Abstract
Obesity increases plasma renin activity (PRA) and angiotensin II (ANG II) levels, leading to vascular damage, elevated blood pressure, diabetes, and renal damage. Because genetic deletion of crucial parts of the renin-angiotensin system (RAS) protect against obesity-related cardiovascular defects, we hypothesized that Dahl salt-sensitive (SS) rats, a model of chronically low PRA and ANG II levels, would be protected against vascular defects during diet-induced obesity (DIO) compared to SS.13BN consomic rats showing normal RAS regulation. We evaluated vascular function in middle cerebral arteries (MCA) of SS or SS.13BN rats fed high fat (HF, 45% kCal from fat) vs. normal fat diet (NFD) for 15–20 weeks from weaning. Endothelium-dependent relaxation in response to acetylcholine (ACh 10−8 to 10−4 M) was restored in MCA of HF SS rats vs. NFD controls while vasodilation to ACh was dramatically reduced in HF SS 13BN rats vs. NFD controls. These findings support the hypothesis that physiological levels of ANG II play an important role in maintaining normal vascular relaxation in cerebral arteries and suggest that the cerebral vasculature of the SS rat model is genetically protected against endothelial dysfunction in DIO.
Keywords: Cardiovascular pathophysiology, Renin, Cardiovascular disease, Endothelial dysfunction, Microcirculation
INTRODUCTION
The renin-angiotensin system (RAS) has long been associated with obesity and the metabolic syndrome. Multiple studies have shown that obesity is frequently accompanied by increased angiotensin II (ANG II), levels, elevated blood pressure, vascular dysfunction, renal damage, and diabetes1–4. High fat diet and obesity have been demonstrated to be associated with endothelial dysfunction, oxidant stress, and stroke in multiple experimental models and in human populations as well5–11.
By contrast, another risk factor for hypertension and vascular damage, increased dietary salt (NaCl) consumption, is characterized by reduced circulating ANG II and PRA 12–15. In a long term follow-up study of salt sensitivity in humans, Weinberger and coworkers 16 found that individuals dying from cardiovascular causes had a significantly lower PRA than survivors or individuals dying from other causes. A commonly used experimental model of chronically lowered RAS activity is the Dahl salt sensitive (SS) rat strain, which bears a striking similarity to salt-sensitive hypertension in humans, particularly in the African-American population.
Endothelium-dependent vascular relaxation mechanisms that are absent even in normotensive SS rats fed low salt diet can be rescued either pharmacologically by chronic i.v. infusion of a subpressor dose of ANG II 17, or genetically by introgressing chromosome 13 containing the renin gene from the Brown Norway rat into the SS genetic background 17–19. The resulting consomic strain (SS.13BN), exhibits normal regulation of the RAS and a significant reduction in the salt-sensitivity of their blood pressure compared to the SS parental strain 19–21. SS.13BN consomic rats 17–19 and congenic strains carrying the BN renin allele in the SS genetic background 22 also exhibit reduced vascular oxidant stress 17, 22, and restored vascular relaxation in response to endothelium-dependent vasodilator stimuli 17–19, 22.
In human studies to investigate potential mechanisms of the paradoxical advantage of obese hypertensive patients for cardiovascular prognosis compared to lean hypertensives in some conditions (obesity paradox), Weber et al. 23 reported that obese hypertensive patients had an attenuated renin response to treadmill exercise compared to either lean hypertensive patients or lean control subjects. Because PRA and plasma ANG II levels are significantly lower in normotensive SS rats fed LS diet compared to SS.13BN consomic rats and because SS rats have an impaired ability to regulate PRA normally 20, 24, 25, these animals may be protected against cardiovascular damage associated with obesity. We hypothesized that the reduced ability of SS rats to increase PRA and ANG II levels 20, 24, 25, as normally occurs with obesity, would protect these animals from the vascular defects and oxidant stress associated with DIO in animals showing normal regulation of the RAS.
In this study, we found DIO in SS rats had the same effect as direct infusion of a low dose of ANG II in these animals, namely to restore endothelium-dependent relaxation of cerebral arteries in response to acetylcholine. The latter observation suggests that the SS rat may provide a novel experimental model that will help separate the systemic effects of altered ANG II levels from the direct effects of obesity on the vasculature.
MATERIALS AND METHODS
Experimental Animal Groups
Male Dahl salt sensitive (SS) or SS BN13 MCW consomic rats were placed on high fat diet (Research Diets D12451, 45% kcal from fat containing 10% Mineral Mix S10026 with final concentrations of 0.3% NaCl and 0.7% K) or normal fat diet (NFD) (11.9% kCal from fat, 0.26% NaCl, 0.36% K;) AIN 76 diet (Dyets, Inc., Bethlehem, PA) for 16–20 weeks after weaning (3–5 weeks of age). Body weight was monitored by bi-weekly weighing, and a subset of the animals was given losartan (20 mg/kg/day for 1 week) in the drinking water prior to the acute vessel experiments. All rats were housed with free access to food and water in an animal care facility at the Medical College of Wisconsin (MCW), which is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the MCW IACUC.
Cannulated Middle Cerebral Artery (MCA) Preparation
On the day of the experiment, animals were anesthetized with a ketamine (78.0 mg/kg) and acepromazine (2.2 mg/kg) cocktail. The brain was removed and immersed in physiological salt solution (PSS). The MCA was carefully excised, cannulated with glass micropipettes, pressurized to 80 mmHg, and perfused and superfused with PSS (37°C) equilibrated with a 21% O2-5% CO2-74% N2 gas mixture as described previously 18. Internal diameter was measured via television microscopy. Vessels that failed to develop intrinsic resting tone because of damage in isolation or mounting (<10% of all attempted experiments) were discarded. No outliers were exuded using the statistical 2 sigma test.
Response to Vasodilator Stimuli
Vascular diameter changes in response to the endothelium-dependent vasodilator agonist ACh and endothelium-independent NO donor sodium nitroprusside (MCA) were determined in each vessel. At the end of the experiment, resting tone and maximum diameter of the artery were assessed by superfusing the vessel with papaverine (100 µM) or Ca2+-free PSS.
Arterial Blood Pressure Measurements
Chronic indwelling catheters were introduced via the femoral artery of anesthetized rats as previously described 26, 27. The animals were allowed a 3-day recovery period before beginning the experiment, and mean arterial pressure was measured in the conscious, freely moving rat.
Western Blots
The expression level of various proteins [Cu/Zn SOD, MnSOD, eNOS, p-eNOS (ser 1177), AT1R, and AT2R] was evaluated by Western blotting in pooled samples of cerebral arteries including MCA and vessels of similar size from the ventral surface of the brain, as described in the Statistical Methods section. Protein expression of Cu/Zn SOD, MnSOD, eNOS, and p-eNOS, was visualized by enhanced chemiluminescence SuperSignal West Pico (Thermo Scientific) and normalized to either β actin (Cu/Zn SOD, MnSOD, eNOS) or eNOS (p-eNOS).
Statistical Methods
Data are presented as mean value ± SEM. For all concentration-response curves, differences between groups at each concentration were determined using a two-way, repeated-measures analysis of variance (ANOVA). A post hoc Student-Newman-Keuls test was used to compare multiple means following ANOVA, and P <0.05 was considered to be statistically significant.
RESULTS
Body Weight Progression and Food Intake
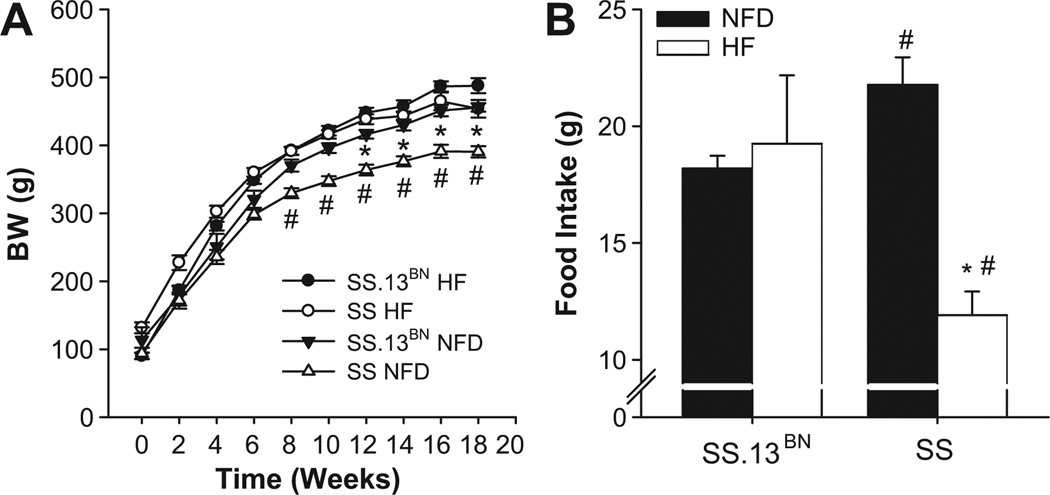
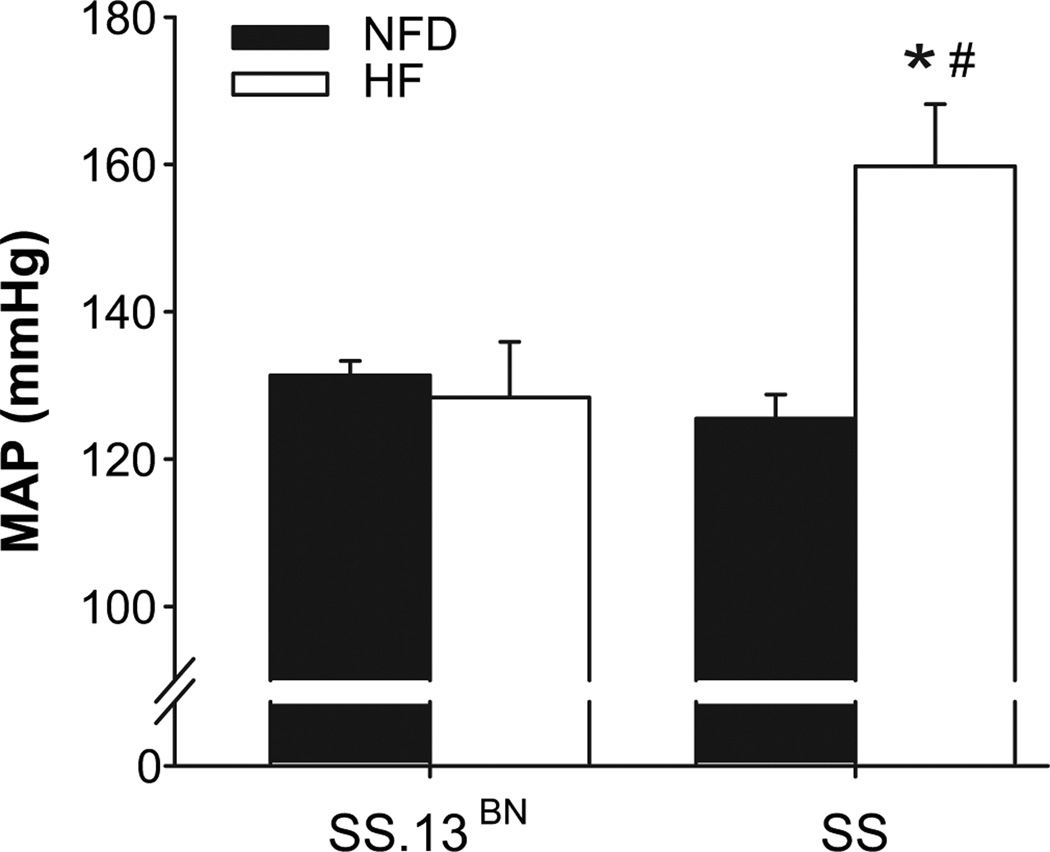
As shown in Figure 1, SS rats fed NFD diet were significantly lighter than SS-13BN rats fed NFD diet. SS HF rats gained weight faster and were significantly heavier than SS rats fed NFD diet, despite a decreased food intake in SS rats fed HF diet. The weight of SS rats fed HF diet was comparable to that of SS-13BN animals on NFD. Conscious blood pressure was significantly elevated in the SS HF group (Figure 2), while blood pressures in the other groups were not significantly different.
Figure 1.
Body weight progression and food intake of SS-13BN vs. SS rats fed high fat (HF) or normal fat diet (NFD) diet. Comparison of increase in body weights over the course of treatment with either high fat (HF) or normal fat (NFD) diet (A) and food intake in g/day (B). * P < 0.05 SS HF vs. NFD control. # P < 0.05 vs. SS-13BN on same diet. Data are expressed as mean ± SEM for n≥ 8.
Figure 2.
Chronic mean arterial blood pressure measured in conscious animals. SS HF rats show significant increase in chronic MAP. *P<0.05 vs. NFD control. # P < 0.05 vs. SS-13BN on same diet (n≥6 per group).
Cerebral Vascular Function
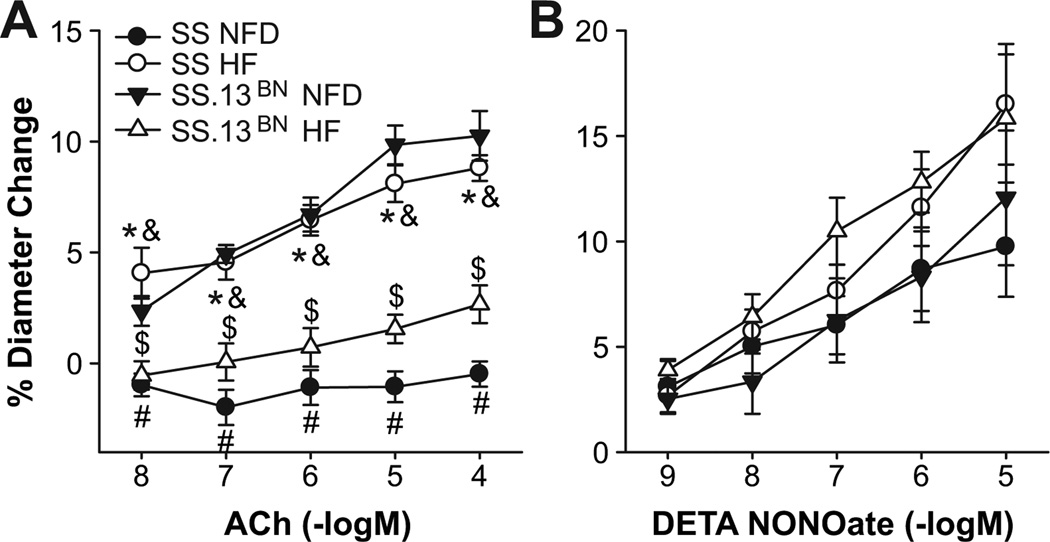
Endothelium-dependent dilation to ACh that was absent in MCA of SS rats fed NF diet was restored in SS rats ingesting a HF diet for 15–20 weeks (Figure 3), but HF diet impaired ACh-induced dilation of MCA in SS-13BN controls. Figure 4 summarizes the effect of AT1 receptor blockade with losartan and acute free radical scavenging with tempol in the PSS on the responses to ACh in MCA from the various groups. Losartan eliminated the restored dilation to ACh in MCA from SS rats fed HF diet and ameliorated endothelial dysfunction in MCA from SS.13BN rats fed HF diet, with no effect on vessel responses to ACh in MCA of SS or SS.13BN rats fed normal fat diet. Tempol restored ACh induced dilation of MCA from SS rats fed normal fat diet and SS.13BN rats fed high fat diet, with no effect on responses to ACh in MCA of SS rats fed HF diet or SS.13BN rats fed normal fat diet.
Figure 3.
Response to acetylcholine (ACh) (A) and DETA-NONOate (B) in cannulated MCA of SS and SS.13BN rats fed NFD or HF diet. Data expressed as mean ± SEM for n ≥ 6. * P < 0.05 SS HF diet vs. SS NFD; $ P < 0.05 SS13BN HF diet vs. SS13BN NFD; #-P < 0.05 SS NFD vs. SS13BN NFD; &- P < 0.05 SS HF diet vs. SS-13BN HF diet.
Figure 4.
Effect of tempol (A, B) or losartan (C, D) on response of cannulated MCA to acetylcholine (ACh) in SS and SS.13BN rats fed NFD (A, C) or HF diet (B,D). Untreated control responses were compared in Figure 3. Data are expressed as mean ± SEM for n ≥ 6, except for SS.13BN + tempol (n=2) on NFD. * P<0.05 SS control vs. SS treated (tempol or losartan); $ P < 0.05 SS13BN control vs. SS.13BN treated (HF tempol, losartan NFD and HF diet); # P<0.05. SS treated vs. SS.13BN treated. Due to the small N, no statistical comparisons were made with SS.13BN + tempol. Previous studies (Reference 19) have shown that ACh-induced dilation in MCA of SS.13BN on NFD is unaffected by tempol.
The protective effect of HF diet to restore endothelium-dependent dilation in SS rats was likely mediated by an increase in NO levels because pre-incubation with L-NAME (100 µM) abolished vasodilation to ACh in SS rats fed HF diet. Endothelium -independent relaxation to the NO donor deta NONOate was similar in all the experimental groups (Figure 3).
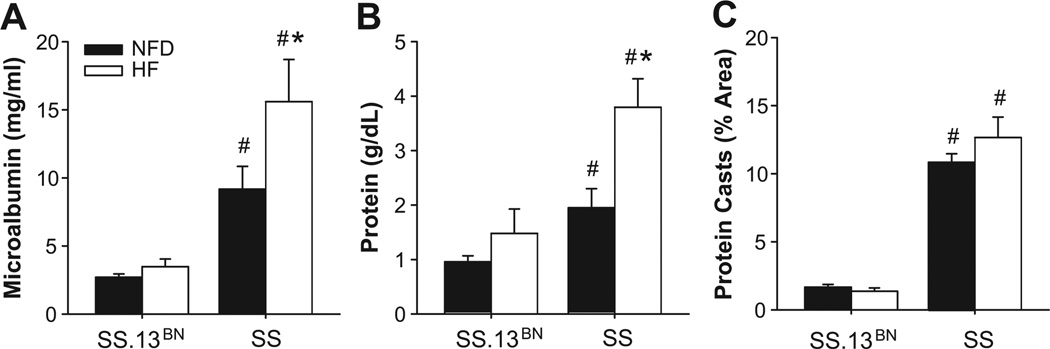
Evaluation of Renal Damage in Dahl SS and SS.13BN Rats
Because the Dahl SS rat is a commonly used model for renal hypertension, we evaluated renal function by measuring microalbumin (Figure 5A) and protein (Figure 5B) levels in the urine, and evaluating protein casts in the renal tubules histologically. High-fat diet led to a significant increase in urinary protein and microalbumin in both groups. Proteinuria and microalbuminuria were significantly greater in SS rats vs. SS13BN rats fed HF diet. Protein casts (Figure 5C) were significantly higher in renal tubules of SS rats fed NF diet and HFD diet compared to SS13BN rats fed the same diet. The latter findings are all consistent with the previously documented renal protective effect of substituting BN chromosome 13 into the SS genetic background, as is the lack of a blood pressure increase in SS-13BN rats fed HF diet vs. SS, where arterial pressure was significantly elevated with HF diet concomitant with elevated protein and microalbumin in the urine and more protein casts in the renal tubules.
Figure 5.
Effect of high fat (HF) diet vs. normal fat diet (NFD) on microalbumin (A), urinary protein (B), and protein casts in renal tubules (C) of SS vs. SS-13BN rats. Protein casts were quantified from. *P<0.05 vs. NFD control and # P<0.05 vs. SS.13BN on same diet. (n≥8 per group).
Protein Expression Using Western Blot
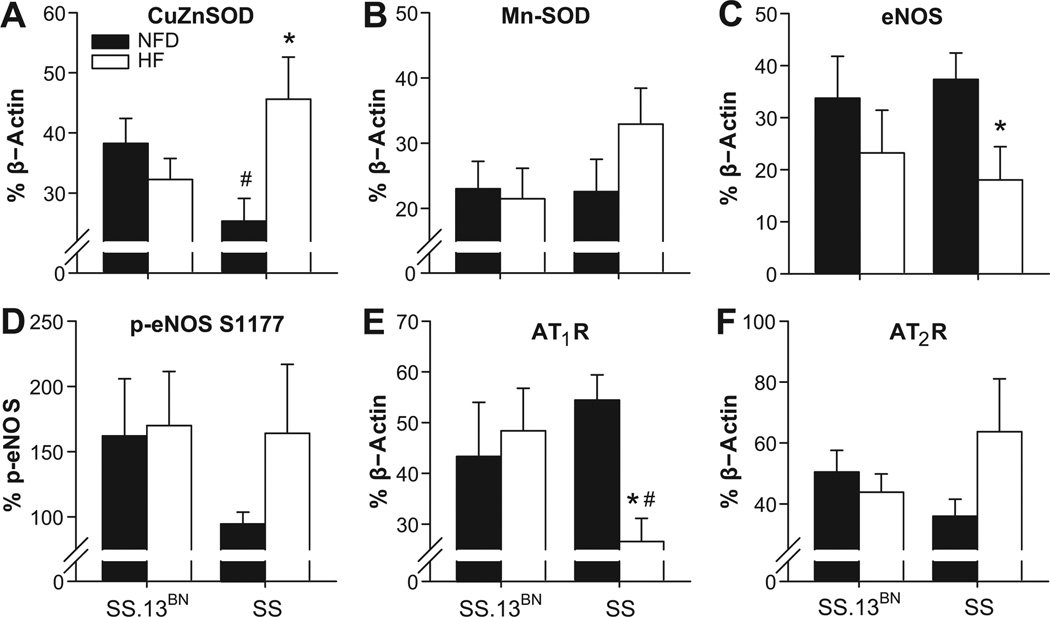
Cu/Zn SOD expression in cerebral arteries of SS rats fed normal fat diet was significantly lower than that of SS.13BN controls (Figure 6A). HF diet led to a significant increase in Cu/Zn SOD expression in arteries of SS rats, but not SS.13BN controls. HF diet also tended to increase MnSOD expression in arteries of SS rats (Figure 6B). In contrast to the vascular phenotype, total eNOS expression was significantly reduced in the SS HF group (Figure 6C), although phosphorylation of eNOS at Ser 1177, which activates eNOS-dependent NO production, appeared to be higher in SS rats fed HF diet compared to SS control rats fed NF diet (Figure 6D).
Figure 6.
Expression of CuZnSOD (A), MnSOD (B), eNOS (C), p-eNOS (D), angiotensin type 1 receptor (AT1R) (E), and angiotensin type 2 receptor (AT2R) (F) in cerebral arteries of SS and SS.13BN rats fed high fat (HF) or normal fat diet (NFD). *P<0.05 vs. NF control and # P<0.05 vs. SS.13BN (n≥6 per group).
ATR1 expression was significantly reduced by HF diet in SS rats (Figure 6E), while expression of the AT2R in the cerebral vasculature of SS HF rats tended to be higher than that of control animals fed NF diet (Figure 6F). The restored dilation to ACh in conjunction with a possible increase in the expression of the AT2 receptor in SS rats fed a high-fat diet is consistent with the results of a recent study 28 showing that chronic activation of the AT2 receptor restores endothelium-dependent vascular relaxation in mesenteric arteries of Sprague-Dawley rats fed high salt diet (another model of endothelial dysfunction and increased oxidant stress).
DISCUSSION
A number of studies 2, 29 have shown that elevated levels of circulating ANG II are associated with increased body weight, body fat, and plasma insulin levels. Relevant to its cardiovascular effects, obesity is closely associated with elevated blood pressure, decreased endothelial function, heart failure, and other cardiovascular risks, which may be caused, at least in part, by an excessive activation of the RAS 30–32.
In mice, genetic deletions of crucial parts of the RAS have a protective effect against diet-induced obesity (DIO) 33. Mice lacking the renin substrate angiotensinogen (Agt) are lean and resistant to diet-induced obesity 34. Ren1c−/− KO mice show increased energy expenditure, dietary fat wasting 33 and similar plasma levels of adiponectin (a commonly used marker for adiposity) as wild type mice, with no effects on blood pressure, food intake, blood glucose, and plasma creatinine 33. Angiotensin AT1A receptor knockout mice (Agtr1a−/−), exhibit increased ANG II levels as part of the feedback loop, but are also lean, resistant to diet-induced obesity, and show high metabolic rate (in line with ANG II levels increasing metabolism) 35.
We previously reported that ANG II suppression with high salt (HS) diet in Sprague-Dawley rats increases vascular oxidant stress 27, 36 and impairs endothelium-dependent vascular relaxation 26, 27, 37, 38. Dahl salt-sensitive rats are a commonly used model to study the effects of HS diet and low renin activity on the cardiovascular and renal system. Dahl SS rats fed normal salt diet are exposed to chronically low levels of circulating ANG II as a result of their inability to regulate PRA normally 20, 24, 25. Decreased activity of the RAS is associated with a protective effect against the effects of obesity 33, 34, 39, and renin inhibitors (which lower PRA and plasma ANG II levels) have been shown to increase the bioavailability of NO, decrease vascular oxidative stress, and protect against atherosclerosis with obesity in WHHL rabbits 40.
SS rats show increased oxidant stress 17, 41, reduced antioxidant defense mechanisms 41 and impaired vascular relaxation to multiple vasodilator stimuli 17–19, 22, 41, even when they are normotensive and maintained on a normal salt diet. As noted above, impaired vascular relaxation and endothelial dysfunction in SS rats can be rescued not only pharmacologically by chronic i.v. infusion of a subpressor dose of ANG II 17, but also genetically by introgression of Brown Norway chromosome 13 containing a normally functioning renin allele into the SS genetic background (SS.13BN consomic rat) 17–19 in order to restore normal circulating levels of ANG II.
Because of the chronically suppressed RAS in SS rats, we hypothesized that the effects of obesity-related increases in RAS activity on vascular function would be ameliorated in SS rats, but would cause a significant decrease in vascular function in SS.13BN rats, which are protected against salt-induced increases in arterial blood pressure 19–21 but have a higher basal RAS activity. Consistent with this hypothesis, SS rats fed HF diet for 15–20 weeks after weaning showed an improved endothelium-dependent dilation to ACh compared to SS rats fed NFD diet and HF-fed SS-13BN consomic control rats (~98% genetically identical to SS, but exhibiting normal activity of the RAS) 42. By contrast, HF diet caused a dramatic reduction in endothelium-dependent dilation to ACh in SS.13BN consomic rats.
The hypothesis that ANG II plays a crucial role in restoring cerebral vascular relaxation in SS rats fed HF diet is supported by the observation that treatment of the animals with the AT1R antagonist losartan blocked the protective effect of the HF diet to restore vascular relaxation. By contrast, in the SS.13BN rats, losartan tended to restore vascular relaxation to ACh in HF fed animals and had no effect on endothelium-dependent dilation to ACh in SS.13BN rats fed normal fat diet.
Interestingly other effects of DIO (e.g. increased BW and a further increase in renal defects) are still observed in both rat strains, in spite of the improved vascular function in SS rats fed HF diet. Glucose and insulin tolerance tests and measurements of fasting blood glucose levels revealed no significant difference between any of the groups (Data not shown), and HF diet did not have a protective effect on renal function in either strain.
In summary, we found that SS rats, a model of low RAS activity, are protected against the deleterious effects of obesity on the cerebral vasculature that are ordinarily mediated via ANG II; and that diet-induced obesity actually restores endothelium-dependent dilation to ACh that is normally absent in MCA of SS rats 17. The findings of the present study support the concept that physiological levels of ANG II play an important role in maintaining normal vascular relaxation mechanisms by showing that a genetic rodent model of low renin, salt-sensitive hypertension exhibits enhanced endothelial function with prolonged HF diet. This mechanism is likely mediated, at least in part, by increased expression of superoxide dismutase; and changes in the expression of the AT1 and possibly AT2 receptors for ANG II occur with ingestion of a high fat diet. The effects of AT1 receptor blockade with losartan are also consistent with a role for ANG II both in leading to endothelial dysfunction in HF-fed SS.13BN rats and in contributing to the protective effect of lower ANG II levels to restore vascular relaxation in HF-fed SS rats. Taken together, the findings of the present study provide further support for the hypothesis that proper levels of circulating ANG II are critically important in maintaining vascular function, as both lowered ANG II levels (HS diet, SS rats)12, 17–19, 22, 26, 27, 36, 37 or increased levels of ANG II [severe Na+ restriction 43 high dose ANG II infusion 44, obesity 2, 4 lead to vascular defects such as endothelial dysfunction. Overall, the results of this study underscore the need for further study of the role of the RAS in obesity-mediated vascular changes, and suggest that the Dahl salt-sensitive rat may be an excellent model system for this undertaking.
Perspectives
The findings of the present study suggest that the SS rat is genetically protected against endothelial dysfunction in DIO despite increased body weight, elevated blood pressure, and reduced renal function. The observation that high fat diet has a protective effect against endothelial dysfunction is, at first glance, surprising. However, the concept that mild obesity has a protective effect on cardiovascular phenotypes is not without precedent. For example, leptin, a marker for adiposity, is a NO-independent coronary artery vasodilator in humans 45, 46; and Okere et al. reported that high fat diet prevents the hypertrophic response to hypertension and improves the contractile performance of the heart in Dahl SS rats 47.
The current findings may be especially relevant to findings in human patient populations with established cardiovascular disease. Although obesity is a known risk factor in the pathogenesis and progression of cardiovascular disease 48, there are numerous reports of an “obesity paradox”, where obesity and higher body mass index (BMI) are associated with lower mortality in patients with established cardiovascular disease 49–52. While the mechanism of this phenomenon remains elusive, attenuated neurohumoral responses, including reduced activation of the renin-angiotensin system have been suggested as a possible factor contributing to the obesity paradox 23, 48, 50. In this regard, it would be interesting to determine whether obesity or higher BMI has any protective effect in individuals with low PRA, who exhibit a significantly higher mortality from cardiovascular causes than survivors in long-term follow-up studies16.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
This study shows that high fat diet to produce diet induced obesity results in a paradoxical restoration of endothelial function in Dahl SS rats that can be prevented by AT1 receptor blockade with losartan.
What is Relevant?
The present study supports the hypothesis that normal physiological levels of ANG II play a crucial role in maintaining normal vascular relaxation while elevated ANG II levels contribute to endothelial dysfunction.
Summary
Ingestion of a high fat diet to produce diet induced obesity ameliorated endothelial dysfunction in Dahl SS rats and abrogated endothelium-dependent dilation to ACh in SS.13BN consomic rats showing normal regulation of plasma renin activity. The protective effect of HF diet to restore vascular relaxation in the SS rats was prevented by AT1 receptor blockade with losartan.
Acknowledgments
We thank the department of Physiology`s assay and microscopy core facilities for measurement of several parameters during the development of this manuscript.
We like to thank Dr. David Mattson for his the help with the interpretation and quantification of the renal phenotypes presented in this work.
Grant Support: NIH #HL65289, #HL-72920, and #HL-92026.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
REFERENCES
- 1.Cassis LA, Marshall DE, Fettinger MJ, Rosenbluth B, Lodder RA. Mechanisms contributing to angiotensin II regulation of body weight. Am J Physiol. 1998;274:E867–E876. doi: 10.1152/ajpendo.1998.274.5.E867. [DOI] [PubMed] [Google Scholar]
- 2.Boustany CM, Bharadwaj K, Daugherty A, Brown DR, Randall DC, Cassis LA. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am J Physiol Regul Integr Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 3.Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, Luft FC, Sharma AM. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45:356–362. doi: 10.1161/01.HYP.0000154361.47683.d3. [DOI] [PubMed] [Google Scholar]
- 4.Rahmouni K, Correia ML, Haynes WG, Mark AL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. 2005;45:9–14. doi: 10.1161/01.HYP.0000151325.83008.b4. [DOI] [PubMed] [Google Scholar]
- 5.Barton M, Baretella O, M M. Obesity and risk of vascular disease: importance of endothelium-dependent vasoconstriction. Br J Pharmacol. 2012;165:591–602. doi: 10.1111/j.1476-5381.2011.01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Yang G, Offer A, Zhou M, Smith M, Peto R, Ge H, Yang L, Whitlock G. Body mass index and mortality in China: a 15-year prospective study of 220 000 men. International Journal of Epidemiology. 2012;41:472–481. doi: 10.1093/ije/dyr208. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Sánchez A, Madrigal-Santillán E, Bautista M, Esquivel-Soto J, Morales-González Á, Esquivel-Chirino C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C, Morales-González JA. Inflammation, oxidative stress, and obesity. International journal of molecular sciences. 2011;12:3117–3132. doi: 10.3390/ijms12053117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, Chade AR, Lerman LO, Lerman A. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. American Journal of Physiology-Heart and Circulatory Physiology. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 9.Ketonen J, Shi J, Martonen E, Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circulation journal: official journal of the Japanese Circulation Society. 2010;74:1479–1479. doi: 10.1253/circj.cj-09-0661. [DOI] [PubMed] [Google Scholar]
- 10.Phillips MCL, Leyden JM, Chong WK, Kleinig T, Czapran P, Lee A, Koblar SA, Jannes J. Ischaemic stroke among young people aged 15 to 50 years in Adelaide, South Australia. The Medical Journal of Australia. 2011;195:610–614. doi: 10.5694/mja11.10558. [DOI] [PubMed] [Google Scholar]
- 11.Towfighi A, Zheng L, Ovbiagele B. Weight of the Obesity Epidemic. Stroke. 2010;41:1371–1375. doi: 10.1161/STROKEAHA.109.577510. [DOI] [PubMed] [Google Scholar]
- 12.McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am J Physiol Heart Circ Physiol. 2009;297:H1296–H1303. doi: 10.1152/ajpheart.01325.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez I, Cowley AW, Jr, Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol. 1992;263:H664–H667. doi: 10.1152/ajpheart.1992.263.3.H664. [DOI] [PubMed] [Google Scholar]
- 14.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol. 2006;291:H114–H120. doi: 10.1152/ajpheart.01116.2005. [DOI] [PubMed] [Google Scholar]
- 15.Cholewa BC, Mattson DL. Role of the renin-angiotensin system during alterations of sodium intake in conscious mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R987–R993. doi: 10.1152/ajpregu.2001.281.3.R987. [DOI] [PubMed] [Google Scholar]
- 16.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 17.Drenjancevic-Peric I, Lombard JH. Reduced angiotensin II and oxidative stress contribute to impaired vasodilation in Dahl salt-sensitive rats on low-salt diet. Hypertension. 2005;45:687–691. doi: 10.1161/01.HYP.0000154684.40599.03. [DOI] [PubMed] [Google Scholar]
- 18.Drenjancevic-Peric I, Lombard JH. Introgression of chromosome 13 in Dahl salt-sensitive genetic background restores cerebral vascular relaxation. American Journal of Physiology-Heart and Circulatory Physiology. 2004;287:H957–H962. doi: 10.1152/ajpheart.01087.2003. [DOI] [PubMed] [Google Scholar]
- 19.Drenjancevic-Peric I, Phillips SA, Falck JR, Lombard JH. Restoration of normal vascular relaxation mechanisms in cerebral arteries by chromosomal substitution in consomic SS. 13BN rats. American Journal of Physiology-Heart and Circulatory Physiology. 2005;289:H188–H195. doi: 10.1152/ajpheart.00504.2004. [DOI] [PubMed] [Google Scholar]
- 20.Cowley AW, Jr, Roman RJ, Kaldunski ML, Dumas P, Dickhout JG, Greene AS, Jacob HJ. Brown Norway chromosome 13 confers protection from high salt to consomic Dahl S rat. Hypertension. 2001;37:456–461. doi: 10.1161/01.hyp.37.2.456. [DOI] [PubMed] [Google Scholar]
- 21.Moreno C, Dumas P, Kaldunski ML, Tonellato PJ, Greene AS, Roman RJ, Cheng Q, Wang Z, Jacob HJ, Cowley AW., Jr Genomic map of cardiovascular phenotypes of hypertension in female Dahl S rats. Physiol Genomics. 2003;15:243–257. doi: 10.1152/physiolgenomics.00105.2003. [DOI] [PubMed] [Google Scholar]
- 22.Durand MJ, Moreno C, Greene AS, Lombard JH. Impaired relaxation of cerebral arteries in the absence of elevated salt intake in normotensive congenic rats carrying the Dahl salt-sensitive renin gene. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299:H1865–H1874. doi: 10.1152/ajpheart.00700.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber MA, Neutel JM, Smith DHG. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. Journal of the American College of Cardiology. 2001;37:169–174. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 24.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension. 2001;37:386–390. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Stec DE, Drummond H, Simon JS, Koike G, Jacob HJ, Roman RJ. Transfer of a salt-resistant renin allele raises blood pressure in Dahl salt-sensitive rats. Hypertension. 1997;29:619–627. doi: 10.1161/01.hyp.29.2.619. [DOI] [PubMed] [Google Scholar]
- 26.Weber DS, Lombard JH. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. American Journal of Physiology-Heart and Circulatory Physiology. 2000;278:H500–H506. doi: 10.1152/ajpheart.2000.278.2.H500. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291:H929–H938. doi: 10.1152/ajpheart.00692.2005. [DOI] [PubMed] [Google Scholar]
- 28.Raffai G, Durand MJ, Lombard JH. Acute and chronic angiotensin-(1–7) restores vasodilation and reduces oxidative stress in mesenteric arteries of salt-fed rats. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301:H1341–H1352. doi: 10.1152/ajpheart.00202.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiéra F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? The International Journal of Biochemistry & Cell Biology. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- 30.Barton M, Carmona R, Ortmann J, Krieger JE, Traupe T. Obesity-associated activation of angiotensin and endothelin in the cardiovascular system. The International Journal of Biochemistry & Cell Biology. 2003;35:826–837. doi: 10.1016/s1357-2725(02)00307-2. [DOI] [PubMed] [Google Scholar]
- 31.Barton M, Carmona R, Morawietz H, d’Uscio LV, Goettsch W, Hillen H, Haudenschild CC, Krieger JE, Münter K, Lattmann T. Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin. Hypertension. 2000;35:329–336. doi: 10.1161/01.hyp.35.1.329. [DOI] [PubMed] [Google Scholar]
- 32.Gorzelniak K, Engeli S, Janke J, Luft FC, Sharma AM. Hormonal regulation of the human adipose-tissue renin-angiotensin system: relationship to obesity and hypertension. Journal of Hypertension. 2002;20:965–973. doi: 10.1097/00004872-200205000-00032. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi N, Li F, Hua K, Deng J, Wang CH, Bowers RR, Bartness TJ, Kim HS, Harp JB. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell metabolism. 2007;6:506–512. doi: 10.1016/j.cmet.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massiera F, Seydoux J, Geloen A, Quignard-Boulange A, Turban S, Saint-Marc P, Fukamizu A, Negrel R, Ailhaud G, Teboul M. Angiotensinogen-deficient mice exhibit impairment of diet-induced weight gain with alteration in adipose tissue development and increased locomotor activity. Endocrinology. 2001;142:5220–5225. doi: 10.1210/endo.142.12.8556. [DOI] [PubMed] [Google Scholar]
- 35.Kouyama R, Suganami T, Nishida J, Tanaka M, Toyoda T, Kiso M, Chiwata T, Miyamoto Y, Yoshimasa Y, Fukamizu A, Horiuchi M, Hirata Y, Ogawa Y. Attenuation of diet-induced weight gain and adiposity through increased energy expenditure in mice lacking angiotensin II type 1a receptor. Endocrinology. 2005;146:3481–3489. doi: 10.1210/en.2005-0003. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries. Journal of vascular research. 2007;44:382–390. doi: 10.1159/000102955. [DOI] [PubMed] [Google Scholar]
- 37.Weber DS, Lombard JH. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280:H2196–H2202. doi: 10.1152/ajpheart.2001.280.5.H2196. [DOI] [PubMed] [Google Scholar]
- 38.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. American Journal of Physiology-Heart and Circulatory Physiology. 2003;284:H1124–H1133. doi: 10.1152/ajpheart.00835.2002. [DOI] [PubMed] [Google Scholar]
- 39.Tallam LS, Jandhyala BS. Significance of exaggerated natriuresis after angiotensin AT1 receptor blockade or angiotensin-converting enzyme inhibition in obese Zucker rats. Clinical and experimental pharmacology and physiology. 2001;28:433–440. doi: 10.1046/j.1440-1681.2001.03457.x. [DOI] [PubMed] [Google Scholar]
- 40.Imanishi T, Tsujioka H, Ikejima H, Kuroi A, Takarada S, Kitabata H, Tanimoto T, Muragaki Y, Mochizuki S, Goto M. Renin inhibitor aliskiren improves impaired nitric oxide bioavailability and protects against atherosclerotic changes. Hypertension. 2008;52:563–572. doi: 10.1161/HYPERTENSIONAHA.108.111120. [DOI] [PubMed] [Google Scholar]
- 41.Durand MJ, Lombard JH. Introgression of the Brown Norway Renin Allele Onto the Dahl Salt-Sensitive Genetic Background Increases Cu/Zn SOD Expression in Cerebral Arteries. American journal of hypertension. 2011;24:563–568. doi: 10.1038/ajh.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cowley A, Jr, Liang M, Roman R, Greene A, Jacob H. Consomic rat model systems for physiological genomics. Acta physiologica scandinavica. 2004;181:585–592. doi: 10.1111/j.1365-201X.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- 43.Huang A, Yan C, Suematsu N, Cuevas A, Yang YM, Kertowidjojo E, Hintze TH, Kaley G, Sun D. Impaired flow-induced dilation of coronary arterioles of dogs fed a low-salt diet: roles of ANG II, PKC, and NAD (P) H oxidase. American Journal of Physiology-Heart and Circulatory Physiology. 2010;299:H1476–H1483. doi: 10.1152/ajpheart.01250.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. Journal of Clinical Investigation. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Momin AU, Melikian N, Shah AM, Grieve DJ, Wheatcroft SB, John L, El Gamel A, Desai JB, Nelson T, Driver C. Leptin is an endothelial-independent vasodilator in humans with coronary artery disease: evidence for tissue specificity of leptin resistance. European heart journal. 2006;27:2294–2299. doi: 10.1093/eurheartj/ehi831. [DOI] [PubMed] [Google Scholar]
- 46.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d'Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- 47.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, Hoit BD, Chandler MP, Stanley WC. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clinical and experimental pharmacology and physiology. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 48.Lavie CJ, Milani RV, Ventura HO. Obesity and Cardiovascular Disease:: Risk Factor, Paradox, and Impact of Weight Loss. Journal of the American College of Cardiology. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 49.Lavie CJ, Milani RV, Ventura HO, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. The American journal of cardiology. 2007;100:1460–1464. doi: 10.1016/j.amjcard.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 50.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. American heart journal. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Ovbiagele B, Bath PM, Cotton D, Vinisko R, Diener HC. Obesity and recurrent vascular risk after a recent ischemic stroke. Stroke. 2011;42:3397–3402. doi: 10.1161/STROKEAHA.111.624957. [DOI] [PubMed] [Google Scholar]
- 52.Galal W, Van Gestel YRBM, Hoeks SE, Sin DD, Winkel TA, Bax JJ, Verhagen H, Awara AMM, Klein J, Van Domburg RT. The obesity paradox in patients with peripheral arterial disease. Chest. 2008;134:925–930. doi: 10.1378/chest.08-0418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.