Abstract
Calpains, a family of Ca2+-dependent cytosolic cysteine proteases, can modulate their substrates' structure and function through limited proteolytic activity. In the human genome, there are 15 calpain genes. The most-studied calpains, referred to as conventional calpains, are ubiquitous. While genetic studies in mice have improved our understanding about the conventional calpains' physiological functions, especially those essential for mammalian life as in embryogenesis, many reports have pointed to overactivated conventional calpains as an exacerbating factor in pathophysiological conditions such as cardiovascular diseases and muscular dystrophies. For treatment of these diseases, calpain inhibitors have always been considered as drug targets. Recent studies have introduced another aspect of calpains that calpain activity is required to protect the heart and skeletal muscle against stress. This review summarizes the functions and regulation of calpains, focusing on the relevance of calpains to cardiovascular disease.
Keywords: Calpain, Intracellular proteolysis, Sarcomere, Transgenic mice, Calcium
1. Introduction
Calpains1–4 (Clan CA, family C02; EC 3.4.22.17) are defined by their amino acid (aa) sequence similar to the calpain-like Cys protease motif CysPc, which is registered as cd00044 in the National Center for Biotechnology Information's conserved domain database. Using this criterion, the database identifies calpain homologues in a wide range of living organisms. The physiological relevance of calpains has been elusive, partly because each calpain species is fairly specific in its function as a critical regulator for cellular functions. On the other hand, calpains are often described as aggravating factors in various pathophysiological phenomena;5–11 therefore, inhibiting calpains is an established research interest. Although little has been elucidated about when and how calpain is activated, fortunately, a new perspective emerges. Improved genetic techniques have revealed cause-and-effect relationships between calpain deficiencies and dysfunctions of tissues and organs.12 These calpain-deficiency diseases, which should generally be called calpainopathies, provide clear evidence of the physiological importance of calpains.
Although calpains have frequently been characterized as deleterious degradative proteases in pathogenic conditions including cardiovascular diseases, calpains are actually processing rather than degradative proteases. Calpains differ from other major intracellular proteolytic components such as proteasomes13 and lysosomal proteases functioning in autophagy;14 these systems eliminate and recycle their substrates by degradation. Calpains act by proteolytic processing, as in the activation of conventional protein kinase C (PKC) (see Section 5.2.1). Calpains are unique in that they directly recognize substrates, whereas proteasomes and autophagy rely on other systems—ubiquitylation and autophagosome formation, respectively—to tag their substrates (Figure 1).
Figure 1.
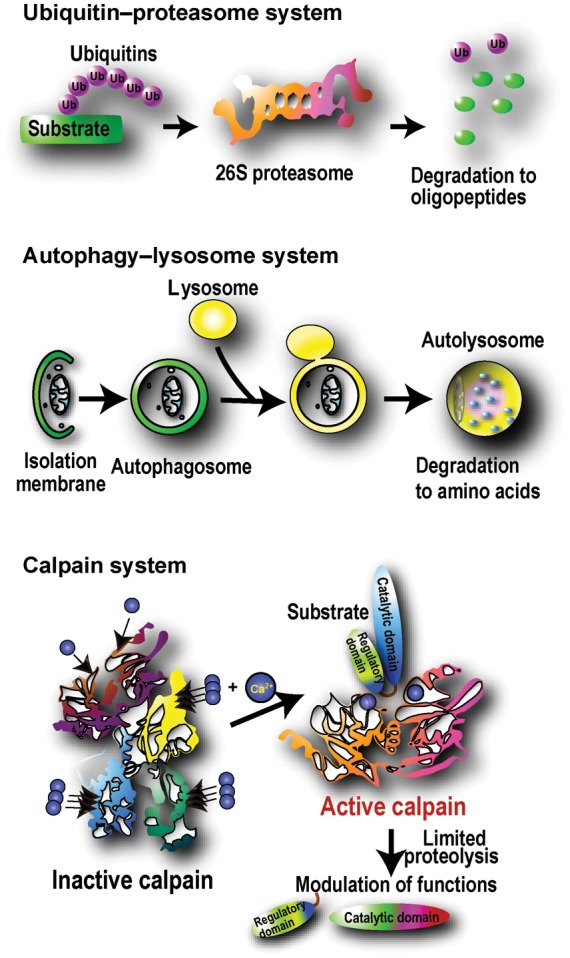
Major intracellular proteolytic systems. The ubiquitin–proteasome system degrades and eliminates specific substrate proteins with an ubiquitin-tagging system consisting of >1000 ubiquitin ligases. The autophagy-lysosome system primarily degrades non-specific cell components, including proteins and microorganisms, contained by isolation membranes. The caspase system (not shown) is a major intracellular proteolytic system with primarily apoptotic functions.114 In contrast, calpains primarily elicit proteolytic processing, rather than degradation, to modulate or modify substrate activity, specificity, longevity, localization, and structure.
After a brief overview of the molecules that comprise the calpain system, we will review calpains' activation mechanisms and physiological and pathophysiological roles, focusing particularly on their relevance in cardiac and skeletal muscle tissues.
2. The calpain system
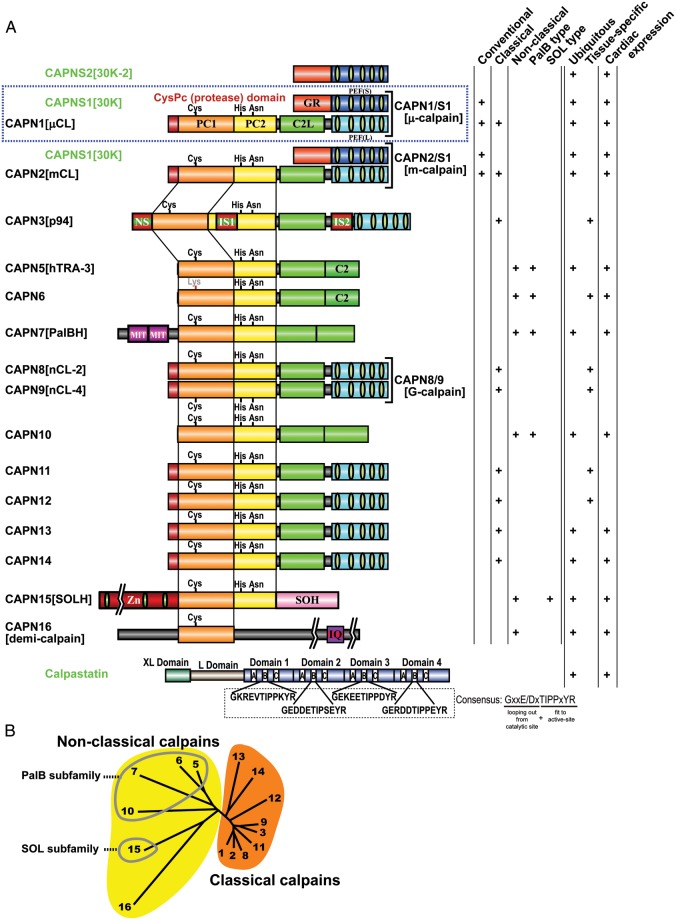
Foundational calpain studies have focused on the mammalian μ- and m-calpains,15 which are thus called the conventional calpains; all other calpains are referred to as unconventional, and their structure is often described relative to that of the conventional calpains (Figure 2).
Figure 2.
Human calpains and their regulatory molecules. (A) Schematic structures. In this review, human calpains are presented in a proposed name (previous name, if any) format. Calpain structural classification and tissue distribution, particularly regarding the heart, is indicated in the right-hand columns. Calpain enzyme complexes (tertiary structures) that have been elucidated in vivo are shown along with their enzyme names (single brackets). Bottom: the domain structure of the longest calpastatin isoform. The four repetitive inhibitory units are labelled as domains 1–4; in each of these, the A–C regions are important for calpastatin's inhibitory activity. The consensus aa sequence in the B-region, which directly interacts with the calpain active site, is shown. Exons encoding the XL and L domains are subject to alternative splicing. Symbols: PC1 and PC2, protease core domains 1 and 2 in the calpain protease (CysPc) domain; C2L, C2-domain-like domain; PEF(L) and PEF(S), penta-EF-hand domains in the larger (L) and smaller (S) subunits, respectively; GR, glycine-rich hydrophobic domain; NS/IS1/IS2, CAPN3[p94]-characteristic sequences; MIT, microtubule interacting and trafficking motif; C2, C2 domain; Zn, Zn-finger motif domain; SOH, SOL-homology domain; IQ, a motif that interacts with calmodulin. (B) Human calpain (represented by its number) phylogenetic tree, drawn using the neighbour-joining and bootstrap method after aligning all sequences.4 Non-classical calpains are divided into further subfamilies.
2.1. Unifying the nomenclature for calpains and their domains
A unified calpain nomenclature was recently proposed based on a web-based discussion among calpain researchers, aiming to overcome hindered development of calpain studies due to complex naming.4 This nomenclature defines mammalian calpain gene products as CAPN1, CAPN2, and so on; this follows the gene product nomenclature defined by the Human Genome Organization Gene Nomenclature Committee. To avoid confusion, this review refers to calpains by their proposed name followed by their previously common name, where applicable, in square brackets (e.g. CAPN1[μCL] or CAPN2[mCL]; μCL and mCL denote μ- and m-calpain larger catalytic subunits, respectively). Accordingly, conventional calpains are heterodimers of CAPN1[μCL] or CAPN2[mCL] and CAPNS1[30 K], and are called CAPN1/S1[μ-calpain] and CAPN2/S1[m-calpain] (CAPN1/S1 is short for CAPN1/CAPNS1).
CAPN1[μCL] and CAPN2[mCL] are divided into four regions/domains: the N-terminal anchor helix; the CysPc protease domain, which is divided into two protease core domains (PC1 and PC2);16,17 a C2 domain-like (C2L) domain; and a penta-EF-hand (PEF(L)) domain.18 CAPNS1[30K], the smaller regulatory subunit, contains an N-terminal Gly-rich (GR) domain and a PEF(S) domain (Figure 2).
2.2. Calpain homologues
Although other papain superfamily proteases (clan CA) have weak local similarities to the CysPc domain, they are clearly differentiated from calpains by their low aa sequence similarity. Accordingly, the human genome has 15 calpain genes, and other mammals have nearly the same number. Calpain genes exist in almost all eukaryotes and a few bacteria,1 and these non-mammalian calpains are also enormously interesting scientific subjects. In this review, we focus on mammalian calpains, which are classified by two criteria: structure and distribution.1,4,19
2.2.1. Classical and non-classical calpains
The domain structure of CAPN1[μCL] and CAPN2[mCL], in which CysPc is followed by C2L and PEF domains (Figure 2), is classical by definition. Accordingly, non-classical calpains are missing C2L and/or PEF domains.20 Both classical and non-classical calpains may have additional domains. The nine human classical calpains—CAPN1–3, 8, 9, and 11–1421—share strong sequence similarities, which, however, does not necessarily indicate functional or biochemical similarities. For example, of all the human classical calpains, only CAPN1[μCL] and CAPN2[mCL] form heterodimers with CAPNS1[30 K] in vivo.
Non-classical calpains are divided into several subfamilies. The PalBH subfamily is the most evolutionarily conserved, being found from humans (CAPN7[PalBH]) to fungi (PalB) and yeast (Rim13[Cpl1]), but not in plants.22 The PalB subfamily has two tandem C2L/C2 domains following the CysPc domain, and may have up to two microtubule interaction and trafficking (MIT) motifs at the N-terminus. The SOL subfamily is also evolutionarily conserved, with orthologues in almost all animal species, including humans (CAPN15[SOLH]), drosophila, and green algae. Its domain structure is characterized by several Zn2+-finger motifs and a specific SOL-homology (SOH) domain at the CysPc N- and C-termini, respectively (Figure 2).
2.2.2. Ubiquitous and tissue-specific calpains
Six human calpain genes are tissue-specific; the others, including conventional calpains, are ubiquitous. Human tissue-specific calpains are CAPN3[p94] in skeletal muscle,23 CAPN6 in the placenta and embryonic muscles,24 CAPN8[nCL-2] and CAPN9[nCL-4] in the gastrointestinal tract,20 CAPN11 in the testis,25 and CAPN12 in hair follicles.26 It is widely assumed that ubiquitous calpains have basic roles in the cell, whereas tissue-specific calpains are involved in specific cell functions. Accordingly, defects in ubiquitous calpains can be lethal, as seen in Capn2−/− and Capns1−/− mice,27–30 whereas defects in tissue-specific calpains may cause tissue-specific phenotypes, such as the muscular dystrophy caused by CAPN3 mutations.31 In conditions such as cardiomyopathy, muscular dystrophies, or traumatic ischaemia, conventional calpain overactivation has been identified as an aggravating factor, probably because the intracellular Ca2+ homeostasis is compromised.32 In such cases, attenuating symptoms by specifically inhibiting conventional calpains is a major objective.33 In contrast, functional loss of a tissue-specific calpain can perturb the tissues in which it is expressed, as with muscular dystrophy and stress-induced gastric ulcers.31,34–36 Such systems might enable the identification of biological events in which calpain is important.
3. Calpain system regulatory components
The calpain system has two essential regulatory components, CAPNS1[30 K] and calpastatin. Notably, however, the effect of these molecules, whether positive or negative, is limited to a few calpains, and mostly, to the conventional calpains. Other molecules may exist that govern other calpains or the calpain activity in specific tissues.
3.1. The conventional calpain smaller regulatory subunit, CAPNS1[30 K]
The PEF(S) domain of CAPNS1[30 K] is significantly similar to PEF(L), and the interaction between the fifth EF-hand motifs of CAPNS1[30 K] and CAPN1[μCL] or CAPN2[mCL] forms a heterodimer, resulting in the conventional calpains. The CAPNS1[30 K] GR domain contains hydrophobic Gly-clusters, most of which are autolysed as conventional calpains are activated. Three-dimensional structural analysis shows this domain to have a very soft structure.
CAPNS1[30 K] is an important chaperone-like component for conventional calpains. Without CAPNS1[30 K], during in vitro renaturation CAPN2[mCL] is very slow to become active, if it does at all.37 Consistent with this, both CAPN1[μCL] and CAPN2[mCL] are almost completely down-regulated in Capns1−/− mice, resulting in embryonic lethality; these mice rarely survive past E11.5.30,38 Thus, CAPNS1[30 K] is absolutely required for the stability of both conventional calpain catalytic subunits. So far, CAPNS1[30 K] has only been shown to be necessary for CAPN1[μCL] and CAPN2[mCL]. Although there is one paralogue, CAPNS2[30K-2], in the human genome, its regulatory effect on calpains is unknown.39 Recent studies showing Capns1 involvement in osteoblasts and chondrocytes29,40 suggest that CAPNSs may have as-yet-unknown functions.
3.2. Calpastatin: the one and only specific endogenous calpain inhibitor
Calpastatin is the only known endogenous-specific inhibitor of the conventional calpains.41 Among calpain homologues so far examined, calpastatin also inhibits CAPN8[nCL-2]42 and CAPN9[nCL-4],43 but not CAPN3[p94],44 in vitro. Calpastatin is effectively proteolysed by CAPN3[p94], implying that CAPN3[p94] helps regulate conventional calpains in skeletal muscle.44 One calpastatin molecule contains four inhibitor units (Figure 2); each unit inhibits one calpain molecule with variable efficiency.45–47 Oligopeptides as short as 20 aa derived from these inhibitory units can inhibit calpain, although with reduced efficacy. Calpastatins have poor primary sequence conservation between species, despite their high specificity even to different species' conventional calpains, which are highly conserved: humans and rat CAPN1[μCL] are 89% identical, whereas calpastatins are only 66% identical. The 3D structure of CAPN2/S1[m-calpain] co-crystallized with a calpastatin fragment and Ca2+ revealed that calpastatin's intrinsically unstructured property enables it to bind calpain tightly (Figure 3) while looping several adjacent aa residues out from the active site to protect itself from proteolysis.48,49
Figure 3.
Inactive and active CAPN2/S1[m-calpain] 3D structures. Surface-type schematic 3D structures (cross-eyed view) of active (Ca2+- and calpastatin-bound) CAPN2/S1[m-calpain], using the PDB data 3BOW.48 Oligopeptides, represented by the yellow surface + ball-and-stick inside, indicate calpastatin tightly bound to CAPN2/S1[m-calpain]; due to its soft structure, some parts of calpastatin are not visible. The active protease domain (CysPc) is formed by the fusion of the PC1 and PC2 subdomains upon the binding of one Ca2+ to each subdomain. Though not visible here, the active site exists deep inside at the site circled in black. None of the 10 Ca2+ is visible here.
4. Calpain activation and regulation
4.1. Insights into the structure of CysPc
The first calpain primary structure to be determined was chicken CAPN11[μ/mCL];50 15 years later, the Ca2+-free 3D structure of CAPN2/S1[m-calpain] was solved.16,17 This revealed that the CysPc domain is split into two halves, keeping the active-site residues and the potential substrate-binding cleft in non-functional conformations. Thus, the CysPc domain is characterized as two protease core (PC1/2) domains within one protease domain.4
Next, 3D structures of the CAPN1[μCL] and CAPN2[mCL] Ca2+-bound CysPc domains brought three major findings. Firstly, two unique Ca2+-binding sites (CBS-1 and -2) exist in the PC1 and PC2 domains, respectively.51,52 Secondly, upon binding Ca2+, the PC1 and PC2 domains move towards each other to form the active site. Thirdly, the active-site cleft is somewhat deeper and narrower than those of other papain-like Cys proteases,53 so the substrates must be in an extended conformation to fit the cleft; this partly explains why calpains preferentially proteolyse inter-domain unstructured regions. Determination of the whole 3D structure of active CAPN2/S1[m-calpain] co-crystallized with calpastatin and Ca2+ confirmed the above activation mechanism48,49 (Figure 3).
4.2. Other mechanisms of calpain activation
In mammalian conventional calpains, CAPNS1[30K] must form a heterodimer with CAPN1[μCL] or CAPN2[mCL] to regulate the calpain activity. What, then, is known about the regulation of other calpains?
CAPN3[p94], which is specific to skeletal muscles, has a unique modification within CysPc. Without any subunit, CAPN3[p94] autodegrades very rapidly under physiological conditions; this process depends on the specific insertion sequences IS1 and IS2, located within PC2 and in the linker region between the C2L and PEF domains, respectively54 (Figure 2). This autolytic activity was recently shown to be Na+-dependent in the absence of Ca2+, making this the first example of an intracellular Na+-dependent enzyme.55 These properties are unique to CAPN3[p94], differentiating it from any other calpains. Some CAPN3 and Capn3 stage-specific alternative splice variants, lacking IS1, IS2, or both, show decreased autolytic activity56 and result in myopathy when overexpressed in muscles.57 Taken together, these findings suggest that additional aspects of CAPN3[p94] contribute to its CysPc domain regulation, and support CAPN3[p94]'s function under conditions specific to skeletal muscles.
CAPN8[nCL-2] and CAPN9[nCL-4] form the heterodimer CAPN8/9[G-calpain] in vivo; heterodimer formation is essential for the stable existence of CAPN8/9[G-calpain], which is the first example of a hybrid heterodimer of two distinct calpain catalytic subunits.36 Another example regulating calpain activation is that CAPN7[PalBH] interacts with the ESCRT-III protein IST1 (increased sodium tolerance-1) via its MIT motifs, enhancing its autolytic activity.58
4.3. Calpain activation mechanism in vivo: the Ca2+ requirement
One of the classical calpain research questions is how the conventional calpains are activated in the cytosol, since their activation in vitro requires a high Ca2+concentration (at least tens of μM) that is seldom available in vivo. One explanation is that the vicinity of plasma and endosomal membranes is a favourable niche for calpain activation; in vitro experiments have shown that phospholipids, a major component of plasma membranes, lower the Ca2+ concentration required to activate calpain.59–61 Another possibility is that a very small number of calpain molecules, localized to a small region with a high local Ca2+ concentration, is sufficient to fulfill calpain's functions.
On the other hand, Ca2+/Na+ concentrations change dynamically at the neuromuscular junction (NMJ). Indeed, calpain was activated at the NMJ in muscle cells from patients with slow-channel myasthenic syndrome,62 in which Ca2+ overload occurs at the NMJ due to mutations in the genes encoding nicotinic acetylcholine receptor subunits. In a mouse model of this syndrome, the transgenic (Tg) overexpression of calpastatin ameliorates the symptoms and neuromuscular transmission. These suggest that under the normal condition, calpains should be tightly regulated to elicit proper functions at the NMJ; further studies will clarify which calpains, conventional and/or muscle-specific calpains, are critical to the phenomenon.
4.4. Substrate specificity of calpains
Another classical research question is how calpains' substrate specificities are defined. The substrate specificities of the two conventional calpain species, CAPN1/S1[μ-calpain] and CAPN2/S1[m-calpain], are almost indistinguishable.63 Some preferences for calpain substrate sequences have been suggested, but a clear rule like that for caspases and trypsins is still elusive. To find such a rule, two major approaches were taken: a recursive method, comparing substrate cleavage-site sequences published thus far, and a deductive method using short oligo-peptide libraries.64–66 Intriguingly, these methods drew somewhat different conclusions; the former studies found T[W > P][L > T > V][K > Y > R]|SPP for the preferred P4-P3-P2-P1-|-P1′-P2′-P3′ sequence (|, cleavage site),64 whereas the latter found F[F > L > P][L > V][L = F]|[M > A > R]E[R > K].66 This difference may indicate that suboptimal sequences make better conventional calpain substrates because of their reduced reaction rates, a possible advantage for precise modulation by calpain-mediated proteolysis.66
In a more recent approach, a prediction tool was constructed using bioinformatics, that is, to analyse experimental data by a machine learning process.67 This assists our understanding of calpain-mediated proteolysis by predicting where the cleavage site, if any, is. This allows efficient speculation about possible functions mediated by calpain substrates. A draft version can be found at http://calpain.org.67
5. Calpain function in the heart and skeletal muscles
The importance of proteolytic systems for maintaining cellular function is increasingly recognized; calpains are no exception. The impact of calpain function depends on the particular substrates and conditions investigated,68–74 as illustrated by the calpain activity in skeletal and cardiac muscles.
5.1. Skeletal muscle homeostasis and CAPN3[p94]
The first tissue-specific calpain, the skeletal muscle-specific CAPN3[p94], was identified in 1989.23 Although CAPN3[p94] is a classical calpain, it contains three additional regions: NS (located at the N-terminus), IS1, and IS2. These give CAPN3[p94] a rapid autolytic activity and specific binding to connectin/titin, an elastic filamentous muscle protein of >3000 kDa. In 1995, CAPN3 mutations were discovered to be responsible for limb-girdle muscular dystrophy type 2A (LGMD2A).31 Accordingly, Capn3−/− mice have an LGMD2A-like phenotype.34,35 So far, CAPN3 mutations and LGMD2A have the only clearly demonstrated cause-and-effect relationship between a calpain gene mutation and human disease; thus, LGMD2A is also called calpainopathy.
Studies using CAPN3[p94] knock-in (Capn3CS/CS) mice, which have a structurally intact but protease-inactive CAPN3[p94]:C129S mutant, showed that compromised CAPN3[p94] protease activity is primarily responsible for LGMD2A, and that Ca2+ release from the sarcoplasmic reticulum (SR) is reduced in Capn3−/−, but not Capn3CS/CS, muscles.75–77 Thus, two independent activities of CAPN3[p94], proteolytic and non-proteolytic, contribute to its physiological functions.76
In Capn3CS/CS mice analysed under exercise conditions, the adaptive up-regulation of heat-shock proteins and of muscle ankyrin-repeat protein-2 (MARP2, also called Ankrd2), a muscle-specific transcriptional regulator, is compromised.75,78 Altogether, it is hypothesized that pathogenic CAPN3 mutations disrupt this calpain's ability to control multiple homeostatic mechanisms in skeletal muscles, resulting in LGMD2A.
CAPN3[p94] is expressed at much lower levels in the heart than in skeletal muscle, to the point of being undetectable.79 However, the proteins it interacts with, such as connectin/titin, MARP2,80 and MARP1 [also called Ankrd1, or cardiac ankyrin-repeat protein (CARP)],81 exist in both cardiac and skeletal muscles. This implies that another protease in the heart, most probably conventional calpains, may undertake the role played by CAPN3[p94] in skeletal muscle. In this context, it is intriguing that gene mutations causing small deletions of the human connectin/titin C-terminus, which contains a CAPN3[p94]-binding site, down-regulate CAPN3[p94] and produce early-onset myopathy with fast progressive dilated cardiomyopathy, leading to death.82 It was recently shown that myospryn/C5orf10/CMYA5 (cardiomyopathy associated 5), which is a filamentous protein of ∼450 kDa with fibronectin type III motifs and a SPRY domain at its C-terminus, interacts with both CAPN3[p94] and the connectin/titin C-terminus.83
CAPN3[p94] binds not only to the connectin/titin C-terminus, but also to its N2A region, where binding sites for MARP1, MARP2, and MARP3 [also called Ankrd23, or diabetes-related ankyrin-repeat protein (DARP)] are located.84 CAPN3[p94] and MARP2 accumulate at the N2A region under stress, suggesting that the connectin/titin N2A region is a molecular base for sensing physical muscle cell stress at the sarcomere level.75,84–86 Notably, CAPN1[μCL] is also reported to localize to the N2A region.87 Although expressed at very low levels in normal skeletal muscle, MARP1 is also a CAPN3[p94] substrate.81 Intriguingly, mutations in MARP1 are found in some cases of dilated cardiomyopathy.88
5.2. Calpains in cardiovascular diseases
It was recently reported that calpain activity is necessary for cardiac cell function (Table 1 and Figure 4). However, as mentioned earlier, increased conventional calpain activity has often been reported as an aggravating factor in cardiovascular diseases and other pathophysiological conditions. Some of candidate calpain substrates related to cardiovascular system are shown in Table 2.
Table 1.
Calpains involved in cardiovascular diseases
| Types of disorders | +/−a | Model system (Animal) | Calpainb | Inhibition or activation of calpain(s) | Substrates | Ref. |
|---|---|---|---|---|---|---|
| Cardiac hypertrophy and cardiomyocyte loss | – | Right ventricular pressure overload (feline) | 1 and/or 2 | ZLNalc | (gelsolin?) | 89 |
| Cardiac contractile dysfunction | – | Right ventricular pressure overload (swine) | 1 and/or 2 | ZVFald | — | 90 |
| Cardiac infarction and DNA damage | – | Coronary artery occlusion/reperfusion (rat) | 1 and/or 2 | ALLNale | — | 91 |
| Cardiac infarction and apoptosis | – | Ischaemia/reperfusion (rabbit and rat) | 1 and/or 2 | ALLNal, ZVFal | Bid | 92 |
| Cardiac contractile dysfunction | – | Ischaemia/reperfusion (rat) | 1 and/or 2 | Leupeptin | SR proteins | 93 |
| Cardiac infarction and contractile dysfunction | – | Coronary artery occlusion/ reperfusion (swine) | 1 and/or 2 | A-705253 | — | 115 |
| Cardiac infarction, dysfunction, and apoptosis | – | Ischaemia/reperfusion (mouse) | 1 | Conditional CAPN1 Tgf, conditional PKCα fragment Tgf, calpastatin Tgf, Capn1−/− | PKCα | 107 |
| Myocardial hypertrophy/ fibrosis (associated with type 1 diabetes) | – | Ove26 Tgg and streptozotocin-injection (mouse) | 1 and/or 2 | Cardiac-specific Capns1−/− | — | 94 |
| Hyperglycaemia | – | Streptozotocin injection (rat) | 1 | ZLLalh, PD150606 | — | 95 |
| Hyperglycaemia with hypoinsulinaemia | – | Zucker diabetic fatty rat | 1 | Anti-sense nucleotide, ZLLal | — | 96 |
| Hypertension, cardiovascular hypertrophy, and perivascular inflammation | – | Angiotensin-II infusion (mouse) | 1 and/or 2 | Calpastatin Tgi | Spectrin | 97 |
| Atherosclerosis and abdominal aortic aneurysms | – | Ldlr−/− and angiotensin-II infusion (mouse) | 1 | BDA-410 | Spectrin-1 | 98 |
| Atherosclerosis | – | Ldlr−/−, Apoe−/− (mouse) | 2 | ALLMalj, ZLNal, siRNA | VE-cadherin | 99 |
| Lethality | – | CAPN1 or CAPN2 Tg over-expression (mouse) | 1 and 2 | CAPN1 Tg, CAPN2 Tgk | 105 | |
| Lethality with cardiomyocyte necrosis | – | CAPN1 Tg over-expression (mouse) | 1 | CAPN1 Tgf | Desmin, PKCα | 105 |
| (No phenotype) | NA | CAPN2 Tg over-expression (mouse) | 2 | CAPN2 Tgf | 105 | |
| Dilated cardiomyopathy and atrial arrhythmias | + | Calpastatin Tg over-expression (mouse) | 1 | Calpastatin Tgk | 105 | |
| Cardiomyopathy, cardiac dysfunction, and plasma membrane damage | + | Transverse aortic constriction, β-adrenergic stress (mouse) | 1 and/or 2 | Cardiac-specific Capns1−/− | 110 |
a+ or – indicates calpain(s) play a roles as an ameliorating or aggravating factor, respectively. NA, not applicable.
b‘1’ and ‘2’ stand for CAPN1/S1[μ-calpain] and CAPN2/S1[m-calpain], respectively.
cBenzyloxycarbonyl-Leu-Norleucinal, also called calpeptin.
dBenzyloxycarbonyl-Val-phenylalaninal, also called calpain inhibitor III or MDL-28170.
eAcetyl-Leu-Leu-Norleucinal, also called calpain inhibitor I.
ftetracycline-suppressible (‘tet-off’) Myh6 promoter-driven conditional Tg mice.
gFVB(Cg)-Tg(ins2-CALM)26OveTg(Cryaa-Tag)1Ove/PneJ Tg.
hBenzyloxycarbonyl-Leu-leucinal.
iCytomegalovirus immediate-early enhancer/promoter-driven conventional Tg mice.
jAcetyl-Leu-Leu-methioninal, also called calpain inhibitor II.
kMyh6 promoter-driven conventional Tg mice.
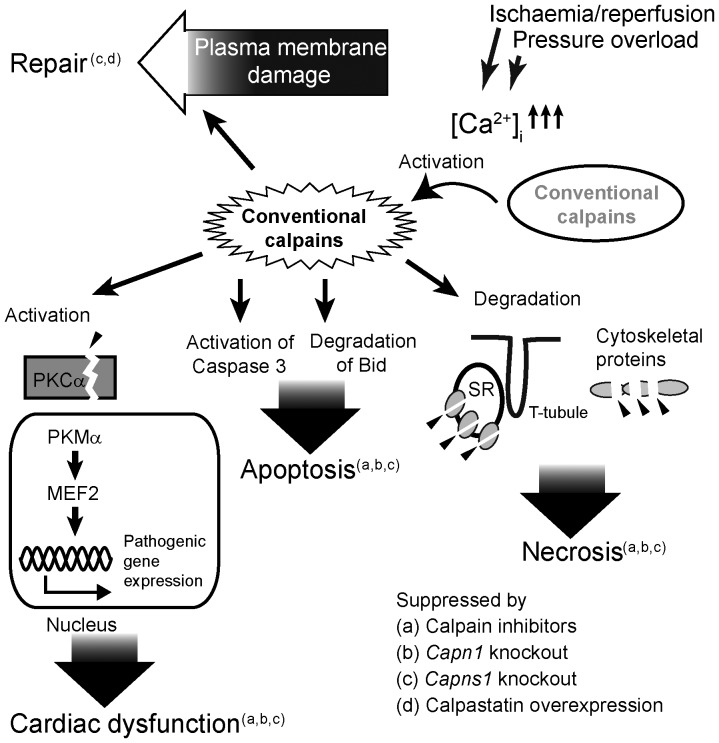
Figure 4.
Multiple involvements in cardiovascular diseases. Schematic of calpains involved in cardiovascular diseases; details of each report are given in Table 1.
Table 2.
Modulation of muscle-related proteins by calpain-mediated proteolysis
| Substrate | Accession No. | Cleavage site(s) after | Calpain(s)a | Effect of proteolysis | Ref.b |
|---|---|---|---|---|---|
| Annexin I | NP_000691 | 26 | 1 | Enhancement of Ca2+ sensitivity | 116 |
| Ezrin | NP_062230 | 467 | 1 | Liberation from apical membrane | 117 |
| Glutamate receptor, ionotropic, NMDA 2A | NP_036705 | 1278, 1329 | 1 | Dissociation from PSD-95 | 118 |
| Insulin-like growth factor-binding protein 4 (IGFBP4) | NP_001543 | 23, 107, 143, 159 | 1 | Reduction in IGF avidity | 119 |
| Interleukin 1α (IL-1α) | NP_000566 | 118 | 1 | Maturation and secretion | 120 |
| Ras homologue gene family, member A (RhoA) | NP_001655 | 180 | 1 | Dominant negative (inhibition of integrin-induced stress fibre assembly) effect | 121 |
| Spectrin β | NP_001020029 | 2058 | 1 | Membrane skeleton reorganization? | 122 |
| Talin 1 | NP_035732 | 433 | 1 | Redistribution of the talin functional domain | 123 |
| Transient receptor potential canonical 6 (TRPC6) | NP_038866 | 16 | 1 | Down-regulation | 124 |
| Troponin T2, cardiac (TnTc) | NP_035749 | 71 | 1 | Altered affinities to TnI and tropomyosin | 125 |
| BH3 interacting domain death agonist (BID) | NP_001187 | 70 | 2 | Induction of apoptosis | 126 |
| Calcineurin | NP_058737 | 421, 422, 423, 425 | 2 | Activation | 127 |
| Caspase 9 | NP_001220 | 115, 330 | 2 | Inactivation | 128 |
| ErbB-1, epidermal growth factor receptor (EGFR) | NP_005219 | 683, 733, 1030, 1059, etc. | 2 | Down-regulation | 129 |
| NFκB inhibitor α (IκBα) | NP_065390 | 50 | 2 | Activation of NFκB | 130 |
| Integrins β1, 2, 3, 7 | NP_002202, etc. | 771, 777, etc. | 2 | Dissociation from cytoskeleton | 131 |
| Phospholipase C β1 | NP_777242 | 880 | 2 | Loss of Gαq interaction | 132 |
| Vimentin | NP_035831 | 18, 20, 32, etc. | 2 | Turnover | 133 |
| BCL2-associated X protein (BAX) | NP_620116 | 28 | 1 or 2 | Pro-apoptotic effect | 134 |
| Caspases 3, 7, 9 | NP_116786, NP_001218, NP_001220 | 7; 36; 115, 120, 143, etc. | 1 or 2 | Activation | 135,136 |
| Filamin A | NP_001447 | 1761 | 1 or 2 | Change in actin avidity | 137 |
| α-Actin-1 | NP_001091 | 39 | 1 and/or 2 | Pro-apoptotic effect | 138 |
| Protein kinase C α, β, γ | XP_001081588, etc. | 309, 316, 324 , etc. | 1 and 2 | Activation | 139 |
| PDLIM1 | NP_066272 | 271 | 3 | Reduced avidities to interacting molecules? | 140 |
| Connectin/titin | NP_596869 | 8563, 8651, 8652; 8506 | 1 and 3 | Myofibril turnover? | 44,80 |
a‘1’, ‘2’, and ‘3’ stand for CAPN1/S1[μ-calpain], CAPN2/S1[m-calpain], and CAPN3[p94], respectively.
bThe referenced reports describe calpain cleavage sites, and do not necessarily describe their relevance to muscles.
5.2.1. Is calpain activity undesirable in the heart?
Many conditions that damage the cardiovascular system appear to be improved by inhibiting calpains. Contractile dysfunction after acute pressure overload, which was reported to be associated with calpain activation, is ameliorated by inhibiting calpains.89,90 Calpain activation, apoptosis, and the proteolysis of several substrates, including Bid and the SR proteins, have been reported in cardiac ischaemia or infarction.91–93 Again, inhibiting calpain activity ameliorates these pathological conditions. Diabetes-associated cardiovascular complications often result in morbidity and mortality. Heart-specific Capns1 disruption mitigated the myocardial hypertrophy and fibrosis in type 1 diabetes model mice.94 In other words, knocking down both CAPN1/S1[μ-calpain] and CAPN2/S1[m-calpain] reduced the cardiac hypertrophy and fibrosis in these mice. Accordingly, inhibition of calpains by inhibitors and/or antisense nucleotides improved hyperglycaemia in streptozotocin-injected rats95 and Zucker diabetic fatty rats,96 animal models for type 1 and 2 diabetes, respectively.
There are other examples where calpain inhibition reduces disease severity. Chronic infusion of angiotensin-II and/or low-density lipoprotein receptor-deficiency [Ldlr−/−] in mice cause cardiovascular disorder such as hypertension and atherosclerosis. Tg overexpression of calpastatin in mice suppressed left ventricle and media hypertrophy, perivascular inflammation and fibrosis associated with angiotensin-II-induced hypertension.97 Atherosclerosis and abdominal aortic aneurysms induced by angiotensin-II infusion in Ldlr−/− mice were also attenuated by administration of the calpain inhibitor.98 CAPN2/S1[m-calpain], but not CAPN1/S1[μ-calpain], is overactivated in the atherosclerosis of endothelial cells (ECs). Miyazaki et al.99 reported that both the CAPN2[mCL] level and proteolysis of vascular endothelial cadherin (VE-cadherin) are increased in aortic ECs from human patients suffering from atherosclerotic lesions, and from Ldlr−/− mice; CAPN2[mCL] siRNA and calpain inhibitors prevented this disorder's progression. Although there is no report on relationship between acute coronary syndrome (ACS) and calpain activity, these findings (exacerbation of atherosclerosis99 as well as angiotensin-II-induced vascular inflammation97 and abdominal aortic aneurysms98 by calpain activity) link calpain activity to ACS, if not directly. As mentioned above, VE-cadherin is one of targets of CAPN2/S1[m-calpain],99 and is also expressed to coronary arteries. Therefore, it is possible that inhibition of calpain ameliorates ACS, or at least suppresses acceleration of ACS.
Another example of the involvement of calpain activity in cardiovascular disorders is myocardial stunning (also called broken-heart syndrome, stress/Takotsubo cardiomyopathy, or transient left-ventricular apical ballooning),100 which is a form of dysfunction caused by post-ischaemic reperfusion. Several reports point to proteolytic degradation of cardiac troponin I (cTnI) by calpain as a possible cellular mechanism underlying the depressed contractile function.101,102 There are, however, some controversial reports, demonstrating that no cTnI degradation was detected during porcine or canine myocardial stunning,103,104 and that myocardial-specific overexpression of CAPN1[μCL] in Tg mice showed no clear cTnI degradation in the heart.105 Although cTnI proteolysis probably affects myocardial contractile functions,106 these counter-evidences suggest that cTnI degradation is not a cause but a result of ischaemia/reperfusion. Ischaemia/reperfusion causes calpain activation resulting in proteolysis of several proteins; however, it is still open to question which protein degradation is responsible for myocardial stunning. As discussed earlier, calpains have certain consensus for preferential sequences for substrates.64,66 Thus, if knock-in mice that have mutated sequence in the calpain cleavage sites of cTnI are generated, they will show whether or not cTnI degradation is essential for myocardial stunning as they only express cTnI mutant unproteolysed by calpains.
In addition to all of these findings, it has been proposed that calpains negatively impact these cardiovascular diseases by overproducing constitutively active PKCα.107 Conventional PKCs, which have an intramolecular regulatory domain, are usually latent. Conventional calpains cut off this regulatory domain to produce activated PKC, which is called PKM, the catalytic fragment of PKC.108 Overactive calpains produce excess PKM in an ischaemic heart; once produced, the kinase remains active regardless of upstream receptor signals, and causes various substrates, such as myosin-binding protein-C and histone deacetylase 5 (HDAC5), to be overphosphorylated. This triggers cycles of morbid cellular responses such as constitutive nuclear HDAC5 export, and subsequently causes cardiomyopathy.107,109
5.2.2. Calpains are essential for the heart!
A few reports have shown that conventional calpain activity is required for cardiovascular health. Galvez et al.105 constructed Tg mice that overexpressed CAPN1[μCL], CAPN2[mCL] or calpastatin. The analysis of these mice demonstrated that calpastatin overexpression using the cardiac α-myosin heavy chain gene (Myh6) conventional promoter, which inhibited 58% of the endogenous CAPN1/S1[μ-calpain] activity in these mice, caused slow-progressing dilated cardiomyopathy. This result indicates that the activity of conventional calpains, especially CAPN1/S1[μ-calpain], is essential to heart function. The phenotypes of other Tg mice overexpressing CAPN1[μCL] or CAPN2[mCL], as summarized in Table 1, show that too much calpain activity appears damaging, although conditional overexpression of CAPN2[mCL] was tolerated under the examined context.
More direct evidence of the requirement for conventional calpains in cardiac functions against haemodynamic stress was published recently. Using a Capns1 flox (flanked by loxP sequences) deletion construct and Myh6 promoter-driven Cre Tg mice, Taneike et al.110 designed a conditional Capns1 knockout and cardiac-specifically disrupted CAPNS1[30K], thus down-regulating both CAPN1[μCL] and CPAN2[mCL]. These cardiac-specific Capns1−/− mice had normal global cardiac structure and function under normal conditions. However, when 10-week-old mice were subjected to pressure overload through transverse aortic constriction (TAC), the resulting fibrosis was significantly larger in the knockout than in the wild-type mice. Intriguingly, both knockout and wild-type mice developed cardiac hypertrophy, indicating that conventional calpain activity was not the primary cause of the TAC-induced hypertrophy. These knockout mice exposed to β-adrenergic stress through isoproterenol infusion developed cardiac dysfunction, again indicating the protective role of conventional calpains under stress. As also seen in cultured fibroblast cells,73,111 cardiomyocytes from the knockout mice showed defective membrane repair,110 indicating that one of the conventional calpains' ubiquitous physiological functions relates to membrane repair.
Another clue to protective/pathogenic roles of calpains may involve matrix metalloproteinase-2 (MMP-2). Historically, MMP-2 was considered to function on extracellular matrix substrates; however, several reports recently showed that the detrimental effect of MMP-2 may occur primarily within the myocytes.112 Furthermore, MMP-2 targets a similar subset of proteins (including cTnI) as calpains, and, surprisingly, calpastatin may inhibit MMP-2 also.112 Therefore, it is possible that at least part of bad reputation of calpains in cardiovascular disorders is responsible for MMP-2.
6. Conclusions and perspectives
Calpain's involvement in skeletal muscle and cardiovascular systems has been the subject of much interest and research. Many studies have shown that the activation of calpains, especially the conventional calpains, exacerbates pathophysiological conditions. However, recent technical advances have allowed us to delve deeper into the true nature of the calpain system. Genetic manipulations to disrupt Capns1 constitutively or conditionally in mice cause embryonic lethality30,38 and cardiac hypertrophy,110 respectively, indicating that calpains are indispensable both to heart-specific functions and to life itself. In short, calpains are a double-edged sword: they are essential for various aspects of life, especially under stress conditions, yet their activation tends to be destructive in cells undergoing pathophysiological chaos.
In the process of revising this article, another review focusing on the role of calpains in multiple aspects of cardiovascular illness had come out.113 As was discussed in the article as well as in this review, regulation of calpain activity stands as an important issue in developing therapeutic reagents for heart failure. In this respect, it should be noted that some pathological states may result from insufficient calpain activity. Genetic defects in tissue-specific calpains cause various diseases, which may be improved by activators or stabilizers for these calpains, and cardiovascular system and calpain functions therein would be no exception. One of the most urgent needs in this research field is the ability to detect real-time calpain activity in vivo at a high resolution for both physiological and pathophysiological conditions. As calpain research enters this new era, both basic science and translational biomedical studies are well positioned to launch comprehensive calpain studies.
Acknowledgments
We thank all the Calpain Project laboratory members for their invaluable support and Drs Leslie Miglietta and Grace Gray for their excellent proof-reading.
Conflict of interest: none declared.
Funding
This work was supported by JSPS KAKENHI (22770139 to Y.O., and 20370055 and 23247021 to H.S.), a Toray Science Foundation grant (to Y.O.), and a Takeda Science Foundation research grant (to H.S.). Open access of this article was funded by the Japan Society for the Promotion of Science.
References
- 1.Sorimachi H, Hata S, Ono Y. Calpain chronicle—an enzyme family under multidisciplinary characterization. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:287–327. doi: 10.2183/pjab.87.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki K, Hata S, Kawabata Y, Sorimachi H. Structure, activation, and biology of calpain. Diabetes. 2004;53(Suppl 1):S12–18. doi: 10.2337/diabetes.53.2007.s12. [DOI] [PubMed] [Google Scholar]
- 3.Croall DE, DeMartino GN. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991;71:813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- 4.Sorimachi H, Hata S, Ono Y. Impact of genetic insights into calpain biology. J Biochem. 2011;150:23–37. doi: 10.1093/jb/mvr070. [DOI] [PubMed] [Google Scholar]
- 5.Carragher NO. Calpain inhibition: a therapeutic strategy targeting multiple disease states. Curr Pharm Des. 2006;12:615–638. doi: 10.2174/138161206775474314. [DOI] [PubMed] [Google Scholar]
- 6.Biswas S, Harris F, Dennison S, Singh J, Phoenix DA. Calpains: targets of cataract prevention? Trends Mol Med. 2004;10:78–84. doi: 10.1016/j.molmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Branca D. Calpain-related diseases. Biochem Biophys Res Commun. 2004;322:1098–1104. doi: 10.1016/j.bbrc.2004.07.126. [DOI] [PubMed] [Google Scholar]
- 8.Yamashima T. Ca2+-dependent proteases in ischemic neuronal death: a conserved ‘calpain-cathepsin cascade’ from nematodes to primates. Cell Calcium. 2004;36:285–293. doi: 10.1016/j.ceca.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Badalamente MA, Stracher A. Delay of muscle degeneration and necrosis in mdx mice by calpain inhibition. Muscle Nerve. 2000;23:106–111. doi: 10.1002/(sici)1097-4598(200001)23:1<106::aid-mus14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 10.Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- 11.Sugita H, Ishiura S, Suzuki K, Imahori K. Ca-activated neutral protease and its inhibitors: in vitro effect on intact myofibrils. Muscle Nerve. 1980;3:335–339. doi: 10.1002/mus.880030410. [DOI] [PubMed] [Google Scholar]
- 12.Sorimachi H, Hata S, Ono Y. Expanding members and roles of the calpain superfamily and their genetically modified animals. Exp Anim. 2010;59:549–566. doi: 10.1538/expanim.59.549. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka K. The proteasome: overview of structure and functions. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85:12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki K, Sorimachi H, Yoshizawa T, Kinbara K, Ishiura S. Calpain: novel family members, activation, and physiologic function. Biol Chem Hoppe Seyler. 1995;376:523–529. doi: 10.1515/bchm3.1995.376.9.523. [DOI] [PubMed] [Google Scholar]
- 16.Hosfield CM, Elce JS, Davies PL, Jia Z. Crystal structure of calpain reveals the structural basis for Ca2+-dependent protease activity and a novel mode of enzyme activation. EMBO J. 1999;18:6880–6889. doi: 10.1093/emboj/18.24.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strobl S, Fernandez-Catalan C, Braun M, Huber R, Masumoto H, Nakagawa K, et al. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc Natl Acad Sci U S A. 2000;97:588–592. doi: 10.1073/pnas.97.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maki M, Narayana SV, Hitomi K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J. 1997;328:718–720. [PMC free article] [PubMed] [Google Scholar]
- 19.Ono Y, Sorimachi H, Suzuki K. Structure and physiology of calpain, an enigmatic protease. Biochem Biophys Res Commun. 1998;245:289–294. doi: 10.1006/bbrc.1998.8085. [DOI] [PubMed] [Google Scholar]
- 20.Sorimachi H, Ishiura S, Suzuki K. A novel tissue-specific calpain species expressed predominantly in the stomach comprises two alternative splicing products with and without Ca(2+)-binding domain. J Biol Chem. 1993;268:19476–19482. [PubMed] [Google Scholar]
- 21.Macqueen DJ, Delbridge ML, Manthri S, Johnston IA. A newly classified vertebrate calpain protease, directly ancestral to CAPN1 and 2, episodically evolved a restricted physiological function in placental mammals. Mol Biol Evol. 2010;27:1886–1902. doi: 10.1093/molbev/msq071. [DOI] [PubMed] [Google Scholar]
- 22.Futai E, Kubo T, Sorimachi H, Suzuki K, Maeda T. Molecular cloning of PalBH, a mammalian homologue of the Aspergillus atypical calpain PalB. Biochim Biophys Acta. 2001;1517:316–319. doi: 10.1016/s0167-4781(00)00256-6. [DOI] [PubMed] [Google Scholar]
- 23.Sorimachi H, Imajoh-Ohmi S, Emori Y, Kawasaki H, Ohno S, Minami Y, et al. Molecular cloning of a novel mammalian calcium-dependent protease distinct from both m- and mu-types. Specific expression of the mRNA in skeletal muscle. J Biol Chem. 1989;264:20106–20111. [PubMed] [Google Scholar]
- 24.Dear N, Matena K, Vingron M, Boehm T. A new subfamily of vertebrate calpains lacking a calmodulin-like domain: implications for calpain regulation and evolution. Genomics. 1997;45:175–184. doi: 10.1006/geno.1997.4870. [DOI] [PubMed] [Google Scholar]
- 25.Dear TN, Moller A, Boehm T. CAPN11: a calpain with high mRNA levels in testis and located on chromosome 6. Genomics. 1999;59:243–247. doi: 10.1006/geno.1999.5859. [DOI] [PubMed] [Google Scholar]
- 26.Dear TN, Meier NT, Hunn M, Boehm T. Gene structure, chromosomal localization, and expression pattern of Capn12, a new member of the calpain large subunit gene family. Genomics. 2000;68:152–160. doi: 10.1006/geno.2000.6289. [DOI] [PubMed] [Google Scholar]
- 27.Dutt P, Croall DE, Arthur SC, De Veyra T, Williams K, Elce JS, et al. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takano J, Mihira N, Fujioka R, Hosoki E, Chishti AH, Saido TC. Vital role of the calpain-calpastatin system for placental-integrity-dependent embryonic survival. Mol Cell Biol. 2011;31:4097–4106. doi: 10.1128/MCB.05189-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimada M, Greer PA, McMahon AP, Bouxsein ML, Schipani E. In vivo targeted deletion of calpain small subunit, Capn4, in cells of the osteoblast lineage impairs cell proliferation, differentiation, and bone formation. J Biol Chem. 2008;283:21002–21010. doi: 10.1074/jbc.M710354200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur JS, Elce JS, Hegadorn C, Williams K, Greer PA. Disruption of the murine calpain small subunit gene, Capn4: calpain is essential for embryonic development but not for cell growth and division. Mol Cell Biol. 2000;20:4474–4481. doi: 10.1128/mcb.20.12.4474-4481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 32.Yamada M, Yoshida Y, Mori D, Takitoh T, Kengaku M, Umeshima H, et al. Inhibition of calpain increases LIS1 expression and partially rescues in vivo phenotypes in a mouse model of lissencephaly. Nat Med. 2009;15:1202–1207. doi: 10.1038/nm.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada M, Hirotsune S, Wynshaw-Boris A. A novel strategy for therapeutic intervention for the genetic disease: preventing proteolytic cleavage using small chemical compound. Int J Biochem Cell Biol. 2010;42:1401–1407. doi: 10.1016/j.biocel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richard I, Roudaut C, Marchand S, Baghdiguian S, Herasse M, Stockholm D, et al. Loss of calpain 3 proteolytic activity leads to muscular dystrophy and to apoptosis-associated IκBα/nuclear factor κB pathway perturbation in mice. J Cell Biol. 2000;151:1583–1590. doi: 10.1083/jcb.151.7.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramerova I, Kudryashova E, Tidball JG, Spencer MJ. Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum Mol Genet. 2004;13:1373–1388. doi: 10.1093/hmg/ddh153. [DOI] [PubMed] [Google Scholar]
- 36.Hata S, Abe M, Suzuki H, Kitamura F, Toyama-Sorimachi N, Abe K, et al. Calpain 8/nCL-2 and calpain 9/nCL-4 constitute an active protease complex, G-calpain, involved in gastric mucosal defense. PLoS Genet. 2010;6:e1001040. doi: 10.1371/journal.pgen.1001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshizawa T, Sorimachi H, Tomioka S, Ishiura S, Suzuki K. A catalytic subunit of calpain possesses full proteolytic activity. FEBS Lett. 1995;358:101–103. doi: 10.1016/0014-5793(94)01401-l. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman UJ, Boring L, Pak JH, Mukerjee N, Wang KK. The calpain small subunit gene is essential: its inactivation results in embryonic lethality. IUBMB Life. 2000;50:63–68. doi: 10.1080/15216540050176610. [DOI] [PubMed] [Google Scholar]
- 39.Schad E, Farkas A, Jekely G, Tompa P, Friedrich P. A novel human small subunit of calpains. Biochem J. 2002;362:383–388. doi: 10.1042/0264-6021:3620383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kashiwagi A, Schipani E, Fein MJ, Greer PA, Shimada M. Targeted deletion of Capn4 in cells of the chondrocyte lineage impairs chondrocyte proliferation and differentiation. Mol Cell Biol. 2010;30:2799–2810. doi: 10.1128/MCB.00157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiss R, Kovacs D, Tompa P, Perczel A. Local structural preferences of calpastatin, the intrinsically unstructured protein inhibitor of calpain. Biochemistry (Mosc) 2008;47:6936–6945. doi: 10.1021/bi800201a. [DOI] [PubMed] [Google Scholar]
- 42.Hata S, Doi N, Kitamura F, Sorimachi H. Stomach-specific calpain, nCL-2/calpain 8, is active without calpain regulatory subunit and oligomerizes through C2-like domains. J Biol Chem. 2007;282:27847–27856. doi: 10.1074/jbc.M703168200. [DOI] [PubMed] [Google Scholar]
- 43.Lee HJ, Tomioka S, Kinbara K, Masumoto H, Jeong SY, Sorimachi H, et al. Characterization of a human digestive tract-specific calpain, nCL-4, expressed in the baculovirus system. Arch Biochem Biophys. 1999;362:22–31. doi: 10.1006/abbi.1998.1021. [DOI] [PubMed] [Google Scholar]
- 44.Ono Y, Kakinuma K, Torii F, Irie A, Nakagawa K, Labeit S, et al. Possible regulation of the conventional calpain system by skeletal muscle-specific calpain, p94/calpain 3. J Biol Chem. 2004;279:2761–2771. doi: 10.1074/jbc.M308789200. [DOI] [PubMed] [Google Scholar]
- 45.Emori Y, Kawasaki H, Imajoh S, Imahori K, Suzuki K. Endogenous inhibitor for calcium-dependent cysteine protease contains four internal repeats that could be responsible for its multiple reactive sites. Proc Natl Acad Sci U S A. 1987;84:3590–3594. doi: 10.1073/pnas.84.11.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maki M, Takano E, Mori H, Kannagi R, Murachi T, Hatanaka M. Repetitive region of calpastatin is a functional unit of the proteinase inhibitor. Biochem Biophys Res Commun. 1987;143:300–308. doi: 10.1016/0006-291x(87)90665-6. [DOI] [PubMed] [Google Scholar]
- 47.Maki M, Takano E, Mori H, Sato A, Murachi T, Hatanaka M. All four internally repetitive domains of pig calpastatin possess inhibitory activities against calpains I and II. FEBS Lett. 1987;223:174–180. doi: 10.1016/0014-5793(87)80531-8. [DOI] [PubMed] [Google Scholar]
- 48.Hanna RA, Campbell RL, Davies PL. Calcium-bound structure of calpain and its mechanism of inhibition by calpastatin. Nature. 2008;456:409–412. doi: 10.1038/nature07451. [DOI] [PubMed] [Google Scholar]
- 49.Moldoveanu T, Gehring K, Green DR. Concerted multi-pronged attack by calpastatin to occlude the catalytic cleft of heterodimeric calpains. Nature. 2008;456:404–408. doi: 10.1038/nature07353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohno S, Emori Y, Imajoh S, Kawasaki H, Kisaragi M, Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984;312:566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- 51.Moldoveanu T, Hosfield CM, Lim D, Elce JS, Jia Z, Davies PL. A Ca2+ switch aligns the active site of calpain. Cell. 2002;108:649–660. doi: 10.1016/s0092-8674(02)00659-1. [DOI] [PubMed] [Google Scholar]
- 52.Moldoveanu T, Hosfield CM, Lim D, Jia Z, Davies PL. Calpain silencing by a reversible intrinsic mechanism. Nat Struct Biol. 2003;10:371–378. doi: 10.1038/nsb917. [DOI] [PubMed] [Google Scholar]
- 53.Moldoveanu T, Campbell RL, Cuerrier D, Davies PL. Crystal structures of calpain-E64 and -leupeptin inhibitor complexes reveal mobile loops gating the active site. J Mol Biol. 2004;343:1313–1326. doi: 10.1016/j.jmb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Sorimachi H, Toyama-Sorimachi N, Saido TC, Kawasaki H, Sugita H, Miyasaka M, et al. Muscle-specific calpain, p94, is degraded by autolysis immediately after translation, resulting in disappearance from muscle. J Biol Chem. 1993;268:10593–10605. [PubMed] [Google Scholar]
- 55.Ono Y, Ojima K, Torii F, Takaya E, Doi N, Nakagawa K, et al. Skeletal muscle-specific calpain is an intracellular Na+-dependent protease. J Biol Chem. 2010;285:22986–22998. doi: 10.1074/jbc.M110.126946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herasse M, Ono Y, Fougerousse F, Kimura E, Stockholm D, Beley C, et al. Expression and functional characteristics of calpain 3 isoforms generated through tissue-specific transcriptional and posttranscriptional events. Mol Cell Biol. 1999;19:4047–4055. doi: 10.1128/mcb.19.6.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spencer MJ, Guyon JR, Sorimachi H, Potts A, Richard I, Herasse M, et al. Stable expression of calpain 3 from a muscle transgene in vivo: immature muscle in transgenic mice suggests a role for calpain 3 in muscle maturation. Proc Natl Acad Sci U S A. 2002;99:8874–8879. doi: 10.1073/pnas.132269299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osako Y, Maemoto Y, Tanaka R, Suzuki H, Shibata H, Maki M. Autolytic activity of human calpain 7 is enhanced by ESCRT-III-related protein IST1 through MIT-MIM interaction. FEBS J. 2010;277:4412–4426. doi: 10.1111/j.1742-4658.2010.07822.x. [DOI] [PubMed] [Google Scholar]
- 59.Saido TC, Shibata M, Takenawa T, Murofushi H, Suzuki K. Positive regulation of mu-calpain action by polyphosphoinositides. J Biol Chem. 1992;267:24585–24590. [PubMed] [Google Scholar]
- 60.Tompa P, Emori Y, Sorimachi H, Suzuki K, Friedrich P. Domain III of calpain is a ca2+-regulated phospholipid-binding domain. Biochem Biophys Res Commun. 2001;280:1333–1339. doi: 10.1006/bbrc.2001.4279. [DOI] [PubMed] [Google Scholar]
- 61.Shao H, Chou J, Baty CJ, Burke NA, Watkins SC, Stolz DB, et al. Spatial localization of m-calpain to the plasma membrane by phosphoinositide biphosphate binding during epidermal growth factor receptor-mediated activation. Mol Cell Biol. 2006;26:5481–5496. doi: 10.1128/MCB.02243-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Groshong JS, Spencer MJ, Bhattacharyya BJ, Kudryashova E, Vohra BP, Zayas R, et al. Calpain activation impairs neuromuscular transmission in a mouse model of the slow-channel myasthenic syndrome. J Clin Invest. 2007;117:2903–2912. doi: 10.1172/JCI30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 64.Tompa P, Buzder-Lantos P, Tantos A, Farkas A, Szilagyi A, Banoczi Z, et al. On the sequential determinants of calpain cleavage. J Biol Chem. 2004;279:20775–20785. doi: 10.1074/jbc.M313873200. [DOI] [PubMed] [Google Scholar]
- 65.du Verle DA, Takigawa I, Ono Y, Sorimachi H, Mamitsuka H. CaMPDB: a resource for calpain and modulatory proteolysis. Genome Inform. 2010;22:202–213. [PubMed] [Google Scholar]
- 66.Cuerrier D, Moldoveanu T, Davies PL. Determination of peptide substrate specificity for mu-calpain by a peptide library-based approach: the importance of primed side interactions. J Biol Chem. 2005;280:40632–40641. doi: 10.1074/jbc.M506870200. [DOI] [PubMed] [Google Scholar]
- 67.du Verle DA, Ono Y, Sorimachi H, Mamitsuka H. Calpain cleavage prediction using multiple kernel learning. PLoS One. 2011;6:e19035. doi: 10.1371/journal.pone.0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pontremoli S, Melloni E. The role of calpain and protein kinase C in activation of human neutrophils. Prog Clin Biol Res. 1988;282:195–208. [PubMed] [Google Scholar]
- 69.Noguchi M, Sarin A, Aman MJ, Nakajima H, Shores EW, Henkart PA, et al. Functional cleavage of the common cytokine receptor γ chain (γc) by calpain. Proc Natl Acad Sci U S A. 1997;94:11534–11539. doi: 10.1073/pnas.94.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 72.Wells A, Huttenlocher A, Lauffenburger DA. Calpain proteases in cell adhesion and motility. Int Rev Cytol. 2005;245:1–16. doi: 10.1016/S0074-7696(05)45001-9. [DOI] [PubMed] [Google Scholar]
- 73.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282:2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 74.Leloup L, Shao H, Bae YH, Deasy B, Stolz D, Roy P, et al. m-Calpain activation is regulated by its membrane localization and by its binding to phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 2010;285:33549–33566. doi: 10.1074/jbc.M110.123604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ojima K, Kawabata Y, Nakao H, Nakao K, Doi N, Kitamura F, et al. Dynamic distribution of muscle-specific calpain in mice has a key role in physical-stress adaptation and is impaired in muscular dystrophy. J Clin Invest. 2010;120:2672–2683. doi: 10.1172/JCI40658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ojima K, Ono Y, Ottenheijm C, Hata S, Suzuki H, Granzier H, et al. Non-proteolytic functions of calpain-3 in sarcoplasmic reticulum in skeletal muscles. J Mol Biol. 2011;407:439–449. doi: 10.1016/j.jmb.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kramerova I, Kudryashova E, Wu B, Ottenheijm C, Granzier H, Spencer MJ. Novel role of calpain-3 in the triad-associated protein complex regulating calcium release in skeletal muscle. Hum Mol Genet. 2008;17:3271–3280. doi: 10.1093/hmg/ddn223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kojic S, Medeot E, Guccione E, Krmac H, Zara I, Martinelli V, et al. The ankrd2 protein, a link between the sarcomere and the nucleus in skeletal muscle. J Mol Biol. 2004;339:313–325. doi: 10.1016/j.jmb.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 79.Fougerousse F, Anderson LV, Delezoide AL, Suel L, Durand M, Beckmann JS. Calpain3 expression during human cardiogenesis. Neuromuscul Disord. 2000;10:251–256. doi: 10.1016/s0960-8966(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 80.Hayashi C, Ono Y, Doi N, Kitamura F, Tagami M, Mineki R, et al. Multiple molecular interactions implicate the connectin/titin N2A region as a modulating scaffold for p94/calpain 3 activity in skeletal muscle. J Biol Chem. 2008;283:14801–14814. doi: 10.1074/jbc.M708262200. [DOI] [PubMed] [Google Scholar]
- 81.Laure L, Daniele N, Suel L, Marchand S, Aubert S, Bourg N, et al. A new pathway encompassing calpain 3 and its newly identified substrate cardiac ankyrin repeat protein is involved in the regulation of the nuclear factor-B pathway in skeletal muscle. FEBS J. 2010;277:4322–4337. doi: 10.1111/j.1742-4658.2010.07820.x. [DOI] [PubMed] [Google Scholar]
- 82.Carmignac V, Salih MA, Quijano-Roy S, Marchand S, Al Rayess MM, Mukhtar MM, et al. C-terminal titin deletions cause a novel early-onset myopathy with fatal cardiomyopathy. Ann Neurol. 2007;61:340–351. doi: 10.1002/ana.21089. [DOI] [PubMed] [Google Scholar]
- 83.Sarparanta J, Blandin G, Charton K, Vihola A, Marchand S, Milic A, et al. Interactions with M-band titin and calpain 3 link myospryn (CMYA5) to tibial and limb-girdle muscular dystrophies. J Biol Chem. 2010;285:30304–30315. doi: 10.1074/jbc.M110.108720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, et al. The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol. 2003;333:951–964. doi: 10.1016/j.jmb.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Witt CC, Ono Y, Puschmann E, McNabb M, Wu Y, Gotthardt M, et al. Induction and myofibrillar targeting of CARP, and suppression of the Nkx2.5 pathway in the MDM mouse with impaired titin-based signaling. J Mol Biol. 2004;336:145–154. doi: 10.1016/j.jmb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 86.LeWinter MM, Wu Y, Labeit S, Granzier H. Cardiac titin: structure, functions and role in disease. Clin Chim Acta. 2007;375:1–9. doi: 10.1016/j.cca.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 87.Raynaud F, Fernandez E, Coulis G, Aubry L, Vignon X, Bleimling N, et al. Calpain 1-titin interactions concentrate calpain 1 in the Z-band edges and in the N2-line region within the skeletal myofibril. FEBS J. 2005;272:2578–2590. doi: 10.1111/j.1742-4658.2005.04683.x. [DOI] [PubMed] [Google Scholar]
- 88.Moulik M, Vatta M, Witt SH, Arola AM, Murphy RT, McKenna WJ, et al. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J Am Coll Cardiol. 2009;54:325–333. doi: 10.1016/j.jacc.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mani SK, Shiraishi H, Balasubramanian S, Yamane K, Chellaiah M, Cooper G, et al. In vivo administration of calpeptin attenuates calpain activation and cardiomyocyte loss in pressure-overloaded feline myocardium. Am J Physiol Heart Circ Physiol. 2008;295:H314–326. doi: 10.1152/ajpheart.00085.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greyson CR, Schwartz GG, Lu L, Ye S, Helmke S, Xu Y, et al. Calpain inhibition attenuates right ventricular contractile dysfunction after acute pressure overload. J Mol Cell Cardiol. 2008;44:59–68. doi: 10.1016/j.yjmcc.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iwamoto H, Miura T, Okamura T, Shirakawa K, Iwatate M, Kawamura S, et al. Calpain inhibitor-1 reduces infarct size and DNA fragmentation of myocardium in ischemic/reperfused rat heart. J Cardiovasc Pharmacol. 1999;33:580–586. doi: 10.1097/00005344-199904000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Chen M, Won DJ, Krajewski S, Gottlieb RA. Calpain and mitochondria in ischemia/reperfusion injury. J Biol Chem. 2002;277:29181–29186. doi: 10.1074/jbc.M204951200. [DOI] [PubMed] [Google Scholar]
- 93.Singh RB, Chohan PK, Dhalla NS, Netticadan T. The sarcoplasmic reticulum proteins are targets for calpain action in the ischemic-reperfused heart. J Mol Cell Cardiol. 2004;37:101–110. doi: 10.1016/j.yjmcc.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Li Y, Ma J, Zhu H, Singh M, Hill D, Greer PA, et al. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–2994. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smolock AR, Mishra G, Eguchi K, Eguchi S, Scalia R. Protein kinase C upregulates intercellular adhesion molecule-1 and leukocyte-endothelium interactions in hyperglycemia via activation of endothelial expressed calpain. Arterioscler Thromb Vasc Biol. 2011;31:289–296. doi: 10.1161/ATVBAHA.110.217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scalia R, Gong Y, Berzins B, Zhao LJ, Sharma K. Hyperglycemia is a major determinant of albumin permeability in diabetic microcirculation: the role of μ-calpain. Diabetes. 2007;56:1842–1849. doi: 10.2337/db06-1198. [DOI] [PubMed] [Google Scholar]
- 97.Letavernier E, Perez J, Bellocq A, Mesnard L, de Castro Keller A, Haymann JP, et al. Targeting the calpain/calpastatin system as a new strategy to prevent cardiovascular remodeling in angiotensin II-induced hypertension. Circ Res. 2008;102:720–728. doi: 10.1161/CIRCRESAHA.107.160077. [DOI] [PubMed] [Google Scholar]
- 98.Subramanian V, Uchida HA, Ijaz T, Moorleghen JJ, Howatt DA, Balakrishnan A. Calpain inhibition attenuates angiotensin II-induced abdominal aortic aneurysms and atherosclerosis in low-density lipoprotein receptor-deficient mice. J Cardiovasc Pharmacol. 2012;59:66–76. doi: 10.1097/FJC.0b013e318235d5ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Miyazaki T, Taketomi Y, Takimoto M, Lei XF, Arita S, Kim-Kaneyama JR, et al. m-Calpain induction in vascular endothelial cells on human and mouse atheromas and its roles in VE-cadherin disorganization and atherosclerosis. Circulation. 2011;124:2522–2532. doi: 10.1161/CIRCULATIONAHA.111.021675. [DOI] [PubMed] [Google Scholar]
- 100.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 101.van der Laarse A. Hypothesis: troponin degradation is one of the factors responsible for deterioration of left ventricular function in heart failure. Cardiovasc Res. 2002;56:8–14. doi: 10.1016/s0008-6363(02)00534-5. [DOI] [PubMed] [Google Scholar]
- 102.Maekawa A, Lee JK, Nagaya T, Kamiya K, Yasui K, Horiba M, et al. Overexpression of calpastatin by gene transfer prevents troponin I degradation and ameliorates contractile dysfunction in rat hearts subjected to ischemia/reperfusion. J Mol Cell Cardiol. 2003;35:1277–1284. doi: 10.1016/s0022-2828(03)00238-4. [DOI] [PubMed] [Google Scholar]
- 103.Thomas SA, Fallavollita JA, Lee TC, Feng J, Canty JM., Jr Absence of troponin I degradation or altered sarcoplasmic reticulum uptake protein expression after reversible ischemia in swine. Circ Res. 1999;85:446–456. doi: 10.1161/01.res.85.5.446. [DOI] [PubMed] [Google Scholar]
- 104.Colantonio DA, Van Eyk JE, Przyklenk K. Stunned peri-infarct canine myocardium is characterized by degradation of troponin T, not troponin I. Cardiovasc Res. 2004;63:217–225. doi: 10.1016/j.cardiores.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 105.Galvez AS, Diwan A, Odley AM, Hahn HS, Osinska H, Melendez JG, et al. Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res. 2007;100:1071–1078. doi: 10.1161/01.RES.0000261938.28365.11. [DOI] [PubMed] [Google Scholar]
- 106.Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, et al. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006;99:1012–1020. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- 107.Kang MY, Zhang Y, Matkovich SJ, Diwan A, Chishti AH, Dorn GW., II Receptor-independent cardiac protein kinase Cα activation by calpain-mediated truncation of regulatory domains. Circ Res. 2010;107:903–912. doi: 10.1161/CIRCRESAHA.110.220772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kishimoto A, Kajikawa N, Shiota M, Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983;258:1156–1164. [PubMed] [Google Scholar]
- 109.Zhang Y, Matkovich SJ, Duan X, Diwan A, Kang MY, Dorn GW., II Receptor-independent protein kinase Cα (PKCα) signaling by calpain-generated free catalytic domains induces HDAC5 nuclear export and regulates cardiac transcription. J Biol Chem. 2011;286:26943–26951. doi: 10.1074/jbc.M111.234757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taneike M, Mizote I, Morita T, Watanabe T, Hikoso S, Yamaguchi O, et al. Calpain protects the heart from hemodynamic stress. J Biol Chem. 2011;286:32170–32177. doi: 10.1074/jbc.M111.248088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mellgren RL. A plasma membrane wound proteome: reversible externalization of intracellular proteins following reparable mechanical damage. J Biol Chem. 2010;285:36597–36607. doi: 10.1074/jbc.M110.110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 113.Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res. 2012;96:38–45. doi: 10.1093/cvr/cvs099. [DOI] [PubMed] [Google Scholar]
- 114.Miura M. Apoptotic and non-apoptotic caspase functions in neural development. Neurochem Res. 2011;36:1253–1260. doi: 10.1007/s11064-010-0341-x. [DOI] [PubMed] [Google Scholar]
- 115.Khalil PN, Neuhof C, Huss R, Pollhammer M, Khalil MN, Neuhof H, et al. Calpain inhibition reduces infarct size and improves global hemodynamics and left ventricular contractility in a porcine myocardial ischemia/reperfusion model. Eur J Pharmacol. 2005;528:124–131. doi: 10.1016/j.ejphar.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 116.Ando Y, Imamura S, Hong YM, Owada MK, Kakunaga T, Kannagi R. Enhancement of calcium sensitivity of lipocortin I in phospholipid binding induced by limited proteolysis and phosphorylation at the amino terminus as analyzed by phospholipid affinity column chromatography. J Biol Chem. 1989;264:6948–6955. [PubMed] [Google Scholar]
- 117.Wang F, Xia P, Wu F, Wang D, Wang W, Ward T, et al. Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells. J Biol Chem. 2008;283:26714–26725. doi: 10.1074/jbc.M800527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guttmann RP, Baker DL, Seifert KM, Cohen AS, Coulter DA, Lynch DR. Specific proteolysis of the NR2 subunit at multiple sites by calpain. J Neurochem. 2001;78:1083–1093. doi: 10.1046/j.1471-4159.2001.00493.x. [DOI] [PubMed] [Google Scholar]
- 119.Ghosh M, Shanker S, Siwanowicz I, Mann K, Machleidt W, Holak TA. Proteolysis of insulin-like growth factor binding proteins (IGFBPs) by calpain. Biol Chem. 2005;386:85–93. doi: 10.1515/BC.2005.011. [DOI] [PubMed] [Google Scholar]
- 120.Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1α. Proc Natl Acad Sci U S A. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kulkarni S, Goll DE, Fox JE. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J Biol Chem. 2002;277:24435–24441. doi: 10.1074/jbc.M203457200. [DOI] [PubMed] [Google Scholar]
- 122.Lofvenberg L, Backman L. Calpain-induced proteolysis of β-spectrins. FEBS Lett. 1999;443:89–92. doi: 10.1016/s0014-5793(98)01697-4. [DOI] [PubMed] [Google Scholar]
- 123.Rees DJ, Ades SE, Singer SJ, Hynes RO. Sequence and domain structure of talin. Nature. 1990;347:685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- 124.Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120:3480–3492. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang Z, Biesiadecki BJ, Jin JP. Selective deletion of the NH2-terminal variable region of cardiac troponin T in ischemia reperfusion by myofibril-associated mu-calpain cleavage. Biochemistry (Mosc) 2006;45:11681–11694. doi: 10.1021/bi060273s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mandic A, Viktorsson K, Strandberg L, Heiden T, Hansson J, Linder S, et al. Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol. 2002;22:3003–3013. doi: 10.1128/MCB.22.9.3003-3013.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, et al. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- 128.Chua BT, Guo K, Li P. Direct cleavage by the calcium-activated protease calpain can lead to inactivation of caspases. J Biol Chem. 2000;275:5131–5135. doi: 10.1074/jbc.275.7.5131. [DOI] [PubMed] [Google Scholar]
- 129.Gregoriou M, Willis AC, Pearson MA, Crawford C. The calpain cleavage sites in the epidermal growth factor receptor kinase domain. Eur J Biochem. 1994;223:455–464. doi: 10.1111/j.1432-1033.1994.tb19013.x. [DOI] [PubMed] [Google Scholar]
- 130.Schaecher K, Goust JM, Banik NL. The effects of calpain inhibition on IkBα degradation after activation of PBMCs: identification of the calpain cleavage sites. Neurochem Res. 2004;29:1443–1451. doi: 10.1023/b:nere.0000026410.56000.dd. [DOI] [PubMed] [Google Scholar]
- 131.Pfaff M, Du X, Ginsberg MH. Calpain cleavage of integrin β cytoplasmic domains. FEBS Lett. 1999;460:17–22. doi: 10.1016/s0014-5793(99)01250-8. [DOI] [PubMed] [Google Scholar]
- 132.Park D, Jhon DY, Lee CW, Ryu SH, Rhee SG. Removal of the carboxyl-terminal region of phospholipase C-1 by calpain abolishes activation by Gq. J Biol Chem. 1993;268:3710–3714. [PubMed] [Google Scholar]
- 133.Fischer S, Vandekerckhove J, Ampe C, Traub P, Weber K. Protein-chemical identification of the major cleavage sites of the Ca2+ proteinase on murine vimentin, the mesenchymal intermediate filament protein. Biol Chem Hoppe Seyler. 1986;367:1147–1152. doi: 10.1515/bchm3.1986.367.2.1147. [DOI] [PubMed] [Google Scholar]
- 134.Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J. Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett. 2003;189:221–230. doi: 10.1016/s0304-3835(02)00552-9. [DOI] [PubMed] [Google Scholar]
- 135.Wolf BB, Goldstein JC, Stennicke HR, Beere H, Amarante-Mendes GP, Salvesen GS, et al. Calpain functions in a caspase-independent manner to promote apoptosis-like events during platelet activation. Blood. 1999;94:1683–1692. [PubMed] [Google Scholar]
- 136.Gafni J, Cong X, Chen SF, Gibson BW, Ellerby LM. Calpain-1 cleaves and activates caspase-7. J Biol Chem. 2009;284:25441–25449. doi: 10.1074/jbc.M109.038174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Villa PG, Henzel WJ, Sensenbrenner M, Henderson CE, Pettmann B. Calpain inhibitors, but not caspase inhibitors, prevent actin proteolysis and DNA fragmentation during apoptosis. J Cell Sci. 1998;111:713–722. doi: 10.1242/jcs.111.6.713. [DOI] [PubMed] [Google Scholar]
- 139.Kishimoto A, Mikawa K, Hashimoto K, Yasuda I, Tanaka S, Tominaga M, et al. Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain) J Biol Chem. 1989;264:4088–4092. [PubMed] [Google Scholar]
- 140.Bertipaglia I, Bourg N, Richard I, Pahlman AK, Andersson L, James P, et al. A proteomic study of calpain-3 and its involvement in limb girdle muscular dystrophy type 2a. Cell Calcium. 2009;46:356–363. doi: 10.1016/j.ceca.2009.10.003. [DOI] [PubMed] [Google Scholar]