Abstract
Objective
Pressure-overload hypertrophy is associated with decreased capillary density in myocardium resulting in impaired substrate delivery. Treatment of hypertrophied hearts with vascular endothelial growth factor (VEGF) induces angiogenesis. Since angiogenesis is associated with extracellular matrix degradation, we sought to determine whether VEGF induced angiogenesis in hypertrophy required matrix metalloproteinases (MMP) activation.
Methods
Newborn rabbits underwent aortic banding. Progression of hypertrophy (mass-to-volume (M/V) ratio) and mid-wall contractility index was monitored by echocardiography. At 4 and 6 weeks, VEGF (2 μg/kg), vehicle or VEGF combined with GM6001 (5 mg/kg), a MMP inhibitor, was administered intrapericardially. CD-31 (indicator of angiogenesis), MMP-2, MT1-MMP and TIMPs (endogenous MMP inhibitors) expression were measured by immunoblotting. MMP-2 activity was determined by gelatin zymography.
Results
Untreated hypertrophied hearts progressed to ventricular dilatation at 7 wks (M/V ratio: 0.75 ± 0.07), but compensatory hypertrophy was maintained with VEGF (0.91±0.07; p<0.05). LV contractility declined in untreated hearts from −0.41 ± 0.9 (5 wks) to −0.73 ± 0.5 (7 wks; p < 0.05) but remained normal with VEGF (+1.61 ± 0.6 vs. +0.47 ± 0.2). MMP-2 expression and activity were significantly elevated in VEGF treated hypertrophied hearts (p < 0.05) and were blocked by concomitant administration of GM6001. VEGF induced neovascularization was inhibited by addition of GM6001. MT1-MMP showed a trend to higher levels in VEGF treated hearts. TIMPs were unchanged in all three groups.
Conclusions
Exogenous VEGF and resultant MMP-2 activation leads to increased capillary formation in severe hypertrophy, preventing progression to ventricular dilation and dysfunction. VEGF and the associated MMP-2 activation play an important and potentially therapeutic role in vascular remodeling of hypertrophied hearts.
Keywords: hypertrophy, angiogenesis, matrix metalloproteinases
Introduction
Alterations at the level of the coronary microcirculation may play a significant role in the genesis and evolution of the detrimental effects of pressure-overload hypertrophy on the heart. With the development of severe myocardial hypertrophy, myocytes enlarge without concomitant, adaptive growth of capillaries. The area of myocardial tissue supplied by one capillary increases and this results in an increase of diffusion distance with limited supply of oxygen and nutrient substrates [16, 32]. The concept of treating perfusion deficits with angiogenic growth factors such as vascular endothelial growth factor (VEGF) has been successfully tested in models of both myocardial and peripheral ischemia [1, 31] and hypertrophy [10]. Angiogenesis is a multi-step process and VEGF, a 38- to 46-kD heparin-binding homodimeric glycoprotein, promotes many of the events necessary for angiogenesis such as proliferation and migration of endothelial cells, remodeling of the extracellular matrix and the formation of capillary tubules [36]. Extracellular matrix degradation is critical during angiogenesis which requires proteolysis of basement membrane and extra-cellular matrix to create space for migration and proliferation of endothelial cells, as well as synthesis of new matrix components. Degradation of matrix components is mediated by specific proteases called matrix metalloproteinases (MMPs), which are produced by endothelial cells, fibroblasts, vascular smooth muscle cells and as recently reported, also by myocytes [4, 34]. MMPs and their endogenous inhibitors TIMPs (tissue inhibitors of matrix metalloproteinases) have been shown to be involved at various stages of the angiogenic process [20], from endothelial cell migration and proliferation, to deposition and remodeling of basement membrane of newly formed blood vessels [24]. Much information regarding the interplay of pro-angiogenic growth factors and MMPs is derived from tumor biology models [15]. Evidence from these models suggests that there is a direct relationship between VEGF expression and MMP expression where either MMPs regulate VEGF expression [12, 26], or there is VEGF-induced upregulation of MMPs [35]. MMP-2 [6, 13] and MT-1-MMP are the enzymes best characterized for their role in angiogenesis [6, 13, 30].
We have previously shown that in a model of pressure-overload hypertrophy, ventricular dilation and decline in myocardial function is associated with impaired nutrient supply to hypertrophying cardiomyocytes due to a decrease in microvascular density [8, 10]. When hypertrophied hearts were treated with a single dose of VEGF, the microvessel count per area of myocardium increased significantly, the compensatory increase in ventricular muscle mass was sustained without ventricular dilatation and myocardial function was maintained for up to two weeks in this model [10]. The present study was designed to determine whether serial VEGF treatment can preserve hypertrophic growth and delay the onset of failure for longer periods and to determine whether MMP activation is an integral part of VEGF-induced neovascularization. We also sought to determine whether the main activator of MMP-2, MT1-MMP, was involved in this process. Since MMP activity is regulated by its endogenous inhibitors, the expression level of the four TIMPs was also determined.
Materials and methods
Left ventricular hypertrophy model
Pressure-overload hypertrophy was achieved by banding the descending aorta in ten-day-old New Zealand White rabbits as previously described [8, 19]. While still in the compensated phase of hypertrophy (4 weeks of age in this model), the animals were treated with an intrapericardial administration of either 2 μg/kg VEGF165 dissolved in PBS/0.1% rabbit serum or vehicle only via sub-xyphoid exposure of the heart. Two weeks later the animals were treated again with VEGF or vehicle. In a separate set of animals, concomitant with VEGF, a broad-range MMP inhibitor, GM6001 (Chemicon, Temecula, CA), was administered in a concentration of 5 mg/kg. The dosage was derived from dose-response experiments using decrease of MMP-2 activity determined by gelatin zymography as the endpoint and lack of side effects from the compound. One week after the second treatment (7 weeks of age), all animals were euthanized by an I.V. injection of ketamine (100 mg/kg) and xylazine (5 mg) with heparin (500 IU) added. Blood was flushed out of the hearts and tissue was frozen in liquid nitrogen and stored for further analysis.
In vivo myocardial function measurements by transthoracic echocardiography
Transthoracic echocardiography was performed using an Accuson 128 or Hewlett-Packard Sonos 1500 Cardiac Imager equipped with a 7–7.5 MHz transducer. Measurements of LV dimensions, wall thickness, and cavity volume were taken with transthoracic echocardiography with simultaneous blood pressure monitoring as we have previously described [8, 19]. Measurements of LV mass to LV volume ratio (M/V ratio) were used as an index for progression of hypertrophy. As a measure of cardiac performance, midwall contractility was determined. Calculation of midwall contractility Z-scores is based on previous experiments where serial echocardiography was performed in non-hypertrophied control animals between the ages of 3 and 7 weeks [19].
Left ventricular myocardial tissue extraction for gelatinase activity measurements
We studied four groups: controls, representing hearts from sham-operated, non-hypertrophied littermates (n = 8/group), untreated hypertrophy hearts (n = 8/group), VEGF treated hypertrophy hearts that received serial VEGF treatment (n = 8/group) and VEGF and MMP inhibitor treated hypertrophy hearts that received serial VEGF treatment concomitant with GM6001 (n = 6/ group). LV myocardial tissue was homogenized on ice in extraction buffer containing NaCl (2 mol/L), Tris (10 mmol/L), NaN3 (0.02%), at pH 7.0 and nutated at 4 °C for 24 hours following a method described by Moses et al. [2]. Samples were then centrifuged for 15 minutes (4 °C, 15,000 g). The supernatant was saved on ice and samples were dialyzed against assay buffer containing Tris (50 mmol/L at pH 7.6), NaCl (0.2 mol/L) and CaCl2 (1 mmol/L) at 4 °C. Total protein content was determined and the myocardial extracts were stored at −80 °C for further analysis. All chemicals were obtained from Sigma-Aldrich, Corp., St. Louis, MO.
Gelatinase activity measurement by zymography
The myocardial extracts were mixed with substrate sample buffer (10% SDS, 4% glycerol, 0.25 mmol/L Tris-HCl at pH 6.8, 0.1% bromophenol blue) without boiling and loaded onto electrophoresis gels (SDS-PAGE) containing 1 mg/ml gelatin (Bio-Rad Laboratories, Hercules, CA) according to previously published methods under non-reducing conditions [2]. After electrophoresis, the gels were soaked in renaturing buffer containing 2.5% Triton X-100 with gentle shaking at room temperature for 30 minutes, rinsed with water, and incubated in substrate buffer (50 mmol/L Tris-HCl at pH 8.0, 5 mmol/L CaCl2, 0.02% NaN3) at 37 °C overnight. After incubation, gels were rinsed with water, stained using 0.5% Coomassie Blue R-250 in acetic acid and isopropyl alcohol mixture (Bio-Rad Laboratories, Hercules, CA), destained in acetic acid and isopropyl alcohol and analyzed. A MMP-2 zymographic standard (R&D Systems Inc., Minneapolis, MN) was included in the gels and served as positive control.
Immunoblot analysis of MMPs and TIMPs
Protein extraction from LV myocardial tissue obtained from non-hypertrophied, age-matched littermates (n = 6), untreated hypertrophied (n = 6) and VEGF treated hypertrophied hearts (n = 6) were immunoprecipitated with the respective antibody by protein A/G-PLUS-agarose as we have previously described in more detail [9]. The immunoprecipitates were separated by gel electrophoresis with 10% SDS-PAGE gels. Proteins were electrophoretically transferred to nitrocellulose membranes, incubated in 5% nonfat dry milk in TBST for 30 minutes at room temperature to block non-specific binding and then incubated with primary antibodies against MMP-2, MT1-MMP, TIMP-1 (R&D Systems Inc., Minneapolis, MN), TIMP-2, TIMP-3 or TIMP-4 (Chemicon International Inc., Temecula, CA), respectively, at a dilution of 1:500 or 1:1000 overnight and this was followed by incubation with horseradish peroxidase-conjugated secondary antibody (Jackson Immuno Research Labs, Inc., West Grove, PA) at a dilution of 1:2500. The bound antibody was detected by the enhanced chemiluminescence method according to the manufacturer’s instruction (Amersham Life Science, Arlington Heights, IL). After exposure on films, quantitative protein analysis was conducted using laser densitometry.
Determination of microvascular density
Microvascularity was quantified by measuring CD-31 (endothelial cell specific marker) expression levels by immunoblotting in protein extracts from LV myocardial tissue obtained from non-hypertrophied, age-matched littermates, untreated hypertrophied animals, VEGF treated hypertrophied animals, and hearts treated with VEGF and GM6001. CD-31 levels are indicative of the degree of neovascularization. In addition, microvascular density was also determined on histological sections obtained from hearts perfused with fluorescein-isothiocyanate-conjugated (FITC) Lycopersicon esculentum lectin (Sigma-Aldrich, St. Louis, MO). The details of this method were previously described by our group [10]. Paraffin-embedded cross-sections of the LV were de-paraffinized and either stained with CD-31 using a red fluorescent secondary antibody (Alexa-594™ fluorophore; Molecular Probes, Eugene, OR) or simply cover-slips were applied to the lectin perfused sections with fluorescent mounting medium (Dako Corporation, Carpinteria, CA). Slides were visualized using an Axiovert 35 Microscope with a Nikon 10× objective (NA = 10×/0.25).
Statistical analysis
Data were analyzed using SPSS software package (version 11.0, SPSS Inc., Chicago, IL) and are reported as mean ± standard error of the mean (SEM). ANOVA was used for comparison among and between groups, followed by Bonferroni’s post-hoc analysis where appropriate. Z-score comparison to the population mean was performed using one-way unpaired t-test. A value of p ≤ 0.05 was considered statistically significant.
Animal care
All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH Publication No. 86–23, revised 1996). The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Children’s Hospital Boston.
Results
Determination of microvascular density
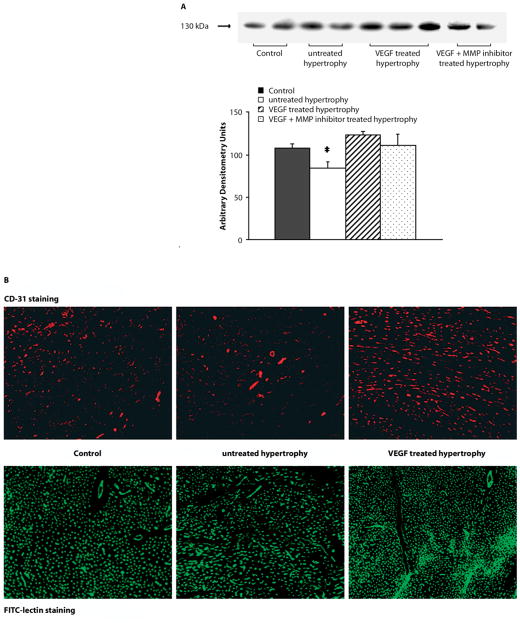
Neoangiogenesis was determined by measuring CD-31 protein levels in controls, untreated hypertrophied, VEGF treated hypertrophied and VEGF+MMP inhibitor (GM6001) treated hypertrophied hearts (see Fig. 1A). Untreated hypertrophied hearts showed a significantly lower expression level of CD-31 compared to all other groups, indicative of decreased microvascular density. VEGF treatment concomitant with MMP inhibition resulted in reduced capillary growth. Histological evaluation of CD-31 immunostaining and lectin labeled LV transverse sections revealed that VEGF treatment induced capillary growth resulting in a significant increase in the number of microvessels confirming the Western blotting data of CD-31.
Fig. 1.
Microvascular density. A Representative immunoblots for CD-31 and summary of the densitometry data are depicted. VEGF treatment of hypertrophied hearts results in significant higher CD-31 levels. Concomitant administration of VEGF with a MMP inhibitor inhibited neoangiogenesis; however, the difference did not reach a level of significance (*p < 0.05 vs. VEGF treated hypertrophy). B Representative immunohistochemical sections of LV tissue are depicted with staining of the microvasculature with CD-31 in red or lectin in green
In vivo myocardial function measurements by transthoracic echocardiography
Cardiac hypertrophy is a compensatory response to sustained elevations in LV wall stress. In this model left ventricular M/V ratio increased, reaching a plateau by 4 to 5 weeks of age. Since the pressure load remained unrelieved, the increase in muscle mass could no longer compensate to reduce peak systolic stress and the left ventricle began to dilate, as indicated by a fall in M/V ratio after week 4 (Fig. 2A). In comparison, serial treatment with VEGF at maximal hypertrophy maintained hypertrophic growth and delayed the onset of ventricular dilation (7 wks: 0.91 ± 0.07) beyond the time point when untreated hypertrophied hearts (7 wks: 0.75 ± 0.07; p < 0.05) had already shown signs of severe dilatation, failure and death.
Fig. 2.

Left ventricular mass to volume ratio and midwall contractility. A LV mass to LV cavity volume measurement as an indicator of hypertrophic growth. VEGF treated hearts maintained a higher ratio of LV mass to cavity volume over an extended period compared to untreated hypertrophied hearts, which showed signs of severe ventricular dilatation (= fall in M/V ratio) (*p < 0.05; versus untreated hypertrophied hearts). B Midwall contractility (depicted as Z-scores) was calculated based on echocardiographic measurements of non-hypertrophied control hearts. VEGF treatment prevented myocardial dysfunction seen in the untreated hypertrophied hearts (*p < 0.05; versus untreated hypertrophied hearts)
The use of midwall indices for contractility measurements better reflects the preservation in global cardiac performance in hypertrophied hearts. In this model, midwall contractility (Z-scores) in the serial VEGF treated group remained within normal range and was significantly higher than the untreated group at 5 weeks (VEGF treated: +1.61 ± 0.6 vs. untreated: −0.41 ± 0.9; p < 0.05) and 7 weeks of age (VEGF treated: +0.47 ± 0.2 vs. untreated: −0.73 ± 0.5; p < 0.05).
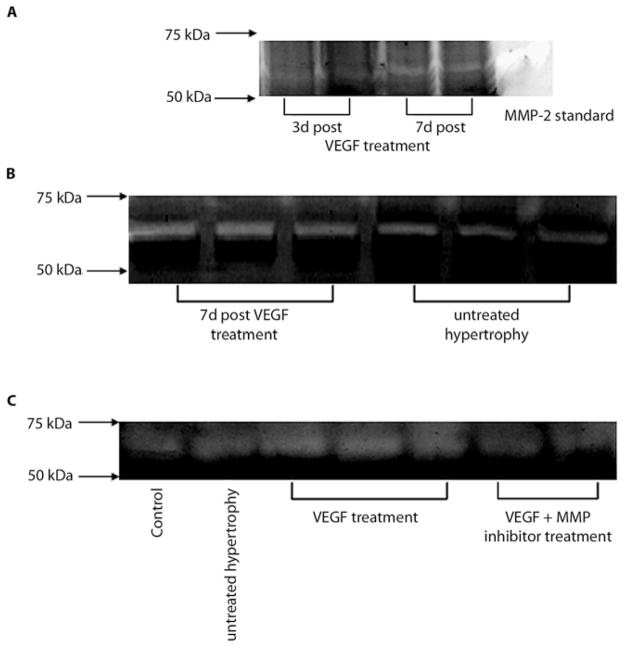
Gelatinase activity
Zymograms revealed the main band at 66 kDa molecular weight, indicative of MMP-2 activity which was also confirmed by a positive MMP-2 control (see Fig. 3A). To determine the time course of MMP activation, different time points following VEGF treatment were investigated and the highest levels in hypertrophied hearts treated with VEGF were seen within a week after VEGF administration (p < 0.05; see representative zymograms in Fig. 3A and B). In order to determine the direct effect of VEGF treatment on MMP activation, we analyzed tissue of hypertrophied hearts treated with VEGF and GM6001 (see Fig. 3C) and showed that GM6001 abolished VEGF induced MMP-2 activation.
Fig. 3.
MMP-2 activity. A, B MMP-2 activity levels were determined by gelatin zymography. Two representative gels are depicted with MMP activity for hypertrophied hearts and VEGF treated hearts at two different time points following treatment. Active MMP-2 was higher in VEGF treated hypertrophied hearts, one week following VEGF administration compared to untreated hypertrophied hearts. C A representative zymogram is shown which indicates that concomitant administration of VEGF and a MMP inhibitor decreased zymographic activity of MMP-2
Immunoblot analysis of MMPs and TIMPs
As indicated by the representative immonoblot (see Fig. 4A), the antibody against MMP-2 detected the latent form of MMP-2 (higher molecular weight) as well as the active enzyme. Protein content of latent MMP-2 was not different between the three groups but the active enzyme portion was significantly higher in hypertrophied hearts following serial VEGF treatment compared to untreated hypertrophied hearts or controls (p < 0.05). A representative immunoblot for MMP-2 is shown in Fig. 4A. Figures 4B and C summarize the densitometry results. At the same time point, MT1-MMP showed the highest expression levels in VEGF treated hypertrophied hearts but the difference did not reach significance compared to controls (see Fig. 5; p = 0.07). MMP effects are also determined by the level of TIMPs. All four TIMP isoforms were detected in the myocardium. As indicated in Fig. 6A, B, C and D (representative immunoblots and summary of densitometry data), there was no difference in the expression of any of the four TIMPs between the groups.
Fig. 4.
Myocardial MMP protein content. A To reconfirm that MMP-2 is activated within one week following VEGF treatment, we performed immunoblot analysis using an antibody identifying total and active MMP-2. A representative immunoblot for this specific MMP species is shown for immunoprecipitates obtained from left ventricular muscle extracts from controls, untreated hypertrophied hearts and VEGF treated hypertrophied hearts. A distinct immunoreactive band could be localized at 72 kDa indicative of the latent, non-active form and a second one at 66 kDa which represents the active form of MMP-2. B, C Quantification of protein content of latent (B) and active (C) form was performed by laser densitometry and values are expressed as arbitrary densitometry units. There is no difference of latent MMP-2 protein content between controls (solid bar) and VEGF treated hypertrophied hearts (shaded bar) and untreated hypertrophied hearts (blank bar) but active MMP-2 levels were significantly higher in hypertrophied hearts following VEGF treatment (*p < 0.05; versus untreated hypertrophy and Control)
Fig. 5.
MT1-MMP protein content. AA representative immunoblot of MT1-MMP protein from left ventricular muscle extracts for all three groups is shown. BA summary of densitometry data showed that VEGF treatment resulted in an increase of MT1-MMP protein levels in hypertrophied hearts compared to untreated hearts but did not reach significance (p = 0.07)
Fig. 6.
TIMP levels in myocardium. A–D Representative immunoblots for all four TIMPs and summary of the densitometry data are depicted. There was no significant difference in TIMP protein levels between the groups
Discussion
This study demonstrates that extracellular matrix turnover, mediated by increased MMP-2 activity is an important mechanism regulating vascular remodeling in the hypertrophying heart following VEGF treatment. Intrapericardial application of VEGF results in activation of MMP-2 within a week following treatment, and upregulation of MT1-MMP protein levels in this model of pressure-overload hypertrophy. Administration of a MMP inhibitor concomitant with VEGF blocked the VEGF induced effect on MMP-2 activation and reduced the effects of VEGF on neovascularization. VEGF-induced neovascularization and associated MMP activation preserves myocardial function beyond the time point when untreated hypertrophied hearts had already developed signs of dilatation and failure.
In angiogenesis, new blood vessels originate from post-capillary venules through a series of events, beginning with the disruption of the vascular basement membrane by proteolytic enzymes. After the disruption of the vessel wall and the degradation of the surrounding extracellular matrix, the extravasation of plasma proteins provides the scaffold for the migration of endothelial cells. Degradation of matrix components is carried out by specific proteases the so called matrix metalloproteinases (MMPs). MMPs contribute to normal as well as pathological tissue remodeling. Endothelial cells synthesize and secrete a variety of these MMPs [20] and at the same time secrete their endogenous inhibitors, the TIMPs (tissue inhibitors of matrix metalloproteinases). Inhibition is provided by formation of tight complexes between TIMPs and activated MMPs. Four different TIMPs have been identified in normal myocardium [29, 33]. TIMP expression is regulated during development and tissue remodeling and under pathological conditions associated with unbalanced MMP activities. Therefore, coordinate expression of MMPs and TIMPs provides the regulatory mechanisms [20] which limits extracellular matrix degradation to endothelial cell migration [23] and proliferation, and also regulates deposition and remodeling of basement membrane in newly formed blood vessels [24]. In our study, we identified all four TIMP isoforms in rabbit myocardium; however, protein levels were not different between any of the groups, indicating that the same level of inhibition was present.
MMP-1, MMP-2, MMP-9 and MT1-MMP have been reported to be the main MMPs involved in the neovascularization process stimulated by growth factors such as VEGF [30]. Interest has been focused on proteases that preferentially degrade basement membrane components such as type IV collagen [18], but also participate in the degradation of other extracellular matrix components. The present study examined MMP-2 activity as well as MMP-2, MT1-MMP and TIMP isoform levels in left ventricular myocardium of normal hearts, hypertrophied myocardium and hypertrophied hearts following VEGF treatment. MMPs can be divided into two structurally distinct groups, secreted MMPs and membrane-type MMPs (MT-MMPs). The former are synthesized and secreted as inactive pro-enzymes that are activated by cleavage of the N-terminal pro-segment by autoactivation, by other MMPs, or by proteases such as trypsin and function at neutral pH [21]. Most MMPs, with the exception of MMP-2, are not constitutively expressed in normal tissues. Endothelial cells have been shown to produce MMP-2 during differentiation into capillary tube-like structures and exogenous addition of MMP-2 has been shown to enhance this process, whereas suppression of MMP-2 alone could inhibit the angiogenic potential as seen in tumors [6]. MMP-2 can degrade intact type IV basement membrane collagen as well as denatured collagens, type I and III, the main components of the myocardial extracellular matrix. In our study, we found that non-hypertrophied, hypertrophied and VEGF treated hypertrophied hearts showed some degree of MMP-2 activation. Since the animals are still growing and developing, some degree of activation has to be expected. However, we also found that increased MMP-2 activation occurs tightly linked to VEGF treatment. Within one week following application of VEGF we found increased activity levels of MMP-2, as measured by zymography and immunoblotting. Our results with concomitant administration of VEGF and MMP inhibition, establish a critical role for MMP activity in the VEGF induced formation of new microvasculature which concurs with a study on skeletal muscle [11].
MMP-2 which is the only isoform also secreted by cardiomyocytes [4], facilitates the degradation of basement membrane type IV collagen, exposes the growth affecting sites on extracellular matrix proteins and increases the bioavailability of matrix-associated growth factors [3]. In comparison to other zymogens, pro-MMP-2 is not readily activated by proteases like MMP-3 but the main activator is located on the cell surface as MT1-MMP which requires the assistance of TIMP-2. MT1-MMP contributes to angiogenesis not only through the activation of MMP-2 [25] but also by cleaving both extracellular matrix, such as type I collagen, fibronectin, laminin, fibrin [22, 25], which promotes cell migration, and cell surface receptors, influencing the bioavailability of growth factors [14]. However, there is a dual action of TIMP-2, which can either inhibit MT1-MMP and MMP-2 by binding to the active site of the enzyme, or it can form a non-covalent complex with pro-MMP-2 [7, 28] creating a tertiary complex of MMP-2/TIMP-2/MT1-MMP. The resulting TIMP-free environment due to complex formation on the cell surface permits effective MMP-2 activation by MT1-MMP [28]. Consistent with our results with regard to VEGF stimulated MMP-2 and MT1-MMP activation, it has been reported that induction of certain MMPs correlates with up-regulation of VEGF [5, 34]. In particular, over-expression of MT1-MMP correlates with increasing endothelial cell proliferation and migration, and subsequent vascularization [26]. The major function of the MMPs is extracellular matrix resorption but the extracellular matrix is not simply an extracellular scaffold but also acts as a reservoir of biologically active molecules, such as growth factors. Degradation of extracellular matrix components by MMPs can alter cellular behavior and phenotypes. There are also a number of non-extra-cellular matrix proteins that serve as substrates for MMPs which demonstrate biological activity [17, 27]. Therefore, it seems likely that MMP-2 activation also provides release of matrix bound VEGF; however this notion remains to be proven in our model.
In summary, the higher capillary density seen in VEGF treated hearts is associated with an increase in MMP-2 activity, suggesting an important and potentially therapeutic role of MMP-2 in the regulation of vascular remodeling in hypertrophied hearts. In addition, MT1-MMP, the main activator of MMP-2, is also upregulated following VEGF treatment. The cumulative effect of VEGF and MMP-2 activation ameliorates the progression of ventricular dysfunction by sustaining hypertrophic growth and thus preventing ventricular dilation. Whether MMP-2 and MT1-MMP activation exerts its major effects by extracellular matrix turnover, clearing the path for migrating and proliferating endothelial cells or whether the matrix degradation induces the release of additional angiogenic growth factors remains to be determined.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-075430 (to I. Friehs) and HL-063095 (to P. J. del Nido)
Contributor Information
Ingeborg Friehs, Department of Cardiac Surgery, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Ave., BADER 279, Boston, MA 02115, USA, Tel.: +1-617/355-8290, Fax: +1-617/730-0214.
Renee E. Margossian, Department of Pediatric Cardiology
Adrian M. Moran, Department of Pediatric Cardiology
Hung Cao-Danh, Department of Cardiac Surgery, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Ave., BADER 279, Boston, MA 02115, USA, Tel.: +1-617/355-8290, Fax: +1-617/730-0214.
Marsha A. Moses, Vascular Biology Program, Children’s Hospital Boston, Harvard Medical School, Boston, MA 02115, USA
Pedro J. del Nido, Email: pedro.delnido@cardio.chboston.org, Department of Cardiac Surgery, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Ave., BADER 279, Boston, MA 02115, USA, Tel.: +1-617/355-8290, Fax: +1-617/730-0214
References
- 1.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89 (5):2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 2.Braunhut SJ, Moses MA. Retinoids modulate endothelial cell production of matrix-degrading proteases and tissue inhibitors of metalloproteinases (TIMP) J Biol Chem. 1994;269:13472–13479. [PubMed] [Google Scholar]
- 3.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3:207–214. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 4.Coker ML, Doscher MA, Thomas CV, Galis ZS, Spinale FG. Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am J Physiol. 1999;277:H777–H787. doi: 10.1152/ajpheart.1999.277.2.H777. [DOI] [PubMed] [Google Scholar]
- 5.Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori Angiopoietin-2 is related to tumor angiogenesis in gastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145–2153. [PubMed] [Google Scholar]
- 6.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci USA. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridman R, Fuerst TR, Bird RE, Hoyhtya M, Oelkut M, Kraus S, Komarek D, Liotta LA, Berman ML, Stetler-Stevenson WG. Domain structure of human 72-kDa gelatinase/type IV collagenase: characterization of proteolytic activity and identification of the tissue inhibitor of metalloproteinase-2 (TIMP-2) binding regions. J Biol Chem. 1992;267:15398–15405. [PubMed] [Google Scholar]
- 8.Friehs I, Moran AM, Stamm C, Colan SD, Takeuchi K, Cao-Danh H, Rader CM, McGowan FX, del Nido PJ. Impaired glucose transporter activity in pressure-overload hypertrophy is an early indicator of progression to failure. Circulation. 1999;100(19 Suppl):II187–II193. doi: 10.1161/01.cir.100.suppl_2.ii-187. [DOI] [PubMed] [Google Scholar]
- 9.Friehs I, Stamm C, Cao-Danh H, McGowan FX, Jr, del Nido PJ. Insulin-like growth factor-1 improves postischemic recovery in hypertrophied hearts. Ann Thorac Surg. 2001;72:1650–1656. doi: 10.1016/s0003-4975(01)03098-3. [DOI] [PubMed] [Google Scholar]
- 10.Friehs I, Moran AM, Stamm C, Choi YH, Cowan DB, McGowan FX, del Nido PJ. Promoting angiogenesis protects severely hypertrophied hearts from ischemic injury. Ann Thorac Surg. 2004;77:2004–2010. doi: 10.1016/j.athoracsur.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Haas TL, Milkiewicz M, Davis SJ, Zhou AL, Egginton S, Brown MD, Madri JA, Hudlicka O. Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol (Heart Circ Physiol) 2000;279:H1540–H1547. doi: 10.1152/ajpheart.2000.279.4.H1540. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactive angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 13.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 14.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kinoh H, Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klagsbrun M, Moses MA. Molecular angiogenesis. Chem Biol. 1999;6:R217–R224. doi: 10.1016/S1074-5521(99)80081-7. [DOI] [PubMed] [Google Scholar]
- 16.Marcus ML, Harrison DG, Chilian WM, Koyanagi S, Inou T, Tomanek RJ, Martins JB, Eastham CL, Hiratzka LF. Alterations in the coronary circulation in hypertrophied ventricles. Circulation. 1987;75:I-19–25. [PubMed] [Google Scholar]
- 17.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Cur Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 18.Mignatti P, Rifkin DB. Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein. 1996;49:117–137. doi: 10.1159/000468621. [DOI] [PubMed] [Google Scholar]
- 19.Moran AM, Friehs I, Takeuchi K, Stamm C, Hammer PE, McGowan FX, del Nido PJ, Colan SD. Non-invasive serial evaluation of myocardial mechanics in pressure overload hypertrophy of rabbit myocardium. Herz. 2003;28:52–62. doi: 10.1007/s00059-003-2392-0. [DOI] [PubMed] [Google Scholar]
- 20.Moses MA. The regulation of neo-vascularization by matrix metalloproteinases and their inhibitors. Stem Cells. 1997;15:180–189. doi: 10.1002/stem.150180. [DOI] [PubMed] [Google Scholar]
- 21.Nagase H. Activation and mechanisms of matrix metalloproteinases. Biol Chem. 1997;78:151–160. [PubMed] [Google Scholar]
- 22.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 23.Puyraimond A, Weitzman JB, Babiole E, Menashi S. Examining the relationship between the gelatinolytic balance and the invasive capacity of endothelial cells. J Cell Sci. 1999;112:1283–1290. doi: 10.1242/jcs.112.9.1283. [DOI] [PubMed] [Google Scholar]
- 24.Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998;8:171–177. doi: 10.1038/cr.1998.17. [DOI] [PubMed] [Google Scholar]
- 25.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumor cells. Nature (London) 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- 26.Sounni NE, Devy L, Hajitou A, Frankenne F, Munaut C, Gilles C, Deroanne C, Thompson EW, Foidart JM, Noel A. MT1-MMP expression promotes tumor growth and angiogenesis through up-regulation of vascular endothelial growth factor expression. FASEB J. 2002;16:555–564. doi: 10.1096/fj.01-0790com. [DOI] [PubMed] [Google Scholar]
- 27.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strongin AY, Collier I, Bannikow G, Marmaer BL, Grant GA, Goldberg GI. Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem. 1995;270:5331–5338. doi: 10.1074/jbc.270.10.5331. [DOI] [PubMed] [Google Scholar]
- 29.Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996;148:1345–1350. [PMC free article] [PubMed] [Google Scholar]
- 30.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomanek RJ. Response of the coronary vasculature to myocardial hypertrophy. J Am Coll Cardiol. 1990;15:528–533. doi: 10.1016/0735-1097(90)90620-5. [DOI] [PubMed] [Google Scholar]
- 33.Tyagi SC, Kumar SG, Banks J, Fortson W. Co-expression of tissue inhibitor and matrix metalloproteinase in myocardium. J Mol Cell Cardiol. 1995;27:2177–2189. doi: 10.1016/s0022-2828(95)91443-9. [DOI] [PubMed] [Google Scholar]
- 34.Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992;153:557–562. doi: 10.1002/jcp.1041530317. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Keiser JA. Vascular endothelial growth factor upregulates the expression of matrix metalloproteinases in vascular smooth muscle cells. Circ Res. 1998;83:832–840. doi: 10.1161/01.res.83.8.832. [DOI] [PubMed] [Google Scholar]
- 36.Wu LW, Mayo LD, Dunbar JD, Kessler KM, Baerwald MR, Jaffe EA, Wang D, Warren RS, Donner DB. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem. 2000;275:5096–5103. doi: 10.1074/jbc.275.7.5096. [DOI] [PubMed] [Google Scholar]