Abstract
We report the palladium-catalyzed, pyrrolidine-mediated α-benzylation of enamines generated from aldehydes and ketones. The method allows for direct coupling of medicinally relevant coumarin moieties with aldehydes and ketones in good yield under mild conditions. The reaction is believed to proceed via a Pd-π-benzyl complex generated from (coumarinyl)methyl acetates.
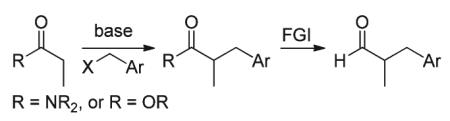
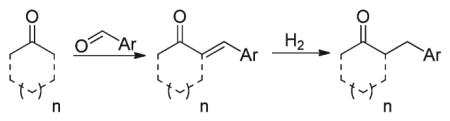
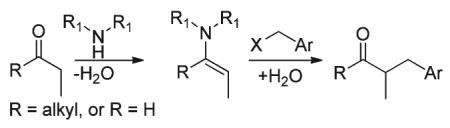
Synthetic methodologies for the catalytic α-benzylation of aldehydes and ketones are relatively rare in the current literature.1 Stoichiometric, base-mediated alkylation of ketones with benzyl halides is well known,2 however over-alkylation by-products can be problematic.3 Procedures for the selective mono-benzylation of aldehydes are typically multi-stepped and involve α-benzylation of the corresponding amide followed by functional group interconversion to obtain the aldehyde (eqn (1)).4 More traditional Knoevenagel-type condensations of carbonyl compounds with benzylaldehyde derivatives followed by selective in situ reduction of the α,β-unsaturated moiety (eqn (2)) also provides the benzylated products.1a,b,5 However these methods pose chemoselectivity challenges between reduction of the carbonyl and the α,β-unsaturated moiety.5c,e,g,6 Another classical synthetic methodology involves the use of amines as organocatalysts to generate nucleophilic enamines from aldehydes and ketones which are subsequently trapped with benzyl halides (eqn (3)).1d,e,7
 |
(1) |
 |
(2) |
 |
(3) |
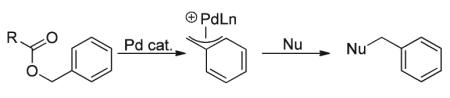
A secondary issue associated with synthesizing α-benzylated ketones and aldehydes involves generation of the benzyl electrophilic coupling partner. Classical methods employ toxic and difficult to handle benzyl halides (eqn (1) and (3)).8 Alternatively, use of activated benzyl alcohol moieties as electrophilic benzylating reagents has received less attention. Legros, Trost, and most recently Rawal, as well as others have reported transition metal-catalyzed benzylation of various nucleophiles via benzyl acetates and carbonates.9 In 2010, our group reported the palladium-catalyzed benzylation of ketone enolates via decarboxylative coupling of benzyl β-ketoesters.1f Unfortunately, the decarboxylative coupling method is not as applicable to the alkylation of aldehyde enolates due to synthetic challenges associated with the synthesis of the requisite reactants. Thus, a remaining challenge is the direct benzylation or arylmethylation of aldehyde enolates.1a-e Herein we report an approach to catalytic aldehyde arylmethylation that utilizes Pd catalysis in conjunction with enamine activation of aldehydes.1c,10
 |
(4) |
 |
(5) |
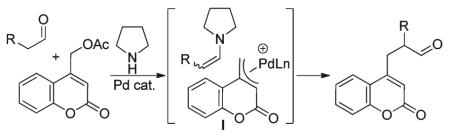
Coumarins are biologically and medicinally relevant compounds that are also widely utilized as dyes.11 In addition to pharmaceutical applications as anti-coagulants and the treatment of asthma12 and lymphedema,13 coumarins possess anti-HIV,14 anti-hypertension, anti-arrhythmia, anti-inflammatory, antiseptic, and analgesic properties.15 For this reason, our group has continued to develop “milder” reaction conditions for the coupling of nucleophiles to coumarin moieties.16 Current literature protocols for the electrophilic introduction of 4-methyl-2H-chromen-2-one involved displacement of halides from the 4-methyl substituent.14,17 To date, there is only a single report for the addition of a ketone nucleophile to an electrophilic Pd-π-benzyl complex generated from the ionization of 4-methyl-coumarin esters (eqn (5), I).1f In addition, there are no reports of the addition of enamines, generated from aldehydes and ketones, to palladium-π-benzyl complexes.9n
Our investigation began by identifying conditions for the palladium-catalyzed, pyrrolidine-mediated benzylation of hexanal (Table 1, II) with coumarinyl acetates.18 On the basis of literature precedent,10 we expected that pyrrolidine would affect the in situ formation of enamine nucleophiles which could react with a palladium-π-benzyl intermediate derived from the coumarinyl(methyl) acetate. Initial studies revealed that 50 mol% of pyrrolidine along with 5 mol% Pd(PPh3)4 at room temperature in DMSO was a sufficient catalytic system to facilitate carbon-carbon bond formation between the (coumarinyl)methyl acetate and the aldehyde. However, the conversion never surpassed 50% (Table 1, entry 1). Under the assumption that turnover of the amine catalyst was responsible for the poor conversion, the loading of pyrrolidine was increased to 1.5 equiv. (50 mol% with respect to aldehyde). Indeed, this modification increased the overall yield to ca. 75% (entry 2), and a minor increase in temperature provided the benzylated aldehyde in sufficient conversion (entry 3, 90%). Introduction of a base into the reaction mixture, or solvent modification from DMSO to NMP, proved to have a negative effect on reactivity resulting in a significant decrease in conversion (entries 4, 6 respectively). Use of acetonitrile as a solvent completely suppressed carbon-carbon bond formation (entry 5). Finally, the bidentate dppf-ligated palladium catalyst was more active, providing the product in good conversion at room temperature (entry 8).
Table 1.
Reaction conditions screen
| |||||
|---|---|---|---|---|---|
|
| |||||
| Entry | Pyrrolidine | Pd cat (5 mol%) | Temp. | Time | Conversion |
| 1 | 0.5 equiv. | Pd(PPh3)4 | rt | 24 h | 50% |
| 2 | 1.5 equiv. | Pd(PPh3)4 | rt | 28 h | 75% |
| 3 | 1.5 equiv. | Pd(PPh3)4 | 50 °C | 16 h | 90% |
| 4a | 1.5 equiv. | Pd(PPh3)4 | rt | 16 h | 50% |
| 5b | 1.5 equiv. | Pd(PPh3)4 | rt | 28 h | 0% |
| 6c | 1.5 equiv. | Pd(PPh3)4 | rt | 28 h | 40% |
| 7 | 1.5 equiv. | (η3-AllylPdCl)2, Xantphos |
rt | 24 h | 50% |
| 8 | 1.5 equiv. | Pd(acac)2, DPPF | rt | 12 h | 90% |
1 equiv. of Et3N.
MeCN as solvent.
NMP as solvent.
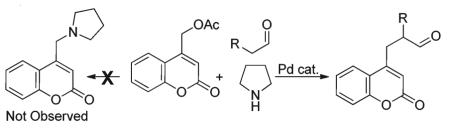
We have recently shown that palladium catalyzes the amination of (coumarinyl)methyl acetates with various amines, including pyrrolidine.19 Thus, it was somewhat surprising that the benzyl amine product resulting from nucleophilic attack of the amine on the intermediate Pd-π-benzyl complex was never observed (eqn (6)). This suggests that enamine formation is faster than ionization of the (coumarinyl)methyl acetate moiety. Such a suggestion is consistent with previous observations that suggest that ionization of benzylic acetates is rate-limiting due to the requirement to partially dearomatize the coumarin when forming the intermediate π-benzyl complex. Alternatively, it is possible that benzylic amination occurs, but is reversible.10l
 |
(6) |
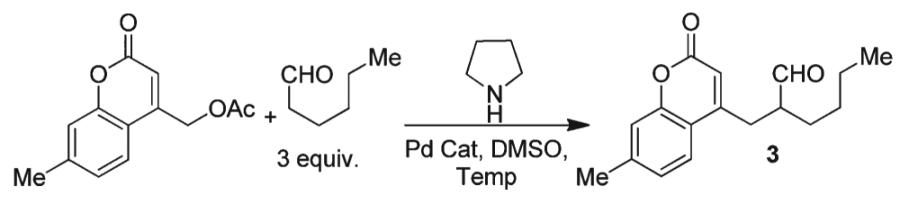
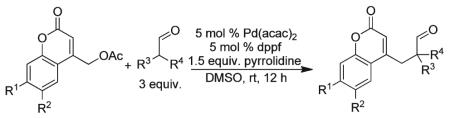
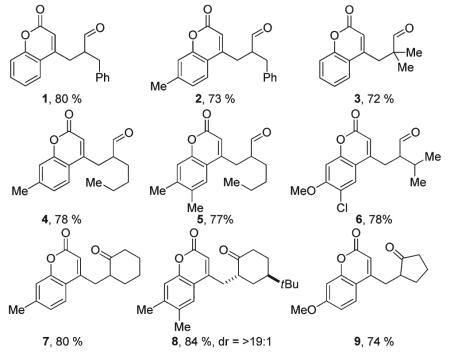
With conditions for carbon-carbon bond formation established we then turned our attention toward investigating the reaction scope. To begin, treatment of 3-phenylpropanal and coumarin methyl acetate with pyrrolidine and a dppf-ligated palladium catalyst resulted in the formation of compounds 1 and 2 (Table 2) in good yield. Increasing the steric bulk of the enamine via subjection of isobutyraldehyde to the reaction conditions revealed little effect on carbon-carbon bond formation and provided the quaternarized product 3 in good yield. In addition, simple substitutions to the coumarin core had little effect on the reaction delivering the products in good yield (Table 2, 2-9).
Table 2.
Palladium-catalyzed benzylation of aldehydes and ketones
|
|---|
|
To complement the benzylated aldehydes, ketones were also subjected to the above reaction conditions. Indeed, cyclohexanone and cyclopentanone, when treated with pyrrolidine, were competent nucleophiles for carbon-carbon bond formation with the (coumarinyl)methyl acetates, generating the arylmethylated ketones 7 and 9, respectively. Enamines obtained from 4-(tert-butyl)cyclohexanone and pyrrolidine readily added to the Pd-π-benzyl electrophile in good yield with >19: 1 diastereo-selectivity (Table 2, 8); the preference for axial delivery of electrophiles to the same enamine is known.20
Conclusions
In conclusion, we have reported the palladium-catalyzed, pyrrolidine-mediated α-benzylation of enamines generated from aldehydes and ketones.‡ The method allows for direct coupling of medicinally relevant coumarin moieties with aldehydes and ketones in good yield under mild conditions. The reaction is believed to proceed via a Pd-π-benzyl complex generated from (coumarinyl)methyl acetates.
Supplementary Material
Acknowledgements
We gratefully acknowledge the National Institutes of Health KU Chemical Methodologies and Library Development Center of Excellence (P50 GM069663) for funding.
Footnotes
Electronic supplementary information (ESI) available: General experimental and characterization of all new compounds. See DOI: 10.1039/c2ob25962a
General procedure for the α-benzylation of ketones and aldehydes with coumarinyl(methyl) acetates: A solution of coumarinyl(methyl) acetate (0.5 mmol), 5 mol% of palladium(ii) acetylacetonate (7.6 mg) and 5.0 mol% of 1,1′-bis(diphenylphosphino)ferrocene (14.4 mg) was prepared in DMSO (3 mL). Next, aldehyde or ketone (1.5 mmol) and pyrrolidine (53 mg, 0.74 mmol) were added. After 12 h stirring at room temperature, the reaction mixture was concentrated in vacuo and the residue was purified by flash column chromatography on silica gel to afford pure product.
References
- 1.(a) Alonso F, Riente P, Yus M. Synlett. 2007:1877. [Google Scholar]; (b) Alonso F, Riente P, Yus M. Eur. J. Org. Chem. 2008:4908–4914. [Google Scholar]; (c) Cozzi PG, Benfatti F, Zoli L. Angew. Chem., Int. Ed. 2009;48:1313–1316. doi: 10.1002/anie.200805423. [DOI] [PubMed] [Google Scholar]; (d) Brown AR, Kuo W-H, Jacobsen EN. J. Am. Chem. Soc. 2010;132:9286–9288. doi: 10.1021/ja103618r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Shih H-W, Vander Wal MN, Grange RL, MacMillan DWC. J. Am. Chem. Soc. 2010;132:13600–13603. doi: 10.1021/ja106593m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Torregrosa RRP, Ariyarathna Y, Chattopadhyay K, Tunge JA. J. Am. Chem. Soc. 2010;132:9280–9282. doi: 10.1021/ja1035557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Gall M, House HO. Org. Synth. 1972;52:39–49. [Google Scholar]; (b) Edwards HN, Wycpalek AF, Corbin NC, McChesney JD. Synth. Commun. 1978;8:563–567. [Google Scholar]; (c) Artaud I, Torossian G, Viout P. Tetrahedron. 1985;41:5031–5037. [Google Scholar]; (d) Brill WF. J. Mol. Catal. 1985;32:17–26. [Google Scholar]; (e) Chapdelaine MJ, Hulce M. Org. React. 1990;38:225–653. [Google Scholar]; (f) Murakata M, Nakajima M, Koga K. J. Chem. Soc., Chem. Commun. 1990:1657–1658. [Google Scholar]; (g) Ponthieux S, Outurquin F, Paulmier C. Tetrahedron. 1997;53:6365–6376. [Google Scholar]; (h) Cahiez G, Chau F, Blanchot B. Org. Synth. 1999;76:239. [Google Scholar]; (i) Cahiez G. Encycl. Reagents Org. Synth. 1995:3227. [Google Scholar]
- 3.Koenig JR, Liu H, Drizin I, Witte DG, Carr TL, Manelli AM, Milicic I, Strakhova MI, Miller TR, Esbenshade TA, Brioni JD, Cowart M. Bioorg. Med. Chem. Lett. 2010;20:1900–1904. doi: 10.1016/j.bmcl.2010.01.131. [DOI] [PubMed] [Google Scholar]
- 4.(a) Oppolzer W, Moretti R, Thomi S. Tetrahedron Lett. 1989;30:5603–5606. [Google Scholar]; (b) Myers AG, Yang BH, Chen H, Gleason JL. J. Am. Chem. Soc. 1994;116:9361–9362. [Google Scholar]; (c) Kummer DA, Chain WJ, Morales MR, Quiroga O, Myers AG. J. Am. Chem. Soc. 2008;130:13231–13233. doi: 10.1021/ja806021y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Cho CS, Kim BT, Lee MJ, Kim T-J, Shim SC. Angew. Chem., Int. Ed. 2001;40:958–960. doi: 10.1002/1521-3773(20010302)40:5<958::AID-ANIE958>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; (b) Cho CS, Kim BT, Kim T-J, Shim SC. Tetrahedron Lett. 2002;43:7987–7989. [Google Scholar]; (c) Taguchi K, Nakagawa H, Hirabayashi T, Sakaguchi S, Ishii Y. J. Am. Chem. Soc. 2004;126:72–73. doi: 10.1021/ja037552c. [DOI] [PubMed] [Google Scholar]; (d) Kwon MS, Kim N, Seo SH, Park IS, Cheedrala RK, Park J. Angew. Chem., Int. Ed. 2005;44:6913–6915. doi: 10.1002/anie.200502422. [DOI] [PubMed] [Google Scholar]; (e) Martinez R, Brand GJ, Ramon DJ, Yus M. Tetrahedron Lett. 2005;46:3683–3686. [Google Scholar]; (f) Lu S-M, Bolm C. Angew. Chem., Int. Ed. 2008;47:8920–8923. doi: 10.1002/anie.200803709. [DOI] [PubMed] [Google Scholar]; (g) Li X, Li L, Tang Y, Zhong L, Cun L, Zhu J, Liao J, Deng J. J. Org. Chem. 2010;75:2981–2988. doi: 10.1021/jo100256t. [DOI] [PubMed] [Google Scholar]
- 6.Cho CS, Kim BT, Kim T-J, Shim SC. J. Org. Chem. 2001;66:9020–9022. doi: 10.1021/jo0108459. [DOI] [PubMed] [Google Scholar]
- 7.For alkylation of enamines via benzyl halides: Stork G, Dowd SR. Org. Synth. 1974;54:46–50. Harvey RG, Pataki J, Cortez C, Di Raddo P, Yang CX. J. Org. Chem. 1991;56:1210–1217. Russell GA, Wang K. J. Org. Chem. 1991;56:3475–3479. Lazny R, Nodzewska A, Sienkiewicz M, Wolosewicz K. J. Comb. Chem. 2004;7:109–116. doi: 10.1021/cc049874y.
- 8.Marchini S, Passerini L, Hoglund MD, Pino A, Nendza M. Environ. Toxicol. Chem. 1999;18:2759–2766. [Google Scholar]
- 9.For catalytic benzylation via benzyl acetates and carbonates: Legros JY, Fiaud JC. Tetrahedron Lett. 1992;33:2509–2510. Legros J-Y, Primault GL, Toffano M, Riviere M-A, Fiaud J-C. Org. Lett. 2000;2:433–436. doi: 10.1021/ol991274y. Kuwano R, Kondo Y, Matsuyama Y. J. Am. Chem. Soc. 2003;125:12104–12105. doi: 10.1021/ja037735z. Kuwano R, Kondo Y, Shirahama T. Org. Lett. 2005;7:2973–2975. doi: 10.1021/ol0509787. Kuwano R, Yokogi M. Org. Lett. 2005;7:945–947. doi: 10.1021/ol050078q. Nakao Y, Ebata S, Chen J, Imanaka H, Hiyama T. Chem. Lett. 2007;36:606–607. Kuwano R, Kusano H. Org. Lett. 2008;10:1979–1982. doi: 10.1021/ol800548t. Kuwano R. Synthesis. 2009:1049–1061. Fields WH, Chruma JJ. Org. Lett. 2010;12:316–319. doi: 10.1021/ol902651j. Mukai T, Hirano K, Satoh T, Miura M. Org. Lett. 2010;12:1360–1363. doi: 10.1021/ol1002576. Trost BM, Czabaniuk LC. J. Am. Chem. Soc. 2010;132:15534–15536. doi: 10.1021/ja1079755. Peng B, Zhang S, Yu X, Feng X, Bao M. Org. Lett. 2011;13:5402–5405. doi: 10.1021/ol2023278. Yuan F-Q, Gao L-X, Han F-S. Chem. Commun. 2011;47:5289–5291. doi: 10.1039/c1cc10953g. Zhu Y, Rawal VH. J. Am. Chem. Soc. 2011;134:111–114. doi: 10.1021/ja2095393. Recio A, III, Heinzman JD, Tunge JA. Chem. Commun. 2012;48:142–144. doi: 10.1039/c1cc16011g. Trost BM, Czabaniuk LC. J. Am. Chem. Soc. 2012;134:5778–5781. doi: 10.1021/ja301461p.
- 10.For Pd-catalyzed allylation of enamines: Hiroi K, Suya K, Sato S. J. Chem. Soc., Chem. Commun. 1986:469–470. Huang Y, Lu X. Tetrahedron Lett. 1988;29:5663–5664. Murahashi S, Makabe Y, Kunita K. J. Org. Chem. 1988;53:4489–4495. Hiroi K, Abe J, Suya K, Sato S. Tetrahedron Lett. 1989;30:1543–1546. Hiroi K, Abe J, Suya K, Sato S, Koyama T. J. Org. Chem. 1994;59:203–213. Ibrahem I, Cordova A. Angew. Chem., Int. Ed. 2006;45:1952–1956. doi: 10.1002/anie.200504021. Liu D, Xie F, Zhang W. Tetrahedron Lett. 2007;48:7591–7594. Mukherjee S, List B. J. Am. Chem. Soc. 2007;129:11336–11337. doi: 10.1021/ja074678r. Usui I, Schmidt S, Breit B. Org. Lett. 2009;11:1453–1456. doi: 10.1021/ol9001812. Vulovic B, Bihelovic F, Matovic R, Saicic RN. Tetrahedron. 2009;65:10485–10494. Zhao X, Liu D, Xie F, Zhang W. Tetrahedron. 2009;65:512–517. Zhao X, Liu D, Guo H, Liu Y, Zhang W. J. Am. Chem. Soc. 2011;133:19354–19357. doi: 10.1021/ja209373k. Zhao X, Liu D, Xie F, Liu Y, Zhang W. Org. Biomol. Chem. 2011;9:1871–1875. doi: 10.1039/c0ob00915f. Afewerki S, Ibrahem I, Rydfjord J, Breistein P, Córdova A. Chem.-Eur. J. 2012;18:2972–2977. doi: 10.1002/chem.201103366.
- 11.(a) Bhide BH, Parikh SP, Patel SJ. Chem. Ind. 1974:306–307. [Google Scholar]; (b) Trenor SR, Shultz AR, Love BJ, Long TE. Chem. Rev. 2004;104:3059–3077. doi: 10.1021/cr030037c. [DOI] [PubMed] [Google Scholar]; (c) Wang B-Y, Liu X-Y, Hu Y-L, Su Z-X. Polym. Int. 2009;58:703–709. [Google Scholar]
- 12.Liu JH. Traditional Herbal Medicine Research Methods: Identification, Analysis, Bioassay, and Pharmaceutical and Clinical Studies. John Wiley and Sons, Inc; New York, NY: 2011. [Google Scholar]
- 13.Farinola N, Piller N. Lymphatic Res. Biol. 2005;3:81–86. doi: 10.1089/lrb.2005.3.81. [DOI] [PubMed] [Google Scholar]
- 14.Neyts J, Clercq ED, Singha R, Chang YH, Das AR, Chakraborty SK, Hong SC, Tsay S-C, Hsu M-H, Hwu JR. J. Med. Chem. 2009;52:1486–1490. doi: 10.1021/jm801240d. [DOI] [PubMed] [Google Scholar]
- 15.(a) Crombie L, Games DE, McCormick A. J. Chem. Soc. C. 1967:2545–2552. [Google Scholar]; (b) Govindachari TR, Pai BR, Subramaniam PS, Rao UR, Muthukumaraswamy N. Tetrahedron. 1967;23:4161–4165. [Google Scholar]; (c) Estevez-Braun A, Gonzalez AG. Nat. Prod. Rep. 1997;14:465–475. doi: 10.1039/np9971400465. [DOI] [PubMed] [Google Scholar]; (d) Horton DA, Bourne GT, Smythe ML. Chem. Rev. 2003;103:893–930. doi: 10.1021/cr020033s. [DOI] [PubMed] [Google Scholar]; (e) Ito C, Itoigawa M, Mishina Y, Cechinel Filho V, Enjo F, Tokuda H, Nishino H, Furukawa H. J. Nat. Prod. 2003;66:368–371. doi: 10.1021/np0203640. [DOI] [PubMed] [Google Scholar]; (f) Ngameni B, Touaibia M, Patnam R, Belkaid A, Sonna P, Ngadjui BT, Annabi B, Roy R. Phytochemistry. 2006;67:2573–2579. doi: 10.1016/j.phytochem.2006.09.017. [DOI] [PubMed] [Google Scholar]; (g) Yang H, Jiang B, Reynertson KA, Basile MJ, Kennelly EJ. J. Agric. Food Chem. 2006;54:4114–4120. doi: 10.1021/jf0532462. [DOI] [PubMed] [Google Scholar]; (h) Chun K, Park S-K, Kim HM, Choi Y, Kim M-H, Park C-H, Joe B-Y, Chun TG, Choi H-M, Lee H-Y, Hong SH, Kim MS, Nam K-Y, Han G. Bioorg. Med. Chem. 2008;16:530–535. doi: 10.1016/j.bmc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 16.(a) Jana R, Trivedi R, Tunge JA. Org. Lett. 2009;11:3434–3436. doi: 10.1021/ol901288r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chattopadhyay K, Jana R, Day VW, Douglas JT, Tunge JA. Org. Lett. 2010;12:3042–3045. doi: 10.1021/ol101042x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Torregrosa RRP, Ariyarathna Y, Chattopadhyay K, Tunge JA. J. Am. Chem. Soc. 2010;132:9280–9282. doi: 10.1021/ja1035557. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Jana R, Partridge JJ, Tunge JA. Angew. Chem., Int. Ed. 2011;50:5157–5161. doi: 10.1002/anie.201100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.For selected references: Hodgkiss RJ, Jones GW, Long A, Middleton RW, Parrick J, Stratford MRL, Wardman P, Wilson GD. J. Med. Chem. 1991;34:2268–2274. doi: 10.1021/jm00111a049. Eckardt T, Hagen V, Schade B, Schmidt R, Schweitzer C, Bendig J. J. Org. Chem. 2002;67:703–710. doi: 10.1021/jo010692p. Khan IA, Kulkarni MV, Gopal M, Shahabuddin MS, Sun C-M. Bioorg. Med. Chem. Lett. 2005;15:3584–3587. doi: 10.1016/j.bmcl.2005.05.063. Fonseca ASC, Gonçalves MST, Costa SPG. Tetrahedron. 2007;63:1353–1359. Pisani L, Muncipinto G, Miscioscia TF, Nicolotti O, Leonetti F, Catto M, Caccia C, Salvati P, Soto-Otero R, Mendez-Alvarez E, Passeleu C, Carotti A. J. Med. Chem. 2009;52:6685–6706. doi: 10.1021/jm9010127. Valente S, Bana E, Viry E, Bagrel D, Kirsch G. Bioorg. Med. Chem. Lett. 2010;20:5827–5830. doi: 10.1016/j.bmcl.2010.07.130. Saleh NI, Al-Soud YA, Al-Kaabi L, Ghosh I, Nau WM. Tetrahedron Lett. 2011;52:5249–5254. Stefanachi A, Favia AD, Nicolotti O, Leonetti F, Pisani L, Catto M, Zimmer C, Hartmann RW, Carotti A. J. Med. Chem. 2011;54:1613–1625. doi: 10.1021/jm101120u.
- 18.Since coumarins are aromatic, the coupling is an arylmethylation or benzylation; Morais VMF, Sousa CCS, Matos MAR. J. Mol. Struct. (THEOCHEM) 2010;946:13–19. Ilic P, Mohar B, Knop JV, Juric A, Trinajstic N. J. Heterocycl. Chem. 1982;19:625–631. The aromatic character of the pyrone ring of the coumarin is certainly lower than that of benzene, so the reactions may also be classified as allylation reactions (ref. 10).
- 19.Chattopadhyay K, Fenster E, Grenning AJ, Tunge JA. Beilstein J. Org. Chem. 2012 doi: 10.3762/bjoc.8.133. accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karady S, Lenfant M, Wolff RE. Bull. Chem. Soc. Fr. 1965;9:2472–2474. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


