact on activity of GRP94 also raises the possibility that such an assay could be used in testing the impact of posttranslational modifications on activity; for comparisons of variants of GRP94, whether as a result of site directed mutagenesis or naturally occurring ones and co-chaperone binding (see sections 6 and 8.4).
Fig. 5. An IGF-dependent cellular assay for the function of GRP94 variants.
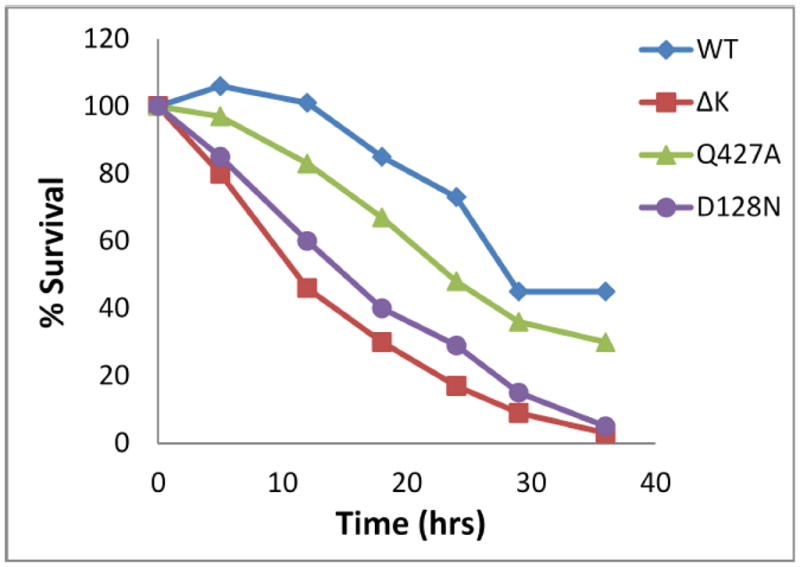
Differential survival of grp94−/− cells in serum-free medium when transiently expressing GRP94-GFP chimeric constructs. WT GRP94-GFP confers the highest survival. ΔK is an in-frame deletion of amino acids 144–488 that is a chaperone-dead negative control. The point mutant D128N is incapable of binding ATP [170] and Q427A is partially deficient in ATP hydrolysis in vitro [86]. This figure is adapted from [108].
9. What does GRP94 do?
9.1. Chaperones protein folding
The most important activity of GRP94 is as a molecular chaperone that directs folding and/or assembly of secreted and membrane proteins. Unlike other ubiquitous luminal chaperones, however, GRP94 is a rather selective chaperone whose client list is much shorter than that of other ubiquitously expressed ER chaperones, like BiP or calreticulin. Many plasma membrane receptors and secreted products, like transferrin receptor and MHC class I proteins, do not require GRP94’s activity for proper folding and traffic to the cell surface, as demonstrated by knockout and knock-down cells (e.g. [15]). Those clients that are not only found in association with GRP94, but also require this association for proper expression are listed in Table II. Listed separately are examples of the many proteins for which there is only association data with GRP94 without other criteria. This distinction is important, because association could arise simply because GRP94 is an abundant luminal constituent and because it is known to form complexes with many proteins, particularly when a mutation causes accumulation in the ER lumen of such itinerant proteins. Thus, a functional consequence of the association with the chaperone is an important criterion.
Table II.
Clients and putative substrates of GRP94.
| Client Protein | Method | Topology | Size (kD) | Binds to | Reference |
|---|---|---|---|---|---|
| MHC class II | IP a, functional b | membrane | ~28K | Unassembled | [105] |
| IgH | IP, KO c, KD d | membrane and soluble | 50–80 | V and C domains | [106] |
| IgL | IP, KO, KD | Soluble | 25 | V domain | [153] |
| BSDL (CEL) | IP, functional | Soluble | 67 | [21] | |
| Toll-like Receptors | KO, functional | membrane | ~90 | [15, 113] | |
| Integrins | IP, KO, functional | membrane | ~150 | [15, 113] | |
| Platelet glycoprotein Ib-IX-V complex | IP, KO, functional | membrane | 24 | GPIX subunit | [115] |
| Thyroglobulin | IP, functional | Soluble | 660 | Region I | [154] |
| Insulin-like growth factor –I | IP, KO, KD | Soluble | 7.5 | Mature domain | [120, 155] |
| Insulin-like growth factor –II | IP, KO, KD | Soluble | 7.5 | Mature domain | [16] |
| Apo B | IP, pulse-chase | 90 | [156] | ||
| Collagen | IP, functional | Soluble | 140 | [157] | |
| Clavata (plants) | KO | 10 | [11] | ||
| p75 (Leishmania) | IP, functional | membrane | 75 | [11] | |
| Others | |||||
| Protein C | IP | Soluble | 52 | [158] | |
| α1-antitrypsin (mutant) | IP | Soluble | 51 | [159] | |
| Thrombospondin (TSP1) | IP | Soluble | 120 | [160] | |
| HSV-1 glycoprotein B (mutant) | IP | membrane | >130 | [161] |
IP, immunoprecipitation;
Functional, assembly or traffic or activity of the protein + GRP94;
KO, knockout;
KD, knockdown
The first GRP94 clients discovered were from the immunoglobulin (Ig) family: folding intermediates of either unassembled MHC class II proteins [127] or Ig heavy or light chains [128], are complexed with GRP94. GRP94 binds to L chain via their Ig-fold variable domains and this feature also illustrates its relative selectivity, because BiP binding peptides have been demonstrated in both the V and C domains of this client [129, 130]. The interaction with GRP94 is functionally important, because secretion of light chains is inhibited when GRP94 is ablated [131]. On the other hand, Li et al. showed that serum Ig levels are not depleted in mice whose GRP94 is deleted in B cells [132], suggesting that there is a by-pass quality control mechanism that enables secretion without GRP94. However, GRP94 is important for lymphocyte development, as in its absence neither B nor T cell lineages can progress beyond an early stage [133].
A second known client of GRP94 is BSDL. As discussed above (section 4), Lombardo et al. showed that BSDL is bound to GRP94 and other, unidentified proteins, and this complex is trafficked together and is secreted from pancreatic cells [21, 134].
Other clients of GRP94 are Toll-like receptors and integrins, [15, 135], which are oligomeric transmembrane proteins. Interestingly, not all TLR or all integrins require chaperoning by GRP94: surface expression of β1 integrins does not require GRP94, while others do [136]. Among TLRs, both family members that are expressed on the cell surface and those that are intracellular require GRP94’s action [135]. The deficiency in TLR expression leads to inefficient innate immunity to select pathogens [15]. In neither the integrin nor the TLR clients has the precise binding site for GRP94 been mapped. Drosophila gp93, when expressed in mammalian cells, can compensate for the loss of mammalian GRP94 in supporting surface expression of several integrins and TLRs [136]. Thus, as expected from the structural homology, there is considerable functional conservation among the GRP94s during evolution. The remarkable insight derived from the functional complementation of clients by orthologues is that despite the evolutionary distance between Toll and mammalian TLRs (~15% identity) or between fly and mammalian integrins (~23% identity) the GRP94s from either can chaperones these clients. There must, therefore, be a common action cycle for GRP94 when it chaperones all proteins, even though we know little of the details of such action cycle.
The platelet glycoprotein Ib-IX-V complex is an oligomeric receptor for von Willebrand factor and its absence leads to impairment of platelets function. GRP94 binds selectively to the GPIX subunit and not to the other two [137]. GRP94 is an obligate chaperone for this glycoprotein complex and without it, there is no surface expression and enhanced ERAD of the complex.
Thyroglobulin is a very large, multi-domain protein that binds GRP94 as well as many other chaperones [138]. Region I of thyroglobulin is a block of 1191 residues that is comprised of ~10 repeating, disulfides-rich units. Conformational maturation of region I is a limiting step in thyroglobulin folding. This region contains binding sites for both GRP94 and BiP [139]. Mutations causing hypothyroidism are known to induce local/regional misfolding or to impede interactions between protein domains that are required for thyroglobulin secretion. Such mutants show higher avidity to GRP94 and to other chaperones [140, 141].
Among the known clients, insulin-like growth factors are distinct both in their small size and their monomeric assembly state. Both insulin-like growth factor-I and – II interact with GRP94 and in the absence of the chaperone do not mature and are not secreted [13, 16]. It is notable that IGF-I is N-glycosylated, while IGF-II is not, yet both depend on GRP94 for their traffic to the Golgi complex, where they are processed proteolytically. Preliminary studies suggest that GRP94 binds to an epitope within the 67 amino acid secreted form of the IGFs (unpublished data). GRP94 is an obligate chaperone for IGF biosynthesis and inhibition of GRP94 activity abrogates its secretion and subsequent IGF receptor signaling. In this respect, the only other known client that requires GRP94 stringently is the platelet glycoprotein complex, whereas most other client proteins (e.g. the Ig superfamily proteins) display a leakier, less stringent dependence on GRP94. The requirement of IGF for GRP94 has profound physiological consequences, since for example, mice whose GRP94 is depleted specifically in the musculature are considerably smaller and have lower IGF levels [142].
Table II illustrates a major enigma of GRP94 – it is not clear what features it could recognize in proteins, because apart from being disulfide-bonded proteins, there is no obvious common denominator among the listed clients. They represent different folds, from the Ig fold to the LRD fold, from all-beta sheet domains to helix-rich domains, monomeric and oligomeric proteins and both single domain and multi-domain clients.
The activity of chaperones in protein folding and in resolving protein aggregation is often considered redundant or non-essential, partly because in many cases folding can proceed even without chaperones altogether, and partly because anti-aggregation activity can be performed by multiple molecular chaperone without any obvious client protein specificity. Furthermore, often there are homologous chaperones in a given folding compartment (for example, yeast has 4 cytosolic HSP70s; mammals have 2 cytosolic HSP90s). Nonetheless, inhibiting or ablating chaperone activities by genetic means shows quite a different picture: many chaperones are essential and provide unique, non-overlapping functions in vivo, even if in vitro they are not essential.
Ablation of GRP94 can be accomplished either pharmacologically or genetically. Many published studies employ the small molecule inhibitors geldanamycin and radicicol (or their derivatives) to inhibit HSP90s and the processes that depend on them. It is important to note that all current such inhibitors target GRP94 just as efficiently as they do HSP90 and bind to the nucleotide-binding site with similar affinities [e.g. [81]]. Since the client spectra of HSP90s and GRP94 are totally non-overlapping, the interpretation of their effects is practically impossible. This point is particularly relevant in the design and/or evaluation of HSP90 inhibitors in clinical trials [143, 144].
9.2. Calcium binding
The activity of GRP94 as a luminal Ca++ binding protein has long been appreciated [4, 6]. Like the other abundant luminal proteins, GRP94 is a low-affinity, high-capacity calcium binding protein, that is estimated to have 15 calcium-binding sites, 4 with moderate affinity (KD~2 μM) and 11 with low affinity (KD~600 μM) [4]. Since there are no “EF hand” binding motifs in the sequence, the Ca++ binding sites are likely to be clusters of negatively charged residues. Several acidic regions are distributed throughout the sequence of GRP94 and are distinct from the homologous regions in HSP90s (Fig. 2). One or two of the higher affinity Ca++ binding sites map to the charged linker domain that follows the N-terminal ATPase domain [92]. Whether the Ca++ binding capacity of GRP94 is essential in vivo is not yet established and it is possible that this function of GRP94 is redundant with other proteins. For example, deletion of GRP94 in skeletal muscle does not lead to an obvious defect in Ca++ homeostasis, presumably because calsequenstrin-1 provides an even larger pool of Ca++ binding in the sarcoplasmic reticulum, which is capable of compensating for GRP94’s loss [142]. Given the abundance of calsequenstrin-1 in the sarcoplasmic reticulum of fast twitch muscle fibers [145, 146] and given that each calsequestrin molecule can bind twice the Ca++ ions as GRP94, calsequestrin has a much higher Ca++ binding capacity and could mask the loss of GRP94 in this respect.
In addition to its apparent function as a calcium carrier, Ca++ binding regulates GRP94 activity. Free Ca++ at 100 nM, within the physiologically relevant range, causes a conformational change, as measured by decreased helical content of GRP94 [4]. Indications that the charged linker itself undergoes conformational changes are found by comparisons of the various crystal structures of GRP94, but unfortunately, no specific structural information is available about this domain. Ca++ binding at physiological concentrations was shown to modulate the peptide binding activity of GRP94 [92]. However, so far all the known functional and structural changes only derive from in vitro experiments. Even though thapsigargin, an inhibitor of the SERCA calcium pumps, induces the expression of GRP94 in cells and tissues, there is yet no direct functional evidence in vivo that calcium affects GRP94 activity.
9.3. Roles in quality control
Cells exercise control over the quality of secretory proteins both by promoting their proper folding and by detection and disposal of misfolded molecules. The disposal of proteins that misfold in the lumen of the ER occurs by degradation by the proteasomes, thus misfolded proteins have to be retro-translocated from the lumen to the cytosol. Many components target misfolded proteins, remove them from the ER, tag them with ubiquitin and direct them to proteasomal degradation, and these components are known collectively as the ER-associated degradation (ERAD) machinery. A major unresolved question of much current interest is the nature of the machinery that triages misfolded from native proteins and selects the former for translocation. More and more data point to a connection between the folding machinery and the retro-translocation machinery. Most directly, mutant α1-antitrypsin that is usually degraded by ERAD is not degraded when GRP94 expression is ablated [124]. GRP94 cooperates with the lectin OS-9 (section 8.4), which somehow binds to GRP94 and delivers the mutant α1-antitrypsin to the Hrd-1 ubiquitin ligase complex in the ER membrane. Both components are needed for this delivery, as ablation of OS-9 also inhibits degradation [124, 125]. It is likely that other chaperones, like BiP/GRP78, and other lectins, like XTP3 also mediate triaging of ERAD substrates [147]. Furthermore, since ERAD of cholera toxin A1 subunit does not involve binding of GRP94, but rather is sensitive to ablation of the cytosolic HSP90, the GRP94-OS-9 complex is not used by all ERAD substrates and its selectivity will need to be elucidated by future studies.
9.4. Peptide binding
There is ample literature about the peptide-binding activity of GRP94 in the context of stimulating T cells to respond to foreign antigens and enhance immunity. GRP94 has been independently identified as gp96, a protein that serves as a potent stimulator of anti-tumor activity [148] and later discovered to possess this immunological activity because it binds peptides, not because its own sequence is mutated in cancers [149]. This led to extensive investigation of the role of GRP94 in “cross-priming” - the process by which dendritic cells capture peptides derived from other cells and then present them to, and activate naive CD8+ T cells ([150] and reviewed in [151, 152]). This pathway of peptide presentation is believed to play an important role in the generation of antiviral and antitumor responses [151, 152]). Most of the studies on cross-priming via GRP94 use extracellular GRP94, either purified or recombinant protein, that is either loaded with a complex mixture of peptides in the tissue of origin, or is deliberately loaded with the desired antigenic peptide. Presentation of peptides via GRP94 is not limited to viral peptides, and a number of cellular peptides, including some derived from oncogenes have been shown to stimulate cellular immunity effectively using this route. It is known that the GRP94-peptide complex is taken up by dendritic cells and other ‘professional’ antigen presenting cells by a process of receptor-mediated endocytosis, which relies on a number of scavenger receptors, including CD91 [153, 154]. The peptide is separated from GRP94 and transferred on MHC class I molecules [104, 155], which then display it secondarily on the cell surface, where it can be recognized by the T cell receptors of the appropriate T cells. There is little doubt that in such scenario potent activation of T cell immunity is possible and when optimized, can be a powerful therapeutic tool [151]. Several molecular aspects of the GRP94-dependent cross-priming process are still not clear, such as why cross-priming is primarily an MHC class I/CD8+ T cell process, even though the peptide binding specificities of GRP94 and MHC class I are quite different.
While immunization with GRP94-peptide complexes to achieve cross-priming has been shown to be powerful in lab models as well as in clinical trials, the physiological role of peptide presentation by GRP94 has been controversial and recent studies argue against such a physiological immune function. We have shown that a mutant GRP94 with a deficient peptide-binding capacity can still mediate the chaperone function [108], thus uncoupling the two activities. More directly, Yewdell et al. showed that cells whose expression of GRP94 is silenced demonstrate no reduction in their abilities to cross-prime antiviral CD8+ T cell responses in vivo [107].
9.5. Roles in ER stress
As discussed in section 5, GRP94 and BiP/GRP78 are canonical hallmarks of the UPR response, in both physiological and pathological contexts. The similarity of their promoters explains at least in part why these two, and other ER stress genes are co-induced. As noted above, GRP94 and BiP are co-induced also by physiological ER stress, for example during differentiation of hepatocytes, acinar cells or B cells [38–43]. Signals that induce such differentiation into professional secretory cells rely on the same signal transduction machinery as the stress responses, and this usage of ER stress signaling in differentiation makes physiological sense, because the major object of such differentiation program is to up-regulate the secretory machinery, including the ER protein folding machinery.
GRP94 is connected to ER stress responses also through its chaperoning of clients, for example insulin-like growth factors (IGFs). Like GRP94 itself, mammalian IGF-I and -II are targets of the ER stress response at the transcriptional level [156]. The up-regulation of GRP94 levels during the UPR augments the increased transcription by promoting the folding of signaling-competent IGF-I and –II [16]. Together, the two effects lead to increased hormone production under stress conditions enables cells to initiate the IGF signaling pathway, which is used to cope with metabolic stresses. Furthermore, administration of IGF-I together with other inducers of ER stress significantly potentiates the UPR, likely boosting the folding capacity of the compartment, as needed to alleviate the initial stress signal [157]. The cellular outcome of this two-way relationship is promotion of recovery from stress, augmentation of nutrient sensing and protein translation mechanisms and suppression of apoptosis [158, 159].
GRP94 (as well as some other ER resident proteins) is often found in multiple tumors as a highly over-expressed protein and many publications imply it as a candidate marker for monitoring tumor progression [160, 161]. This line of work points to the exciting relation between ER stress and cancer, a relation whose underlying mechanisms should be investigated further.
9.6. Interactions with other ER chaperones
Both protein folding and quality control require coordinate activity of many ER components. The folding of many secretory proteins, particularly the multi-domain oligomeric ones, requires more than one chaperone, presumably because there are multiple bottlenecks in each folding pathway. Because different chaperones recognize their clients based on distinct structural features (e.g. linear peptides, carbohydrates, disulfide bonds), they often work either in parallel or in sequence through the assembly of transient complexes [162, 163]. GRP94 and BiP are often reported to associate with secretory proteins (e.g. [138]) and sometimes at a consistent stoichiometry, implying such transient complexes. During the folding of Ig, GRP94 and BiP work sequentially, with BiP binding preferentially to earlier folding intermediates and GRP94 – to later intermediates. The two chaperones also form a ternary complex with the folding client, presumably to transfer it from BiP to GRP94 [128, 164]. This sequence of interaction fits well with the broad peptide specificity of BiP and the more selective recognition by GRP94. Apparently, GRP94 does not always work in concert with BiP, since GRP94 association is detected during the biosynthesis of IGF without a corresponding interaction with BiP. Interestingly, GRP94 has not been reported to act in concert with calnexin or calreticulin, suggesting that there is functional separation between sets of chaperones, perhaps based on the rules proposed by Molinari [165].
The coordination of ER folding factors is most apparent in the massive UPR transcriptional activation, where expression of components of the protein folding and degradation machinery is coordinately up-regulated. Such orchestrated activation was first discovered by Lee et al, using pharmacological treatments as UPR inducers [46, 166, 167], and then shown to be relevant for physiological processes, such as terminal plasma cell differentiation [38, 40]. The coordinate transcriptional regulation of GRP94, BiP, PDI and other components of the machinery, together with their biochemical functions suggest that they form a functional network that both increases the efficiency of folding and reduces the chances of misfolded proteins escaping. Such an ER network would be equivalent to the cytosolic folding factors that are known to work in concert by forming dynamic complexes [162, 163].
One prediction of the putative quality control network is that depletion of one chaperone would affect either the activity or the level of expression of another. Indeed, several studies address this prediction. In C. elegans, there is compensatory and mutual regulation among nine ER chaperones, including BiP (hsp4) and GRP94 (T05E11.3) [54]). Suppression of GRP94 by RNAi activates the IRE-1/XBP-1 branch of the UPR and induces BiP expression. BiP is also induced when C. elegans E3 ubiquitin ligase HRD-1 is silenced [168], showing that components of the ERAD pathway are integral parts of the quality control network. Such compensatory relationship extends to mammalian cells: knockdown or inactivation of BiP induces the expression of GRP94, PDI, calreticulin and ERp57, and silencing of calreticulin stimulates the expression of GRP94 and PDI [169]. Calreticulin-null embryonic fibroblasts also show constitutively higher level of GRP94 and other ER proteins (Eletto and Argon, unpublished). On the other hand, this phenomenon is not universal: GRP94-null cells do not display increased expression of other chaperones [13]. Thus, there is conserved regulatory relationship, whereby the loss of some quality control components leads to compensatory upregulation of others, but the effects are not always mutual.
10. Conclusions
GRP94 has long been among the more enigmatic chaperones. Despite many similarities to HSP90, not long ago it was still not known whether it is an ATPase, how it binds clients and whether it has co-chaperones. In addition, since it is not necessary for the function of yeast ER, it was not clear what important functions lead to its ubiquitous expression, abundance and sequence conservation. Much progress in recent years has started uncovering parts of the mystery. The expansion of the known client spectrum show that GRP94 is a general chaperone. The abilities to knockout and knockdown its expression have uncovered clients for which GRP94 interaction is obligatory and shown that it is an essential chaperone in the development of metazoans as well as for the functions of several cell types and tissues. The development of cell-based assays (Fig. 5) have started to reveal the critical molecular activities of the protein, enabled teasing them apart and relating the in vitro activities of GRP94 domains to its overall in vivo actions. These advances revealed that GRP94 acts more similarly to HSP90 than had been thought previously, and the discovery of the first co-chaperones, albeit different types of factors than those found in the cytosol, suggests that the proteome associated with GRP94 may soon be characterized. Some of the important remaining challenges are to identify the client binding site of the chaperone, determine how it affects the folding process and elucidate how the ER variations on the HSP90 action cycle lead to recognition, binding and release of client proteins.
Highlights.
GRP94 is an endoplasmic reticulum resident chaperone that belongs to HSP90 family
GRP94 chaperones secreted and membrane proteins
GRP94 is essential in development and physiology of multi-cellular organisms
GRP94 shares biochemical features with HSP90 but displays distinct activities
Acknowledgments
We thank Dr. J. Kim (Univ. Penn) for help with phylogenetics and performing the bootstrapping analysis, D. Dersh, E. Barton and K. Gao for communicating unpublished data, and O. Ostrovsky for careful reading of the manuscript. Work in the authors’ lab was supported by NIH grants AG18001, AG040153 and GM07748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 2.van der Vlies D, Makkinje M, Jansens A, Braakman I, Verkleij AJ, Wirtz KW, Post JA. Oxidation of ER resident proteins upon oxidative stress: effects of altering cellular redox/antioxidant status and implications for protein maturation. Antioxid Redox Signal. 2003;5:381–387. doi: 10.1089/152308603768295113. [DOI] [PubMed] [Google Scholar]
- 3.Sevier CS, Kaiser CA. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta. 2008;1783:549–556. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Van PN, Peter F, Soling HD. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J Biol Chem. 1989;264:17494–17501. [PubMed] [Google Scholar]
- 5.Lee AS. The accumulation of three specific proteins related to glucose-regulated proteins in a temperature-sensitive hamster mutant cell line K12. J Cell Physiol. 1981;106:119–125. doi: 10.1002/jcp.1041060113. [DOI] [PubMed] [Google Scholar]
- 6.Koch G, Smith M, Macer D, Webster P, Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. J Cell Sci. 1986;86:217–232. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava PK. Peptide-binding heat shock proteins in the endoplasmic reticulum: role in immune response to cancer and in antigen presentation. Adv Cancer Res. 1993;62:153–177. doi: 10.1016/s0065-230x(08)60318-8. [DOI] [PubMed] [Google Scholar]
- 8.Stechmann A, Cavalier-Smith T. Phylogenetic analysis of eukaryotes using heat-shock protein Hsp90. J Mol Evol. 2003;57:408–419. doi: 10.1007/s00239-003-2490-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen B, Zhong D, Monteiro A. Comparative genomics and evolution of the HSP90 family of genes across all kingdoms of organisms. BMC Genomics. 2006;7:156. doi: 10.1186/1471-2164-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baviskar SN, Shields MS. RNAi silenced Dd-grp94 (Dictyostelium discoideum glucose-regulated protein 94 kDa) cell lines in Dictyostelium exhibit marked reduction in growth rate and delay in development. Gene Expr. 15:75–87. doi: 10.3727/105221611x12973615737587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiguro S, Watanabe Y, Ito N, Nonaka H, Takeda N, Sakai T, Kanaya H, Okada K. SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J. 2002;21:898–908. doi: 10.1093/emboj/21.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maynard JC, Pham T, Zheng T, Jockheck-Clark A, Rankin HB, Newgard CB, Spana EP, Nicchitta CV. Gp93, the Drosophila GRP94 ortholog, is required for gut epithelial homeostasis and nutrient assimilation-coupled growth control. Dev Biol. 2010;339:295–306. doi: 10.1016/j.ydbio.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wanderling S, Simen BB, Ostrovsky O, Ahmed NT, Vogen S, Gidalevitz T, Argon Y. GRP94 is essential for mesoderm induction and muscle development because it regulates IGF secretion. Mol Biol Cell. 2007;18:3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audouard C, Le Masson F, Charry C, Li Z, Christians ES. Oocyte-targeted deletion reveals that hsp90b1 is needed for the completion of first mitosis in mouse zygotes. PLoS One. 2011;6:e17109. doi: 10.1371/journal.pone.0017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 16.Ostrovsky O, Ahmed NT, Argon Y. The chaperone activity of GRP94 towards of insulin-like growth factor II is necessary for the stress response to serum deprivation. Mol Biol Cell. 2009;20:1855–1864. doi: 10.1091/mbc.E08-04-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci. 2001;26:504–510. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 18.Descoteaux A, Avila HA, Zhang K, Turco SJ, Beverley SM. Leishmania LPG3 encodes a GRP94 homolog required for phosphoglycan synthesis implicated in parasite virulence but not viability. EMBO J. 2002;21:4458–4469. doi: 10.1093/emboj/cdf447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cala SE, Jones LR. GRP94 resides within cardiac sarcoplasmic reticulum vesicles and is phosphorylated by casein kinase II. J Biol Chem. 1994;269:5926–5931. [PubMed] [Google Scholar]
- 20.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 21.Bruneau N, Lombardo D, Bendayan M. Participation of GRP94-related protein in secretion of pancreatic bile salt-dependent lipase and in its internalization by the intestinal epithelium. J Cell Sci. 1998;111:2665–2679. doi: 10.1242/jcs.111.17.2665. [DOI] [PubMed] [Google Scholar]
- 22.Nganga A, Bruneau N, Sbarra V, Lombardo D, Le Petit-Thevenin J. Control of pancreatic bile-salt-dependent-lipase secretion by the glucose-regulated protein of 94 kDa (Grp94) Biochem J. 2000;352(Pt 3):865–874. [PMC free article] [PubMed] [Google Scholar]
- 23.Takemoto H, Yoshimori T, Yamamoto A, Miyata Y, Yahara I, Inoue K, Tashiro Y. Heavy chain binding protein (BiP/GRP78) and endoplasmin are exported from the endoplasmic reticulum in rat exocrine pancreatic cells, similar to protein disulfide-isomerase. Arch Biochem Biophys. 1992;296:129–136. doi: 10.1016/0003-9861(92)90554-a. [DOI] [PubMed] [Google Scholar]
- 24.Peter F, Nguyen Van P, Soling HD. Different sorting of Lys-Asp-Glu-Leu proteins in rat liver. J Biol Chem. 1992;267:10631–10637. [PubMed] [Google Scholar]
- 25.Altmeyer A, Maki RG, Feldweg AM, Heike M, Protopopov VP, Masur SK, Srivastava PK. Tumor-specific cell surface expression of the-KDEL containing, endoplasmic reticular heat shock protein gp96. Int J Cancer. 1996;69:340–349. doi: 10.1002/(SICI)1097-0215(19960822)69:4<340::AID-IJC18>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Frasson M, Vitadello M, Brunati AM, La Rocca N, Tibaldi E, Pinna LA, Gorza L, Donella-Deana A. Grp94 is Tyr-phosphorylated by Fyn in the lumen of the endoplasmic reticulum and translocates to Golgi in differentiating myoblasts. Biochim Biophys Acta. 2008;1793:239–252. doi: 10.1016/j.bbamcr.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Wiest DL, Burgess WH, McKean D, Kearse KP, Singer A. The molecular chaperone calnexin is expressed on the surface of immature thymocytes in association with clonotype-independent CD3 complexes. EMBO J. 1995;14:3425–3433. doi: 10.1002/j.1460-2075.1995.tb07348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzarella RA, Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94) J Biol Chem. 1987;262:8875–8883. [PubMed] [Google Scholar]
- 29.Hoshino T, Wang J, Devetten MP, Iwata N, Kajigaya S, Wise RJ, Liu JM, Youssoufian H. Molecular chaperone GRP94 binds to the Fanconi anemia group C protein and regulates its intracellular expression. Blood. 1998;91:4379–4386. [PubMed] [Google Scholar]
- 30.Csermely P, Miyata Y, Schnaider T, Yahara I. Autophosphorylation of grp94 (endoplasmin) J Biol Chem. 1995;270:6381–6388. doi: 10.1074/jbc.270.11.6381. [DOI] [PubMed] [Google Scholar]
- 31.Miyata Y, Yoneda Y, Yahara I. Stimulation of CK2-dependent Grp94 phosphorylation by the nuclear localization signal peptide. Mol Cell Biochem. 2010 doi: 10.1007/s11010-011-0944-9. [DOI] [PubMed] [Google Scholar]
- 32.Shaffer KL, Sharma A, Snapp EL, Hegde RS. Regulation of protein compartmentalization expands the diversity of protein function. Dev Cell. 2005;9:545–554. doi: 10.1016/j.devcel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Ni M, Zhou H, Wey S, Baumeister P, Lee AS. Regulation of PERK signaling and leukemic cell survival by a novel cytosolic isoform of the UPR regulator GRP78/BiP. PLoS One. 2009;4:e6868. doi: 10.1371/journal.pone.0006868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shiu RP, Pouyssegur J, Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteins in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci USA. 1977;74:3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spindler SR, Crew MD, Mote PL, Grizzle JM, Walford RL. Dietary energy restriction in mice reduces hepatic expression of glucose-regulated protein 78 (BiP) and 94 mRNA. J Nutr. 1990;120:1412–1417. doi: 10.1093/jn/120.11.1412. [DOI] [PubMed] [Google Scholar]
- 36.Subjeck JR, Shyy TT. Stress protein systems of mammalian cells. Am J Physiol. 1986;250:1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- 37.Lee AS. Mammalian stress response: induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–273. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- 38.Wiest DL, Burkhardt JK, Hester S, Hortsch M, Meyer DI, Argon Y. Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J Cell Biol. 1990;110:1501–1511. doi: 10.1083/jcb.110.5.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gass JN, Gifford NM, Brewer JW. Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J Biol Chem. 2002;277:49047–49054. doi: 10.1074/jbc.M205011200. [DOI] [PubMed] [Google Scholar]
- 40.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 41.Kondo S, Murakami T, Tatsumi K, Ogata M, Kanemoto S, Otori K, Iseki K, Wanaka A, Imaizumi K. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat Cell Biol. 2005;7:186–194. doi: 10.1038/ncb1213. [DOI] [PubMed] [Google Scholar]
- 42.Nakai A, Kawatani T, Ohi S, Kawasaki H, Yoshimori T, Tashiro Y, Miyata Y, Yahara I, Satoh M, Nagata K. Expression and phosphorylation of BiP/GRP78, a molecular chaperone in the endoplasmic reticulum, during the differentiation of a mouse myeloblastic cell line. Cell Struct Funct. 1995;20:33–39. doi: 10.1247/csf.20.33. [DOI] [PubMed] [Google Scholar]
- 43.van Anken E, Romijn EP, Maggioni C, Mezghrani A, Sitia R, Braakman I, Heck AJ. Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity. 2003;18:243–253. doi: 10.1016/s1074-7613(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 44.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 45.Little E, Ramakrishnan M, Roy B, Gazit G, Lee AS. The glucose-regulated proteins (GRP78 and GRP94): functions, gene regulation, and applications. Crit Rev Eukaryot Gene Expr. 1994;4:1–18. doi: 10.1615/critreveukargeneexpr.v4.i1.10. [DOI] [PubMed] [Google Scholar]
- 46.Chang SC, Erwin AE, Lee AS. Glucose-regulated protein (GRP94 and GRP78) genes share common regulatory domains and are coordinately regulated by common trans-acting factors. Mol Cell Biol. 1989;9:2153–2162. doi: 10.1128/mcb.9.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshida H, Haze K, Yanagi H, Yura T, Mori K. Identification of the cis-Acting Endoplasmic Reticulum Stress Response Element Responsible for Transcriptional Induction of Mammalian Glucose- regulated Proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem. 1998;273:33741–33749. doi: 10.1074/jbc.273.50.33741. [DOI] [PubMed] [Google Scholar]
- 48.Roy B, Lee AS. The mammalian endoplasmic reticulum stress response element consists of an evolutionarily conserved tripartite structure and interacts with a novel stress-inducible complex. Nucleic Acids Res. 1999;27:1437–1443. doi: 10.1093/nar/27.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 51.Brewer JW, Cleveland JL, Hendershot LM. A pathway distinct from the mammalian unfolded protein response regulates expression of endoplasmic reticulum chaperones in non- stressed cells. EMBO J. 1997;16:7207–7216. doi: 10.1093/emboj/16.23.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paris S, Denis H, Delaive E, Dieu M, Dumont V, Ninane N, Raes M, Michiels C. Up-regulation of 94-kDa glucose-regulated protein by hypoxia-inducible factor-1 in human endothelial cells in response to hypoxia. FEBS Lett. 2005;579:105–114. doi: 10.1016/j.febslet.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 53.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 54.Kapulkin V, Hiester BG, Link CD. Compensatory regulation among ER chaperones in C. elegans. FEBS Lett. 2005;579:3063–3068. doi: 10.1016/j.febslet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 55.Zhao YG, Gilmore R, Leone G, Coffey MC, Weber B, Lee PW. Hsp90 phosphorylation is linked to its chaperoning function. Assembly of the reovirus cell attachment protein. J Biol Chem. 2001;276:32822–32827. doi: 10.1074/jbc.M105562200. [DOI] [PubMed] [Google Scholar]
- 56.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melnick J. PhD thesis. 1995. Structural maturation of immunoglobulins in vivo: Characterization of their interactions with the chaperones BiP and GRP94, Duke Univ. [Google Scholar]
- 58.Trujillo R, Miro F, Plana M, Jose M, Bollen M, Stalmans W, Itarte E. Substrates for protein kinase CK2 in insulin receptor preparations from rat liver membranes: identification of a 210-kDa protein substrate as the dimeric form of endoplasmin. Arch Biochem Biophys. 1997;344:18–28. doi: 10.1006/abbi.1997.0155. [DOI] [PubMed] [Google Scholar]
- 59.Riera M, Roher N, Miro F, Gil C, Trujillo R, Aguilera J, Plana M, Itarte E. Association of protein kinase CK2 with eukaryotic translation initiation factor eIF-2 and with grp94/endoplasmin. Mol Cell Biochem. 1999;191:97–104. [PubMed] [Google Scholar]
- 60.Lasa-Benito M, Marin O, Meggio F, Pinna LA. Golgi apparatus mammary gland casein kinase: monitoring by a specific peptide substrate and definition of specificity determinants. FEBS Lett. 1996;382:149–152. doi: 10.1016/0014-5793(96)00136-6. [DOI] [PubMed] [Google Scholar]
- 61.Brunati AM, Contri A, Muenchbach M, James P, Marin O, Pinna LA. GRP94 (endoplasmin) co-purifies with and is phosphorylated by Golgi apparatus casein kinase. FEBS Lett. 2000;471:151–155. doi: 10.1016/s0014-5793(00)01378-8. [DOI] [PubMed] [Google Scholar]
- 62.Pasqualini E, Caillol N, Valette A, Lloubes R, Verine A, Lombardo D. Phosphorylation of the rat pancreatic bile-salt-dependent lipase by casein kinase II is essential for secretion. Biochem J. 2000;345(Pt 1):121–128. [PMC free article] [PubMed] [Google Scholar]
- 63.Ramakrishnan M, Schonthal AH, Lee AS. Endoplasmic reticulum stress-inducible protein GRP94 is associated with an Mg2+-dependent serine kinase activity modulated by Ca2+ and GRP78/BiP. J Cell Physiol. 1997;170:115–129. doi: 10.1002/(SICI)1097-4652(199702)170:2<115::AID-JCP3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 64.Reed RC, Zheng T, Nicchitta CV. GRP94-associated enzymatic activities. Resolution by chromatographic fractionation. J Biol Chem. 2002;277:25082–25089. doi: 10.1074/jbc.M203195200. [DOI] [PubMed] [Google Scholar]
- 65.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, Ploegh HL. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 66.Hadari YR, Haring HU, Zick Y. p75, a member of the heat shock protein family, undergoes tyrosine phosphorylation in response to oxidative stress. J Biol Chem. 1997;272:657–662. doi: 10.1074/jbc.272.1.657. [DOI] [PubMed] [Google Scholar]
- 67.Donella-Deana A, James P, Staudenmann W, Cesaro L, Marin O, Brunati AM, Ruzzene M, Pinna LA. Isolation from spleen of a 57-kDa protein substrate of the tyrosine kinase Lyn. Identification as a protein related to protein disulfide-isomerase and localisation of the phosphorylation sites. Eur J Biochem. 1996;235:18–25. doi: 10.1111/j.1432-1033.1996.00018.x. [DOI] [PubMed] [Google Scholar]
- 68.Bhat GJ, Samikkannu T, Thomas JJTJ. Thekkumkara, alpha-thrombin rapidly induces tyrosine phosphorylation of a novel, 74–78-kDa stress response protein(s) in lung fibroblast cells. J Biol Chem. 2004;279:48915–48922. doi: 10.1074/jbc.M409043200. [DOI] [PubMed] [Google Scholar]
- 69.Gaut JR. In vivo threonine phosphorylation of immunoglobulin binding protein (BiP) maps to its protein binding domain. Cell Stress Chaperones. 1997;2:252–262. doi: 10.1379/1466-1268(1997)002<0252:ivtpoi>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yu X, Guo ZS, Marcu MG, Neckers L, Nguyen DM, Chen GA, Schrump DS. Modulation of p53, ErbB1, ErbB2, and Raf-1 expression in lung cancer cells by depsipeptide FR901228. J Natl Cancer Inst. 2002;94:504–513. doi: 10.1093/jnci/94.7.504. [DOI] [PubMed] [Google Scholar]
- 71.Murphy PJ, Morishima Y, Kovacs JJ, Yao TP, Pratt WB. Regulation of the dynamics of hsp90 action on the glucocorticoid receptor by acetylation/deacetylation of the chaperone. J Biol Chem. 2005;280:33792–33799. doi: 10.1074/jbc.M506997200. [DOI] [PubMed] [Google Scholar]
- 72.Kovacs JJ, Murphy PJ, Gaillard S, Zhao X, Wu JT, Nicchitta CV, Yoshida M, Toft DO, Pratt WB, Yao TP. HDAC6 regulates Hsp90 acetylation and chaperone-dependent activation of glucocorticoid receptor. Mol Cell. 2005;18:601–607. doi: 10.1016/j.molcel.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 73.Kekatpure VD, Dannenberg AJ, Subbaramaiah K. HDAC6 modulates Hsp90 chaperone activity and regulates activation of aryl hydrocarbon receptor signaling. J Biol Chem. 2009;284:7436–7445. doi: 10.1074/jbc.M808999200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 74.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 75.Rao R, Nalluri S, Kolhe R, Yang Y, Fiskus W, Chen J, Ha K, Buckley KM, Balusu R, Coothankandaswamy V, Joshi A, Atadja P, Bhalla KN. Treatment with panobinostat induces glucose-regulated protein 78 acetylation and endoplasmic reticulum stress in breast cancer cells. Mol Cancer Ther. 9:942–952. doi: 10.1158/1535-7163.MCT-09-0988. [DOI] [PubMed] [Google Scholar]
- 76.Ko MH, Puglielli L. Two endoplasmic reticulum (ER)/ER Golgi intermediate compartment-based lysine acetyltransferases post-translationally regulate BACE1 levels. J Biol Chem. 2009;284:2482–2492. doi: 10.1074/jbc.M804901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ko MH, Puglielli L. The sterol carrier protein SCP-x/pro-SCP-2 gene has transcriptional activity and regulates the Alzheimer disease gamma-secretase. J Biol Chem. 2007;282:19742–19752. doi: 10.1074/jbc.M611426200. [DOI] [PubMed] [Google Scholar]
- 78.Richter K, Moser S, Hagn F, Friedrich R, Hainzl O, Heller M, Schlee S, Kessler H, Reinstein J, Buchner J. Intrinsic inhibition of the Hsp90 ATPase activity. J Biol Chem. 2006;281:11301–11311. doi: 10.1074/jbc.M510142200. [DOI] [PubMed] [Google Scholar]
- 79.Schulte TW, Akinaga S, Murakata T, Agatsuma T, Sugimoto S, Nakano H, Lee YS, Simen BB, Argon Y, Felts S, Toft DO, Neckers LM, Sharma SV. Interaction of radicicol with members of the heat shock protein 90 family of molecular chaperones. Mol Endocrinol. 1999;13:1435–1448. doi: 10.1210/mend.13.9.0339. [DOI] [PubMed] [Google Scholar]
- 80.Vogen SM, Gidalevitz T, Biswas C, Simen BS, Stein E, Gulmen F, Argon Y. Radicicol-sensitive peptide binding to the N-terminal portion of GRP94. J Biol Chem. 2002;277:40742–40750. doi: 10.1074/jbc.M205323200. [DOI] [PubMed] [Google Scholar]
- 81.Frey S, Leskovar A, Reinstein J, Buchner J. The ATPase cycle of the endoplasmic chaperone Grp94. J Biol Chem. 2007;282:35612–35620. doi: 10.1074/jbc.M704647200. [DOI] [PubMed] [Google Scholar]
- 82.Soldano KL, Jivan A, Nicchitta CV, Gewirth DT. Structure of the N-terminal domain of GRP94: Basis for ligand specificity and regulation. J Biol Chem. 2003;278:48330–48338. doi: 10.1074/jbc.M308661200. [DOI] [PubMed] [Google Scholar]
- 83.Bergerat A, de Massy B, Gadelle D, Varoutas PC, Nicolas A, Forterre P. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 84.Dutta R, Inouye M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem Sci. 2000;25:24–28. doi: 10.1016/s0968-0004(99)01503-0. [DOI] [PubMed] [Google Scholar]
- 85.Immormino RM, Dollins DE, Shaffer PL, Soldano KL, Walker MA, Gewirth DT. Ligand-induced conformational shift in the N-terminal domain of GRP94, an Hsp90 chaperone. J Biol Chem. 2004;279:46162–46171. doi: 10.1074/jbc.M405253200. [DOI] [PubMed] [Google Scholar]
- 86.Dollins DE, Warren JJ, Immormino RM, Gewirth DT. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28:41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schulte TW, Akinaga S, Soga S, Sullivan W, Stensgard B, Toft D, Neckers LM. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones. 1998;3:100–108. doi: 10.1379/1466-1268(1998)003<0100:arbttn>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Immormino RM, Metzger LEt, Reardon PN, Dollins DE, Blagg BS, Gewirth DT. Different poses for ligand and chaperone in inhibitor-bound Hsp90 and GRP94: implications for paralog-specific drug design. J Mol Biol. 2009;388:1033–1042. doi: 10.1016/j.jmb.2009.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marcu MG, Doyle M, Bertolotti A, Ron D, Hendershot L, Neckers L. Heat shock protein 90 modulates the unfolded protein response by stabilizing IRE1alpha. Mol Cell Biol. 2002;22:8506–8513. doi: 10.1128/MCB.22.24.8506-8513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wearsch PA, Voglino L, Nicchitta CV. Structural transitions accompanying the activation of peptide binding to the endoplasmic reticulum Hsp90 chaperone GRP94. Biochemistry. 1998;37:5709–5719. doi: 10.1021/bi9801006. [DOI] [PubMed] [Google Scholar]
- 91.Hainzl O, Lapina MC, Buchner J, Richter K. The charged linker region is an important regulator of Hsp90 function. J Biol Chem. 2009;284:22559–22567. doi: 10.1074/jbc.M109.031658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biswas C, Ostrovsky O, Makarewich CA, Wanderling S, Gidalevitz T, Argon Y. The peptide binding activity of GRP94 is regulated by Calcium. Biochem J. 2007;405:233–241. doi: 10.1042/BJ20061867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cochran AG, Skelton NJ, Starovasnik MA. Tryptophan zippers: stable, monomeric beta -hairpins. Proc Natl Acad Sci U S A. 2001;98:5578–5583. doi: 10.1073/pnas.091100898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Richter K, Buchner J. hsp90: twist and fold. Cell. 2006;127:251–253. doi: 10.1016/j.cell.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Yamada S, Ono T, Mizuno A, Nemoto TK. A hydrophobic segment within the C-terminal domain is essential for both client-binding and dimer formation of the HSP90-family molecular chaperone. Eur J Biochem. 2003;270:146–154. doi: 10.1046/j.1432-1033.2003.03375.x. [DOI] [PubMed] [Google Scholar]
- 96.Chu F, Maynard JC, Chiosis G, Nicchitta CV, Burlingame AL. Identification of novel quaternary domain interactions in the Hsp90 chaperone, GRP94. Protein Sci. 2006;15:1260–1269. doi: 10.1110/ps.052065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wearsch PA, Nicchitta CV. Endoplasmic reticulum chaperone GRP94 subunit assembly is regulated through a defined oligomerization domain. Biochemistry. 1996;35:16760–16769. doi: 10.1021/bi962068q. [DOI] [PubMed] [Google Scholar]
- 98.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 99.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 100.Matts RL, Dixit A, Peterson LB, Sun L, Voruganti S, Kalyanaraman P, Hartson SD, Verkhivker GM, Blagg BS. Elucidation of the Hsp90 C-Terminal Inhibitor Binding Site. ACS Chem Biol. 2011 doi: 10.1021/cb200052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ratzke C, Mickler M, Hellenkamp B, Buchner J, Hugel T. Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle. Proc Natl Acad Sci U S A. 2010;107:16101–16106. doi: 10.1073/pnas.1000916107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Freeman BC, Myers MP, Schumacher R, Morimoto RI. Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14:2281–2292. doi: 10.1002/j.1460-2075.1995.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brinker A, Scheufler C, Von Der Mulbe F, Fleckenstein B, Herrmann C, Jung G, Moarefi I, Hartl FU. Ligand discrimination by TPR domains. Relevance and selectivity of EEVD-recognition in Hsp70 x Hop x Hsp90 complexes. J Biol Chem. 2002;277:19265–19275. doi: 10.1074/jbc.M109002200. [DOI] [PubMed] [Google Scholar]
- 104.Reed RC, Nicchitta CV. Chaperone-mediated cross-priming: a hitchhiker’s guide to vesicle transport (review) Int J Mol Med. 2000;6:259–264. doi: 10.3892/ijmm.6.3.259. [DOI] [PubMed] [Google Scholar]
- 105.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18:201–205. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 106.Biswas C, Sriram U, Ciric B, Ostrovsky O, Gallucci S, Argon Y. The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional APCs. Int Immunol. 2006;18:1147–1157. doi: 10.1093/intimm/dxl049. [DOI] [PubMed] [Google Scholar]
- 107.Lev A, Dimberu P, Das SR, Maynard JC, Nicchitta CV, Bennink JR, Yewdell JW. Efficient cross-priming of antiviral CD8+ T cells by antigen donor cells is GRP94 independent. J Immunol. 2009;183:4205–4210. doi: 10.4049/jimmunol.0901828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ostrovsky O, Makarewich C, Snapp EL, Argon Y. An essential role for ATP binding and hydrolysis in the chaperone activity of GRP94 in cells. Proc Natl Acad Sci U S A. 2009;106:11600–11605. doi: 10.1073/pnas.0902626106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 110.Krukenberg KA, Street TO, Lavery LA, Agard DA. Conformational dynamics of the molecular chaperone Hsp90. Q Rev Biophys. 2011;44:229–255. doi: 10.1017/S0033583510000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Krukenberg KA, Bottcher UM, Southworth DR, Agard DA. Grp94, the endoplasmic reticulum Hsp90, has a similar solution conformation to cytosolic Hsp90 in the absence of nucleotide. Protein Sci. 2009 doi: 10.1002/pro.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dollins DE, Immormino RM, Gewirth DT. Structure of unliganded GRP94, the endoplasmic reticulum Hsp90. Basis for nucleotide-induced conformational change. J Biol Chem. 2005;280:30438–30447. doi: 10.1074/jbc.M503761200. [DOI] [PubMed] [Google Scholar]
- 113.Wassenberg JJ, Reed RC, Nicchitta CV. Ligand interactions in the adenosine nucleotide binding domain of the Hsp90 chaperone, GRP94. II. Ligand-mediated activation of GRP94 molecular chaperone and peptide binding activity. J Biol Chem. 2000;275:22806–22814. doi: 10.1074/jbc.M001476200. [DOI] [PubMed] [Google Scholar]
- 114.Cunningham CN, Krukenberg KA, Agard DA. Intra- and intermonomer interactions are required to synergistically facilitate ATP hydrolysis in Hsp90. J Biol Chem. 2008;283:21170–21178. doi: 10.1074/jbc.M800046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Prodromou C, Roe SM, Piper PW, Pearl LH. A molecular clamp in the crystal structure of the N-terminal domain of the yeast Hsp90 chaperone. Nat Struct Biol. 1997;4:477–482. doi: 10.1038/nsb0697-477. [DOI] [PubMed] [Google Scholar]
- 116.Panaretou B, Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17:4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol. 2001;154:267–273. doi: 10.1083/jcb.200104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li J, Richter K, Buchner J. Mixed Hsp90-cochaperone complexes are important for the progression of the reaction cycle. Nat Struct Mol Biol. 2010;18:61–66. doi: 10.1038/nsmb.1965. [DOI] [PubMed] [Google Scholar]
- 119.Wakabayashi Y, Kobayashi M, Akashi-Takamura S, Tanimura N, Konno K, Takahashi K, Ishii T, Mizutani T, Iba H, Kouro T, Takaki S, Takatsu K, Oda Y, Ishihama Y, Saitoh S, Miyake K. A protein associated with toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J Immunol. 2006;177:1772–1779. doi: 10.4049/jimmunol.177.3.1772. [DOI] [PubMed] [Google Scholar]
- 120.Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Hao B, Bona R, Han D, Li Z. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun. 2010;1 doi: 10.1038/ncomms1070. doi:10 1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Stefanovic S, Hegde RS. Identification of a targeting factor for posttranslational membrane protein insertion into the ER. Cell. 2007;128:1147–1159. doi: 10.1016/j.cell.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 122.Kao G, Nordenson C, Still M, Ronnlund A, Tuck S, Naredi P. ASNA-1 positively regulates insulin secretion in C. elegans and mammalian cells. Cell. 2007;128:577–587. doi: 10.1016/j.cell.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 123.Bhamidipati A, Denic V, Quan EM, Weissman JS. Exploration of the topological requirements of ERAD identifies Yos9p as a lectin sensor of misfolded glycoproteins in the ER lumen. Mol Cell. 2005;19:741–751. doi: 10.1016/j.molcel.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 124.Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10:272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bernasconi R, Pertel T, Luban J, Molinari M. A dual task for the Xbp1-responsive OS-9 variants in the mammalian endoplasmic reticulum: inhibiting secretion of misfolded protein conformers and enhancing their disposal. J Biol Chem. 2008;283:16446–16454. doi: 10.1074/jbc.M802272200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Szathmary R, Bielmann R, Nita-Lazar M, Burda P, Jakob CA. Yos9 protein is essential for degradation of misfolded glycoproteins and may function as lectin in ERAD. Mol Cell. 2005;19:765–775. doi: 10.1016/j.molcel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 127.Schaiff WT, Hruska KA, Jr, McCourt DW, Green M, Schwartz BD. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells. J Exp Med. 1992;176:657–666. doi: 10.1084/jem.176.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Melnick J, Dul JL, Argon Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum. Nature. 1994;370:373–375. doi: 10.1038/370373a0. [DOI] [PubMed] [Google Scholar]
- 129.Knarr G, Gething MJ, Modrow S, Buchner J. BiP binding sequences in antibodies. J Biol Chem. 1995;270:27589–27594. doi: 10.1074/jbc.270.46.27589. [DOI] [PubMed] [Google Scholar]
- 130.Davis DP, Khurana R, Meredith S, Stevens FJ, Argon Y. Mapping the major interaction between BiP and immunoglobulin light chains to sites within the variable domain. J Immunol. 1999;163:3842–3850. [PubMed] [Google Scholar]
- 131.Gidalevitz T, Simen BB, Ostrovsky O, Eletto D, Vogen SM, Dul JL, Argon Y. GRP94 activity is necessary for completion of immunoglobulin light chain folding. 2011 In Revision. [Google Scholar]
- 132.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112:1223–1230. doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Staron M, Yang Y, Liu B, Li J, Shen Y, Zuniga-Pflucker JC, Aguila HL, Goldschneider I, Li Z. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115:2380–2390. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bruneau N, Lombardo D. Chaperone function of a Grp 94-related protein for folding and transport of the pancreatic bile salt-dependent lipase. J Biol Chem. 1995;270:13524–13533. doi: 10.1074/jbc.270.22.13524. [DOI] [PubMed] [Google Scholar]
- 135.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morales C, Wu S, Yang Y, Hao B, Li Z. Drosophila glycoprotein 93 Is an ortholog of mammalian heat shock protein gp96 (grp94, HSP90b1, HSPC4) and retains disulfide bond-independent chaperone function for TLRs and integrins. J Immunol. 2009;183:5121–5128. doi: 10.4049/jimmunol.0900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Staron M, Wu S, Hong F, Stojanovic A, Du X, Bona R, Liu B, Li Z. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117:7136–7144. doi: 10.1182/blood-2011-01-330464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muresan Z, Arvan P. Thyroglobulin transport along the secretory pathway. Investigation of the role of molecular chaperone, GRP94, in protein export from the endoplasmic reticulum [published erratum appears in J Biol Chem 1997 Nov 28;272(48):30590] J Biol Chem. 1997;272:26095–26102. doi: 10.1074/jbc.272.42.26095. [DOI] [PubMed] [Google Scholar]
- 139.Lee J, Arvan P. Repeat motif-containing regions within thyroglobulin. J Biol Chem. 2011 doi: 10.1074/jbc.M111.242099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu M, Hodish I, Haataja L, Lara-Lemus R, Rajpal G, Wright J, Arvan P. Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol Metab. 2010;21:652–659. doi: 10.1016/j.tem.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim PS, Lee J, Jongsamak P, Menon S, Li B, Hossain SA, Bae JH, Panijpan B, Arvan P. Defective protein folding and intracellular retention of thyroglobulin-R19K mutant as a cause of human congenital goiter. Mol Endocrinol. 2008;22:477–484. doi: 10.1210/me.2007-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barton E, Park S, James JK, Makarewich CA, Eletto D, Philippou A, Lei H, Brisson B, Ostrovsky O, Li Z, Argon Y. Muscle-specific deletion of GRP94 leads to small muscles and impaired organismal growth due to inhibition of muscle-derived IGF production submitted. 2011 [Google Scholar]
- 143.Goetz MP, Toft DO, Ames MM, Erlichman C. The Hsp90 chaperone complex as a novel target for cancer therapy. Ann Oncol. 2003;14:1169–1176. doi: 10.1093/annonc/mdg316. [DOI] [PubMed] [Google Scholar]
- 144.Zhang H, Chung D, Yang YC, Neely L, Tsurumoto S, Fan J, Zhang L, Biamonte M, Brekken J, Lundgren K, Burrows F. Identification of new biomarkers for clinical trials of Hsp90 inhibitors. Mol Cancer Ther. 2006;5:1256–1264. doi: 10.1158/1535-7163.MCT-05-0537. [DOI] [PubMed] [Google Scholar]
- 145.Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Park H, Park IY, Kim E, Youn B, Fields K, Dunker AK, Kang C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J Biol Chem. 2004;279:18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- 147.Bernasconi R, Galli C, Calanca V, Nakajima T, Molinari M. Stringent requirement for HRD1, SEL1L, and OS-9/XTP3-B for disposal of ERAD-LS substrates. J Cell Biol. 2010;188:223–235. doi: 10.1083/jcb.200910042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83:3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991;167:109–123. doi: 10.1007/978-3-642-75875-1_7. [DOI] [PubMed] [Google Scholar]
- 150.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 151.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 152.Podack ER, Raez LE. Allogeneic tumor-cell-based vaccines secreting endoplasmic reticulum chaperone gp96. Expert Opin Biol Ther. 2007;7:1679–1688. doi: 10.1517/14712598.7.11.1679. [DOI] [PubMed] [Google Scholar]
- 153.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–155. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 154.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–6136. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ackerman AL, Kyritsis C, Tampe R, Cresswell P. Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci U S A. 2003;100:12889–12894. doi: 10.1073/pnas.1735556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hu MC, Gong HY, Lin GH, Hu SY, Chen MH, Huang SJ, Liao CF, Wu JL. XBP-1, a key regulator of unfolded protein response, activates transcription of IGF1 and Akt phosphorylation in zebrafish embryonic cell line. Biochem Biophys Res Commun. 2007;359:778–783. doi: 10.1016/j.bbrc.2007.05.183. [DOI] [PubMed] [Google Scholar]
- 157.Novosyadlyy R, Kurshan N, Lann D, Vijayakumar A, Yakar S, LeRoith D. Insulin-like growth factor-I protects cells from ER stress-induced apoptosis via enhancement of the adaptive capacity of endoplasmic reticulum. Cell Death Differ. 2008;15:1304–1317. doi: 10.1038/cdd.2008.52. [DOI] [PubMed] [Google Scholar]
- 158.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 160.Ma Y, Hendershot LM. The role of the unfolded protein response in tumour development: friend or foe? Nat Rev Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 161.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 163.Buchberger A, Schroder H, Hesterkamp T, Schonfeld HJ, Bukau B. Substrate shuttling between the DnaK and GroEL systems indicates a chaperone network promoting protein folding. J Mol Biol. 1996;261:328–333. doi: 10.1006/jmbi.1996.0465. [DOI] [PubMed] [Google Scholar]
- 164.Melnick J, Argon Y. Molecular chaperones and the biosynthesis of antigen receptors. Immunol Today. 1995;16:243–250. doi: 10.1016/0167-5699(95)80167-7. [DOI] [PubMed] [Google Scholar]
- 165.Molinari M, Helenius A. Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science. 2000;288:331–333. doi: 10.1126/science.288.5464.331. [DOI] [PubMed] [Google Scholar]
- 166.Drummond IA, Lee AS, Resendez E, Jr, Steinhardt RA. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987;262:12801–12805. [PubMed] [Google Scholar]
- 167.Lee AS. Coordinated regulation of a set of genes by glucose and calcium ionophores in mammalian cells. Trends Biochem Sci. 1987;12:20–23. [Google Scholar]
- 168.Sasagawa Y, Yamanaka K, Ogura T. ER E3 ubiquitin ligase HRD-1 and its specific partner chaperone BiP play important roles in ERAD and developmental growth in Caenorhabditis elegans. Genes Cells. 2007;12:1063–1073. doi: 10.1111/j.1365-2443.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 169.Maruri-Avidal L, Lopez S, Arias CF. Endoplasmic reticulum chaperones are involved in the morphogenesis of rotavirus infectious particles. J Virol. 2008;82:5368–5380. doi: 10.1128/JVI.02751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gidalevitz T, Biswas C, Ding H, Schneidman-Duhovny D, Wolfson HJ, Stevens F, Radford S, Argon Y. Identification of the N-terminal peptide binding site of Glucose-regulated Protein 94. J Biol Chem. 2004;279:16543–16552. doi: 10.1074/jbc.M313060200. [DOI] [PubMed] [Google Scholar]