Abstract
Global genome nucleotide excision repair (GG-NER) removes DNA damage from nontranscribing DNA. In Saccharomyces cerevisiae, the RAD7 and RAD16 genes are specifically required for GG-NER. We have reported that autonomously replicating sequence-binding factor 1 (ABF1) protein forms a stable complex with Rad7 and Rad16 proteins. ABF1 functions in transcription, replication, gene silencing, and NER in yeast. Here we show that binding of ABF1 to its DNA recognition sequence found at multiple genomic locations promotes efficient GG-NER in yeast. Mutation of the I silencer ABF1-binding site at the HMLα locus caused loss of ABF1 binding, which resulted in a domain of reduced GG-NER efficiency on one side of the ABF1-binding site. During GG-NER, nucleosome positioning at this site was not altered, and this correlated with an inability of the GG-NER complex to reposition nucleosomes in vitro. We discuss how the GG-NER complex might facilitate GG-NER while preventing unregulated gene transcription during this process.
ABF1 is an essential and abundant DNA-binding protein in Saccharomyces cerevisiae (1). Structurally it is comprised of a N-terminal DNA-binding domain and a C-terminal activation domain. In this respect ABF1 resembles many site-specific transcription factors (2). However, this protein is distinct from other site-specific transcription factors in that it is very abundant, and its ability to bind specifically to multiple sites in DNA can mediate a variety of nuclear functions (3). Notably whereas binding of ABF1 at the autonomously replicating sequence designated ARS1 is required for efficient replication from this origin of replication (4), ABF1 binding at the HMR-E silent mating type locus is one of three site-specific DNA binding events required for proper silencing in this region of the genome (5). Additionally binding of ABF1 at sites located within transcriptional promoters mediates either activation or repression of transcription in over 100 different genes with diverse metabolic activities (6). More recently it has been shown that ABF1 plays a role during nucleotide excision repair (NER)2 of transcriptionally silent regions of the genome, so-called global genome NER (GG-NER), in yeast (7). Based on this multiplicity of functions, ABF1 is one of three yeast proteins referred to as general regulatory factors (8). General regulatory factors appear to have little intrinsic regulatory activity. Rather they apparently amplify the activity of other regulatory factors with which they interact. This property has prompted some to refer to general regulatory factors as “obligate synergizers” (8).
The DNA-binding domain of ABF1 recognizes a large number of specific DNA sequences scattered throughout the yeast genome, including the silent mating type loci, ARSs, telomeric X-regions, and the promoter regions of over a hundred genes. The DNA sequence RTCRYNNNNNACG has been proposed as a consensus ABF1-binding site (9–12), and yeast genome pattern matching of this consensus sequence reveals several thousand potential ABF1-binding sites. Recent work suggests that this is a conservative estimate (6, 12). However, ABF1 binding has been observed at many sites that do not fit this consensus sequence. At the present time genomics and other analytical approaches designed to elucidate the biological significance of ABF1 binding to DNA have assigned functions to only a small fraction of the large number of potential DNA-binding sites in the yeast genome.
We previously demonstrated that ABF1 protein forms a stable complex with the yeast Rad7 and Rad16 NER proteins and plays a functional role in NER in yeast (7). Rad7 and Rad16 are required for GG-NER, i.e. NER of nontranscribed regions of the genome and of the nontranscribed strands of actively transcribing genes (13–16). Our studies additionally demonstrated that like the well characterized yeast chromatin modification protein Snf2, the Rad16 component of the Rad7-Rad16-ABF1 complex generates superhelical torsion in DNA (17), an event that is central to oligonucleotide excision during NER in vitro. Consistent with a functional role of ABF1 protein during NER in yeast ABF1 is required for survival of yeast cells following exposure to UV radiation and for GG-NER (17). Notably a temperature-sensitive abf1 allele that inactivates the DNA-binding domain of Abf1 is also UV radiation-sensitive and defective in NER.
Here we report that the binding of ABF1 to its cognate DNA recognition sequence promotes efficient Rad7- and Rad16-dependent GG-NER following UV irradiation of yeast cells. Our results additionally reveal that the binding of Rad7-Rad16-Abf1 complexes to DNA does not promote significant nucleosome repositioning in regions where GG-NER is enhanced following UV irradiation of cells.
EXPERIMENTAL PROCEDURES
Plasmid and Strain Construction—All S. cerevisiae strains are in the SX46a background. The details for the construction of plasmids and strains can be found in the supplemental experimental procedures.
DNA Probes for Electrophoretic Mobility Shift Assays (EMSAs)—A 480-bp ABF1-binding site containing DNA fragments cut from pUC18-ABF1-HIS3 or pUC18-ABF1bs-HIS3 with PvuII restriction enzyme was isolated from a 1.5% agarose gel. To generate labeled DNA probes, the gel-purified DNA fragments were end-labeled with T4 DNA polynucleotide kinase and [γ-32P]dATP, and the unincorporated dATP was removed on G25 columns.
EMSAs of DNA Binding by ABF1—ABF1 was produced as described previously (7). ABF1-DNA binding activity was measured by EMSAs by incubation of 10 ng of ABF1 with 15 fmol of labeled DNA in a buffer containing 10 mm Tris-HCl, pH 7.5, 50 mm NaCl, 0.5 mm EDTA, and 0.5 mm dithiothreitol for 30 min at room temperature and separation by electrophoresis using 4% polyacrylamide gels in a buffer of 22.5 mm Tris borate, pH 8.3, and 0.5 mm EDTA for 1 h at 150 V at 4 °C. Competition assays were performed using unlabeled competitor DNA with wild-type or mutant ABF1 binding sequence. The gel was exposed against a phosphor screen and subsequently analyzed (GE Healthcare).
EMSAs of DNA Binding by GG-NER Complex—Rad7/Rad16-containing GG-NER protein complex was purified as described previously (16). GG-NER complex was analyzed for binding activity in 10 μl with 25 ng of GG-NER complex and 15 fmol of [γ-32P]dATP-labeled DNA probe in a binding buffer containing 20 mm Hepes, pH 7.6, 40 mm KCl, 1.0 mm MgCl2, 0.1 mm EGTA, 0.5 mm dithiothreitol, and 1.0 μg of poly(dI-dC) left for 30 min at room temperature. Supershift assays were carried out using rabbit polyclonal anti-Rad16 or anti-Rad7 antibodies (16). Sample mixtures were incubated at room temperature with antibody at 1:20 dilutions for 30 min. Competition experiments are described above under “EMSAs of DNA Binding by ABF1.” Samples were fractionated by electrophoresis on a 4% polyacrylamide gel and electrophoresed for 1.5 h at 300 V at 4 °C. The gel was dried and exposed against a phosphor screen.
Preparation of UV Irradiation-damaged DNA and in Vitro NER Assay—In vitro NER was undertaken as described previously (7). Details for the preparation of UV irradiation-damaged pUC18-ABF1-HIS3 and pUC18-ABF1bs-HIS3 ABF1 binding sequence for in vitro NER assays are noted in the supplemental experimental procedures. We estimated that on average five to six cyclobutane pyrimidine dimers (CPDs) per plasmid are induced in the DNA substrates used following UV irradiation. This lesion density falls well within the linear range of NER activity based on previous dose range studies (18, 19).
Chromatin Extraction, Micrococcal Nuclease Treatment, and Low Resolution Nucleosome Mapping—These were undertaken as described previously (20).
Chromatin Immunoprecipitation (ChIP) and qPCR—ChIP and qPCR were performed as described previously (21) with modifications described in the supplemental experimental procedures. Primers were designed to amplify the inner nucleosomal region of N1, N2, and N3 (see Fig. 1) for histone H3 ChIP analysis to assess the histone level in these nucleosomal regions. To assess the occupancy of ABF1 and Rad7 in the HMLα I silencer region, primers for the ABF1 binding region (qPCR4), the upstream (qPCR1) and downstream regions (qPCR5) of the ABF1-binding site were used. The sequences of primers can be found in supplemental Table 1.
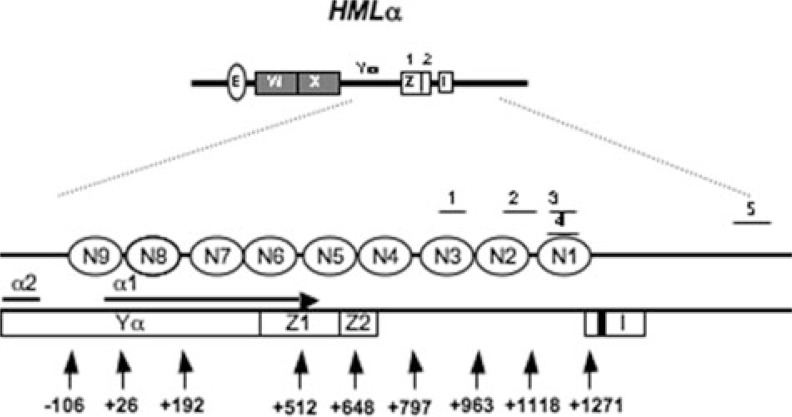
FIGURE 1.
Schematic representation of the physical map of HMLα locus (adapted from Weiss and Simpson (25)). The I silencer is indicated by a white box labeled “I.” The ABF1 binding sequence is shown by a black bar; open ellipses indicate positioned nucleosomes (N1–N9). The α1 coding region is identified by the horizontal arrow. The numbered vertical arrows mark the relative nucleotide positions from the α1 ATG start codon; A is +1. qPCR regions (1–5) are as follows: qPCR1, +871 to +959; qPCR2, +1056 to +1156; qPCR3, +1163 to +1248; qPCR4, +1162 to +1278; qPCR5, +1884 to +2095.
UV Treatment of Yeast Cells, DNA Isolation, and the End Labeling Procedure for Detecting DNA Damage and Repair at the Level of Nucleotide in Vivo—These were undertaken as described previously (22).
Nucleosome Reconstitution—Nucleosomes were assembled by mixing equimolar amounts of histone octamer and DNA in high salt concentrations and performing stepwise dialysis into low salt concentrations as described previously (23). DNA for nucleosome assembly was generated by PCR using a template sequence derived from a murine mammary tumor virus isolate. Nucleosomes were assembled on DNA fragments based on the murine mammary tumor virus nucleosome A positioning sequence. The sequences of primers used to generate the 105A64 fragment are provided in supplemental Table 1. The PCR fragments were purified by ion exchange chromatography on a 1.0-ml RESOURCE 1Q column using an ÄKTA FPLC system (Amersham Biosciences).
Nucleosome Redistribution Assays—All reactions were performed in a final volume of 10 μl containing 2 pmol of nucleosomes and 25 ng of GG-NER complex. An ATP-dependent remodeling reaction was carried out in 50 mm Tris-HCl, pH 7.5, 50 mm NaCl, 3 mm MgCl2, and 1 mm Mg-ATP for 30 min at 30 °C. The reactions were terminated using 0.5 μg of λ DNA/HindIII competitor DNA, 3.5 μl of 20% sucrose were added, and the reactions were placed on ice. A thermal shifting reaction was performed by incubating nucleosomes in 50 mm Tris-HCl, pH 7.5, and 50 mm NaCl at 47 °C for 1 h in a PCR machine. The samples were resolved on a 5% native polyacrylamide gel for 3 h at 300 V at 4 °C. Gels were scanned by a Typhoon imaging system (Amersham Biosciences).
Triple Helix Displacement Assay—A triple helix displacement assay was performed as described previously (24) with modifications as described in the supplemental experimental procedures.
RESULTS
Mutating the ABF1-binding Site at HMLα Locus Impairs GG-NER Complex Binding—We previously demonstrated that abf1 mutants that harbor mutations in the DNA-binding domain of the protein and hence are defective in their ability to bind the ABF1 DNA recognition sequence are UV radiation-sensitive and defective in NER (7). In contrast, abf1 mutants that can bind ABF1-binding sites but are defective in DNA replication because of mutations in other regions of the protein are not abnormally sensitive to killing by UV radiation and are NER-proficient.
The HMLα ABF1-binding site in the yeast genome has been studied extensively with respect to ABF1 function, chromatin structure, transcription, and NER (25–30). This site is located within the I silencer region and promotes gene silencing at the HMLα locus. However, inhibition of ABF1 binding at this site does not alter nucleosome positioning, silencing, or transcription of HMLα (27). Furthermore the site is not associated with an ARS and is not an origin of DNA replication (31). In the present studies we probed the molecular basis of the requirement for ABF1 protein for NER at this well characterized region of the genome (see Fig. 1).
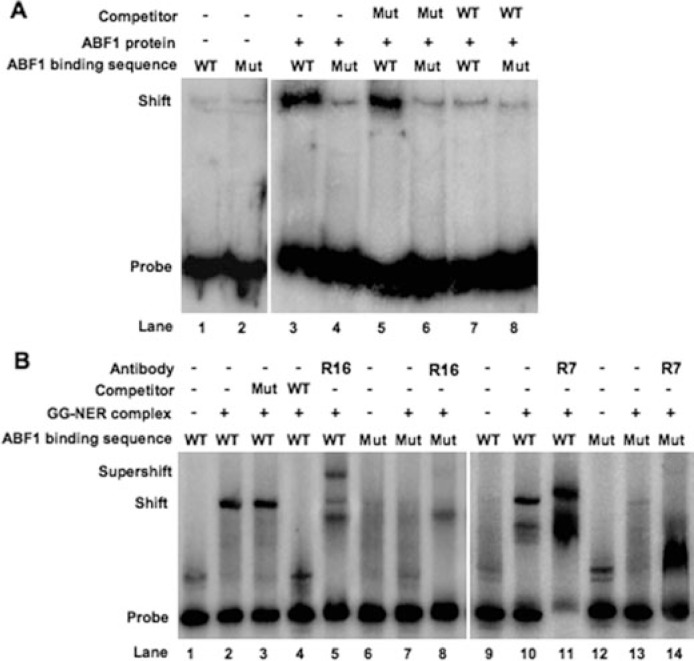
In initial experiments we investigated the effect of mutating the ABF1-binding site on NER by comparing ABF1 binding at the wild-type and a mutated HMLα locus using EMSAs. Purified ABF1 protein was incubated with radioactively labeled undamaged double-stranded DNA probes containing either the wild-type or mutated ABF1 binding sequences. The specificity of the protein-DNA interactions was verified by control experiments in which wild-type and mutated unlabeled DNA fragments were compared for their ability to compete with labeled probe for ABF1 binding. As shown in Fig. 2A, ABF1 efficiently bound the wild-type probe (lane 3). The observed band shift was significantly competed by an excess of wild-type cold DNA (lane 7). However, such competition was not observed in the presence of up to a 50-fold molar excess of unlabeled mutant DNA (lane 5).
FIGURE 2.
EMSAs to detect ABF1 and GG-NER complex DNA binding activity. A, EMSAs with ABF1. [γ-32P]dATP-labeled 480-bp duplex DNA containing wild-type ABF1 binding sequence (WT; lanes 1, 3, 5, and 7) or triple point-mutated ABF1 binding sequence (Mut; lanes 2, 4, 6, and 8) was incubated at room temperature for 30 min either with (lanes 3– 8) or without recombinant ABF1 (lanes 1 and 2). A competition experiment was performed in the presence of unlabeled DNAs containing three point-mutated (lanes 5 and 6) or wild-type (lanes 7 and 8) ABF1 binding sequence. B, EMSAs with GG-NER complex. [γ-32P]dATP-labeled 480-bp duplex DNA containing wild-type ABF1 binding sequence (lanes 1–5 and 9–11) or triple point-mutated ABF1 binding sequence (Mut; lanes 6–8 and 12–14) was incubated at room temperature for 30 min either with (lanes 2–5, 7, 8, 10, 11, 13, and 14) or without GG-NER complex (lanes 1, 6, 9, and 12). Competition was performed in the presence of unlabeled DNAs containing triple point-mutated (Mut; lane 3) and normal (WT; lane 4) ABF1 binding sequence. In supershift assays, antibodies against Rad16 (R16) (lanes 5 and 8) and Rad7 (R7) (lane 11 and 14) were subsequently added to the mixture and incubated for another 30 min at room temperature.
In contrast to these results we observed defective ABF1 binding to probes containing point mutations in conserved nucleotides in the ABF1-binding site (lane 4), and the addition of cold competitor DNA merely inhibited background ABF1-DNA interactions (lane 8). We therefore conclude that mutations in the ABF-binding site in the HMLα locus severely impair the binding ability of ABF1 protein to the site.
In light of our previous observations that ABF1 protein interacts with the Rad7-Rad16 GG-NER protein complex (7) we asked whether defective binding of ABF1 protein weakens the recruitment of Rad7 and Rad16 to the undamaged DNA probes used above. As shown in Fig. 2B, purified Rad7-Rad16 GG-NER complex bound to the wild-type probe (lane 2) more efficiently than to the mutant probe (lane 7). The specificity of the DNA-protein interactions was demonstrated by competition assays with cold competitor DNA probes (lanes 3 and 4). Furthermore the addition of anti-Rad7 and anti-Rad16 antibodies resulted in a supershift of the protein-DNA complex (lanes 5 and 11). We conclude that mutations in the ABF1-binding site that preclude the binding of ABF1 protein interfere with efficient recruitment of Rad7 and Rad16 proteins, known components of the GG-NER complex.
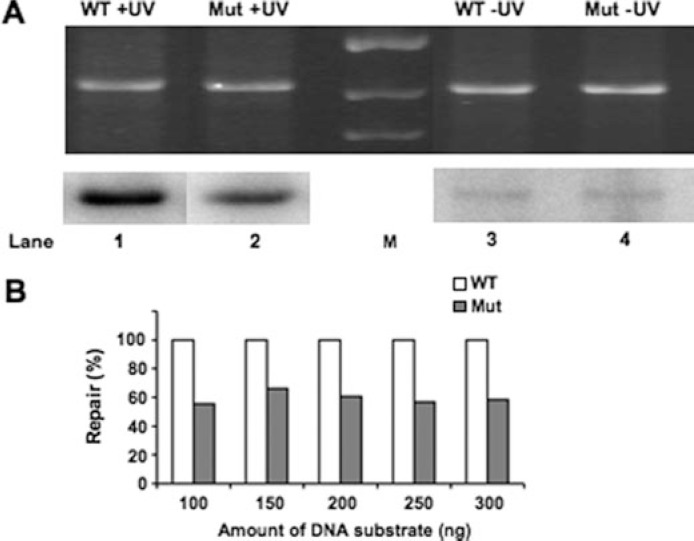
Loss of ABF1 Binding Reduces NER Efficiency in Vitro—To establish that the failure to observe efficient recruitment of Rad7 and Rad16 proteins indeed affects GG-NER we compared repair synthesis of UV irradiated plasmids carrying either wild-type (pUC18-ABF1-HIS3) or mutated (pUC18-ABF1bs-HIS3) ABF1 binding sequences (Fig. 3, A and B). Ethidium bromide staining of the gel (Fig. 3A, top) shows the total amount of plasmid DNA loaded on the gel. The autoradiograph of the dried gel (Fig. 3A, bottom) indicates the amount of incorporated radiolabeled dATP during repair synthesis. Control experiments using undamaged plasmids were routinely included to determine background levels of radiolabel incorporation (Fig. 3A, lanes 3 and 4). The low level of background radiolabel incorporation in the undamaged plasmid was subtracted from the specific signal incorporated in the damaged plasmid to determine the level of DNA repair synthesis. The damaged plasmid DNA containing triple point mutations in the ABF1-binding site (Fig. 3A, lane 2) was consistently repaired about 50% less efficiently than the wild-type plasmid (Fig. 3A, lane 1). This occurred over a range of initial damaged DNA substrate amounts as shown in Fig. 3B. This indicates that the presence of an ABF1-binding site in the damaged DNA plasmid DNA template consistently enhanced the repair synthesis in the plasmid over the range of DNA substrate amounts indicated.
FIGURE 3.
In vitro NER analysis to monitor repair synthesis. NER was performed at 26 °C for 2 h using UV radiation-damaged (+UV; 300 J/m2) or undamaged (−UV) plasmid pUC18-ABF1-HIS3 (WT; lanes 1 and 3, respectively) or pUC18-ABF1bs-HIS3 (Mut; lanes 2 and 4, respectively) in the presence of wild-type yeast whole cell extract. A, ethidium bromide-stained gel represents the total DNA loading (top), and an autoradiograph of the gel indicates the level of DNA repair synthesis or background radiolabel incorporation for the damaged (+UV) or undamaged (−UV) plasmids (bottom). M, DNA marker lane. B, relative levels of DNA repair radiolabel incorporation for the damaged (+UV) or undamaged (−UV) plasmids at the range of DNA substrate amounts indicated.
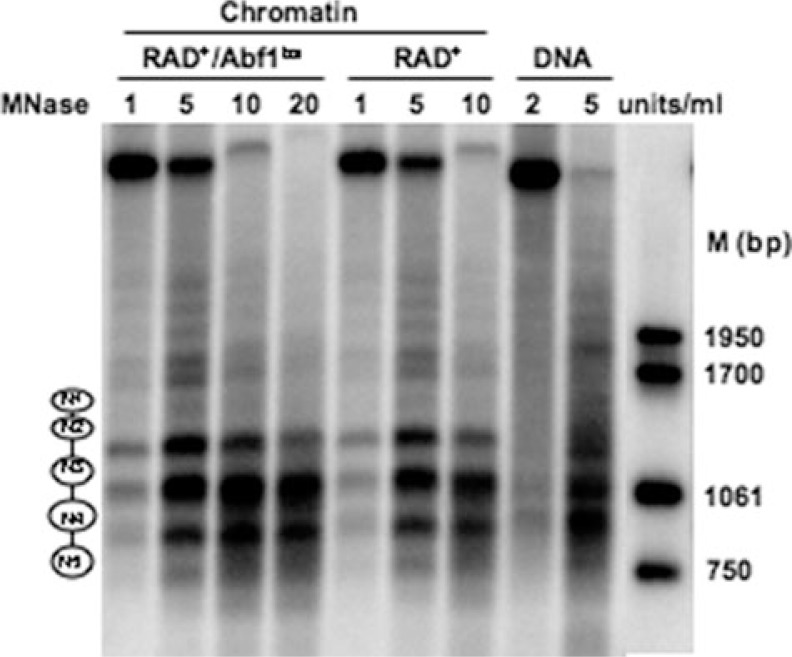
Loss of ABF1 Binding at HMLα I Silencer Does Not Alter Nucleosome Positioning in the Region—It has been suggested that the binding of ABF1 protein to its cognate sites in DNA alters nucleosome positioning (3, 6, 32). To determine whether the function of ABF1 protein in GG-NER involves altered nucleosome positioning we introduced the triple point-mutated ABF1-binding site into the I silencer region of HMLα on chromosome III and examined micrococcal nuclease sensitivity in chromatin extracted from wild-type cells (RAD+) and those carrying a mutated ABF1-binding site (RAD+/ABF1bs) (20). Stretches of DNA separated by 140–160 bp in length that are protected in chromatin but not in naked DNA are diagnostic of nucleosomes (33, 34). Fig. 4 displays micrococcal nuclease-sensitive sites at low resolution and reveals that the wild-type and mutated strains exhibited similar sensitivity. Nucleosomes were found positioned at N1, N2, N3, N4, and N5, which is consistent with previous reports of nucleosome positioning in the region (25).
FIGURE 4.
Low resolution micrococcal nuclease nucleosome mapping. The amount of micrococcal nuclease (MNase) used is indicated at the top of each lane. Molecular weight markers (M) on the right indicate the size (bp) of the bands. The positioned nucleosomes (N1–N5) are shown as ellipses on the left.
To confirm that no major changes in chromatin structure transpire following loss of ABF1 binding we measured total histone H3 levels at the HMLα1 locus by ChIP at the N1, N2, and N3 nucleosome sites. Our results show that in each position examined the level of total histone H3 is similar in the wild type and ABF1-binding site mutant (supplemental Fig. 1). Collectively our results demonstrate that mutation of the ABF1-binding site does not significantly alter chromatin structure in the region examined.
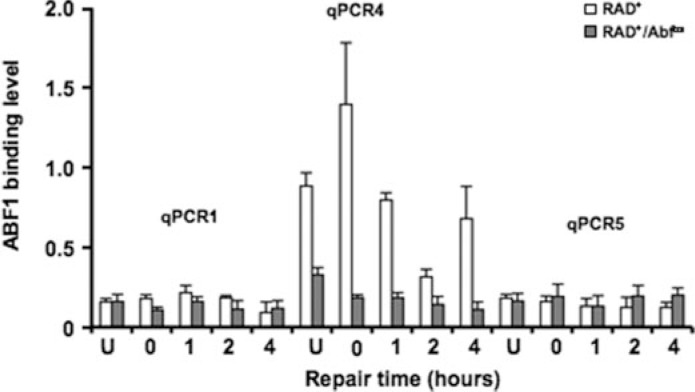
ABF1 and GG-NER Complex Binding at the ABF1 I Silencer Site in Vivo—The experiments described above were carried out using purified proteins or protein complexes and cell-free extracts. To confirm the effect of altering the consensus ABF1-binding site sequence on ABF1 binding and hence on the efficiency of GG-NER in vivo, we examined the occupancy of ABF1 and the Rad7-Rad16 GG-NER complex in the vicinity of the ABF1-binding site at the HMLα I silencer by in vivo ChIP analysis. This was accomplished using specific antibodies against ABF1 and Rad7 proteins. First we measured ABF1 occupancy at the ABF1-binding site both in RAD+ and RAD+/ABF1bs strains by mapping the binding of ABF1 using primer pairs spanning the region surrounding the HMLα I silencer. Fig. 5 shows that ABF1 bound to the wild-type ABF1 consensus site at the I silencer (qPCR4) in the absence of DNA damage (U) consistent with the expected presence of ABF1 at the I silencer. Following exposure of cells to UV radiation we observed a small initial increase (0 h) in ABF1 occupancy followed by a loss of ABF1 occupancy at later times (1 and 2 h). Occupancy of ABF1 at the I silencer returned to levels detected in unirradiated cells after 4 h. ABF1 occupancy was not detected at positions upstream (+871 to +959) or downstream (+1884 to +2095) of the I silencer (measured by using the qPCR1 and qPCR5 primers in either the RAD+ or RAD+/ABF1bs strains), indicating the specificity of ABF1 binding at the I silencer site. Furthermore ABF1 occupancy was significantly reduced at the HMLα I silencer regions tested in the RAD+/ABF1bs strain containing a triple point mutation in the ABF1-binding site of the I silencer, confirming the importance of this site for ABF1 binding.
FIGURE 5.
ABF1 binding to HMLα I silencer requires an ABF1-binding site. Immunoprecipitation with ABF1 antibody was performed in RAD+ and RAD+/Abf1bs cells. The occupancy of ABF1 was measured by qPCR with the primers spanning the HMLα I silencer region (qPCR1, qPCR4, and qPCR5). Data are represented as average of three experiments ±S.D.
We also confirmed the occupancy of Rad7 protein at the I silencer using qPCR3 primers, which span the same region of the genome as qPCR4 primers, and antibodies raised against Rad7 protein (supplemental Fig. 2). We did not detect a significant change of Rad7 occupancy after exposure to UV radiation at 1 h (supplemental Fig. 2) or later times (data not shown). In summary, our results indicate that mutations in the ABF1-binding site at the I silencer inhibit ABF1 binding to the site and that changes in the occupancy of individual components of the GG-NER complex occur at the binding site in response to DNA damage associated with UV radiation exposure.
Repair of UV Radiation-induced Damage at the I Silencer ABF1-binding Site of HMLα1 in Vivo—In subsequent experiments we directly examined GG-NER under the experimental conditions described above. Yeast cells were irradiated with UV light at a dose of 100 J/m2, and DNA was extracted as described previously (29). NER was examined at nucleotide resolution by measuring the removal of CPDs from DNA using a 3′-end labeling technique described previously (22). In this analysis digestion of DNA with the restriction enzyme NdeI yields a 742-bp DNA fragment containing the HMLα1 coding region. Double digestion with AvaII and HinfI generates a 852-bp fragment downstream of HMLα1 containing the ABF1-binding site, and digestion with HinfI generates a 1118-bp fragment that encompasses the region generated by AvaII and HinfI double digestion.
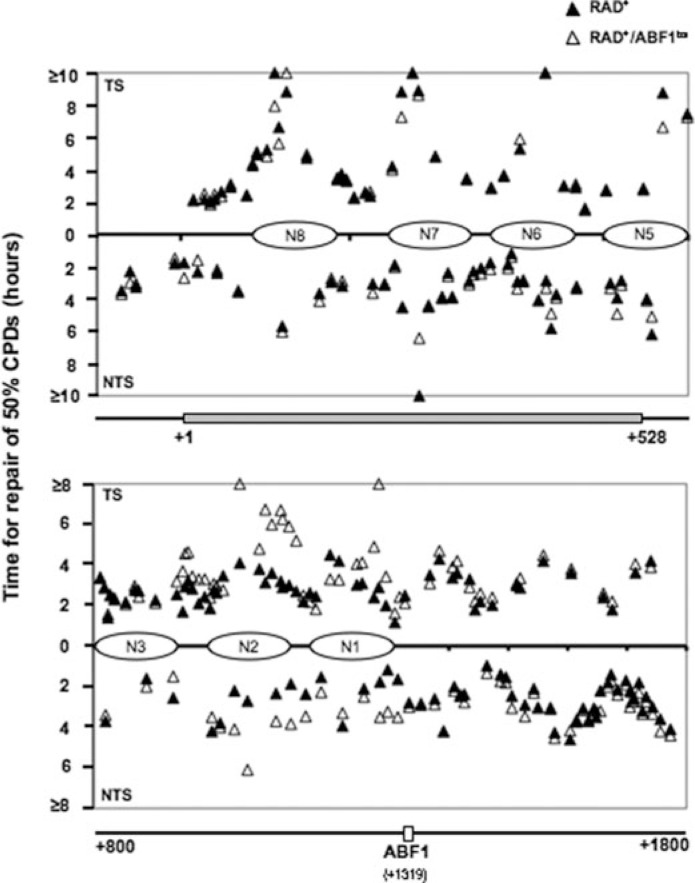
Supplemental Figs. 3–7 show the presence of CPD in transcribed strands (TS) and nontranscribed strands (NTS) of DNA. The coding sequence of HMLα1 (supplemental Fig. 3) and the ABF1 binding sequence (supplemental Figs. 4 and 5) are indicated in gray bars and unfilled bars, respectively. The quantified results are represented in Fig. 6 as the time taken to remove 50% (t50%) of the initial load of CPDs induced at each site. As demonstrated in the figure, the repair rates for the RAD+ and RAD+/ABF1bs both in TS and NTS are similar in HMLα1, indicating that loss of ABF1 binding does not alter NER efficiency in this region of the ABF1-binding site (+1 to +528) (Fig. 6 and supplemental Fig. 3). In contrast, reduced repair efficiency was observed in the strain containing the mutated ABF1-binding site on the same side of the ABF1-binding site but downstream of HMLα1 (+900 to ABF1-binding site) and extending for several hundred base pairs from the ABF1-binding site (Fig. 6 and supplemental Figs. 4 and 5). No difference in repair rates was observed on the opposite side of the ABF1-binding site (ABF1-binding site to +1800). The DNA repair rates plotted in Fig. 6 represent the average values obtained from at least three independent experiments. The average (t50%) in hours and the S.D. for the following regions on the TS and NTS are: TS: nucleotide position 820–1067: WT, 2.87 ± 0.58; mutant, 2.99 ± 0.54; nucleotide position 1068–1464: WT, 2.97 ± 0.39; mutant, 3.77 ± 0.25; nucleotide position 1326–1559: WT, 2.69 ± 0.39; mutant, 2.67 ± 0.55; NTS: nucleotide position 822–1067: WT, 3.06 ± 0.05; mutant, 2.80 ± 0.13; nucleotide position 1109–1464: WT, 2.16 ± 0.36; mutant, 2.97 ± 0.36; nucleotide position 1487–1773: WT, 2.91 ± 0.26; mutant, 2.85 ± 0.60. These results indicate that ABF1 binding at the HMLα locus promotes efficient NER in a single direction from the ABF1-binding site.
FIGURE 6.
CPD repair in RAD+ and RAD+/ABF1bs strains. The gels (supplemental Figs. 4–6) were quantified with ImageQuant software. Repair time for 50% of CPDs (t50%) at a site was calculated or extrapolated. Closed triangles represent CPD repair in the RAD+ strain; open triangles represent CPD repair in the RAD+/ABF1bs. Horizontal bars at the bottom of each graph show regions examined, and numbers indicate nucleotide positions.
It is well established that the Rad7 and Rad16 proteins are required for GG-NER of the transcriptionally repressed HMLα locus but not for repair of the same sequence when present at the transcriptionally active MATα locus (35). To confirm that we were indeed observing GG-NER in the ABF1-binding site mutant, the RAD7 gene was deleted in both RAD+ and RAD+/ABF1bs to generate strains Δrad7 and Δrad7/ABF1bs, and the repair of CPDs was examined. Supplemental Figs. 6 and 7 show no repair within 4 h in the TS or NTS both in the NdeI and AvaII/HinfI fragments examined in the Δrad7/ABF1bs and the Δrad7 strains. Hence NER observed at the HMLα is unequivocally GG-NER.
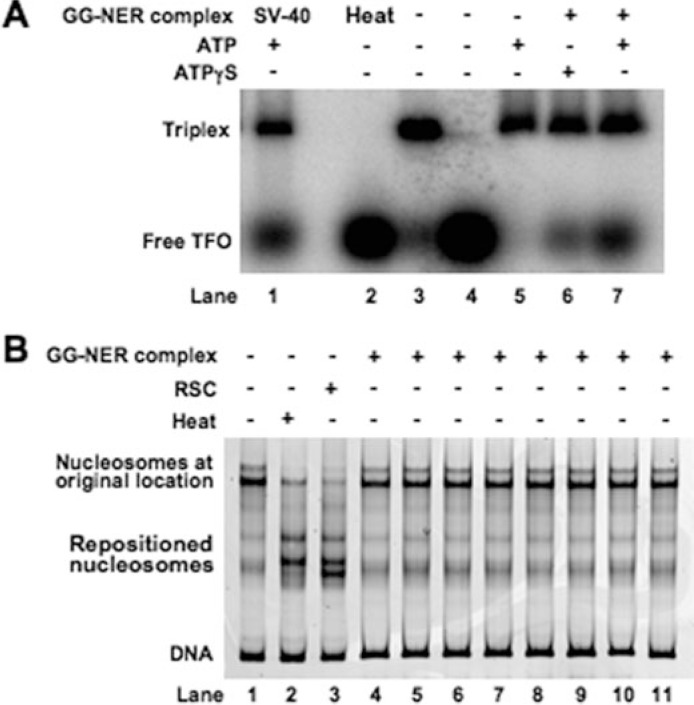
DNA Translocase Activity of the GG-NER Complex—The Rad7-Rad16 GG-NER complex has been shown to generate negative superhelical torsion in damaged DNA that is Rad16-dependent (17). Rad16 is a member of the Snf2 family of chromatin-remodeling proteins, many of which have also been observed to generate torsion (36). It has been substantiated for several Snf2 family members that such negative superhelical torsion is generated as a result of DNA translocation (24, 37–43). To investigate whether Rad16 has DNA translocating activity we established a triplex strand displacement assay using a triple helix formed by annealing a homopyrimidine oligonucleotide to a DNA duplex at low pH. Such triplexes are stable at neutral pH, but if the triplex strand is displaced it will not reanneal (44). The triplex has a distinct electrophoretic mobility, and when the homopyrimidine oligonucleotide is displaced either by thermal incubation (Fig. 7A, lane 2) or incubation with SV-40 T-antigen (Fig. 7A, lane 1) an accumulation of free oligonucleotide is observed. When the GG-NER complex was incubated with the triplex in the presence of ATP, oligonucleotide displacement was observed (Fig. 7A, lane 7). The extent of displacement was reduced to a level similar to that observed in the absence of ATP (Fig. 7A, lane 3) when ATP was substituted with the poorly hydrolyzable analogue ATPγS (Fig. 7A, lane 6). We conclude that the GG-NER complex promotes DNA triple helix strand displacement as a result of DNA translocase activity.
FIGURE 7.
DNA translocase and nucleosome sliding activity of GG-NER complex. A, triple helix substrates consisting of a 40-nucleotide triple helical region were prepared as described under “Experimental Procedures.” Substrates were incubated with (lanes 6–7) or without (lane 3) GG-NER complex at 30 °C for 30 min in the presence of ATP (lanes 5 and 7) or ATPγS (lane 6) or heated for 5 min at 90 °C (lane 2). SV-40 T-antigen-treated sample (lane 1) is included as a positive control. Lane 4 contains [γ-32P]dATP-labeled third strand (indicated as Free TFO) only. A control fraction from the final step of the purification known not to contain GG-NER complex was also tested (lane 5). Reaction mixtures were separated on a 1% agarose gel, and displacement of the free triplex-forming oligonucleotide (TFO) was monitored. B, movement of nucleosomes on the Cy3-labeled DNA fragment 105A64 was monitored following heating at 47 °C for 1 h (lane 2) or treatment with different GG-NER complex-containing fractions from the final step of the purification as indicated previously (16) (lanes 4–11) or 0.12 pmol of RSC complex (lane 3) for 30 min by native gel electrophoresis.
The GG-NER Complex Has Little Effect on Nucleosome Mobility—Many Snf2 family proteins that share with Rad16 the ability to translocate along DNA and generate superhelical torsion are also efficient in redistributing nucleosomes (45). To determine whether the GG-NER complex can redistribute nucleosomes we used an in vitro assay (see “Experimental Procedures” for details). In this assay mononucleosomes were reconstituted using a fluorescently labeled 316-bp DNA fragment in which the murine mammary tumor virus NucA positioning sequence was flanked by 105 and 64 bp of additional DNA sequence on either side (46). The position of a nucleosome on a DNA fragment affects its mobility during native gel electrophoresis, and this provides a means of assessing nucleosome redistribution. Following either thermal treatment or incubation with the RSC chromatin-remodeling complex nucleosomal DNA migrated further into the gel consistent with movement of nucleosomes toward the ends of this fragment (Fig. 7B, lanes 2 and 3). In contrast, addition of purified GG-NER complex showed no effect (Fig. 7B, lanes 4–11). We conclude that, unlike many other members of the Snf2 family such as the RSC complex, Rad16 as a component of the GG-NER complex does not promote efficient nucleosome redistribution under the conditions tested.
DISCUSSION
Previous studies have shown that ABF1 protein forms a stable complex with the yeast Rad7 and Rad16 GG-NER proteins and plays a role in NER (7, 17). The present studies were directed at demonstrating that the role(s) of ABF1 in NER is dependent on specific binding to ABF1 consensus-binding sites in DNA in contrast to general nonspecific DNA binding. This was achieved by inhibiting ABF1 binding at a specific ABF1 DNA-binding site located at the I silencer of the HMLα locus and examining NER in the vicinity of this site. We cloned a DNA fragment containing the ABF1-binding site in the I silencer of HMLα into a plasmid and introduced three point mutations into conserved nucleotides in the ABF1-binding site. When we examined the ability of purified ABF1 protein to bind to the plasmid DNA substrate in competitive bandshift assays in vitro we observed efficient binding to the wild-type ABF1-binding site, whereas mutation of the binding site severely inhibited binding. Similarly supershift experiments using antibodies against the Rad7 or Rad16 components of the GG-NER complex revealed that it bound to the plasmid containing the wild-type ABF1-binding site but not when the ABF1 consensus sequence was mutated. Hence the binding efficiency of both purified ABF1 and the GG-NER complex to plasmid DNA is dependent on an intact ABF1 consensus binding sequence.
When wild-type and mutated plasmids were used as substrates for measuring NER activity in vitro, we observed that the plasmid containing the wild-type ABF1-binding site was repaired twice as efficiently as that with the mutated ABF1-binding site, suggesting that the specific binding of ABF1 to its DNA consensus sequence promotes efficient GG-NER in vitro. Similar conclusions were derived from studies that examined the effect of mutating the ABF1 DNA-binding site on chromatin structure and NER in vivo. Notably we confirmed that loss of ABF1 binding at the I silencer ABF1 consensus sequence does not significantly alter chromatin structure at the ABF1 site, the nucleosome content in the region, or the silencing of HMLα.
Our analysis of the effect of I silencer ABF1-binding site mutations on binding of the Rad7-Rad16 GG-NER complex is particularly instructive. Both ABF1 and Rad7 proteins are present at the ABF1-binding site in the absence of DNA damage, and occupancy of the Rad7-Rad16 GG-NER complex at the site is dependent on an intact ABF1 consensus DNA-binding site. Following UV radiation we observed a small initial increase followed by a loss of occupancy of ABF1 protein. This returned to normal levels several hours after irradiation. In contrast, Rad7 occupancy did not change following DNA damage. The significance of the differential occupancy of GG-NER components at the I silencer-binding site after UV radiation exposure is not clear.
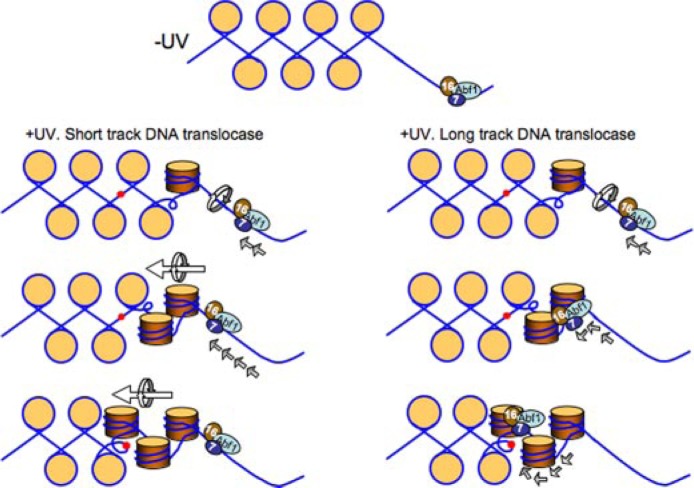
Failure of the GG-NER complex to bind to the mutated ABF1 consensus sequence resulted in a domain of reduced GG-NER efficiency extending for ∼400 base pairs in one direction from the ABF1-binding site. We suggest that the GG-NER complex binds to the ABF1-binding site in an orientation-specific manner, and in response to DNA damage, subsequent activities of the complex promote efficient GG-NER within a defined region extending from the ABF1-binding site. This notion is supported by the results of experiments not reported here that showed that switching the orientation of the ABF1-binding site at the I silencer results in reduced GG-NER rates in the affected repair domain similar to the repair rate observed when ABF1 fails to bind to the mutated ABF1-binding site. However, normal levels of ABF1 occupancy were observed at the switched ABF1-binding site. Thus, the orientation of ABF1 binding to DNA significantly affects its function during GG-NER. We speculate that the binding of the GG-NER complex to multiple ABF1-binding sites throughout the genome positions the GG-NER complex at specific locations in the genome and facilitates the formation of GG-NER domains in response to DNA damage. We are currently investigating the significance of the loss of ABF1 occupancy from the I silencer in response to UV radiation to determine how this relates to the efficiency of GG-NER.
How might putative GG-NER domains be generated by the GG-NER complex following DNA damage? Our previous studies explored the biochemical properties of the purified GG-NER complex (17). These experiments showed that the action of the complex generates superhelical torsion in DNA. Furthermore the generation of torsion is necessary to promote excision of DNA damage-containing oligonucleotides during NER. We suggest that in response to UV radiation the GG-NER complex promotes the formation of a domain of increased superhelical torsion in DNA following unidirectional translocation initiated at the ABF1-binding site. A constrained domain of increased torsion could conceivably be generated by the DNA translocase activity of the complex acting over a very short distance or by more extensive translocase activity essentially tracking along the DNA throughout the domain. A similar activity for a complex of Rad7 and Rad16 proteins has been suggested previously (47).
Although DNA translocation by many Snf2 family proteins can facilitate the translational repositioning of nucleosomes, the present study indicates that the GG-NER complex does not significantly affect nucleosome sliding in vitro. Furthermore the GG-NER complex does not promote nucleosome repositioning during GG-NER in vivo. Thus, the ability to translocate and generate negative helical torsion in DNA is not sufficient to promote nucleosome repositioning. Our studies also highlight the fact that some Snf2 family proteins such as SSO1653 and Mot1 (and now Rad16) do not efficiently alter nucleosome positioning (36). We therefore speculate that during a global process such as GG-NER it is important to ensure that chromatin structure is not compromised because this could result in unregulated gene transcription from repressed regions of the genome. Our results are summarized and shown as a model in Fig. 8.
FIGURE 8.
Proposed model of GG-NER complex function at the HMLα locus ABF1-binding site. In the absence of UV radiation the GG-NER complex binds in a specific orientation to the ABF1-binding site (top panel). Following UV radiation the directional DNA translocase activity of the complex results in changes in occupancy of the GG-NER complex at the ABF1-binding site. This generates a domain of altered superhelical torsion in one direction from the ABF1-binding site. This action promotes efficient GG-NER within the domain. The GG-NER complex could generate superhelical torsion by DNA translocation over a short distance from the ABF1-binding site, for example tens of base pairs generating a domain of torsion over hundreds of base pairs (left panel), or by long distance tracking of the complex throughout the domain (right panel). Note that the translational setting of the nucleosomes (beige circles) on the DNA is not perturbed by this process. DNA damage is represented by red spots. 7, Rad7; 16, Rad16.
Footnotes
The abbreviations used are: NER, nucleotide excision repair; GG-NER, global genome nucleotide excision repair; ABF1, autonomously replicating sequence-binding factor 1; ARS, autonomously replicating sequence; EMSA, electrophoretic mobility shift assay; CPD, cyclobutane pyrimidine dimer; ChIP, chromatin immunoprecipitation; qPCR, quantitative PCR; FPLC, fast protein liquid chromatography; TS, transcribed strands; NTS, nontranscribed strands; WT, wild type; ATPγS, adenosine 5′-O-(thiotriphosphate).
This work was supported by a Medical Research Council (MRC) career development award and career establishment grant (to S. H. R.) and an MRC program award (to R. W.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental experimental procedures, Figs. 1–7, and Table 1.
Supplemental material: http://www.jbc.org/content/suppl/2008/11/14/M806830200.DC1.html
REFERENCES
- 1.Diffley JF, Stillman B. Science. 1989;246:1034–1038. doi: 10.1126/science.2511628. [DOI] [PubMed] [Google Scholar]
- 2.Reid JL, Iyer VR, Brown PO, Struhl K. Mol. Cell. 2000;6:1297–1307. doi: 10.1016/s1097-2765(00)00128-3. [DOI] [PubMed] [Google Scholar]
- 3.Yarragudi A, Miyake T, Li R, Morse RH. Mol Cell Biol. 2004;24:9152–9164. doi: 10.1128/MCB.24.20.9152-9164.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao H, Stillman B. Proc Natl Acad Sci U S A. 1995;92:2224–2228. doi: 10.1073/pnas.92.6.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou Y, Yu Q, Bi X. Mol Cell Biol. 2006;26:7806–7819. doi: 10.1128/MCB.01197-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarragudi A, Parfrey LW, Morse RH. Nucleic Acids Res. 2007;35:193–202. doi: 10.1093/nar/gkl1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed SH, Akiyama M, Stillman B, Friedberg EC. Genes Dev. 1999;13:3052–3058. doi: 10.1101/gad.13.23.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fourel G, Miyake T, Defossez PA, Li R, Gilson E. J Biol Chem. 2002;277:41736–41743. doi: 10.1074/jbc.M202578200. [DOI] [PubMed] [Google Scholar]
- 9.Rhode PR, Elsasser S, Campbell JL. Mol Cell Biol. 1992;12:1064–1077. doi: 10.1128/mcb.12.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beinoraviciute-Kellner R, Lipps G, Krauss G. FEBS Lett. 2005;579:4535–4540. doi: 10.1016/j.febslet.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 11.McBroom LD, Sadowski PD. J Biol Chem. 1994;269:16455–16460. [PubMed] [Google Scholar]
- 12.Mukherjee S, Berger MF, Jona G, Wang XS, Muzzey D, Snyder M, Young RA, Bulyk ML. Nat Genet. 2004;36:1331–1339. doi: 10.1038/ng1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhage RA, van Gool AJ, de Groot N, Hoeijmakers JH, van de Putte P, Brouwer J. Mol Cell Biol. 1996;16:496–502. doi: 10.1128/mcb.16.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verhage RA, Zeeman AM, Lombaerts M, van de Putte P, Brouwer J. Mutat Res. 1996;362:155–165. doi: 10.1016/0921-8777(95)00045-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Wei S, Reed SH, Wu X, Svejstrup JQ, Feaver WJ, Kornberg RD, Friedberg EC. Mol Cell Biol. 1997;17:635–643. doi: 10.1128/mcb.17.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed SH, You Z, Friedberg EC. J Biol Chem. 1998;273:29481–29488. doi: 10.1074/jbc.273.45.29481. [DOI] [PubMed] [Google Scholar]
- 17.Yu S, Owen-Hughes T, Friedberg EC, Waters R, Reed SH. DNA Repair (Amst) 2004;3:277–287. doi: 10.1016/j.dnarep.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Wu X, Friedberg EC. Methods. 1995;7:177–186. [Google Scholar]
- 19.Wang Z, Wu X, Friedberg EC. Biochemistry. 1992;31:3694–3702. doi: 10.1021/bi00129a019. [DOI] [PubMed] [Google Scholar]
- 20.Teng Y, Yu S, Waters R. Nucleic Acids Res. 2001;29:E64. doi: 10.1093/nar/29.13.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu Y, Teng Y, Liu H, Reed SH, Waters R. Proc Natl Acad Sci U S A. 2005;102:8650–8655. doi: 10.1073/pnas.0501458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teng Y, Li S, Waters R, Reed SH. J Mol Biol. 1997;267:324–337. doi: 10.1006/jmbi.1996.0908. [DOI] [PubMed] [Google Scholar]
- 23.Flaus A, Richmond TJ. Methods Mol Biol. 1999;119:45–60. doi: 10.1385/1-59259-681-9:45. [DOI] [PubMed] [Google Scholar]
- 24.Saha A, Wittmeyer J, Cairns BR. Genes Dev. 2002;16:2120–2134. doi: 10.1101/gad.995002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss K, Simpson RT. Mol Cell Biol. 1998;18:5392–5403. doi: 10.1128/mcb.18.9.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bi X, Braunstein M, Shei GJ, Broach JR. Proc Natl Acad Sci U S A. 1999;96:11934–11939. doi: 10.1073/pnas.96.21.11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shei GJ, Broach JR. Mol Cell Biol. 1995;15:3496–3506. doi: 10.1128/mcb.15.7.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney DJ, Broach JR. Mol Cell Biol. 1989;9:4621–4630. doi: 10.1128/mcb.9.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed SH, McCready S, Boiteux S, Waters R. Mol. Gen. Genet. 1996;250:515–522. doi: 10.1007/BF02174040. [DOI] [PubMed] [Google Scholar]
- 30.Reed SH, Boiteux S, Waters R. Mol. Gen. Genet. 1996;250:505–514. doi: 10.1007/BF02174039. [DOI] [PubMed] [Google Scholar]
- 31.Vujcic M, Miller CA, Kowalski D. Mol Cell Biol. 1999;19:6098–6109. doi: 10.1128/mcb.19.9.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lascaris RF, Groot E, Hoen PB, Mager WH, Planta RJ. Nucleic Acids Res. 2000;28:1390–1396. doi: 10.1093/nar/28.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson RT. Prog Nucleic Acids Res Mol Biol. 1991;40:143–184. doi: 10.1016/s0079-6603(08)60841-7. [DOI] [PubMed] [Google Scholar]
- 34.Thoma F. Biochim. Biophys. Acta. 1992;1130:1–19. doi: 10.1016/0167-4781(92)90455-9. [DOI] [PubMed] [Google Scholar]
- 35.Terleth C, Schenk P, Poot R, Brouwer J, van de Putte P. Mol Cell Biol. 1990;10:4678–4684. doi: 10.1128/mcb.10.9.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Nucleic Acids Res. 2006;34:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whitehouse I, Stockdale C, Flaus A, Szczelkun MD, Owen-Hughes T. Mol Cell Biol. 2003;23:1935–1945. doi: 10.1128/MCB.23.6.1935-1945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durr H, Korner C, Muller M, Hickmann V, Hopfner KP. Cell. 2005;121:363–373. doi: 10.1016/j.cell.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Cosma MP, Tanaka T, Nasmyth K. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 40.Xue Y, Gibbons R, Yan Z, Yang D, McDowell TL, Sechi S, Qin J, Zhou S, Higgs D, Wang W. Proc Natl Acad Sci U S A. 2003;100:10635–10640. doi: 10.1073/pnas.1937626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaskelioff M, Peterson CL. Nat Cell Biol. 2003;5:395–399. doi: 10.1038/ncb0503-395. [DOI] [PubMed] [Google Scholar]
- 42.Lia G, Praly E, Ferreira H, Stockdale C, Tse-Dinh YC, Dunlap D, Croquette V, Bensimon D, Owen-Hughes T. Mol. Cell. 2006;21:417–425. doi: 10.1016/j.molcel.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Smith CL, Saha A, Grill SW, Mihardja S, Smith SB, Cairns BR, Peterson CL, Bustamante C. Mol. Cell. 2006;24:559–568. doi: 10.1016/j.molcel.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Firman K, Szczelkun MD. EMBO J. 2000;19:2094–2102. doi: 10.1093/emboj/19.9.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Becker PB, Horz W. Annu Rev Biochem. 2002;71:247–273. doi: 10.1146/annurev.biochem.71.110601.135400. [DOI] [PubMed] [Google Scholar]
- 46.Flaus A, Owen-Hughes T. Mol Cell Biol. 2003;23:7767–7779. doi: 10.1128/MCB.23.21.7767-7779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzder SN, Sung P, Prakash L, Prakash S. J Biol Chem. 1998;273:6292–6296. doi: 10.1074/jbc.273.11.6292. [DOI] [PubMed] [Google Scholar]