Abstract
We investigated the vascular transport properties of exogenously applied proteins to Brassica oleracea plants and compared their delivery to various aerial parts of the plant with carboxy fluorescein (CF) dye. We identified unique properties for each protein. Alexafluor-tagged bovine serum albumin (Alexa-BSA) and Alexafluor-tagged Histone H1 (Alexa-Histone) moved slower than CF dye throughout the plant. Interestingly, Alexa-Histone was retained in the phloem and phloem parenchyma while Alexa-BSA moved into the apoplast. One possibility is that Alexa-Histone sufficiently resembles plant endogenous proteins and is retained in the vascular stream, while Alexa-BSA is exported from the cell as a foreign protein. Both proteins diffuse from the leaf veins into the leaf lamina. Alexa-BSA accumulated in the leaf epidermis while Alexa-Histone accumulated mainly in the mesophyll layers. Fluorescein-tagged hepatitis C virus core protein (fluorescein-HCV) was also delivered to B. oleracea plants and is larger than Alexa-BSA. This protein moves more rapidly than BSA through the plant and was restricted to the leaf veins. Fluorescein-HCV failed to unload to the leaf lamina. These combined data suggest that there is not a single default pathway for the vascular transfer of exogenous proteins in B. oleracea plants. Specific protein properties appear to determine their destination and transport properties within the phloem.
Keywords: fluorescent proteins, phloem, protein transport, symplastic transport, vascular loading, vascular transport
Introduction
The vasculature of higher plants provides interorgan transfer of nutrients and signaling molecules needed for plant development or environmental responses. The vascular system consists of phloem and xylem. The phloem provides unidirectional transport of photo assimilates, proteins, mRNAs, small RNAs, signaling molecules and other micronutrients to young developing tissues and meristematic regions.1,2 The xylem mainly carries water and micronutrients which can also circulate to the phloem.
Plants in Brassica family, with close relationship to the fully sequenced model plant Arabidopsis thaliana, are employed for studying phloem biology and there are large numbers of vascular bundles throughout the stem and this enables researchers to collect significant volumes of phloem sap for proteomic studies.3 Phloem exudates are often collected through aphid stylets and have been shown to contain several classes of proteins that are important for sugar transport, detoxifying reactive oxygen species, regulating protein turnover and defense to pathogens and insects.4 While, the presence of proteins and nucleic acids in phloem sap has been revealing, we do not know if they are mobile through the sieve elements or if their translocation are essential.3,5 FT is one example of an endogenous protein that is transported long distance to the apical meristems where they contribute to cell fate determination. Such a mechanism of transport is “selective”, since FT is specifically targeted to the meristem and not all phloem proteins unload there.6
More studies employed phloem specific promoters such as the AtSUC-2 to express GFP or GFP fusions in transgenic plants.7 These studies have provided valuable information concerning local translocation of proteins in leaf veins and post-phloem transport.7,8 Plant viral movement proteins are also exogenous proteins and carry RNA across companion cells (CC) into phloem sieve elements (SE).9,10 Unlike GFP, plant viral movement proteins (MPs) engage with plasmodesmata and gate open these channels to move from cell-to-cell and then enter the CC-SE complex. While GFP diffuses across most cell layers, viral MPs are selectively excluded from certain tissues. For example, phloem limited viruses traffic from the CC-SE complex to phloem parenchyma and bundle sheath cells, but cannot enter the mesophyll layer.11,12 The bundle sheath provides a cellular boundary for protein export. Therefore exogenous proteins have properties that affect their ability to enter and exit the phloem.
In this study we decided to compare the phloem transport of three exogenous proteins applied to B. oleracea petioles and roots. The B. oleracea petiole is triangular in shape and has a single U-shaped layer of collateral vascular bundles. We decided to use B. oleracea to assess whether the arrangement of vascular bundles in the leaf petioles is a factor in the rate and quality of mass flow from the stem into the leaf. We recently published research using N. benthamiana, which has a central vascular stele. In this work,13 we compared the transport of carboxy fluorescein dye, green fluorescent protein (GFP), plant viral movement proteins, commercially available bovine serum albumin, histone H1 (from calf thymus) and hepatitis virus C (HCV) core protein. The outcomes suggest that not all exogenous proteins have similar abilities to exit the translocation stream or share the same fate in sink tissues. Given that the vascular arrangement of B. oleracea is unlike N. benthamiana, the goal of this work was to learn if the ability to differentiate exogenously applied proteins with respect to transport and post phloem sorting is conserved across plant genera regardless of vascular arrangement.
Results
Measuring the transfer of fluorescence intensity from the loading site into upper leaves
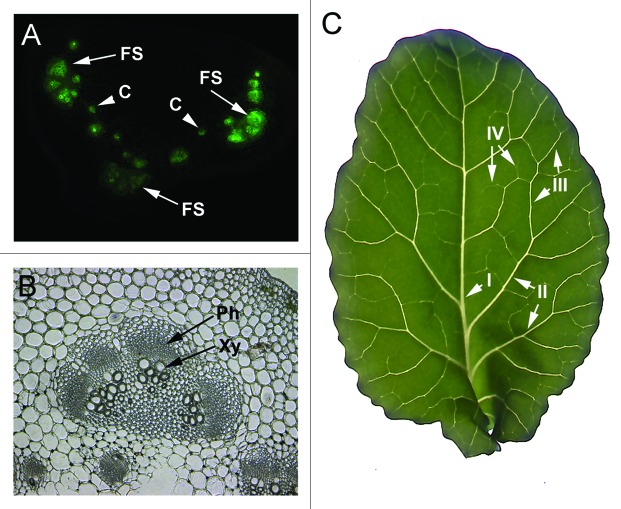
B. oleracea has two classes of vascular bundles in the petiole. There are usually three to four large fan-shaped bundles found in all leaf petioles (Fig. 1A), and this does not vary with leaf maturation. There are two to seven smaller circular bundles per cross section (Fig. 1A) and these vary in number with maturation. The phloem occurs on the abaxial side of the xylem (Fig. 1B and C).
Figure 1.
The vascular pattern of B. oleracea plants. (A) L 4 petiole cross sections following treatment with CF dye. Fluorescence highlights the vascular bundles. Arrows point to examples of fan-shaped vascular bundles (FS) and arrowheads point to examples of small circular bundles (C). (B) Light microscopic image of FS vascular bundle. The bottom of the panel shows two small C bundles. The arrows point to the phloem (Ph) and xylem (Xy). (C) Image of B. oleracea leaf shows pinnate venation patterns with a prominent midrib that extends from the petiole to the top leaf apex. The mid rib is also known as class I vein. Class II veins branch from class I, class III branch from class II and class IV branch from class III. Leaf vein classes I and II intersect with the leaf margins, which is known as a craspedodromous subtype of venation.
Commercially available Alexafluor488-BSA, Alexafluor488-Histone H1 (0.3 mg/ml) (here called Alexa-BSA and Alexa-Histone) and CF dye (60 μg/ml) were applied to either the L1 petiole14 or roots of B. oleracea plants. Importantly, we employed concentrations of dye and protein which produced similar absorbance values. Dyes were placed in small eppendorf tubes and affixed to the cut petiole surface or roots using parafilm to hold the tubes in place. Leaves are numbered L1 to L5 in their order of emergence above the soil, L1 is the mature source leaf that lies closest to the soil surface and L5 is the youngest sink leaf to emerge. In reports when CF dye is applied to the cut L1 petiole, the dye follows the same route through the phloem as photo assimilates (Fig. 1C) and unloads in sink leaves.14 On occasions CF dye enters the xylem.14
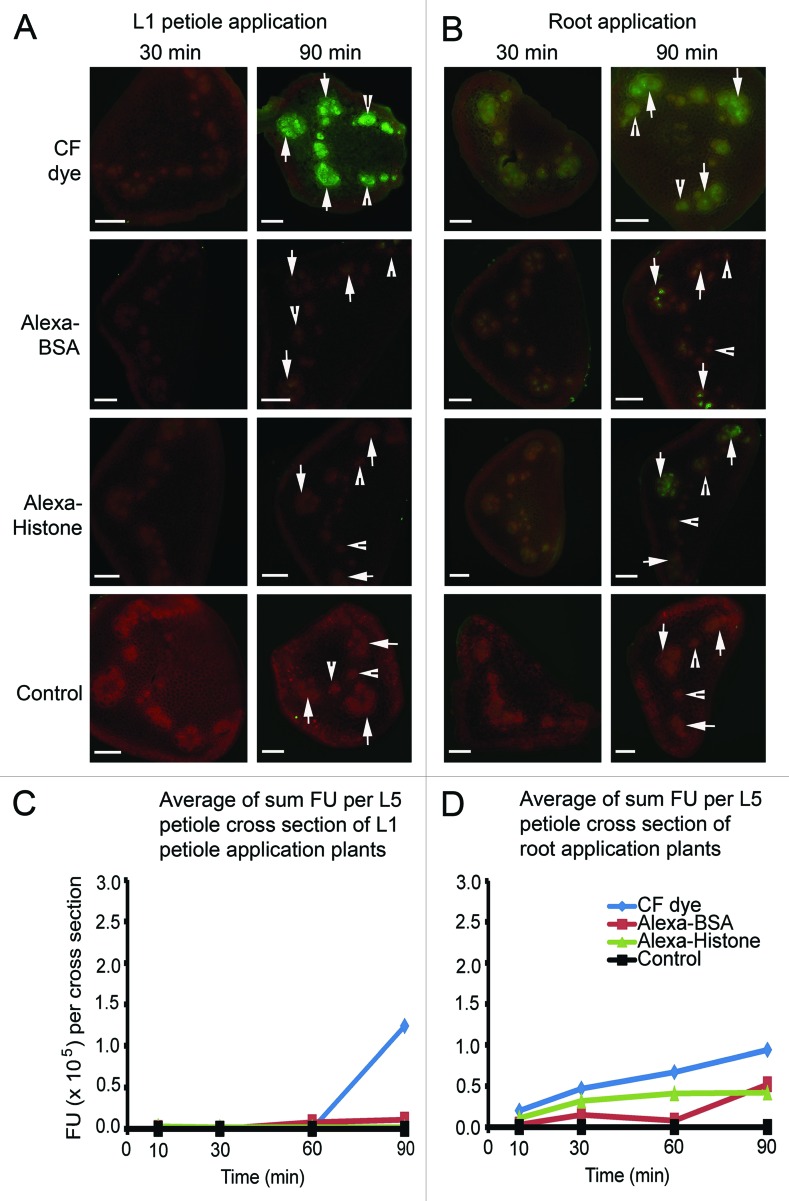
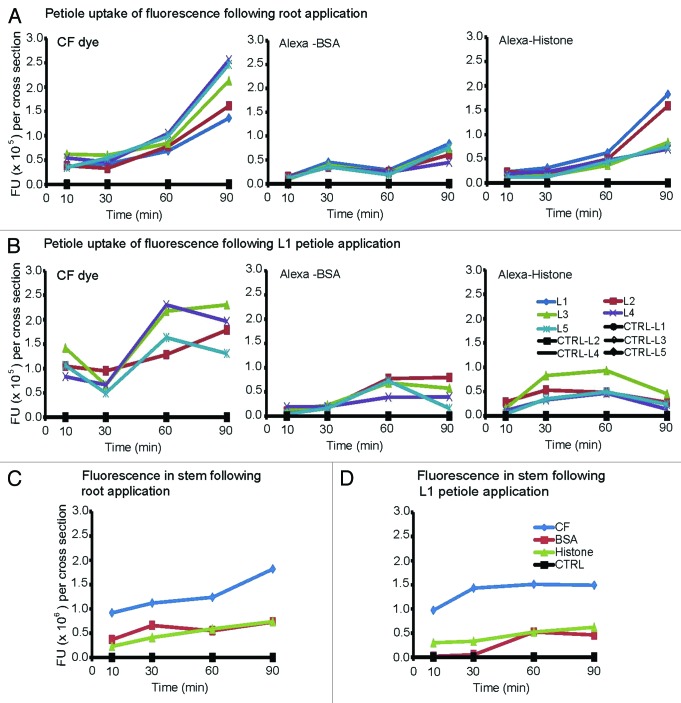
To record protein and dye transfer to the upper leaves of B. oleracea plants, 0.5 mm sections were cut from the petioles of each upper leaf at 10, 30, 60 and 90 min. Digital images of the cross sections were recorded using epifluorescence microscopy. We monitored fluorescence in large fan-shaped bundles (Fig. 2A and B arrows) and small circular bundles (Fig. 2A and B arrowheads). The pattern of fluorescence was specific to the dye or proteins applied to the plant. For the initial comparison we focused our attention on the L5 petioles (Fig. 2C and D) and at 30 and 90 min there were important differences among the treatments (Fig. 2A and B).
Figure 2.
Images show the average fluorescence intensity of L5 petiole cross sections following L1 petiole (A) or root (B) application at 30 and 90 min. Plants were treated with CF dye, Alexa Fluor 488 BSA and Alexa Fluor 488 Histone H1. Bar = 500 μm. Arrows point to fan-shaped bundles and arrowheads point to small circular bundles. (C) and (D) graphically depict the average fluorescence values per cross section (n = 4). Fluorescence values of three fan-shaped bundles and two circular bundles per petiole cross section were measured using ImageJ software and were added as a sum measure of fluorescence. All values reach a maximum at 90 min. Uptake of Alexa-BSA and Alexa-Histone are higher when delivered to the roots than to the L1 petiole. All protein values were lower than CF dye.
When CF dye is applied to L1 petioles, dim fluorescence appears at 30 min in upper leaf petioles but reaches a maximum at 90 min in excised L5 petioles. Regardless of whether CF dye is applied to the L1 petiole or root, equal fluorescence intensities occur in all vascular bundles,15,16 irrespective of their dimensions (Fig. 2A and B, Table 1). Alexa-BSA and Alexa-Histone are distributed among fan shaped and circular bundles (Table 1) in the treated plants although the levels of fluorescence following L1 petiole application were lower than with CF dye (Fig. 2). Following application of Alexa-BSA or Alexa-Histone to B. oleracea roots, fluorescence spreads more rapidly to aerial parts of the plant (Fig. 2B and Table 1) but the values were less than reported for CF dye. These data suggest that diffusion of Alexa-BSA and -Histone in the phloem of B. oleracea is slower than CF dye, perhaps because they are significantly larger molecules. There does not appear to be significant fluorescence in surrounding nonvascular tissues (Fig. 2A and B). Table 1 compares the average fluorescence values of fan-shaped and circular bundles obtained from petiole cross sections at 30, 60 and 90 min. There was no evidence of preferred transport via the fan-shaped or circular bundles suggesting that the veins are functionally equivalent for dye and protein transport
Table 1. Average FU per mm3 of fan shaped bundles and circular bundles of L5 petiole at different time points. ( × 105 FU mm-3).
| Fan shaped bundles | Circular bundles | ||||||
|---|---|---|---|---|---|---|---|
| |
|
30 min |
60 min |
90 min |
30 min |
60 min |
90 min |
| Soil-petiole Appl. |
CF dye |
0.0 ± 0.0 |
0.0 ± 0.0 |
6.4 ± 1.3 |
0.1 ± 0.1 |
0.1 ± 0.1 |
8.8 ± 2.5 |
| Alexa-BSA |
0.2 ± 0.2 |
0.1 ± 0.1 |
0.8 ± 0.4 |
0.2 ± 0.2 |
0.9 ± 0.2 |
0.4 ± 0.4 |
|
| Alexa-Histone |
0.2 ± 0.0 |
0.2 ± 0.1 |
0.1 ± 0.1 |
0.1 ± 0.1 |
0.1 ± 0.1 |
0.2 ± 0.2 |
|
| Soil-root Appl. |
CF dye |
2.6 ± 1.8 |
4.6 ± 0.9 |
4.1 ± 1.7 |
2.6 ± 0.6 |
4.4 ± 1.6 |
3.9 ± 1.9 |
| Alexa-BSA |
0.8 ± 0.4 |
0.2 ± 0.2 |
3.4 ± 1.3 |
1.1 ± 0.1 |
0.9 ± 0.1 |
2.5 ± 1.1 |
|
| Alexa-Histone | 2.4 ± 1.4 | 2.7 ± 1.0 | 2.8 ± 0.1 | 0.8 ± 0.4 | 2.1 ± 0.9 | 1.2 ± 0.5 | |
The fluorescent values of three fan-shaped bundles and two circular bundles per petiole cross section were measured using ImageJ software and were added as a sum measure of fluorescence in each cross section. The average (n = 5) fluorescence units (FU) per cross section were plotted (Fig. 2C and D). Movement of CF dye, Alexa-BSA, or Alexa-Histone from the site of application in the L1 petiole to L5 sink leaves was slow. CF dye accumulation in L5 vascular strands at 90 min (1.24 × 105 FU) and was higher than Alexa-BSA or Alexa-Histone (0.11 × 105 FU) (Fig. 2C). Notably, if we compare these values reported in Figure 1C with images in Figure 1A, we can conclude that saturating levels fluorescence measure > 1 × 105 FU.
This is contrasted by the higher levels of CF dye or proteins at 30 min (0.47 × 105 FU) in the L5 petiole following application to plant roots (Fig. 1D). The CF dye values continued to increase until 90 min to 0.94 × 105 FU, which is slightly less than the values reported following petiole application. Alexa-BSA fluorescence was low following delivery to the roots and biphasic kinetics is also noted. Interestingly, the first phase of Alexa-BSA transfer to the leaves is within the first 30 min (0.15 × 105 FU) and then a second phase of transfer is between 60 and 90 min (0.52 × 105 FU). Alexa-Histone transfer to upper leaves also occurs within the first 30 min and remains at a plateau (0.42 × 105 FU) (Fig. 2D).
CF dye, Alexa-BSA and Alexa-Histone vary in their accumulation in petiole sections. Alexa-BSA follows a slow biphasic uptake in the vascular bundles while Alexa-Histone uptake reaches an earlier maximum. Both proteins accumulate to a level that is approximately 50% of CF dye following root application (Fig. 2D) while phloem transport following L1 petiole application is more limited. These data suggest that neither phyllotaxy nor sap flow are the sole factors governing their accumulation in leaf petioles. Perhaps the physical properties of the fluorescent dye and proteins (tertiary structure, charge, molecular mass) influence their mobility.
Protein accumulation in symplast and apoplast
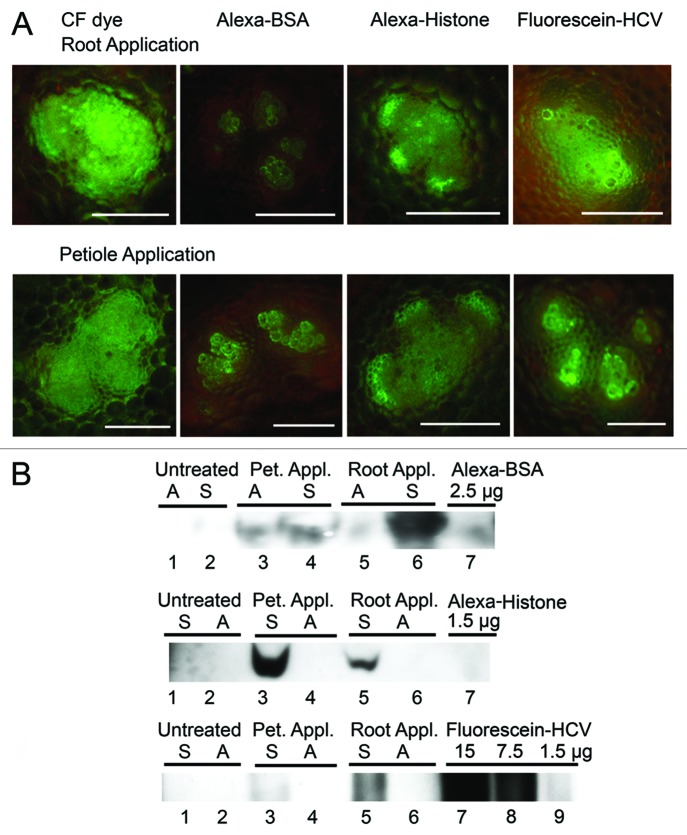
The obvious plateau or decline phases during the 90 min interval (Fig. 2C and D) led us to examine whether fluorescence diffuses into neighboring parenchyma and xylem. Epifluorescence microscopic images of individual fan-shaped vascular bundles were taken using petiole cross sections following application of fluorophores to plant roots or L1 petioles (Fig. 3A and B). Following root application, the images showed some movement of CF dye beyond the vascular bundle into surrounding parenchyma cells although much greater fluorescence was seen surrounding cells following L1 petiole application (Fig. 3A). Alexa-BSA were seen mainly in xylem tracheary elements when it is applied to either the roots or L1 petioles (Fig. 3A and B). These observations also correlate with the low level of trafficking depicted graphically in Figure 2C and D. Alexa-Histone fluorescence occurs throughout the vascular bundle with some fluorescence in surrounding parenchyma (Fig. 3A and B). Fluorescence does not appear in intercellular spaces suggesting that Alexa-Histone follows a symplastic route for long-distance transport.
Figure 3.
Analysis of symplastic and apoplastic accumulation of fluorescent dyes and proteins in B. oleracea leaves. (A) Confocal microscopic images of CF dye, Alexa-BSA and Alexa-Histone H1 in fan-shaped bundles in petiole cross sections following root application or L1 petiole application. Bar = 50μm. CF dye fills the vascular bundle and spreads to surrounding tissues. Alexa-BSA was mainly in the tracheary elements of the xylem. Alexa-Histone spreads beyond the phloem into parenchyma cells. Fluorescence-HCV spreads further than Alexa-BSA but not as far as Alexa-Histone throughout the vascular bundle. Control samples were treated with buffer and show no fluorescence (not shown here). (B) Immunoblot probed with polyclonal antisera detecting BSA and Histone. Pet. Appl, petiole application; Root Appl, root application; M, marker; Plant endogenous histone H1 is not also detected by antisera. Apoplastic wash fluids (A) and cell extracts (S) were pooled from L3 and L4 leaves. The top immunoblot detects Alexa-BSA in both the A and S extracts. Alexa-BSA was directly loaded to gel and produces a band around 66 kDa (lane 7). The middle immunoblot detects Alexa-Histone in the S but not A wash fluid. Alexa-Histone was directly loaded to the gel and produces a band that is around 34 kD. The bottom immunoblot detects fluorescein-HCV in the S but not A fraction. Lanes 7–9 contain samples of fluorescein-HCV that were directly loaded to the gel. Loading amounts (μg) for Alexa-BSA and -Histone and fluorescein-HCV are indicated above the lanes.
To discover if Alexa-BSA or -Histone are exported to the apoplast, L3 and L4 leaves were infiltrated with 2-[N-morpholino]ethansesulfonic acid (MES) buffer and the apoplast wash fluids were pooled. Remaining tissue was ground and both samples were analyzed by SDS-PAGE (Fig. 3B) and silver staining or immunoblot. Alexa-BSA associated with both apoplast wash fluids and cellular extracts (Fig. 3B). Both the silver stained gel and immunoblot show that Alexa-BSA reaches the L3 and L4 petiole via a symplastic or apoplastic route. The distribution between the apoplast and symplast is similar following petiole application, but more protein is detected in the symplast following root application. This is hard to explain. In contrast, Alexa-Histone is mainly in symplastic tissue extracts and not in the apoplastic wash fluids (Fig. 2B). The antisera did not react with untreated samples indicating that it does not detect endogenous plant histone proteins (Fig. 3B).
Interestingly, Alexa-BSA was exported to the apoplast while Alexa-Histone was restricted to the symplast. These data suggest a sorting mechanism in the phloem. One possibility is that Alexa-Histone resembles plant endogenous histones and is recognized as an endogenous protein, whereas Alexa-BSA is clearly exogenous and this could trigger a mechanism for export to the apoplast. Further research is needed to accurately compare the destinations of endogenous and exogenous proteins.
Protein transfer to upper leaf petioles of soil grown B. oleracea plants
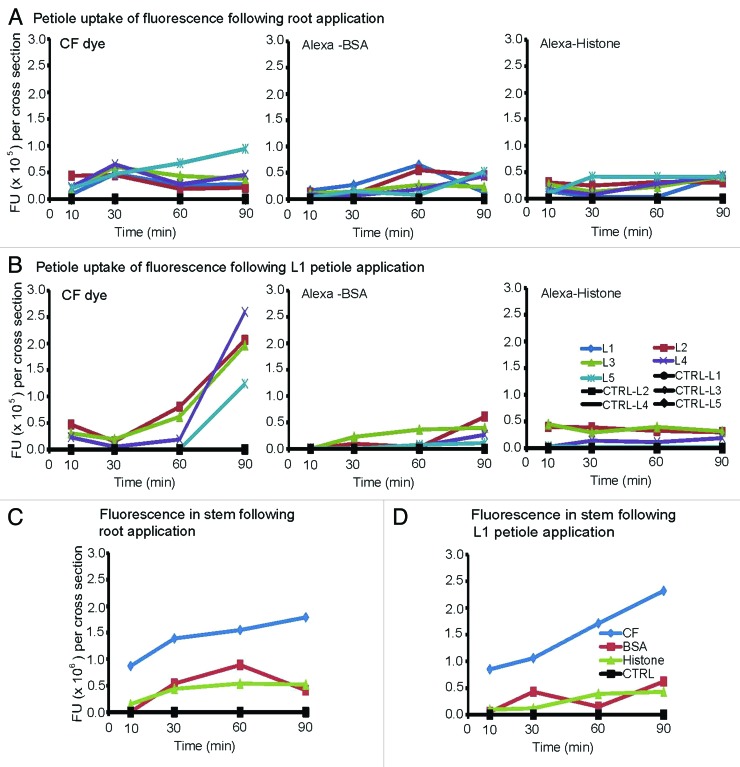
It is well established that phloem delivery of CF dye to an importing leaf is governed by phyllotaxy in N. benthamiana plants, whereby carbon-based molecules transferred via the phloem reaches the closest leaves sooner than distal leaves (Roberts et al., 1997).14 We undertook experiments to determine if this same principle applies in B. oleracea and whether exogenous proteins move to specific destinations or if their transport is also governed by phyllotaxy. Here the transport of CF dye, Alexa-BSA and Alexa-Histone was monitored at 10, 30, 60 and 90 min by examining cross sections of L1-L5 petioles. Control plants were treated with buffer only. The transport of CF dye, Alexa-BSA and Alexa-Histone was monitored at 10, 30, 60 and 90 min post-delivery by examining cross sections of L1-L5 petioles. Control plants were treated with buffer only. We measured the average FU per mm3 of three fan-shaped bundles and two circular bundles per petiole cross section using ImageJ software and plotted these values in Figure 4. We also measure the fluorescence intensity of stem cross sections.
Figure 4.
Fluorescence intensity of petiole and stem cross sections of soil-grown B. oleracea plants. Graphs depict the average fluorescence values in (n = 4) petiole or stem cross sections. The fan-shaped and circular vascular bundles were selected as ROIs using Image J software and fluorescence values were recorded. The data were collected at 10, 30, 60 and 90 min after application of CF dye, Alexa-BSA or -Histone.
The first observation is that CF dye, Alexa-BSA, or Alexa-Histone reached all leaves simultaneously when they are applied to B. oleracea roots. With respect to CF dye, fluorescence transfer to the youngest L5 leaf is continuous during the 90 min period (Fig. 4A and B). For most other leaves, fluorescence reaches a peak at 30 min followed by a slight decline. For Alexa-BSA fluorescence reaches a peak at 60 min in L1 and L2, which are closest to the soil surface. Alexa-BSA reaches younger leaves somewhat later, although all petiole values reach a maximum 0.65 × 105 FU. For Alexa-Histone, the youngest L5 leaf reaches a maximum at 30 min while all other leaves show maximum uptake by 10 min. The values remain fairly stable for the duration of the experiment. Thus transport of BSA is slower than CF dye in both phases.
Phyllotaxy mainly governs transport following root application and further investigations indicate that it is also a factor following L1 petiole application. For CF dye, fluorescence reaches L2, L3 and L4 leaves within 10 min following application (0.23–0.47 × 105 FUs) (Fig. 4B). There is a slight decline in all leaves and then a second increase in L2 and L3 between 30 and 90 min reaching 2.07 × 105 FUs. Fluorescence lags in the younger L4 and L5 leaves and a steep increase in transport is seen between 60 and 90 min with L4 reaching a greater maximum (2.6 × 105 FUs) than L5 (1.24 × 105 FUs) at 90 min. L2 is located on the opposite of L1 and L3 is located on the same side as L1 suggesting that the dye moves into the next neighboring leaves and is not governed by orthostichy.14,17 Alexa-BSA does not show significant improvement in its ability to move into distal leaves. Alexa-BSA has a low maximum (0.61–0.40 × 105 FUs) at 90 min with minimal protein reaching L5 leaves (0.11 × 105 FU). Movement of Alexa-Histone also produces low maximums with the greatest uptake occurring at 10 min following delivery. Fluorescence reaches L2 and L3 leaves first (0.3 × 105 FUs) and slowing increases in L4 and L5 leaves to a level that is barely detectable (0.19 and 0.02 × 105 FUs).
To assess the flow of fluorescence from the stems into the petioles, we compared the fluorescence intensities in both locations. Stem sections were cut just below the L1 petiole and the average fluorescence intensities were determined from several individual vascular rays. We predicted that similar levels in the stems and petioles would suggest that the flow is constant, not interrupted, between these two organs. If the fluorescence is lower in the petioles than in the stem, then dye or protein import into the leaves is regulated along the vascular traces diverging from the stem. If the fluorescence is higher in the petioles than in the stems, then the petioles and leaves likely present higher sink strength and the flow is increased into the petioles.
Figure 4C and D shows the fluorescence intensities of CF dye, Alexa-BSA and Alexa-Histone in stem cross sections. At 30 min, the levels of CF dye was 2- to 3-fold greater in the stem than in petioles following root and L1 petiole delivery. CF dye uptake was greater over time in the stems suggesting that the petiole restricts movement into the leaves. The levels of Alexa-BSA and Alexa-Histone were comparable to the maximal values reported in petioles following root or L1 petiole regardless of root or petiole application. These data argue that protein movement through the vasculature is not as extensive as dyes.
Protein transfer to upper leaves of hydroponically grown B. oleracea plants
Plants kept in hydroponic medium have greater water content and sap flow through the vasculature than plants grown in soil.18 If sap flow controls the timing and extent of dyes and proteins moving into the petioles then, we expect to see higher fluorescence in the petioles of plants maintained in hydroponic medium. We observed higher CF fluorescent values between 30 and 90 min in hydroponically grown plants when the dye is applied to the roots compared with soil grown plants. CF dye fluorescence reached the highest levels at 90 min in L4 and L5 petioles, followed by L3, L2 and L1 in that order (Fig. 5A). These data indicate CF dye moves with the sap from the roots the youngest leaves and then into progressively more mature leaves. Following root delivery of Alexa-BSA, the fluorescence trend lines were also changed although it appears to reach all leaf petioles at the same time. Among hydroponically grown plants, Alexa-BSA uptake is bi-phasic. There is an initial increase in fluorescence until 30 min followed by a slight decline and then a second increase occurs between 60 and 90 min. At 90 min the level of Alexa-BSA is higher in leaf petioles in hydroponically grown than soil grown plants. We also observed higher Alexa-Histone fluorescence values between 30 and 90 min in hydroponically grown plants compared with soil grown plants (Fig. 5A). Unlike CF-dye, Alexa-histone reached higher levels in the mature L1 and L2 leaves which are located closest to the soil surface with lesser amounts reaching younger leaves. The fluorescence values for Alexa-Histone in younger L3, L4 and L5 leaves are 3-fold less than in the mature leaves. Overall these data suggest that higher water application influences the pattern of CF dye, Alexa-BSA and Alexa-Histone transfer to all petioles although each presented unique distribution patterns that are likely to reflect properties of the dye and proteins.
Figure 5.
Fluorescence intensity of petiole cross sections of B. oleracea plants which were transferred to hydroponic medium. Graphs depict the average fluorescence values in the vascular bundles of (n = 4) petiole or stem cross sections. The fan-shaped and circular vascular bundles were selected as ROIs using Image J software and fluorescence values were recorded. The data were collected at 10, 30, 60 and 90 min after application of CF dye, Alexa-BSA or -Histone.
When CF dye was applied to L1 petioles of hydroponically grown plants, 2- to 3-fold greater fluorescence was seen at 10 and 60 min compared with when the dye is applied to the roots (Fig. 5B). Alexa-BSA also reaches a peak at 60 min in most leaves, which is earlier than following root application of hydroponically grown plants. Alexa-Histone reaches maximum fluorescence in leaves at 30 min, although these values are significantly lower than seen at 90 min following root application. If we compare the graphs in Figure 3B and 4B it appears that the hydroponic conditions influence the trends in fluorescence delivery to aerial parts of the plant following L1 petiole application. These data argue that water content in the vasculature enables the initial transfer of dye and proteins from source leaves to younger petioles.
The fluorescence intensities in stem cross sections of hydroponic plants were similar to the levels reported for soil grown plants. Although BSA is a larger protein compare with Histone, the uptake levels of Alexa-BSA was higher than Alexa-Histone during 0–60 min. Between 60 and 90 min the intensities of both proteins were comparable (Fig. 5C). The fluorescence intensity in stem already reached 5.0 × 106 FU during 0–60 min, but the intensity levels in petiole cross sections were low as 2.5 × 105 FU. These data suggest that protein unloading to the petioles is regulated following root application. Following L1 petiole application the levels of CF dye, Alexa-BSA and Alexa-Histone show similar trends between stem and petioles (Fig. 5B and D).
Unloading of CF dye, Alexa-BSA and Alexa-Histone in leaf veins
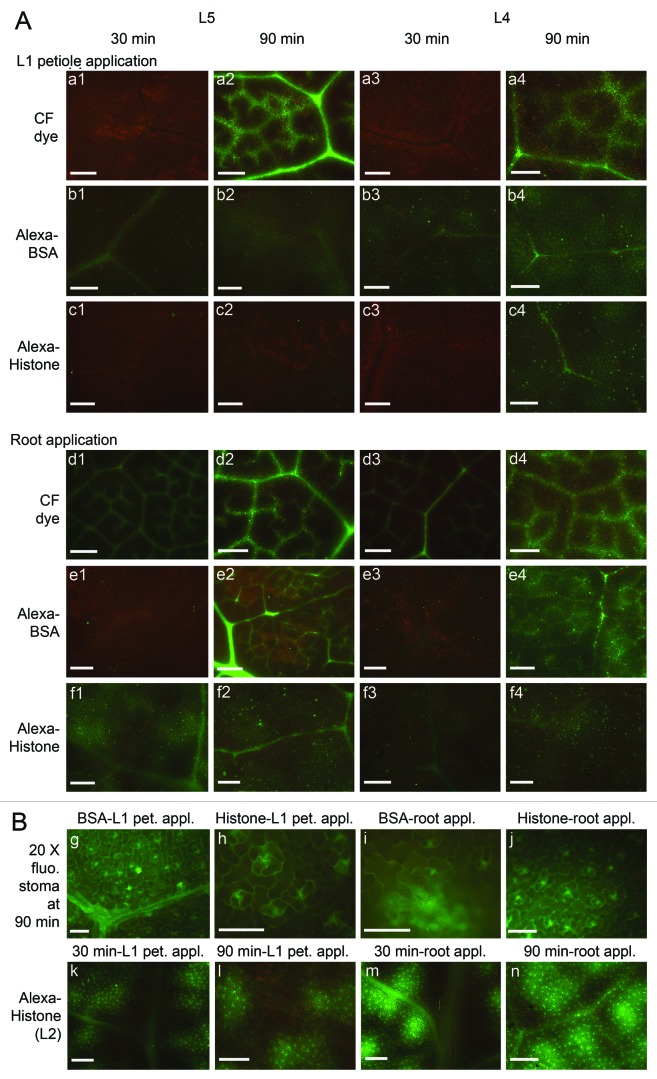
In broccoli leaves, class I vein, which is also called midrib, extends from the petiole to the leaf apex. The netted vein pattern comprises smaller veins which have several distinctive vein size classes that branch from the larger veins (Fig. 1C). Class III veins are major veins that branch from class II veins. Class IV is a minor vein that branch from class III and can be quite small in diameter.14,19,20 After dye or fluorescent proteins were applied, the leaves were detached from plant at various times for observation. Figure 6A shows the unloading patterns produced by CF dye, Alexa-BSA and Alexa-Histone fluorescence in L5 and L4 at 30 and 90 min. L4 is a sink/source transitional leaf and L5 is a true sink leaf. Both leaves were examined to learn if the proteins and dye unload from the veins differently in sink and source tissues.
Figure 6.
Unloading pattern of fluorescence L5 sink and L4 source/sink transition leaves. Images were taken at 30 and 90 min following L1 petiole and root application. (A) CF dye (a1–4 and d1–4), Alexa Fluor 488 BSA (b1–4, e1–4) and Alexa Fluor 488 Histone (c1–4 and f1–4). At 90 min strong CF dye fluorescence unloads into neighboring cells and produces a “bleeding” pattern around the class II and III veins. Bars in images taken at 4x magnification = 500 μm; (Bg-j) Alexa-BSA and Alexa-Histone are seen in leaf lamina and produce prominent patterns around stomata. Higher magnification is used to demonstrate the presence of Alexa-BSA fluorescence in epidermal cells and Alexa-Histone is mainly in mesophyll cells. (Bk-n) Alexa-Histone in L2 leaves forms the foci of fluorescence in the mesophyll that are quite distinct and appear to bleed from class II and III veins. Both produce a prominent pattern around stomata Bars = 100 μm.
Interestingly, CF dye fluorescence appeared in leaf veins by 30 min following root application of the dye but was not prominent in leaf veins following L1 petiole application until 90 min. Regardless of whether CF dye was applied to the roots or L1 petiole, class III and IV veins of L5 and L4 were labeled at 90 min (Fig. 6Aa1–4 and d1–4). Following L1 petiole or root application, the CF dye fluorescence at 90 min was greater than at 30 min. Fluorescence was primarily in the veins of L5 leaves but was bleeding into the mesophyll of the L4 leaves. Thus there is greater unloading of fluorescence in source tissues (Fig. 5Aa2, a4, d2 and d4). In N. benthamiana leaves the unloading capacity of veins in sink and source leaves was reported to be similar.13
Alexa-BSA produces a unique unloading pattern that does not resemble CF dye. Following petiole application, Alexa-BSA was evident in L4 and L5 leaf veins at 30 min (Fig. 6Ab1) and in the leaf lamina at 90 min (Fig. 6Ab). This pattern is consistent with the distribution of fluorescence to L4 and L5 petioles reported in Figure 4. Following L1 petiole application, fluorescence was seen in class III veins but not minor veins. Following root application, Alexa-BSA is seen in vein class I-V. Alexa-BSA appears to unload preferentially to the leaf epidermal cell layer and the fluorescence intensity in the L4 leaf lamina is more profound than in L5 leaves (Fig. 6Ab1–4). Interestingly, upon examination at higher magnification the appearance of fluorescence in the epidermis is obvious (Fig. 6Bg and i). If we compare the pattern of Alexa-BSA in L5 leaves at 90 min following L1 petiole and root application, it seems that more fluorescence remains in the leaf veins following root application (Fig. 6Ab2 and e2). This suggests that the location of protein loading might influence its unloading in upper leaves.
Alexa-Histone also produces a unique pattern that does not resemble CF dye or Alexa-BSA in L5 and L4 leaves. Following L1 petiole application fluorescence is obvious in the leaf veins I–III. Fluorescence never appears in class IV and V vein but does appear in the lamina in L4 and L5 leaves at 90 min (Fig. 6Ac1–4). This pattern is consistent with the low accumulation in L4 and L5 petioles reported in Figure 4. As with CF dye, Alexa-Histone appears in leaf veins and lamina at 30 min following root application, which is earlier than when the samples are applied to the L1 petiole (Fig. 6Af1–4). Alexa-Histone appears to unload to the mesophyll as well as epidermal layers. Curiously, L2 leaves show obvious density of fluorescent foci that appear to be bleeding from class III veins. Fluorescence associates with mesophyll but not epidermal cells (Fig. 6Bk–n).
Fluorescence intensity of Fluorescein-HCV in petiole cross sections and leaf veins
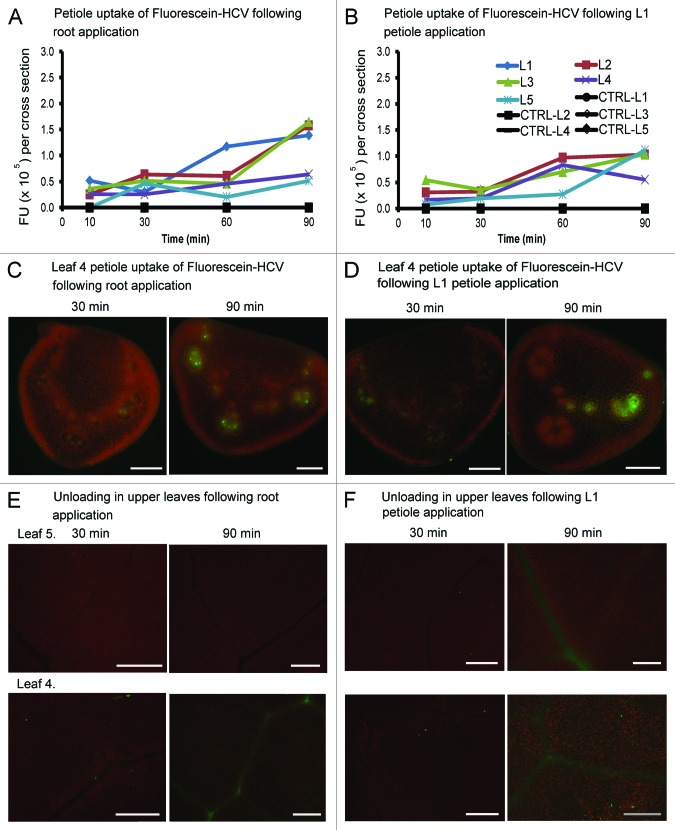
The association of virus cores with plant material has garnered attention in recent years as outbreaks of human enteroviruses associating with raw vegetables have been reported.21 Research has shown that Hepatitis A virus can survive on the surface of fruits and vegetables.22 Contamination occurs either by irrigating plants with fecal contaminated groundwater or by infected food handlers. While Hepatitis C virus (HCV) is not considered to be foodborne, we decided to test commercially available HCV core antigen [2–192]-galactosidase-tagged, fluorescein conjugate (Fluorescein-HCV) to learn if it can be taken up by the plant vasculature, in a manner similar to other exogenous proteins. Fluorescein-HCV has a molecule weight of 136 kDa which is comprised of the 22 kDa HCV core antigen plus 114 kDa galactosidase-tagged. Given that the molecule is larger than the 66 kDa Alexa-BSA, we predicted that if size were the determining factor for phloem transport, then its ability to move in the phloem should be restricted in comparison to Alexa-BSA.
When Fluorescein-HCV was applied to B. oleracea roots or L1 petioles, there was a fairly slow increase of fluorescence to all leaves. Fluorescence intensities at 90 min were approximately 2-fold higher than reported for Alexa-BSA (compare Fig. 7A and B with Fig. 4A). Fluorescence was greater in leaves closest to the soil surface and less accumulated in leaves furthest from the roots. Thus root application leads to a greater general increase that is sustained over time.
Figure 7.
The uptake of fluorescein-HCV core antigen by B. oleracea petioles and leaves. (A and B) Graphs depict the average fluorescence values in petiole cross sections. The fan-shaped and circular vascular bundles were selected as ROIs using Image J software and fluorescence values were recorded. The data were collected at 10, 30, 60 and 90 min after application of Ffluorescein-HCV. (C and D) Petiole uptake of Fluorescein-HCV following L1 petiole delivery. Images of L4 petiole cross sections of B. oleracea following L1 petiole (A) or root (B) application at 30 and 90 min. Bars = 500 μm. (E and F) Leaf vascular pattern in L4 source/sink transition leaf or L5 sink leaf. Images were taken at 30 or 90 min following root or petiole application of fluorescent markers. Bars = 500 μm.
Surprisingly, the low fluorescence levels in L4 and L5 petioles implies that the Fluorescein-HCV is not taken up by sink leaves (Fig. 7A and B).14 However, microscopic examination of L4 petiole segments confirmed that the intensity of fluorescence increased in the vascular bundles between 30 and 90 min (Fig. 7C and D). Interestingly, fluorescein-HCV does not unload from the veins. This is unlike Alexa-BSA or Alexa-Histone suggesting that there is a specific restriction in HCV core antigen movement (Fig. 7E and F).
Given the low accumulation of fluorescein-HCV we hypothesized that this protein performed similar to Alexa-BSA and might be exported to the apoplast. Leaves were infiltrated with 2-[N-morpholino]ethansesulfonic acid (MES) buffer and the apoplast wash fluids were pooled. Remaining tissue was ground and both samples were analyzed by SDS-PAGE and immunoblot (Fig. 3B). Bands were detected mainly in tissue extracts not in the apoplastic wash fluids. We noted greater accumulation of fluorescein-HCV following root loading than loading of the L1 petiole.
Interestingly, the cumulative data shows that each protein is sorted differently in the phloem: Alexa-BSA was exported to the apoplast while Alexa-Histone was restricted to the symplast. Alexa-Histone and -BSA unload into the leaf lamina while fluorescein-HCV is restricted to leaf veins. These data suggest that there is a sorting mechanism for proteins in the phloem of B. oleracea plants.
Discussion
We employed four different size fluorescent molecules to study transfer from the site of application in the roots and L1 petiole to the L4 and L5 leaves: CF dye (460 Da), Alexa-Histone (34 kDa), Alexa-BSA (66 kDa) and fluorescein-HCV (136 kDa). When CF-dye and fluorescence-HCV were applied to the roots, they moved extensively throughout the plant, but Alexa-BSA and Alexa-Histone showed minimal movement into aerial parts of the plant, as determined in petiole cross sections. While the transfer rates over 90 min for the larger proteins appear to be slow, the intensity of fluorescence is adequate to detect proteins in leaf veins and lamina. Alexa-BSA and Alexa-Histone produce low levels of fluorescence in major veins and appear to unload into the leaf lamina. Thus regardless of the protein dimensions and the intensity of fluorescence in distal parts of the plant, there does not appear to be an obvious physical barrier preventing extensive movement of exogenously applied proteins within the vasculature.
It is interesting to note that fluorescein HCV, which is much larger than Alexa-Histone and Alexa-BSA, moves more extensively than the latter two proteins during the 90 min observation period. Following root application, greater fluorescein-HCV is seen in L1, L2 and L3 petioles than CF dye. CF dye moves more extensively than all other proteins when it is applied to the L1 petiole. These data suggest that protein size relative to CF dye is not the defining feature for uptake. We cannot consider apoplastic transport as a positive factor for long distance movement of Alexa-Histone or fluorescein-HCV since they appeared to be restricted to symplastic domain and was not recovered in the apoplastic wash fluid. Perhaps the ability of Alexa-Histone and fluorescein-HCV (Fig. 2A) to unload from the phloem into neighboring cell layers affects the pressure gradient within the sieve tube and increasing its uptake. Alexa-Histone showed less movement into the upper leaf petioles when it was applied to plants maintained in hydroponic medium. These data suggest that water potential is not the driving force for Alexa-Histone movement. We noted that Alexa-BSA reached peak uptake by 60 min following root application but peak uptake was at 90 min following L1 petiole application. Furthermore, when Alexa-Histone was applied to the L1 petioles or roots, there was little change in uptake over time. These observations are hard to explain. Perhaps the vascular dimensions of the L1 petiole are different than from the roots and this changes the transfer potential or rate to distal parts of the plant. Another possibility is that the vascular trace at the juncture of the L1 petiole and stem downregulates the rate export from the leaf in comparison to phloem traces moving from the roots into the stem.
The notion that the petiole regulates flow to and from the stem is supported by data in Figures 3 and 4. In panels C and D of both figures we see that the amount of fluorescence in the stem vascular rays is higher than in the leaf petioles. Assuming that these vascular rays branch into the petioles we would expect to see similar intensities if there was a seamless transition. It is possible that there is a change in the dimension of the phloem strands that transition from the stem into the petiole and this controls the amount of proteins that enter or exit the petiole. There might also be a regulatory mechanism that has not yet been described that sorts proteins to different destinations. Similar observations were made in experiments using N. benthamiana. While the vascular arrangements of these two plant species are significantly different, clearly the petiole in both plant species regulates the transition of proteins into the leaves. Currently we lack the investigative tools to examine the existence of such a mechanism in B. oleracea and N. benthamiana plants. But the correlation suggests that the petiole provides a change in dimension which has a function conserved across plant species.
Transgenic approaches have been used in the past to study phloem and post-phloem transport of proteins and RNA.7 The green fluorescent protein (GFP) has been expressed in companion cells in mature leaves of Arabidopsis and N. benthamiana and its ability to unload in sink tissues has led researchers to conclude that protein unloading is not regulated.1 Thus the approach used here enabled us to identify the petiole as a regulatory component, which may not be made obvious by a transgenic approach. GFP can also unload in roots and spread unrestricted into surrounding cells. However GFP fused to sporamin or other cellular proteins show little movement beyond the phloem and appear to be restricted from exiting the protophloem domain or entering the root tip of transgenic Arabidopsis.7 These outcomes were suggested to indicate that size is often a limit for nonspecific protein unloading. This interpretation could explain why the pattern of movement for fluorescein-HCV core protein is unlike the other exogenous proteins. Fluorescein-HCV resided mainly in major and minor veins in sink leaves. One could argue that the size of the protein restricts phloem unloading since there was little evidence of fluorescence moving into neighboring cells.
Among plant viruses, there are numerous reports that viruses or coat proteins are restricted to the phloem and can only unload with the aid of a viral movement protein.23-25 Furthermore, the insect infecting flock house virus can spread systemically in plants if aided by a plant viral movement protein.25 These data suggest that there is a mechanism in plants that specifically regulates viral proteins in the phloem. Once the virus core protein is loaded into the phloem it can then spread throughout the plant vasculature. We do not know whether restriction in protein unloading is a defense mechanism limiting virus spread, or if complete vascular unloading, as for BSA and Histone, represents a defense mechanism that clears foreign proteins from the plant vasculature. However the contrasting data raises intriguing questions for future research.
In a prior investigation using N. benthamiana, MRI technology was employed to assess phloem transport rates.13 We showed that MRI and fluorescence data produced similar outcomes with respect to BSA transport through the stem, and provided credibility to the approach of measuring fluorescence intensities in cross sections over time. The velocity measurements show significant rates of transport near the site where the contrast agent and/or protein is applied, and as the ROIs move further away the rates drop. MRI technology is powerful technology and has the ability to obtain valuable profiles of protein movement through the phloem although the costs of the technology can be preclusive. The ability to rely on fluorescence technology to obtain similar data are more cost effective and produces reasonable examination of protein mobility in the phloem.
We noted differences in protein and dye transport via adaxial and abaxial vascular bundles in N. benthamiana plants. Specifically, adaxial vascular bundles showed preferential transport of solutes and not proteins.13 This led us to examine whether there are functional differences in the transport capacity of architecturally dissimilar vascular bundles in B. oleracea. We know very little about the vascular continuity between the leaf petiole and leaf lamina in B. oleracea. Both N. benthamiana and B. oleracea have pinnate venation patterns with a prominent midrib that extends from the petiole to the top leaf apex and smaller veins of distinct size classes branching from the larger veins. For B. oleracea leaf vein classes I and II intersect with the leaf margins, which is known as a craspedodromous subtype of venation. B. oleracea veins are light colored and this could suggest less photosynthetic parenchyma in the vascular bundle than N. benthamiana leaves. While the photosynthetic characteristics of cells surrounding the vascular system has been described in tobacco, comparisons have not been made with Brassica spp and so we do not know if there are differences in tissue specific photosynthetic CO2 fixation and if such differences might affect the sink properties and unloading veins in maturing leaves. Furthermore the trace of each fan-shaped and circular bundle from the petiole to the midrib and how it divides into the leaf veins has not been mapped. Thus, given that there is very little known about the vascular continuity between the petiole and leaf lamina in B. oleracea, and the evidence in N. benthamiana that architecturally different vascular bundles have different capacities for solute and protein transport, we were surprised to learn that there were no obvious functional differences between the transport properties of fanleaf-shaped and circular bundles. We failed to note differences between the distribution of CF dye or proteins between the fan-shaped and circular bundles (Table 1). Both classes of vascular bundles appeared to have similar transport capacities in B. oleracea.
The leaf patterns for Alexa-BSA, -Histone and fluorescein-HCV were unique to each of these proteins and they did not display the same pattern as CF dye for movement into minor veins or unloading to the mesophyll. These data suggest that there is a protein sorting mechanism associated with leaf veins. CF dye is an indicator used to differentiate sink, source/sink transition and source leaves. In sink leaves, CF dye enters major and minor veins, and can bleed into surrounding tissues. Alexa-BSA and -Histone unloaded in L4 and L5 leaves into the surrounding leaf lamina but this pattern is unlike the pattern reported in N. benthamiana leaves. In N. benthamiana both proteins completely unloaded into the leaf lamina and there were minimal proteins left in small regions of the leaf veins. Fluorescence was prominent in the epidermal and mesophyll cells and on rare occasions we could see some fluorescence highlighting major veins. In B. oleracea the vascular accumulation of the proteins was evident throughout the experiment, similar to CF-dye suggesting that protein unloading is less aggressive than in N. benthamiana. Thus while we previously suggested that N. benthamiana leaf veins utilize a mechanism that clears exogenous proteins, there is no evidence here to suggest B. oleracea has a similar capacity. Thus B. oleracea seems to have a greater capacity to tolerate foreign proteins in the plant vascular system.
Researchers have reported enteric viruses and hepatitis A are common in vegetables and that contaminated groundwater could be a source for viral outbreaks. These contaminating viruses have been identified using diagnostic criteria and have not fully investigated the penetrance of the viruses into internal tissues. Evidence of HCV core in the B. oleracea vasculature sheds new light on the potential uptake of foodborne contaminants. It might be reassuring to note that HCV core accumulation in the leaves is less than BSA or histone suggesting that contamination is restricted to the vasculature. However the general flow of HCV core through the plant to various leaf petioles is comparable or greater than the other exogenous proteins. These data are intriguing for two reasons. First, they show that a single viral protein can easily be taken up by an edible plant just by adding the proteins to a hydroponic system. This could be a source of edible vaccines that has never been tested. Second, these data suggest that exogenous proteins, including viral proteins, can follow the flow of assimilates throughout the plant but that there is a mechanism that differentiates foreign proteins for transport to the apoplast, unloading to leaf lamina, or restriction to leaf veins. The pattern of unloading in B. olaeracea is unlike the pattern in N. benthamiana thus we cannot draw global rules of how proteins perform in the plant vasculature. Differences could be due to architectural differences in the vascular bundles which may affect flow rates and sorting at the leaf petiole, or there may be additional machinery that regulates vascular metabolism that is divergent among these plant genera.
Methods
Plant materials
Brassica oleracea seeds were sown in Metro-Mix and grown in a growth room with 16 h light and 25°C. The age of plants was characterized by the number of expanding leaves, which is five in all the experiments. Some plants were removed from soil, the roots were rinsed in water and then transplanted to growth pouches to analyze transport under hydroponic conditions.13,26,27 Plants were maintained in growth pouches for 24 h prior to each experiment to ensure plants were adapted to the liquid growing environment to ensure maximal water conductivity in all tissue for experiments.
Fluorescence imaging
Sixty micrograms/milliliters 6 (5)-carboxyfluorescein (CF; Sigma) dye was prepared according to standard protocols.14,28 Alexa-Histone H1 (32 kDa; Invitrogen), Alexa-BSA (66 kDa; Invitrogen) and fluorescein-HCV (136 kDa, Sigma-Aldrich) were diluted to 0.3mg/ml, 0.3 mg/ml and 0.09 mg/ml respectively. Nanodrop spectrophotometer was used to normalize fluorescence intensities of CF dye, Alexa-BSA and Alexa-Histone to compare the levels of fluorescence detected in leaf veins. The fluorescent compounds were introduced into the roots or petiole of first source leaf (Leaf 1; L1) as described previously.13
The progression of CF dye or proteins to the upper leaves was monitored using a hand held UV Blue-Ray lamp (UVP, LLC). To quantify CF dye or proteins transport in petiole vasculature, 50 μm B. oleracea petiole cross sections were cut with a vibrating microtome (Vibratome) and collected in phosphate buffered saline (PBS). Cross sections of the source and sink petioles were imaged using a Nikon E600 epifluorescence microscopy (Nikon Corp.) which contains a 470–490 nm excitation filter, a DM505 dichroic mirror and a BA520 barrier filter with a built-in Magnafire camera. Image J software was used for cross section image analysis, including quantifying the fluorescence units per dimensional vol (FU/mm3).
Intercellular wash fluid (IWF) and protein extractions
Leaves from control, Alexa-BSA or -Histone treated plants were cut and carefully washed with deionized water and blotted dry. Leaves were infiltrated by pushing the plunger of a syringe which contain 1ml 180 mM 2-[N-morpholino]ethane-sulphonic acid (MES) and then blotted again. Leaves were transferred to centrifuge tubes and centrifuged immediately at 230 g, for 4 min at 4°C. The liquid (intercellular wash fluid) in the centrifuge tube was collected for SDS-PAGE and immunoblot analysis. The remaining leaf tissue was ground with grinding buffer [100 mM TRIS-HCl (pH7.5), 10 mM KCl, 5 mM MgCl2, 400mM sucrose, 10% glycerol and 10 mM β-mercaptoethanol] at a ratio of 0.1 g leaf tissue per 100 μl buffer. The intercellular wash fluid and leaf extracts were each diluted with equal volume of Tris-glycine SDS gel loading buffer (Invitrogen) was added. Samples were boiled for 5 min in water-bath, transferred to ice for 3 min, then centrifuged at 13200 rpm for 5 min before loading to SDS-PAGE.
SDS-PAGE and immunoblot analysis
Intercellular wash fluids and leaf extracts were separated by SDS-PAGE and electroblotted to Hybond-P (GE Healthcare) using standard protocols.29 Blots were incubated with Histone antibody [1:200 (v/v); Santa Cruz Biotechnology Inc.] or BSA antibody [1:3000 (v/v); Invitrogen] for 1 h. HRP-conjugated goat anti-rabbit IgG (GE healthcare) was diluted 1:20000 (for Histone) or 1:50000 (for BSA) served as the secondary antiserum using ECL Advanced Western Blotting Kit (GE Healthcare). Blots were exposed to film for 10–60 sec. Films were scanned and images are cropped with scanner CanonScan 9950F and associated program Arcsoft Photo Studio 5 (Canon USA).
Acknowledgments
This work was supported by Oklahoma Center for Advancement of Science and Technology (OCAST) Plant Biology Program contract no. 7331.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/19020
References
- 1.Oparka KJ, Cruz SS. THE GREAT ESCAPE: Phloem Transport and Unloading of Macromolecules1. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:323–47. doi: 10.1146/annurev.arplant.51.1.323. [DOI] [PubMed] [Google Scholar]
- 2.Lough TJ, Lucas WJ. Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu Rev Plant Biol. 2006;57:203–32. doi: 10.1146/annurev.arplant.56.032604.144145. [DOI] [PubMed] [Google Scholar]
- 3.Giavalisco P, Kapitza K, Kolasa A, Buhtz A, Kehr J. Towards the proteome of Brassica napus phloem sap. Proteomics. 2006;6:896–909. doi: 10.1002/pmic.200500155. [DOI] [PubMed] [Google Scholar]
- 4.Atkins CA, Smith PM, Rodriguez-Medina C. Macromolecules in phloem exudates--a review. Protoplasma. 2011;248:165–72. doi: 10.1007/s00709-010-0236-3. [DOI] [PubMed] [Google Scholar]
- 5.Buhtz A, Springer F, Chappell L, Baulcombe DC, Kehr J. Identification and characterization of small RNAs from the phloem of Brassica napus. Plant J. 2008;53:739–49. doi: 10.1111/j.1365-313X.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu J, Warthmann N, Küttner F, Schmid M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol. 2007;17:1055–60. doi: 10.1016/j.cub.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Stadler R, Wright KM, Lauterbach C, Amon G, Gahrtz M, Feuerstein A, et al. Expression of GFP-fusions in Arabidopsis companion cells reveals non-specific protein trafficking into sieve elements and identifies a novel post-phloem domain in roots. Plant J. 2005;41:319–31. doi: 10.1111/j.1365-313X.2004.02298.x. [DOI] [PubMed] [Google Scholar]
- 8.Imlau A, Truernit E, Sauer N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 1999;11:309–22. doi: 10.1105/tpc.11.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ. Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development. 1999;126:4405–19. doi: 10.1242/dev.126.20.4405. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Yoo BC, Rojas MR, Gomez-Ospina N, Staehelin LA, Lucas WJ. Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science. 2003;299:392–6. doi: 10.1126/science.1077813. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JR, Garcia-Arenal F. The bundle sheath-phloem interface of Cucumis sativus is a boundary to systemic infection by Tomato aspermy virus. Mol Plant Microbe Interact. 1998;11:109–14. doi: 10.1094/MPMI.1998.11.2.109. [DOI] [Google Scholar]
- 12.Peleg G, Malter D, Wolf S. Viral infection enables phloem loading of GFP and long-distance trafficking of the protein. Plant J. 2007;51:165–72. doi: 10.1111/j.1365-313X.2007.03128.x. [DOI] [PubMed] [Google Scholar]
- 13.Niu C, Smith N, Garteiser P, Towner R, Verchot J. Comparative analysis of protein transport in the N. benthamiana vasculature reveals different destinations. Plant Signal Behav. 2011;6 doi: 10.4161/psb.6.11.17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts AG, Cruz SS, Roberts IM, Prior D, Turgeon R, Oparka KJ. Phloem Unloading in Sink Leaves of Nicotiana benthamiana: Comparison of a Fluorescent Solute with a Fluorescent Virus. Plant Cell. 1997;9:1381–96. doi: 10.1105/tpc.9.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo L, Yu Y, Xia X, Yin W. Identification and functional characterisation of the promoter of the calcium sensor gene CBL1 from the xerophyte Ammopiptanthus mongolicus. BMC Plant Biol. 2010;10:18. doi: 10.1186/1471-2229-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maksymowych AB, Orkwiszewski JAJ, Maksymowych R. Vascular Bundles in Petioles of Some Herbaceous and Woody Dicotyledons. Am J Bot. 1983;70:1289–96. doi: 10.2307/2443419. [DOI] [Google Scholar]
- 17.Joy KW. Translocation in Sugar-Beet. I. Assimilation of 14co2 + Distribution of Materials from Leaves. J Exp Bot. 1964;15:485. doi: 10.1093/jxb/15.3.485. [DOI] [Google Scholar]
- 18.Li Y, Fuchs M, Cohen S, Cohen Y, Wallach R. Water uptake profile response of corn to soil moisture depletion. Plant Cell Environ. 2002;25:491–500. doi: 10.1046/j.1365-3040.2002.00825.x. [DOI] [Google Scholar]
- 19.Avery GS. Structure and development of the tobacco leaf. Am J Bot. 1933;20:565–92. doi: 10.2307/2436259. [DOI] [Google Scholar]
- 20.Ding B, Parthasarathy MV, Niklas K, Turgeon R. A morphometric analysis of the phloem-unloading pathway in developing tobacco leaves. Planta. 1988;176:307–18. doi: 10.1007/BF00395411. [DOI] [PubMed] [Google Scholar]
- 21.Cheong S, Lee C, Song SW, Choi WC, Lee CH, Kim SJ. Enteric viruses in raw vegetables and groundwater used for irrigation in South Korea. Appl Environ Microbiol. 2009;75:7745–51. doi: 10.1128/AEM.01629-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Satter S, Tetro J, Bidawid S, Faber J. Foodborne spread of hepatitis A: Recent studies on virus survival, transfer and inactivation. Can J Infect Dis Med Microbiol. 2000;11:159–63. doi: 10.1155/2000/805156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HL, Wang Y, Giesman-Cookmeyer D, Lommel SA, Lucas WJ. Mutations in viral movement protein alter systemic infection and identify an intercellular barrier to entry into the phloem long-distance transport system. Virology. 1998;245:75–89. doi: 10.1006/viro.1998.9154. [DOI] [PubMed] [Google Scholar]
- 24.Ding X, Shintaku MH, Carter SA, Nelson RS. Invasion of minor veins of tobacco leaves inoculated with tobacco mosaic virus mutants defective in phloem-dependent movement. Proc Natl Acad Sci U S A. 1996;93:11155–60. doi: 10.1073/pnas.93.20.11155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dasgupta R, Garcia BH, 2nd, Goodman RM. Systemic spread of an RNA insect virus in plants expressing plant viral movement protein genes. Proc Natl Acad Sci U S A. 2001;98:4910–5. doi: 10.1073/pnas.081288198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driskel BA, Hunger RM, Payton ME, Verchot-Lubicz J. Response of Hard Red Winter Wheat to Soilborne wheat mosaic virus Using Novel Inoculation Methods. Phytopathology. 2002;92:347–54. doi: 10.1094/PHYTO.2002.92.4.347. [DOI] [PubMed] [Google Scholar]
- 27.Verchot J, Driskel BA, Zhu Y, Hunger RM, Littlefield LJ. Evidence that soilborne wheat mosaic virus moves long distance through the xylem in wheat. Protoplasma. 2001;218:57–66. doi: 10.1007/BF01288361. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Ding B, Baulcombe DC, Verchot J. Cell-to-cell movement of the 25K protein of potato virus X is regulated by three other viral proteins. Mol Plant Microbe Interact. 2000;13:599–605. doi: 10.1094/MPMI.2000.13.6.599. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Press, 1989. [Google Scholar]