Abstract
Study Objectives:
Children with obstructive sleep apnea have blunted baroreflex sensitivity and increased blood pressure variability. The aim of the study was to test the hypothesis that treatment of sleep apnea by adenotonsillectomy results in significant improvement of baroreflex sensitivity, lowering of blood pressure and blood pressure variability and increase vagal heart rate modulation.
Study Design:
One hundred ninety-four children aged 9.6 ± 2.3 years were enrolled; 133 had obstructive sleep apnea and 61 were healthy controls. For children with sleep apnea, polysomnography with 3-lead electrocardiography and continuous blood pressure was performed before adenotonsillectomy, then 6 weeks and 6 months postoperatively. Controls underwent the same assessment at study entry and 6 months later. Spontaneous baroreflex sensitivity was measured in the time and frequency domains. Data analyses were performed for available and complete cases.
Results:
Children with sleep apnea experienced postoperatively an increase in baroreflex sensitivity and decrease in blood pressure variability during wakefulness and sleep. A decrease in blood pressure during sleep and in heart rate during wakefulness was also measured. The improvement in baroreflex sensitivity was predicted by the change in the apnea-hypopnea and arousal indices. A normal pattern of rising baroreflex sensitivity during the night was restored in children with severe apnea after surgery. However, baroreceptor sensitivity did not completely normalize after treatment.
Conclusion:
Treatment of obstructive sleep apnea in children by adenotonsillectomy is associated with gradual improvement in known risk factors for cardiovascular disease. Complete normalization of baroreceptor sensitivity was not achieved 6 months postoperatively.
Citation:
Crisalli JA; McConnell K; VanDyke RD; Fenchel MC; Somers VK; Shamszumann A; Chini B; Daniels SR; Amin RS. Baroreflex sensitivity after adenotonsillectomy in children with obstructive sleep apnea during wakefulness and sleep. SLEEP 2012;35(10):1335-1343.
Keywords: Sleep apnea, baroreflex, tonsillectomy, hypertension, blood pressure variability
INTRODUCTION
Obstructive sleep apnea (OSA) in adults is associated with an increased risk of cardiovascular morbidity. Hypertension, stroke, heart failure, and coronary artery disease have been closely linked to OSA. 1,2 Most studies of the mechanisms leading to cardiovascular morbidity in OSA have been conducted in subjects with preexisting cardiovascular risk factors and/or disease. As a result, our understanding of the early mechanisms of cardiovascular disease in OSA is incomplete. In the absence of this knowledge, the causal relationship between OSA and cardiovascular disease remains inconclusive.
There is compelling evidence that autonomic dysfunction precedes the onset of hypertension 3,4 and is an independent risk factor for end-organ damage and cardiovascular morbidity. 5,6 Impaired vagal control of the sinoatrial node, as expressed by reduced baroreflex sensitivity and an increase in sympathetic tone, characterizes the initial stages of autonomic dysfunction in patients at risk for cardiovascular disease. 7 The baroreflex buffers changes in blood pressure (BP) through the negative feedback regulation of heart rate (HR), cardiac contractility, and vascular resistance.
In previous work, we demonstrated that BP variability is increased in normotensive children with OSA and is associated with left ventricular remodeling. 5,6 The analysis of nighttime baroreflex control of HR revealed decreased baroreflex sensitivity in children with moderate to severe OSA compared to healthy controls. 8 Furthermore, rising baroreflex sensitivity during the night, which was observed in healthy controls, was absent in children with moderate to severe OSA. These findings are consistent with studies demonstrating baroreflex dysfunction and increased BP variability in adults with OSA. 9,10
In light of these data, we hypothesized that treatment of OSA through surgical removal of the adenoids and tonsils would result in improvement in nighttime and daytime baroreflex sensitivity in children with moderate to severe OSA, and would restore the pattern of rising baroreflex sensitivity during the night. We additionally hypothesized that declines in measures of OSA severity after adenotonsillectomy would predict improvement in baroreflex sensitivity. To elucidate the early stages of autonomic impairment in patients with OSA, we focused our investigation on children with the disorder who were free from other chronic illnesses.
METHODS
Subjects
Children ranging in age from 7 to 13 years with hypertrophy of palatine tonsils who were scheduled for adenotonsillectomy due to nightly snoring were recruited for this study from the otolaryngology clinics and the community. The inclusion criteria for the OSA group were: (1) absence of chronic medical conditions or genetic syndromes, and (2) overnight polysomnography (PSG) consistent with the diagnosis of OSA (defined as an obstructive apnea-hypopnea index [AHI] > 1/h of sleep). These children were subdivided into those with mild OSA, defined as 1 < obstructive AHI < 5, and those with moderate to severe OSA, defined as an obstructive AHI ≥ 5. Age- and gender-matched healthy children comprised a control group. Inclusion criteria for the control group were: (1) absence of habitual snoring, and (2) absence of OSA or alveolar hypoventilation on PSG. Children receiving chronic medications were excluded if they were unable to temporarily discontinue their use. Parents provided informed consent and children provided assent. Results of pretreatment baroreceptor sensitivity from the same study population were previously published. 8 The institutional review board approved this study and written informed consent/assent was obtained.
Study Design
Evaluation at the time of enrollment included a history and physical examination and determination of body mass index (BMI). Children with OSA underwent adenotonsillectomy within 1 month of this baseline assessment. Overnight PSG and continuous BP and ECG monitoring were repeated at 6 weeks and 6 months after surgery. Children in the control group underwent the same assessment only at baseline and 6 months later. All children underwent overnight PSG performed according to American Thoracic Society standards 11 using a computerized system (Grass, Telefactor, West Warwick, RI). Simultaneous continuous BP monitoring was obtained through finger arterial photoplethysmography (Portapres, TNO-TPD Biomedical Instrumentation, Amsterdam, The Netherlands). Electrocardiography (ECG) was recorded using a Grass Telefactor system with standard ECG electrodes. RR-intervals were calculated from the ECG data using custom software employing a derivative and peak detection algorithm. The RR-interval data were manually reviewed to identify and remove artifact and ectopic beats. Systolic (SBP) and diastolic (DBP) blood pressures were determined from the photoplethysmography data as the maximum and minimum pressure, respectively, during each heartbeat. Data were acquired at 500 Hz per channel.
PSG interpretation was performed by the principal investigator (RA), who was blinded to subjects' medical history. Sleep staging, arousals and obstructive apneas/hypopneas (AHI) were defined and scored according to the standard criteria as previously described. 5 The obstructive AHI was defined as the number of obstructive apneas, obstructive hypopneas, and mixed apneas per hour of sleep. The arousal index was defined as the number of arousals per hour of sleep. The oxyhemoglobin desaturation index was defined as the number of episodes per hour that SaO2 decreased by ≥ 3%. The percent of sleep time spent with end tidal CO2 above 50 (EtCO2 > 50 mm Hg) was calculated.
Data Analysis
Spontaneous baroreflex sensitivity was analyzed in the time domain through the sequence method. 12,9 The sequence method is based on the assumption that concurrent linearly related increases or decreases in SBP and RR-interval reflect baroreflex activity. Open loop computer analyses were employed to detect sequences of 3 or 4 heartbeats in which SBP increased by ≥ 1 mm Hg and RR-interval increased by ≥ 5 milliseconds between each beat. Three- or 4-beat sequences in which SBP and RR-interval concurrently decreased to this degree were also identified. The slope of the regression of SBP against RR-interval describes baroreflex sensitivity in the time domain. Baroreflex sensitivity, or sensitivity (BRS), was assessed separately for ascending (+SBP/+RR-interval) and descending (-SBP/-RR-interval) sequences.
Baroreflex sensitivity was analyzed in the frequency domain through the alpha (α) method. 10 The α method is a spectral analysis technique based on the finding that variability in SBP and RR-interval has a high degree of linear correlation at approximately 0.1 Hz and at the respiratory frequency. The α method involves Fourier analysis of beat-to-beat SBP and RR-interval as follows: for each study the complete recordings of SBP and RR-interval were separated into 300-sec time series with a single heartbeat offset. Uniformly sampled data sets were created from each 300-sec time series segment by cubic spline interpolation at 2 Hz, de-trended (using a fifth-order polynomial), and Hanning filtered. The power spectrum of each data set was calculated as the square of the Fourier transform. Coherence between the SBP and RR-interval power spectra was assessed. The α index was calculated as the square root ratio of the integration of the RR-interval and SBP power spectra where the signal coherence was greater than 0.5. The α index was calculated within a low-frequency band of 0.04 to 0.15 Hz and within a high-frequency band of 0.15 to 0.5 Hz.
SBP and RR-interval variability were defined as the periodicity of the beat-to-beat SBP and RR-interval as measured through spectral analysis and integrated over the low- and high-frequency bands. BP variability in the low frequency is primarily a marker of sympathetic and vagal modulation, while variability in the high frequency (also called respiratory frequency) is a marker of vagal modulation.
Cardiovascular data during wakefulness was obtained in a 30-min session preceding the PSG. During sleep, data that coincided with obstructive apneas and hypopneas were excluded from analysis. This approach enabled comparison of baroreflex sensitivity parameters between control subjects and children with OSA without the confounding effect of obstructive events.
Statistical Analysis
BMI measurements were converted into Z scores (BMIZ) according to the standards published by the CDC. 13 Chi-square tests were used to test for group differences in gender and race frequencies. Group differences in continuous variables were tested with the Kruskal-Wallis nonparametric test. The Wilcoxon signed-rank nonparametric test was used to test for within-group differences in all variables between study assessments. Spectral data, time domain measures of baroreflex sensitivity, RR-interval, and BP parameters were normalized by natural log transformation (noted as “log” throughout).
The primary analyses were conducted using the available case method. 14 Standard modeling assumptions for this approach are consistent with the intention to treat principle.
To ensure that the conclusion was unbiased, missing data, attrition patterns for each of the groups with respect to baseline, clinical and demographic characteristics of subjects were compared. A mixed effects model was used for analysis of unbalanced or incomplete outcome data; this method has been recommended as a principled approach in prospective sleep disorder trials. 15 The estimated effects of treatment of OSA on trend of baroreflex were measured in available and complete cases. Available cases included subjects who completed the baseline visit and either the 6-week or 6-month visits for the OSA group and the baseline visit for the controls. Complete cases included subjects who completed all 3 time points for the OSA group and the baseline and 6-month visits for the controls.
Inferential Analysis
Three-step analysis plan was conducted to determine the effect of OSA treatment by adenotonsillectomy on baroreflex sensitivity and BP parameters. The first step aimed to determine whether children with OSA experience an increase in baroreflex sensitivity and a decrease in BP variability after adenotonsillectomy. Baroreflex and other cardiovascular parameters were modeled through nested repeated-measures analysis of covariance controlling for age, BMIZ, gender, and race. A linear trend analysis was used to examine the association of time (in weeks—0, 6, and 24) since adenotonsillectomy with each parameter of interest for sleep apnea subjects. The second step sought to determine whether adenotonsillectomy resulted in changes in the pattern of baroreflex sensitivity over the course of the night. Testing for monotonic increasing behavior of the baroreflex parameters was performed at each assessment using isotonic contrasts for ordered alternatives. 16 Isotonic contrast is a statistical technique aimed at assessing monotone increasing patterns of responses, to determine whether or not baroreflex parameters demonstrated any consistent increases during the night. Baroreflex sensitivity was considered to be consistently rising if a monotonic increasing pattern was observed through at least the sixth hour of the night. The third step aimed to determine whether declines in measures of OSA severity after adenotonsillectomy predicted improvement in baroreflex sensitivity. Baroreflex parameters at the 6-month assessment were modeled against changes in the obstructive AHI, DI, and arousal index as well as baseline demographic, PSG, and baroreceptor parameters over the 6-month period. Significance level for all tests was set as α = 0.05. SAS software (SAS Institute, version 9.2, Cary, NC) was used for all analyses.
RESULTS
Study Population
One hundred ninety-four children were enrolled in the study. This included 61 healthy controls with normal polysomnographic studies, 63 children with mild OSA, and 70 children with moderate to severe OSA. Among the 133 children with OSA, 9 (6.8%) missed only the 6 week-visit, 21 (15.8%) missed only the 6-month visit, and 44 (33.1%) missed both visits for a total 65 subjects (49%). Thirty-two children with mild OSA and 36 with severe OSA completed all components of the study. Among the 61 healthy controls, 15 (24.6%) missed the 6-month visit. Children with mild OSA who missed the 6-week visit did not differ in demographic or polysomnographic characteristics from those who did not. In children with severe OSA, those who missed the 6-week visit did not differ in any characteristic from those who did not—except for larger percentage of REM sleep—21% versus 18% (P = 0.03)
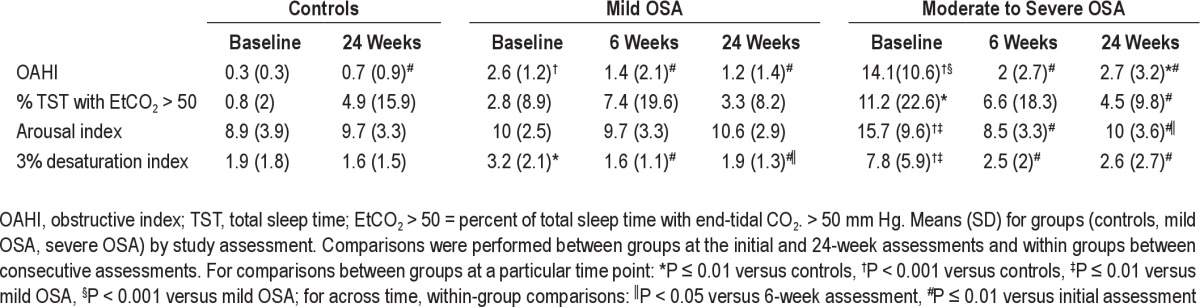
Baseline comparisons between those who missed the 6-month visit and those who did not showed that healthy controls who missed the visit had larger percentage of males (80% versus 43%; P = 0.01) and a lower percentage of whites (47% versus 80%; P = 0.01). There were no significant differences between those who missed and those who did not in children with mild OSA. In children with severe OSA, those who missed the 6-month visit did not differ in any characteristic from those who did not except for larger percentage of REM sleep (21% versus 17%; P = 0.002). Demographic and PSG characteristics of the study participants reported as available and complete cases are shown in Table 1. Adenotonsillectomy in children with OSA resulted in significant declines in measures of OSA severity (Table 2).
Table 1.
Demographic and polysomnographic characteristics at baseline assessment of available and complete cases
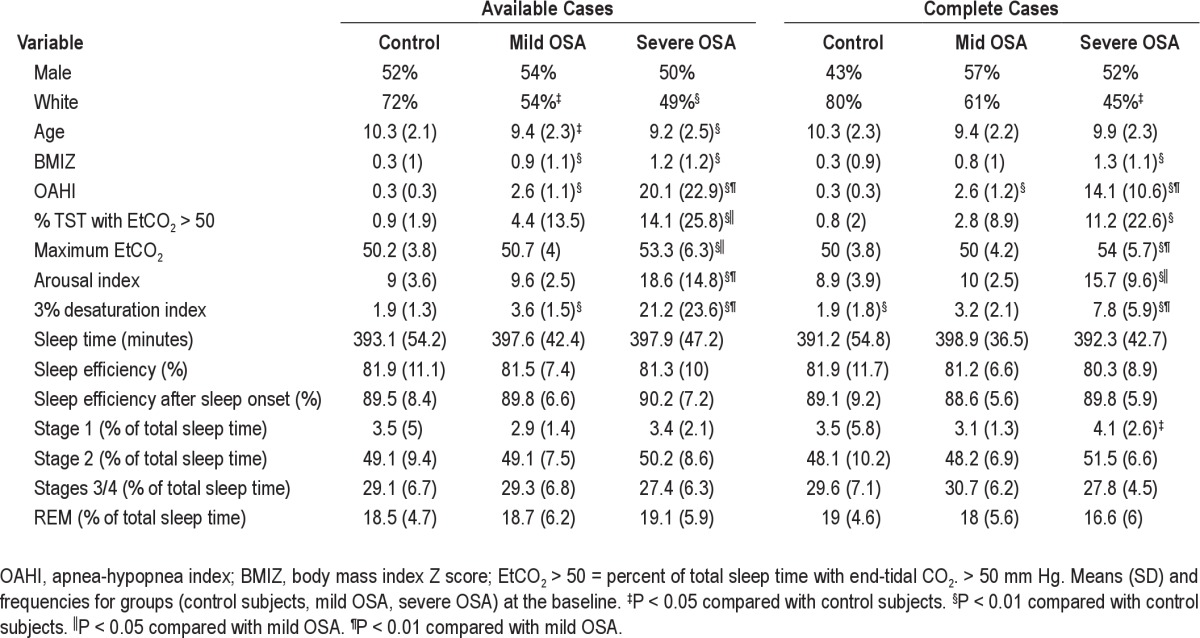
Table 2.
Polysomnographic characteristics at baseline, 6-week, and 24-week assessments
Changes in Baroreflex Sensitivity after Adenotonsillectomy
Severe OSA
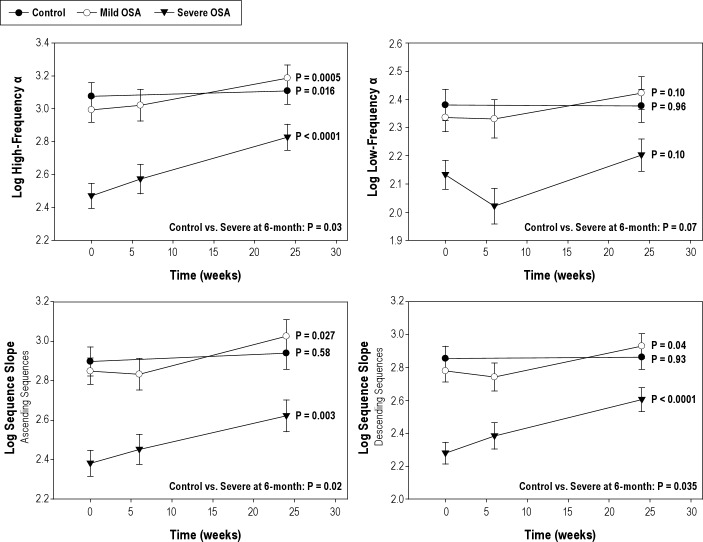
During wakefulness, BRS significantly improved across the 3 time points (baseline, 6 weeks, and 6 months) for ascending (β = 0.008 ± 0.003, P = 0.006) and descending sequences (β = 0.007 ± 0.003, P = 0.02) as well as for high-frequency α (β = 0.012 ± 0.003, P = 0.0001) and low-frequency α (β = 0.004 ± 0.002, P = 0.024). During sleep, children with severe OSA demonstrated significant improvement in BRS for ascending and descending sequences and in the high-frequency α. There were no significant changes observed in low-frequency α (Table 3, Figure 1).
Table 3.
Linear trend analysis examining the association in (weeks—0, 6, and 24) since adenotonsillectomy (β = slope) during sleep derived from available and complete cases
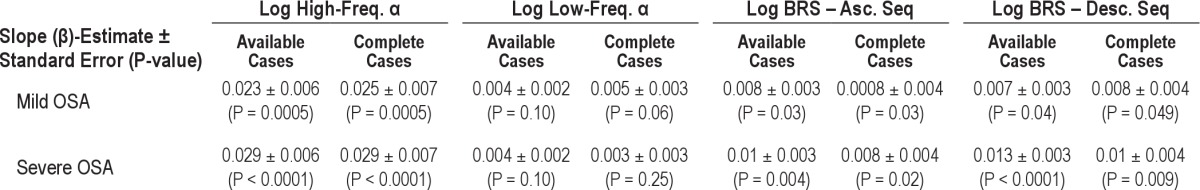
Figure 1.
Baroreflex sensitivity during sleep in the time and frequency domains before, 6 weeks, and 6 months after adenotonsillectomy. Separate P-values for each Group represent test of significance for linear trend. Comparison between Control and Severe groups at 6 months given at the bottom of each graph.
Mild OSA
There were no measured significant changes during wakefulness at the 6-monh follow-up time point in BRS in the high frequency α (β = 0.006 ± 0.004, P = 0.14), in the low-frequency range (β = 0.002 ± 0.003, P = 0.39) and in the time domain analysis for ascending (β = 0.003 ± 0.003, P = 0.35) and descending sequences (β = 0.003 ± 0.003, P = 0.35). During sleep, children with mild OSA demonstrated significant improvement in BRS for ascending and descending sequences and in the high-frequency range in the spectral analysis (Table 3, Figure 1).
Healthy Controls
There were no measured significant changes during wakefulness at the 6-month follow-up time point in BRS in the high-frequency α (β = 0.004 ± 0.003, P = 0.29), in the low-frequency range (β = 0.004 ± 0.003, P = 0.13), and in the time domain analysis for ascending (β = 0.001 ± 0.003, P = 0.7) and descending sequences (β = 0.003 ± 0.003, P = 0.3). During sleep, only high-frequency α significantly increased at the 6-month time point (Figure 1).
Trend analyses of BRS in available and complete cases did yield similar results demonstrating the internal validity of the study (Table 3).
Degree of Normalization of Baroreceptor Sensitivity after Adenotonsillectomy
At the 6-month visit, while a significant increase in high-frequency α was demonstrated in children with severe OSA, they continued to have lower high-frequency α than healthy controls (difference = −0.28 ± 0.11, P = 0.03) and children with mild OSA (−0.35 ± 0.1, P = 0.005). Children with mild OSA did not differ from healthy controls at the 6-month visit. Low-frequency α did not differ in either group of children with OSA from healthy controls. Analysis in the time domain also showed failure of complete normalization of BRS at the 6-month visit in children with severe OSA. Baroreceptor sensitivity derived from ascending sequences in children with severe OSA differed from healthy controls (difference = −0.3 ± 0.12, P = 0.02) and from children with mild OSA (−0.4 ± 0.11, P = 0.002). Equally, BRS derived from descending sequences in children with severe OSA differed from healthy controls (difference = −0.25 ± 0.1, P = 0.035) and from children with mild OSA (−0.3 ± 0.1, P = 0.007).
Determinants of Improvement in Baroreflex Sensitivity after Adenotonsillectomy
In a model which included age, gender, race, BMI Z, baseline obstructive AHI, delta AHI and baseline BRS, the decrease in the obstructive AHI after adenotonsillectomy predicted significant improvement in high-frequency α and in BRS for ascending and descending sequences over the 6-month period (Table 4).
Table 4.
Repeated-measures analysis of covariance predicting baroreflex sensitivity measures at the 6-month assessment
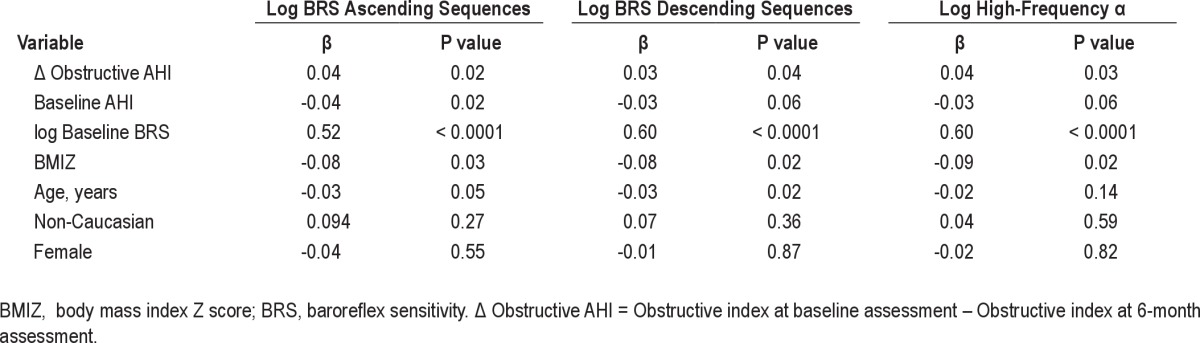
A model including age, gender, race, BMIZ score baseline arousal index, delta arousal index, and baseline BRS showed similar results to delta AHI. Delta arousal index predicted an increase in high-frequency α (P = 0.029), an increase in BRS for ascending (P = 0.014) and descending (P = 0.005) sequences. The parameters of oxygen desaturation did not explain the changes in BRS.
Cardiovascular Variability after Adenotonsillectomy
During wakefulness, SBP variability in the high-frequency range decreased only in subjects with severe OSA after adenotonsillectomy (β = −0.21 ± 0.004, P < 0.0001). SBP variability in the low-frequency range did not change over the 6-month period in either OSA group or in control subjects. Spectral measures of RR-interval variability in the low-frequency range increased in subjects with severe OSA (β = 0.008 ± 0.003, P = 0.02)
During sleep, SBP variability in the high-frequency range decreased in subjects with severe OSA (β = −0.02 ± 0.004, P < 0.0001). SBP variability in the low-frequency range did not change over the 6-month period in either OSA group or in control subjects. Spectral measures of RR-interval variability in the high-frequency range increased in all 3 groups at the end of 6-month period (β = 0.076 ± 0.014, P < 0.0001) for controls, (β = 0.75 ± 0.014, P < 0.0001) for mild OSA, and (β = 0.68 ± 0.014, P < 0.0001) for severe OSA.
Mean BP and HR after Adenotonsillectomy
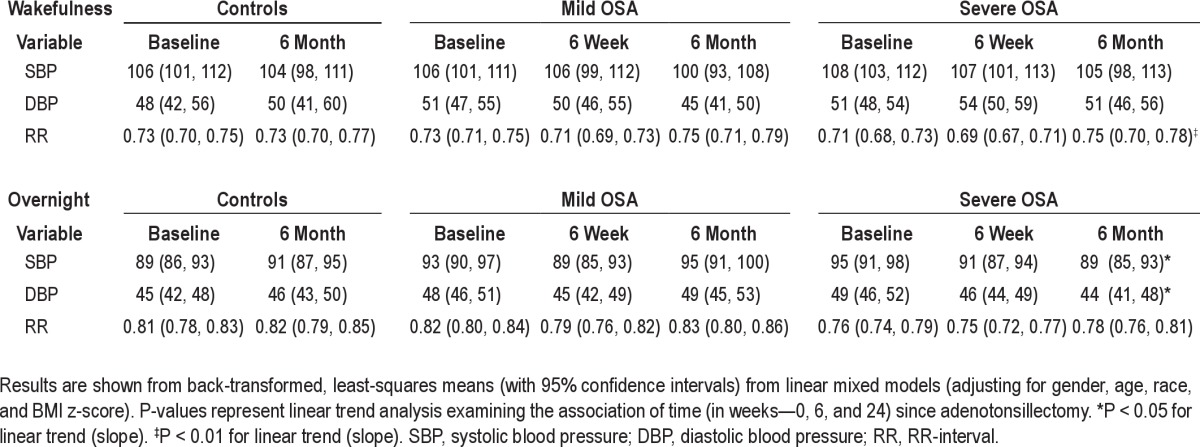
After adenotonsillectomy, a significant decrease in SBP and DBP during sleep was observed only in subjects with severe OSA. A significant decrease in heart rate was observed during wakefulness in subjects with severe OSA. There was no change however, in BP during wakefulness in any of the 3 groups (Table 5).
Table 5.
Estimated means for cardiovascular variables for baseline, 6-week, and 6-month assessment
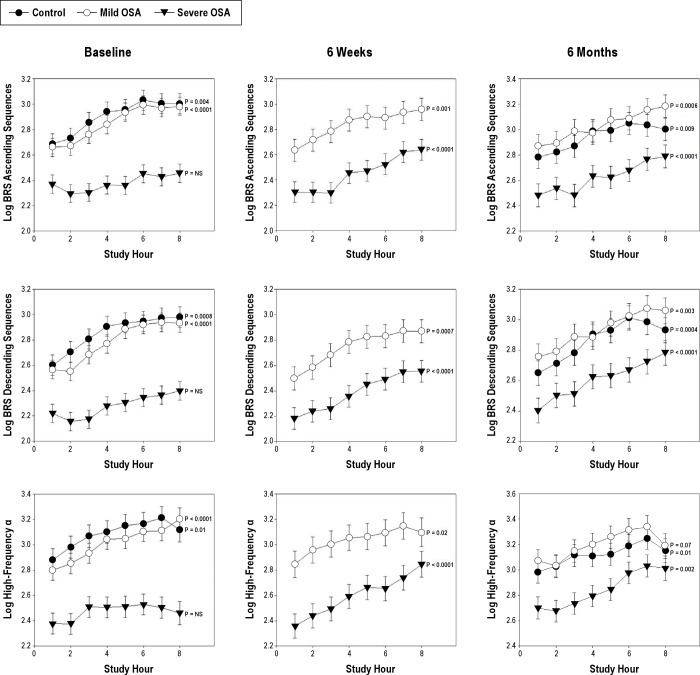
Pattern of Baroreflex Sensitivity during Sleep
Prior to adenotonsillectomy, children with severe OSA did not exhibit a monotonic increase in baroreflex sensitivity during the night. In contrast, healthy controls and children with mild OSA demonstrated monotonic increases in BRS for ascending and descending sequences and in high-frequency α (Figure 2). At 6 weeks and 6 months after adenotonsillectomy, subjects with mild and severe OSA demonstrated significant monotonic increases in ascending and descending sequences and in high-frequency α (Figure 2).
Figure 2.
BRS for ascending and descending sequences and high-frequency α at the baseline, 6 weeks and 6-month assessments for children with severe OSA, mild OSA, and control subjects. Data are hourly means (SEM). P values reflect the results of testing for monotonic increasing behavior during the night.
Controls continued to show monotonic increases for ascending sequences descending sequences and high-frequency α at 6 weeks and 6 months following the baseline evaluation (Figure 2). Low-frequency α did not demonstrate consistent changes during the night in any group at either the baseline or 6-month assessments
DISCUSSION
Our results demonstrate that children with severe OSA experience after adenotonsillectomy improvement in BRS and decrease in BP variability in the high-frequency range during wakefulness and during sleep. An improvement in BRS in the low frequency range was also demonstrated during wakefulness. Both systolic and diastolic BP also decreased during sleep postoperatively. The increase in baroreflex sensitivity was predicted by declines in measures of OSA severity after surgery, namely the change in apnea hypopnea and arousal indices. Moreover, a pattern of rising baroreflex sensitivity over the course of the night, similar to that observed in healthy controls, was restored after surgery in children with severe OSA. These findings, observed in the absence of any changes in baroreflex sensitivity among control subjects during the study period, support the concept that OSA might play a causal role in the development of baroreflex dysfunction in children. Despite the significant improvement in baroreceptor sensitivity over a period of 6 months, it remained lower than healthy controls.
The changes in baroreceptor sensitivity and prolongation of RR interval after treatment of OSA suggest that both reflex and vagal modulation of heart rate increase with the resolution of sleep apnea. The final product of the control of heart rate observed in this study suggests that the balance between the sympathetic and parasympathetic favors parasympathetic predominance with the resolution of sleep apnea. Several models of baroreceptors modulation of sympathetic output have been proposed. One model that has general acceptance is that baroreceptor stimulation has a direct inhibitory effect of sympathetic discharge. 17,18 It is therefore plausible that in our study population, decrease in baroreceptor sensitivity contributed to sympathetic dominance in the presence of sleep apnea and parasympathetic predominance after adenotonsillectomy. Muzumdar et al. 19 and Kaditis et al. 20 described an increase in parasympathetic predominance after treatment of sleep apnea, leading to an overall decrease in heart rate and increase in heart rate variability in the high-frequency range. Their findings concur with our own observation, which showed an increase in vagal tone modulation of heart rate after adenotonsillectomy. However, the magnitude of the decrease in heart rate seen in our study population was smaller than that described by both authors. Likely reasons for the disagreement in the findings during sleep might be due to the younger age and the more severe sleep apnea and hypoxia in their study population and the statistical analysis approach based on paired t-tests.
In previous report, we have shown that baroreceptor sensitivity in the low-frequency range in children with moderate to severe sleep apnea was significantly lower during sleep than that of healthy controls. 8 While the change after adenotonsillectomy in baroreceptor sensitivity in the low frequency range described in this report was small and not statistically significant, it was enough to approximate that of healthy controls.
The results of the spectral analysis indicate that the baroreceptor sensitivity in the respiratory frequency (high-frequency range) is the predominant abnormality observed in children with OSA, and that the main improvement in cardiovascular reflex with the resolution of sleep apnea occurs within that frequency. These data reinforce previous findings about the coupling between the triad that includes respiratory drive, baroreceptor sensitivity, and sympathetic discharge. 21–24 The presence of a respiratory rhythm in sympathetic nerve activity has been well described and documented. Sympathetic neurons to the heart are activated during early inspiration with a post-inspiratory or early expiratory inhibition. The magnitude of the respiratory modulation is proportional to respiratory drive. 22,25 However, the sympathetic excitability by the central respiratory drive is opposed by vagal lung inflation that exerts an opposite effect on central sympathetic excitability. 24 Thus, the dynamic changes in baroreceptor-sympathetic coupling during the respiratory cycle are due to the continuous change of central respiratory drive and vagal lung inflation afferent activity. Based on our findings, we postulate that disturbance in the respiratory-baroreceptor coupling is likely explanation for baroreceptor dysfunction in children with OSA.
The abnormalities in baroreceptor sensitivity described in this report both during wakefulness and during sleep are present during periods of spontaneous respiration that are free from any obstructive events. Further, baroreceptor sensitivity continues to gradually improve from 6 weeks to 6 months postoperatively but fail to completely normalize. These seminal findings raise the question about whether OSA simply causes resetting of the baroreceptor or whether it induces structural remodeling in the network of neurons, which regulate this cardiovascular reflex. Further investigation is needed to determine whether the degree of severity of OSA described in this report actually contributed to abnormal structure of the baroreceptor centers in the brain.
Although there is considerable evidence supporting the association between OSA and autonomic dysfunction in adults, 26–28 only a small number of studies have investigated this relationship in children. In a study assessing heart rate variability during sleep through spectral analysis, Baharav et al. demonstrated impaired vagal control of HR and a shift towards sympathetic predominance in children with OSA compared to control subjects. 29 Increased sympathetic vasomotor tone has also been described in children with OSA. 30 Blunted baroreflex sensitivity has been demonstrated during wakefulness and sleep in children with moderate to severe OSA. 8,31 The present study is the first in children to demonstrate the clinical course over 6-month period of the improvement of baroreceptor sensitivity after resolution of OSA.
The significance of our results stems from evidence that blunted baroreflex sensitivity and associated increases in BP variability are risk factors for cardiovascular morbidity. In a prospective study investigating risk factors for the development of hypertension in healthy adults, lower baroreflex sensitivity at study entry was found to independently predict the onset of hypertension over a 5-year period. 32 In another prospective population-based study of ambulatory BP in more than 1500 adults, a linear relationship was observed between daytime SBP variability and relative risk for cardiovascular mortality. 33 In children, decreased baroreflex sensitivity has also been described in pre-hypertensive children, 3 a group at risk for progressing to hypertension in adulthood. Taken together, these findings suggest that baroreflex dysfunction may predispose to the development of hypertension.
Others and we have demonstrated that increased BP variability is associated with end-organ remodeling independently of mean 24-hour BP. 34,8 We previously demonstrated an association between increasing BP and BP variability and left ventricular remodeling in normotensive children with OSA. 8 Collectively, these observations suggest that baroreflex dysfunction in children with OSA could plausibly increase risk for the early development of cardiovascular disease.
Some of our findings differ from animal and human studies. Specifically, we found that the decrease in the frequency of episodes of 3% oxygen desaturation after surgery was not associated with changes in baroreflex sensitivity. This finding is at odds with results from animal studies demonstrating that chronic intermittent hypoxia leads to decreased baroreflex sensitivity. 35,36 In the majority of these studies, episodes of hypoxia were more frequent and more severe compared to those experienced by most children with OSA. Additionally, Ryan et al. 37 observed that the frequency of 4% desaturations predicts decreased nocturnal baroreflex sensitivity in adults with OSA. Compared to the study from Ryan and colleagues, subjects in our study had a much lower frequency of hypoxic events. We therefore speculate that a threshold exists for the frequency and/or severity of hypoxic events that is necessary to result in depressed baroreflex sensitivity.
Several strengths of our study merit discussion. In our analyses, we adjusted for confounding factors, such as age and BMI, which are known to impact baroreflex function. 38 In this manner, we were able to discern the independent effect of OSA treatment on baroreflex sensitivity. Control subjects underwent assessment of baroreflex sensitivity on two occasions, at a similar interval as children with OSA before and after adenotonsillectomy. We thereby accounted for any normal changes in baroreflex function that may occur over time. Finally, all subjects had PSG performed, thus enabling determination of changes in measures of OSA severity over the study period. This information permitted analyses demonstrating that declines in OSA severity predict improvement in baroreflex sensitivity.
An inherent limitation in assessing baroreflex sensitivity during sleep is the inability to control for differences in respiration between individuals and across sleep-wake states. 39 In an attempt to minimize the effect of differences in respiration between groups and in children with OSA before versus after surgery, we excluded segments of the recordings that coincided with obstructive events. However, we cannot exclude the possibility that persistent differences in respiratory pattern may have confounded our results. Another limitation that is not uncommon in sleep disorders clinical trials is the presence of missing data due to attrition. 15 In order to remedy this limitation, we performed sensitivity analyses to investigate whether the intervention effect of those who missed visits may be similar to the effect of subjects who remained in the study. We first compared attrition patterns for each of the groups with respect to baseline clinical and demographic characteristics of subjects. Secondly, we analyzed the changes in baroreceptor sensitivity in both available and complete cases. The finding of identical results from both analyses demonstrate the internal validity of the study
CONCLUSION
This study demonstrates that children with OSA experience significant but only partial improvement in baroreflex sensitivity after treatment of OSA by adenotonsillectomy. This improvement was accompanied by decreases in BP and BP variability and decrease in heart rate. Our results suggest that autonomic impairment may be an important early mechanism leading to cardiovascular morbidity in OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Drs. Somers and Daniels have received funding from the National Institute of Health. Dr. Somers has received consulting fees from NeuroPro, Johnson and Johnson, Apria Medical, Respicardia, ResMed, Medtronics, Philips Respironics Foundation, and Deshum. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by grants 1R01HL070907, 1R01HL080670, MOI RR 08084, and an ATS Fellow Career Development Award.
Footnotes
A commentary on this article appears in this issue on page 1311.
REFERENCES
- 1.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. New Engl J Med. 2000;342:1378–84. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Genovesi S, Pieruzzi F, Giussani M, et al. Analysis of heart period and arterial pressure variability in childhood hypertension: key role of baroreflex impairment. Hypertension. 2008;51:1289–94. doi: 10.1161/HYPERTENSIONAHA.107.109389. [DOI] [PubMed] [Google Scholar]
- 4.Lucini D, Mela GS, Malliani A, Pagani M. Impairment in cardiac autonomic regulation preceding arterial hypertension in humans: insights from spectral analysis of beat-by-beat cardiovascular variability. Circulation. 2002;106:2673–9. doi: 10.1161/01.cir.0000039106.89299.ab. [DOI] [PubMed] [Google Scholar]
- 5.Amin RS, Carroll JL, Jeffries JL, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–6. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 6.Amin RS, Kimball TR, Bean JA, et al. Left ventricular hypertrophy and abnormal ventricular geometry in children and adolescents with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;165:1395–9. doi: 10.1164/rccm.2105118. [DOI] [PubMed] [Google Scholar]
- 7.Lucini D, Zuccotti G, Malacarne M, et al. Early progression of the autonomic dysfunction observed in pediatric type 1 diabetes mellitus. Hypertension. 2009;54:987–94. doi: 10.1161/HYPERTENSIONAHA.109.140103. [DOI] [PubMed] [Google Scholar]
- 8.McConnell K, Somers VK, Kimball T, et al. Baroreflex gain in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180:42–8. doi: 10.1164/rccm.200808-1324OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254:H377–83. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- 10.Pagani M, Malfatto G, Pierini S, et al. Spectral analysis of heart rate variability in the assessment of autonomic diabetic neuropathy. J Auton Nerv Syst. 1988;23:143–53. doi: 10.1016/0165-1838(88)90078-1. [DOI] [PubMed] [Google Scholar]
- 11.Standards and indications for cardiopulmonary sleep studies in children. American Thoracic Society. Am J Respir Crit Care Med. 1996;153:866–78. doi: 10.1164/ajrccm.153.2.8564147. [DOI] [PubMed] [Google Scholar]
- 12.Parati G, Di Rienzo M, Bertinieri G, et al. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–22. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999-2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 14.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–92. [Google Scholar]
- 15.Olsen MK, Stechuchak KM, Edinger JD, Ulmer CS, Woolson RF. Move over LOCF: principled methods for handling missing data in sleep disorder trials. Sleep Med. 2012;13:123–32. doi: 10.1016/j.sleep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Hirotsu C, Srivastava MS. Simultaneous confidence intervals based on one-sided max t test. Stat Probab Lett. 2000;49:25–37. [Google Scholar]
- 17.McAllen RM, Malpas SC. Sympathetic burst activity: characteristics and significance. Clin Exp Pharmacol Physiol. 1997;24:791–9. doi: 10.1111/j.1440-1681.1997.tb02693.x. [DOI] [PubMed] [Google Scholar]
- 18.Malpas SC, Bendle RD, Head GA, Ricketts JH. Frequency and amplitude of sympathetic discharges by baroreflexes during hypoxia in conscious rabbits. Am J Physiol. 1996;271:H2563–74. doi: 10.1152/ajpheart.1996.271.6.H2563. [DOI] [PubMed] [Google Scholar]
- 19.Muzumdar HV, Sin S, Nikova M, Gates G, Kim D, Arens R. Changes in heart rate variability after adenotonsillectomy in children with obstructive sleep apnea. Chest. 2011;139:1050–9. doi: 10.1378/chest.10-1555. [DOI] [PubMed] [Google Scholar]
- 20.Kaditis AG, Chaidas K, Alexopoulos EI, Varlami V, Malakasioti G, Gourgoulianis K. Effects of adenotonsillectomy on R-R interval and brain natriuretic peptide levels in children with sleep apnea: a preliminary report. Sleep Med. 2011;12:646–51. doi: 10.1016/j.sleep.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 21.Kocsis B, Gebber GL, Barman SM, Kenney MJ. Relationships between activity of sympathetic nerve pairs: phase and coherence. Am J Physiol. 1990;259:R549–60. doi: 10.1152/ajpregu.1990.259.3.R549. [DOI] [PubMed] [Google Scholar]
- 22.Boczek-Funcke A, Dembowsky K, Habler HJ, Janig W, McAllen RM, Michaelis M. Classification of preganglionic neurones projecting into the cat cervical sympathetic trunk. J Physiol. 1992;453:319–39. doi: 10.1113/jphysiol.1992.sp019231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebber GL, Das M, Barman SM. An unusual form of phase walk in a system of coupled oscillators. J Biol Rhythms. 2004;19:542–50. doi: 10.1177/0748730404268053. [DOI] [PubMed] [Google Scholar]
- 24.Gebber GL, Das M, Barman SM. Dynamic changes in baroreceptor-sympathetic coupling during the respiratory cycle. Brain Res. 2005;1046:216–23. doi: 10.1016/j.brainres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Barman SM, Gebber GL. Basis for synchronization of sympathetic and phrenic nerve discharges. Am J Physiol. 1976;231:1601–7. doi: 10.1152/ajplegacy.1976.231.5.1601. [DOI] [PubMed] [Google Scholar]
- 26.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonsignore MR, Parati G, Insalaco G, et al. Continuous positive airway pressure treatment improves baroreflex control of heart rate during sleep in severe obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:279–86. doi: 10.1164/rccm.2107117. [DOI] [PubMed] [Google Scholar]
- 28.Belozeroff V, Berry RB, Sassoon CS, Khoo MC. Effects of CPAP therapy on cardiovascular variability in obstructive sleep apnea: a closed-loop analysis. Am J Physiol Heart Circ Physiol. 2002;282:H110–21. doi: 10.1152/ajpheart.2002.282.1.H110. [DOI] [PubMed] [Google Scholar]
- 29.Baharav A, Kotagal S, Rubin BK, Pratt J, Akselrod S. Autonomic cardiovascular control in children with obstructive sleep apnea. Clin Auton Res. 1999;9:345–51. doi: 10.1007/BF02318382. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien LM, Gozal D. Autonomic dysfunction in children with sleep-disordered breathing. Sleep. 2005;28:747–52. doi: 10.1093/sleep/28.6.747. [DOI] [PubMed] [Google Scholar]
- 31.Chaicharn J, Lin Z, Chen ML, Ward SL, Keens T, Khoo MC. Model-based assessment of cardiovascular autonomic control in children with obstructive sleep apnea. Sleep. 2009;32:927–38. doi: 10.1093/sleep/32.7.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ducher M, Fauvel JP, Cerutti C. Risk profile in hypertension genesis: A five-year follow-up study. Am J Hypertens. 2006;19:775–80. doi: 10.1016/j.amjhyper.2005.07.019. discussion 81. [DOI] [PubMed] [Google Scholar]
- 33.Kikuya M, Hozawa A, Ohokubo T, et al. Prognostic significance of blood pressure and heart rate variabilities: the Ohasama study. Hypertension. 2000;36:901–6. doi: 10.1161/01.hyp.36.5.901. [DOI] [PubMed] [Google Scholar]
- 34.Tatasciore A, Renda G, Zimarino M, et al. Awake systolic blood pressure variability correlates with target-organ damage in hypertensive subjects. Hypertension. 2007;50:325–32. doi: 10.1161/HYPERTENSIONAHA.107.090084. [DOI] [PubMed] [Google Scholar]
- 35.Lai CJ, Yang CC, Hsu YY, Lin YN, Kuo TB. Enhanced sympathetic outflow and decreased baroreflex sensitivity are associated with intermittent hypoxia-induced systemic hypertension in conscious rats. J Appl Physiol. 2006;100:1974–82. doi: 10.1152/japplphysiol.01051.2005. [DOI] [PubMed] [Google Scholar]
- 36.Soukhova-O'Hare GK, Cheng ZJ, Roberts AM, Gozal D. Postnatal intermittent hypoxia alters baroreflex function in adult rats. Am J Physiol Heart Circ Physiol. 2006;290:H1157–64. doi: 10.1152/ajpheart.00767.2005. [DOI] [PubMed] [Google Scholar]
- 37.Ryan S, Ward S, Heneghan C, McNicholas WT. Predictors of decreased spontaneous baroreflex sensitivity in obstructive sleep apnea syndrome. Chest. 2007;131:1100–7. doi: 10.1378/chest.06-2165. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich A, Riese H, van Roon AM, et al. Spontaneous baroreflex sensitivity in (pre)adolescents. J Hypertens. 2006;24:345–52. doi: 10.1097/01.hjh.0000200517.27356.47. [DOI] [PubMed] [Google Scholar]
- 39.Jo JA, Blasi A, Valladares E, Juarez R, Baydur A, Khoo MC. Model-based assessment of autonomic control in obstructive sleep apnea syndrome during sleep. Am J Respir Crit Care Med. 2003;167:128–36. doi: 10.1164/rccm.200202-096OC. [DOI] [PubMed] [Google Scholar]