Abstract
This work examines the involvement of haem oxygenase-1 (HO-1) in salicylic acid (SA)-induced alleviation of oxidative stress as a result of cadmium (Cd) stress in alfalfa (Medicago sativa L.) seedling roots. CdCl2 exposure caused severe growth inhibition and Cd accumulation, which were potentiated by pre-treatment with zinc protoporphyrin (ZnPPIX), a potent HO-1 inhibitor. Pre-treatment of plants with the HO-1 inducer haemin or SA, both of which could induce MsHO1 gene expression, significantly reduced the inhibition of growth and Cd accumulation. The alleviation effects were also evidenced by a decreased content of thiobarbituric acid-reactive substances (TBARS). The antioxidant behaviour was confirmed by histochemical staining for the detection of lipid peroxidation and the loss of plasma membrane integrity. Furthermore, haemin and SA pre-treatment modulated the activities of ascorbate peroxidase (APX), superoxide dismutase (SOD), and guaiacol peroxidase (POD), or their corresponding transcripts. Significant enhancement of the ratios of reduced/oxidized homoglutathione (hGSH), ascorbic acid (ASA)/dehydroascorbate (DHA), and NAD(P)H/NAD(P)+, and expression of their metabolism genes was observed, consistent with a decreased reactive oxygen species (ROS) distribution in the root tips. These effects are specific for HO-1, since ZnPPIX blocked the above actions, and the aggravated effects triggered by SA plus ZnPPIX were differentially reversed when carbon monoxide (CO) or bilirubin (BR), two catalytic by-products of HO-1, was added. Together, the results suggest that HO-1 is involved in the SA-induced alleviation of Cd-triggered oxidative stress by re-establishing redox homeostasis.
Key words: Cadmium, haem oxygenase-1, Medicago sativa, oxidative stress, redox state homeostasis, salicylic acid
Introduction
A plant’s survival often depends on its ability to acclimate rapidly to various abiotic and biotic environmental stresses by adjusting its cellular homeostasis and balancing multiple pathways in different cellular compartments. It has been well established that the maintenance of homeostatic redox levels in cells is essential for plant adaptive responses to biotic and abiotic stresses, and that failure to establish redox homeostasis usually leads to the phenomenon known as oxidative stress (Dutilleul et al., 2003; Noctor et al., 2007). Salicylic acid (SA) is an endogenous regulator or signal of various physiological processes such as thermogenesis and defence against harmful microorganisms in plants (Raskin, 1992; Chen et al., 1993; Delaney et al., 1994; Durner et al., 1997; Shen et al., 1999; Xie and Chen, 1999). It is also involved in the alleviation of oxidative stress caused by ageing as well as by biotic and abiotic stresses (Yang et al., 2004). Increased SA levels in response to heavy metal stress (Metwally et al., 2003) demonstrate a link between the degree of plant tolerance to heavy metals mediated by the SA signal and redox homeostasis (Sharma and Dietz, 2009). Meanwhile, signalling substances, such as calcium (Ca2+), nitric oxide (NO), hydrogen peroxide (H2O2), and their interactions, have been identified as being potentially involved in the cellular responses of plants to heavy metal toxicity (Rodríguez-Serrano et al., 2009). However, the molecular events involved in SA signalling responsible for the alleviation of heavy metal-induced oxidative stress are still poorly understood (Metwally et al., 2003).
Haem oxygenase (HO; EC 1.14.99.3), the rate-limiting enzyme in the breakdown of haem into carbon monoxide (CO), iron, and biliverdin (BV), has attracted much recent research interest (Ryter et al., 2002; Otterbein et al., 2003; Cao et al., 2011). To date, three HO isozymes have been identified in animals. One of these, HO-1, is a stress response protein induced by various oxidative agents, while the HO-2 and HO-3 genes are constitutively expressed (Ryter et al., 2002). In Arabidopsis thaliana, a family of four genes (HY1, HO2, HO3, and HO4) encodes HO, and they play a major role in phytochrome chromophore biosynthesis (Muramoto et al., 2002; Shekhawat and Verma, 2010). Furthermore, the antioxidant behaviour of the HO-1/CO system has been demonstrated in Arabidopsis (Xie et al., 2011, 2012), soybean (Noriega et al., 2004; Yannarelli et al., 2006), alfalfa (Han et al., 2008), and wheat (Huang et al., 2006;Xie et al., 2008; Wu et al., 2011), and these responses might exhibit interactions with reactive oxygen species (ROS) metabolism or signalling.
Cadmium (Cd) is a heavy metal with a long biological half-life, and is present as a pollutant in agricultural soils due mainly to anthropogenic activities. Normally, Cd induces genetic and biochemical changes in plant metabolism that are related to general and Cd-specific stress responses (Ortega-Villasante et al., 2005; Krantev et al., 2008; Sharma and Dietz, 2009; Brunetti et al., 2011). For example, Cd causes oxidative stress in plants resulting in lipid peroxidation of the plasma membrane in plant tissues (Ortega-Villasante et al., 2007). Interestingly, the more Cd-sensitive pea genotypes showed decreased concentrations of glutathione (GSH; γ-glutamyl-cysteinyl-glycine) in their roots, whereas the more tolerant genotypes had increased GSH levels (Metwally et al., 2005). However, homoglutathione (hGSH; γ-glutamyl-cysteinyl-β-alanine) is more abundant than GSH in soybean and alfalfa plants (Matamoros et al., 1999; Baldacci-Cresp et al., 2012). The hGSH is synthesized by homoglutathione synthetase (hGS) and catalyses the conjugation of β-alanine to γ-glutamylcysteine; the latter is synthesized from l-glutamate and l-cysteine by γ-glutamylcysteine synthetase (ECS), the first enzyme in glutathione synthesis. The second enzyme involved in glutathione synthesis is glutathione synthetase (GS, also named GSHS). It was further known that the balance between GSH and oxidized GSH (GSSG) and/or their homologues reduced/oxidized homoglutathione (hGSH/hGSSGh), as well as reduced ascorbic acid (ASA) and its oxidized forms [monodehydroascorbate and dehydroascorbate (DHA)], is crucial for the efficiency of plant antioxidant systems (Noctor et al., 2002). Recently, it has also been found that exposure to Cd at low doses induces HO-1 production which plays a cytoprotective role both in vitro, and especially in vivo (Noriega et al., 2004). The CO released by HO-1 catalysis might act as a signal element for the alleviation of Cd-induced oxidative stress by modulating GSH homeostasis (Han et al., 2008).
In animals, previous studies have shown that aspirin, the acetylated derivative of SA, targets HO-1, presumably via the NO-dependent pathway. Induction of HO-1 expression and activity may be a novel mechanism by which aspirin prevents cellular injury under inflammatory conditions and in cardiovascular disease (Grosser et al., 2003). However, little information is known about the specific role of HO-1 in SA-induced antioxidant behaviour in plants. To investigate the hypothesis that a tight link between HO-1-mediated and SA-dependent signalling exists in the alleviation of Cd toxicity, SA-mediated HO-1 up-regulation is first investigated and then its relationship to SA-induced antioxidative behaviour in the root tissues of alfalfa plants is elucidated. Plants were pre-treated with SA, the HO-1 inducer haemin, and a potent HO-1 inhibitor, zinc protoporphyrin (ZnPPIX), alone or in various combinations, and then exposed to Cd. Various redox homeostasis parameters were determined, such as: antioxidative enzyme expression and activities; lipid peroxidation; ROS distribution; and the hGSH/hGSSGh, ASA/DHA, and NAD(P)H/NAD(P)+ ratios, etc. The possible mechanisms driving these parameters, and their significance, are discussed.
Materials and methods
Chemicals
SA was purchased from Shanghai Medical Instrument, Co., Ltd., China National Medicine (Group), Shanghai, China. Haemin, purchased from Fluka, was used as an HO-1 inducer. ZnPPIX, a potent inhibitor of HO-1 (Noriega et al., 2004; Lang et al., 2005; Wu et al., 2011), was obtained from Sigma. Cysteine, γ-glutamylcysteine (γ-EC, the precursors of GSH), and GSH were purchased from Sigma-Aldrich. hGSH was obtained from Shanghai RD BIOSCIENCES, Co., Ltd. The preparation of CO aqueous solution was carried out according to the method described in a previous report (Han et al., 2008).
Plant materials, growth conditions, and treatments
Commercially available alfalfa (Medicago sativa L. cv Zhongmu No.1) seeds were surface-sterilized with 5% NaClO for 10min, and rinsed extensively in distilled water before being germinated for 1 d at 25 °C in darkness. Uniform seedlings were then selected and transferred to plastic chambers and cultured in nutrient medium (quarter-strength Hoagland’s solution). Alfalfa seedlings were grown in an illuminating incubator at 25±1 °C, with a light intensity of 200 µmol m–2 s–1 and 14h photoperiod. After growing for 5 d, the seedlings were then incubated in quarter-strength Hoagland’s solution with or without 10 µM or the indicated concentrations of SA, 100 µM ZnPPIX, 20 µM haemin, 20 µM Fe (II) citrate (Fe), 100 µM ZnSO4 alone, or the combination treatments for 12h, and/or exposed to 0 or 50 µM CdCl2, 50% saturated CO aqueous solution, 20 µM bilirubin (BR), 20 µM Fe alone, or the indicated combination treatments for another 24h or the indicated times. Sample without chemical treatments was used as the control. The pH for both nutrient medium and treatment solutions was adjusted to 6.0 by using NaOH or HCl. After various treatments, the seedlings were sampled, then used immediately or frozen in liquid nitrogen, and stored at –80 °C for further analysis.
Determination of thiobarbituric acid-reactive substances (TBARS), and ASA, DHA, and chlorophyll contents
Lipid peroxidation was estimated by measuring the amount of TBARS as previously described (Han et al., 2008). The contents of ASA and DHA, and chlorophyll a/b were measured according to previous methods (Law et al., 1983; Xie et al., 2012).
Thiol analysis by HPLC
Low molecular weight thiols and their corresponding disulphide contents were measured according to the methods previously reported, with minor modification (Herschbach et al., 2002; Meyer et al., 2007; Queval and Noctor, 2007). Frozen root tissues were ground to a fine powder under liquid nitrogen and then extracted into 1ml of 0.2M HCl. The combined extracts were centrifuged at 13 000 g for 15min at 4 °C. For determination of thiols plus disulphides (cysteine+cysteine disulphide, γ-EC+γ-EC disulphide, GSH+GSSG, and hGSH+hGSSGh), following neutralization, a 0.2ml aliquot of the extract supernatant was mixed with 100 µl of 500mM 2-(N-cyclohexylamino)ethane-sulphonic acid (CHES) buffer (pH 8.5), then 20 µl of 10mM dithiothreitol (DTT) was added and incubated for 30min, followed by the addition of 20 µl of 30mM monobromobimane (mBBr) to derivatize thiols. For determination of disulphides (cysteine disulphide, γ-EC disulphide, GSSG, and hGSSGh), following neutralization, 0.2ml extracts were blocked with N-ethylmaleimide (NEM). After removal of NEM, 100 µl of 500mM CHES was added, followed by reduction of disulphides to thiols with DTT and labelling of thiol groups with mBBr as above. After 15min under dim light, conjugation of thiols with mBBr was completed and 660 µl of 10% (v/v) acetic acid was added to stabilize the mBBr derivatives. After centrifugation at 10 000 g for 10min, the supernatant was filtered through a 0.22 µm filter, and 50 µl of the mixture was subjected to HPLC analysis (Agilent Technologies, 1200 series Quaternary, Foster City, CA, USA). Thiol derivatives were separated on an Agilent Eclipse XDB-C18 column (4.6×250mm, 5 µm) at a flow rate of 0.8ml min–1. The linear gradient was from 0% solution B to 10% (v/v) solution B (90% methanol, 0.25% acetic acid, pH 4.3) within 25min. This composition was maintained for 2min; thereafter the column was washed with 100% solution B for 10min and re-equilibrated with 100% solution A (10% methanol, 0.25% acetic acid, pH 4.3) for 5min. Thiol derivatives were quantified by fluorescence detection (excitation at 380nm, emission at 480nm). A standard solution of 0.1mM cysteine, γ-EC, GSH, and hGSH was used for quantification. Corresponding retention times were 10, 12, 15, and 22min, respectively.
Pyridine nucleotide analyses
NAD and NADP pool sizes and reduction state were measured in acid and alkaline extracts using the protocol described in Wang and Pichersky (2007). The assays involve the phenazine methosulphate-catalysed reduction of thiazolyl blue tetrazolium bromide (MTT) in the presence of ethanol and alcohol dehydrogenase (for NAD and NADH) or glucose-6-phosphate and glucose-6-phosphate dehydrogenase (for NADP and NADPH). Reduced and oxidized forms are distinguished by preferential destruction in acid or base.
Histochemical analyses
Histochemical detection of lipid peroxidation was performed with Schiff’s reagent as described by Pompella et al. (1987). Histochemical detection of loss of plasma membrane integrity in root apexes was performed with Evans blue as described by Yamamoto et al. (2001).
Determination of Cd content in plant tissues
Cd in root tissues was extracted and measured by graphite furnace atomic absorption spectrophotometry (180-80 Hitachi, Tokyo, Japan) as described by Brune and Dietz (1995).
Enzymatic activities assays
HO activity was analysed using the method described in our previous report (Xuan et al., 2008). Frozen alfalfa seedling roots (~200mg) were homogenized in 3ml of 50mM potassium phosphate buffer (pH 7.0) containing 1mM EDTA and 1% polyvinylpyrrolidone (PVP) for superoxide dismutase (SOD) and guaiacol peroxidase (POD) assay, or their combination, with the addition of 1mM ASC in the case of ascorbate peroxidase (APX) assay. SOD and guaiacol POD activities were analysed by the methods described in previous reports (Huang et al., 2006; Liu et al., 2007). APX activity was measured as described by Nakano and Asada (1981). Protein was determined by the method of Bradford (1976).
Native gradient PAGE, and SOD and POD activity staining
Native gradient PAGE (5–20%) was performed for 15h at 4 °C at a constant voltage of 150V in Tris-glycine buffer, pH 8.3. SOD and POD isozymatic activities on the gel were visualized (Beauchamp and Fridovich, 1971; Janda et al., 1999). For the determination of the relative activity of different isozymes, gels were scanned in the transmission black-and-white mode and the intensity of bands was calculated by using the Quantity One v4.4.0 software (Bio-Rad, Hercules, CA, USA). Then the band intensities of the individual isozymes were expressed as a percentage of the control (C) value.
Western blot analysis for MsHO1
Rabbit polyclonal antibody was prepared against the mature MsHO1 (Fu et al., 2011). A 50 µg aliquot of protein from homogenates was subjected to SDS–PAGE using a 12.5% acrylamide resolving gel (Mini Protean II System, Bio-Rad). Finally, the developed films were scanned (Uniscan B700+, Tsinghua Unigroup Ltd, Beijing, China) and analysed by using Quantity One v4.4.0 software (Bio-Rad, USA).
Real-time RT-PCR analysis
Total RNA of root tissues was isolated using the RNeasy mini kit (Qiagen, Valencia, CA, USA). Real-time quantitative reverse transcription-PCRs (RT-PCRs) were performed using a Mastercycler® ep realplex real-time PCR system (Eppendorf, Hamburg, Germany) with SYBR® Pre-mix Ex Taq™ (TaKaRa Bio Inc., China) according to the manufacturer’s instructions (Xie et al., 2012). Using specific primers (Supplementary Table S1 available at JXB online), the expression levels of the genes are presented as values relative to the corresponding control samples under the indicated conditions, with normalization of data to the geometric average of two internal control genes MSC27 and Actin2 (Vandesompele et al., 2002).
Confocal analysis of ROS production
Production of ROS was assayed by confocal microscopy with 20 µM 2',7'-dichlorofluorescin diacetate (H2DCFDA; Calbiochem, La Jolla, CA, USA). Alfalfa seedling roots were loaded with H2DCFDA for 30min before being washed in 20mM HEPES buffer (pH 7.8) three times for 15min (Mazel et al., 2004; Leshem et al., 2007). All images were obtained by a TCS-SP2 confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg, Germany; with excitation at 488nm, and emission at 500–530nm). All manipulations were performed at 25±1 ºC. Production of ROS in root tips was quantified with the Leica software package.
Statistical analysis
Values are shown as the means ±SE of three independent experiments with at least three replicates for each. Differences among treatments were analysed by one-way analysis of variance (ANOVA) combined with Duncan’s multiple range test, taking P < 0.05 as the thresholds.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers HM212768, DQ122791, AY054988, AM407888, AM411123, AM411122, AM407889, AM407890, JN979555, X63872, and JQ028730.
Results
Lipid peroxidation
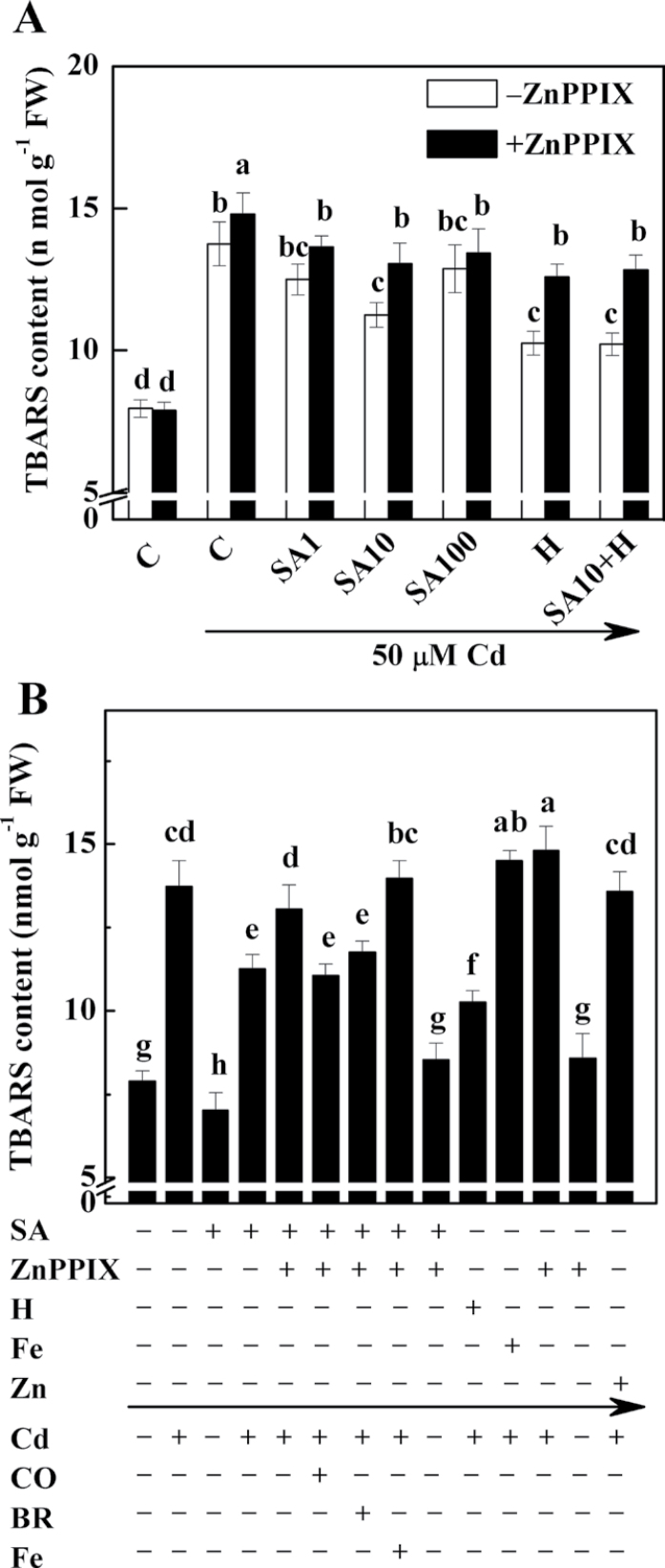
Figure 1A shows that the content of TBARS decreased by 9.0% and 18.1% in seedlings pre-treated with 1 µM and 10 µM SA, respectively, before exposure to 50 µM CdCl2, compared with samples treated with CdCl2 alone. However, treatments with SA concentrations up to 100 µM resulted in a slight decrease but no significant effect on lipid peroxidation. Therefore, 10 µM SA was used in the following experiment.
Fig. 1.
Effects of salicylic acid (SA), ZnPPIX, haemin, Fe (II) citrate, and ZnSO4 pre-treatment on the concentration of thiobarbituric acid-reactive substance (TBARS) in alfalfa seedling roots subjected to 50 µM CdCl2. Five-day-old seedlings were pre-treated or not with 1, 10, or 100 µM SA (SA1, SA10, and SA100; A), 10 µM SA (SA; B), 20 µM haemin (H), 20 µM Fe (II) citrate (Fe), 100 µM ZnSO4 (Zn) alone, or the combination of treatments, in the presence (+) or absence (–) of 100 µM ZnPPIX for 12h, and then exposed to 50 µM CdCl2 with or without 50% saturated aqueous CO solution (CO), 20 µM bilirubin (BR), or 20 µM Fe (II) citrate (Fe) for another 24h. The sample without chemicals was the control (C). Values are means ±SE of three independent experiments with at least three replicates for each. Bars with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test.
Previous results suggested that ZnPPIX, a potent inhibitor of HO-1, might be another substrate for methylation by S-adenosyl-l-methionine:magnesium protoporphyrin IX methyltransferase (MgPMT) in the synthesis of chlorophyll (Gibson et al., 1963). However, the pilot experiment showed that at least under the research conditions used here, pre-treatment with 100 µM ZnPPIX failed to influence the chlorophyll content in alfalfa seedlings significantly regardless of whether Cd was added or not (Supplementary Fig. S1 at JXB online). Similarly (Noriega et al., 2004), further results in this study confirmed that ZnPPIX is a potent inhibitor of MsHO1 (Supplementary Fig. S2). To investigate the physiological role of HO-1 in plant responses to Cd exposure, the changes in content of TBARS in seedlings simultaneously pre-treated with SA and ZnPPIX were investigated (Fig. 1A). As expected, treatment with ZnPPIX significantly increased the content of TBARS (9.2, 16.0, and 4.2%) compared with the values produced by treatment with SA at concentrations of 1, 10, and 100 µM. Meanwhile, seedlings pre-treated with haemin, an HO-1 inducer (Xuan et al., 2008), also showed progressive reductions in the content of TBARS (data not shown), with the maximal response at 20 µM haemin. This response was reversed by the addition of ZnPPIX (22.7%; Fig. 1A). Interestingly, these values, conferred by SA or haemin plus ZnPPIX, were equivalent to the control sample followed by Cd treatment alone (without ZnPPIX pre-treatment), indicating the possible protective role of HO-1. However, no significant additive effects of haemin (20 µM) plus SA (10µM) were observed in the presence or absence of ZnPPIX.
Histochemical staining
Histochemical staining showed that, compared with the Cd-free control sample, root tips of alfalfa plants treated with Cd alone stained extensively with Schiff’s reagent and Evans blue (Supplementary Fig. S3 at JXB online), and stained more strongly following pre-treatment with ZnPPIX. In contrast, root tips pre-treated with SA or haemin regardless of whether they were treated with Cd later (in particular) or not showed only light staining, which was differentially reversed when ZnPPIX was added together with SA or haemin. Combined with the results on the contents of TBARS (Fig. 1), these results further support the hypothesis that the SA- and haemin-induced cytoprotective effects were HO-1 specific.
SA-induced cytoprotective response is HO-1-specific
If HO-1 was really involved in the SA-mediated cytoprotective response, feeding plants with exogenous CO or BR (two catalytic by-products of HO-1) might, at least partially, block the increase of TBARS caused by SA plus ZnPPIX followed by the addition of Cd. As expected, the increase in the content of TBARS was considerably reduced in an aqueous 50% saturated solution of CO or BR (Fig. 1B). Under similar conditions, however, there was an increase in the content of TBARS when Fe (as a control for haemin decomposition) was added. It was also found that adding Fe or ZnSO4 (as a control for ZnPPIX decomposition) followed by Cd stress could not alleviate the TBARS overproduction caused by Cd stress alone.
Similarly, when CO was applied together with Cd, the heavy staining of lipid peroxidation and the loss of plasma membrane integrity in the roots of alfalfa seedlings pre-treated with SA plus ZnPPIX were relieved (Supplementary Fig. S3 at JXB online). Simultaneously added BR produced a modest alleviation response, in comparison with the addition of Fe. The addition of CO, BR, or Fe alone, however, did not change the staining pattern with respect to the chemical-free control samples. The above results further confirmed that the effect of SA on the alleviation of Cd-induced oxidative stress is mediated specifically by HO-1.
Cd toxicity
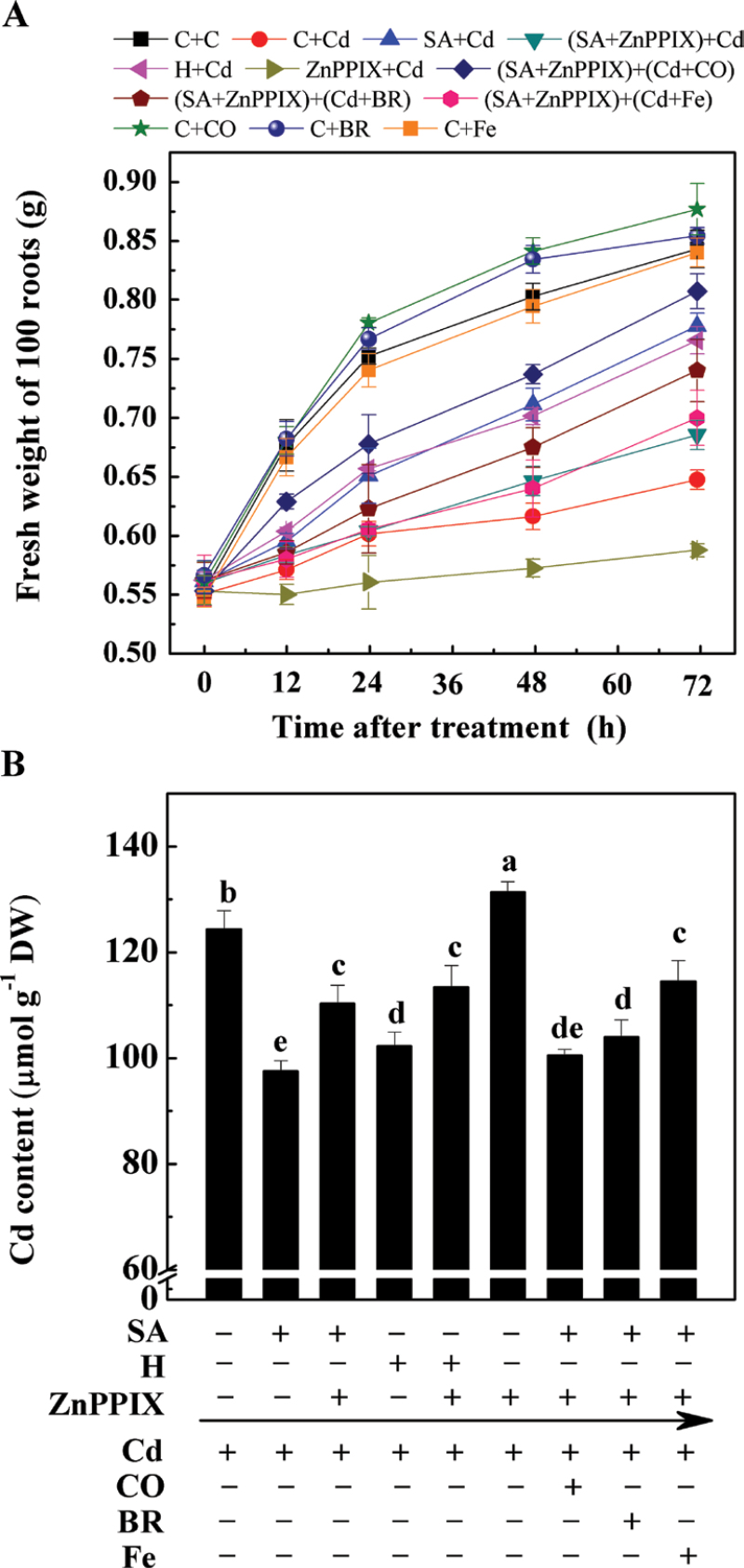
In the following experiment, it was discovered that plants treated with Cd at an external concentration of 50 µM exhibited a time-dependent tendency to a decrease in the fresh weight of 100 seedling roots compared with Cd-free control samples (Fig. 2A). The improved root growth with SA or haemin pre-treatment was also shown to be time dependent. However, it was noticed that during the whole treatment period, the reduction effects on root growth of the combination of SA and ZnPPIX were stronger than those of the SA treatment alone. Meanwhile, an obvious decrease in seedling root growth appeared in the sample pre-treated with ZnPPIX followed by Cd exposure, as compared with the Cd-stressed alone sample. When a 50% saturated aqueous solution of CO (in particular) or BR (modestly) was separately applied together with Cd, the inhibition of seedling root growth caused by the combination of SA and ZnPPIX pre-treatment was differentially alleviated. However, no alleviation role of Fe was observed. It was also noticed that the application of CO (in particular) or BR alone brought about the stimulation of seedling growth.
Fig. 2.
Effects of salicylic acid (SA), ZnPPIX, and haemin (H) pre-treatment on the CdCl2-induced inhibition of 100 seedling roots (A) and Cd contents (B) in root tissues of alfalfa plants. Five-day-old seedlings were pre-treated or not with 10 µM SA, 20 µM haemin, 100 µM ZnPPIX alone, or the combination treatments for 12h, and then exposed to 50 µM CdCl2, 20 µM Fe (II) citrate (Fe), 50% saturated aqueous CO solution (CO), 20 µM bilirubin (BR) alone, or the combination treatments for another 72h. The sample without chemicals was the control (C). Values are means ±SE of three independent experiments with at least three replicates for each. Bars with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test.
The Cd content in plant seedling roots was simultaneously investigated (Fig. 2B). The uptake of Cd in the SA- and haemin-pre-treated roots exhibited a significant tendency to decrease in comparison with samples subjected to a Cd stress alone. In contrast, the combination of the HO-1 potent inhibitor ZnPPIX blocked the above responses. It was also noticed that the addition of CO (in particular) or BR together with Cd could obviously reverse the above ZnPPIX response, although no significant difference was observed in an Fe-treated sample. Interestingly, a significant increase of Cd accumulation was observed in the sample pre-treated with ZnPPIX followed by the addition of Cd, in comparison with the sample subjected to Cd stress alone. Taken together, the above results strongly indicated the involvement of HO-1 in the SA-mediated modulation of Cd toxicity to root growth.
SA- or haemin-induced MsHO1 expression issensitive to ZnPPIX
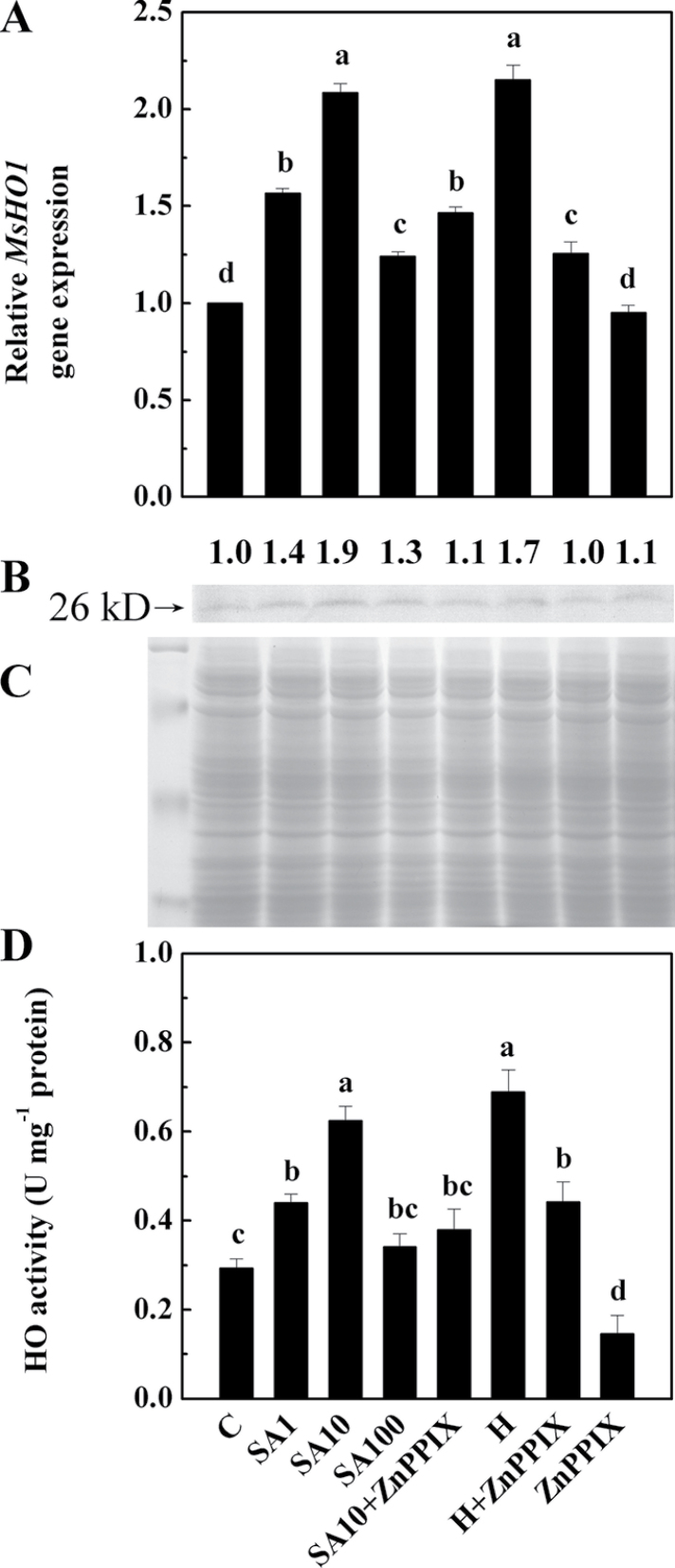
To confirm further whether alfalfa HO-1 (MsHO1; Fu et al., 2011) is associated with the above SA responses, a detailed study of the SA-induced expression of this enzyme was undertaken. Further results revealed that when treated with 1, 10, and 100 µM SA, induction of the MsHO1 gene and its corresponding protein levels peaked at 10 µM SA (Fig. 3A–C). Enzyme activity analysis (Fig. 3D) revealed a similar tendency. Furthermore, the induction of MsHO1 expression, protein level, and HO activity by 10 µM SA or 20 µM haemin was clearly inhibited by ZnPPIX. Additionally, an obvious decrease in HO activity was observed when ZnPPIX was added alone.
Fig. 3.
Comparisons of MsHO1 transcripts (A), HO-1 protein levels (B and C), and HO activity (D) in alfalfa seedling roots. Five-day-old seedlings were treated or not with 1, 10, and 100 µM SA (SA1, SA10, and SA100), 20 µM haemin (H), 100 µM ZnPPIX alone, or the combination treatments for 12h. The sample without chemicals was the control (C). The transcript quantification test was carried out after 12h of treatment, normalized against expression of two internal reference genes in each sample (A). HO-1 protein expression was analysed by western blotting (B). The number above the band indicates the relative abundance of the corresponding MsHO1 protein compared with that of the control sample. Coomassie Brilliant Blue-stained gels were present to show that equal amounts of proteins were loaded (C). HO activity was also determined (D). Three independent experiments were performed, and the results showed similar trends.
Antioxidant enzyme activities and their transcripts
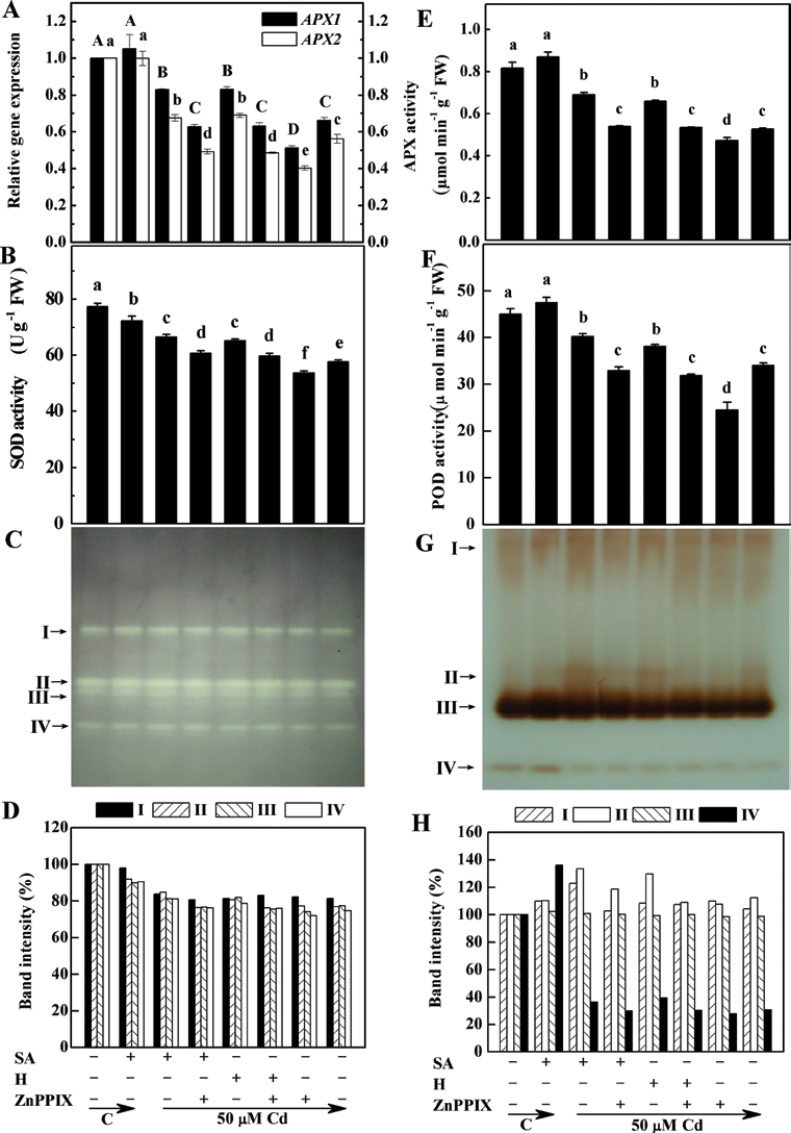
In order to determine whether there is a link between the HO-1 and SA-induced alleviation of oxidative stress, the changes in several antioxidant enzyme activities and corresponding transcripts in alfalfa seedling roots were investigated. Using real-time RT-PCR, it was shown that pre-treatment with 100 µM ZnPPIX differentially blocked the SA-induced enhancement of the transcription levels of the APX1/2 genes, and the total activity of APX in Cd-stressed plants (Fig. 4A, 4E). A similar inhibition was observed in samples pre-treated with ZnPPIX, in comparison with samples subjected to Cd stress alone. Similarly, compared with the samples treated with Cd alone, haemin caused increases in the transcription levels (Fig. 4A) and the total activity of APX (Fig. 4E), and these increases were substantially reduced by pre-treatment with ZnPPIX. These results clearly indicated that HO-1 itself could induce the up-regulation of APX in plants.
Fig. 4.
Effects of salicylic acid (SA), ZnPPIX, and haemin (H) pre-treatment on the expression and activities of ascorbate peroxidase (APX), and total and isozyme activities of superoxide dismutase (SOD) and guaiacol peroxidase (POD) in the root tissues of alfalfa upon Cd stress. Five-day-old seedlings were pre-treated or not with 10 µM SA, 20 µM haemin (H), 100 µM ZnPPIX alone, or the combination treatments for 12h, and then exposed to 50 µM CdCl2 for another 24h. The sample without chemicals was the control (C). Then, the APX1/2 transcript quantification test was carried out, normalized against expression of two internal reference genes in each sample (A). SOD (B), APX (E), and POD (F) activity was also determined. Values are means ±SE of three independent experiments with at least three replicates for each. Bars with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test. For the determination of the in-gel activity of SOD (C) and POD isozymes (G), extracts of root apices containing 100 µg of protein were loaded onto native gradient PAGE (5–20%) and, following electrophoresis, the gels were stained. Relative activities of different SOD and POD isozymes are also shown in (D) and (H), respectively. Band intensities of the individual isozymes are expressed as a percentage of the control values. The arrows indicate the bands corresponding to various isozymes.
Analysis of another two antioxidant enzymes revealed that after 24h of exposure to Cd, the activity levels of SOD and POD in alfalfa seedling roots fell to 25.5% and 24.5% lower than the control sample, respectively (Fig. 4B, 4F). In contrast, SA and haemin pre-treatment resulted in significantly increased SOD and POD activities, being 15.4% and 13.1% (SOD), 18.1% and 11.9% (POD) higher, respectively, than the sample treated with Cd alone. Furthermore, when the HO-1 inhibitor ZnPPIX was also added, the above observed effects were significantly reversed (P < 0.05). Four clear SOD isozymes were detected in the root tissues (Fig. 4C, 4D). Among these, only the SOD-I isozyme was Mn-SOD (as confirmed by the inhibitor test, data not shown; located in the mitochondria and peroxisome), and the rest of the isozymes belonged to the Cu/Zn-SODs (located in the cytosol). Treatment with Cd generally resulted in a decrease in band size. However, the decrease in the amount of SOD isozymes could be partially reversed by pre-treatment with SA and haemin. Surprisingly, slight decreases of SOD isozyme activities were observed when ZnPPIX was added together with SA or haemin (except for the SOD-I isozyme in the sample treated with haemin). Testing another H2O2-scavening enzyme, POD, four bands of isozymes could be detected, and the POD-III isozyme contributed the most activity (Fig. 4G, 4H). Among these, the increase in POD-II isozyme activity due to the SA and haemin pre-treatment was most clearly observed in the gel. Pre-treatment of seedlings with ZnPPIX inhibited the accumulation of this isozyme.
Re-establishing redox homeostasis
It was well known that alfalfa plants contained more hGSH than GSH (Cruz de Carvalho et al., 2010). An experiment using HPLC with fluorescence detection showed that under normal growth conditions, the major glutathione pool obtained from alfalfa seedling roots was hGSH (Table 1); the concentration of hGSH is ~8-fold that of GSH, similar to findings of a previous report (Ortega-Villasante et al., 2007). By using the HPLC assay, it was observed that treatment with Cd for 24h significantly reduced the content of hGSH and increased hGSSGh and GSH contents (GSSG was not detected) in alfalfa seedling roots. However, pre-treatment with SA or haemin reduced or significantly eliminated the effects of Cd treatment alone on the changes of hGSH and hGSSGh. When SA or haemin was added together with the potent HO-1 inhibitor, ZnPPIX, before treatment with Cd, the changes in the content of hGSH and hGSSGh, induced by SA or haemin, were totally prevented. Similarly, a high ratio of hGSH/hGSSGh was also observed in the sample pre-treated with SA or haemin, compared with the sample treated with Cd alone. Furthermore, treatment with ZnPPIX before Cd exposure obviously enhanced the hGSSGh content and decreased the GSH and hGSH contents, leading to a decreased hGSH/hGSSGh ratio. Additionally, the application of SA alone increased the GSH and hGSH contents and the hGSH/hGSSGh ratio. Similar tendencies were also observed in the responses of the ascorbic acid pool (ASA and DHA). These results were consistent with changes in Cd toxicity (Fig. 2) and lipid peroxidation (Fig 1; Supplementary Fig. S3 at JXB online). Comparatively, similar to the responses of GSH, Cd-induced cysteine and γ-EC contents were strengthened by the addition of SA or haemin, which was reversed by ZnPPIX, respectively.
Table 1.
Cysteine (cysteine and cysteine disulphide), γ-EC (γ-EC and γ-EC disulphide), glutathione (GSH and GSSG), homoglutathione (hGSH and hGSSGh), and ascorbic acid (ASA and DHA), and the hGSH/hGSSGh and ASA/DHA ratios in alfalfa seedling roots. Five-day-old seedlings were treated with 0 or 50 µM CdCl2 for 24h with or without 12h pre-treatment with 10 µM SA, 20 µM haemin (H), 100 µM ZnPPIX alone, or their combination treatments. The sample without chemicals was the control (C).
| Treatment | Cysteine (nmol g–1 FW) | Cysteine disulphide (nmol g–1 FW) | γ-EC(nmol g–1 FW) | γ-EC disulphide (nmol g–1 FW) | GSHa(nmol g–1 FW) | hGSH (nmol g–1 FW) | hGSSGh (nmol g–1 FW) | hGSH/hGSSGh | ASA(nmol g–1 FW) | DHA (nmol g–1 FW) | ASA/ DHA |
| C→C | 52±1 c | 2±1 b,c | 15±2 d | 1±0 a,b | 35±4 d | 282±6 a | 60±4 d | 4.69 | 319±7 c | 95±8 d | 3.36 |
| SA→C | 55±3 b,c | 3±0 a,b | 14±2 d | 0±0 b | 42±1 c,d | 310±12 a | 57±7 d | 5.44 | 587±7 a | 68±7 e | 8.63 |
| C→Cd | 63±3 b | 1±0 c | 39±6 b | 2±1 a,b | 60±10 b | 96±7 c | 112±11 b | 0.86 | 294±4 c,d | 222±6 b | 1.32 |
| SA→Cd | 77±1 a | 2±0 b,c | 49±1 a | 2±1 ab | 79±7 a | 142±24 b | 76±3 c | 1.88 | 452±6 b | 177±9 c | 2.55 |
| SA+ZnPPIX→Cd | 65±3 b | 4±0 a | 35±3 b,c | 3±2 a,b | 59±3 b | 86±29 c | 111±5 b | 0.77 | 283±4 d | 291±8 a | 0.97 |
| H→Cd | 76±1 a | 1±0 c | 49±3 a | 2±1 a,b | 79±8 a | 155±17 b | 83±13 c | 1.87 | 426±9 b | 208±10 b | 2.05 |
| H+ZnPPIX→Cd | 63±5 b | 3±0 a,b | 34±3 b,c | 2±1 a,b | 58±5 b | 96±14 c | 106±10 b | 0.91 | 291±6 c,d | 294±8 a | 0.99 |
| ZnPPIX→Cd | 58±4 b,c | 2±0 b,c | 29±2 c | 3±1 a | 52±7 b,c | 49±23 d | 128±8 a | 0.39 | 230±4 e | 270±14 a,b | 0.85 |
Values are means ±SE of three independent experiments. Different letters within columns indicate significant differences (P < 0.05) according to Duncan’s multiple range test.
aGSSG was not detected.
A wealth of evidence has accumulated that NAD (including NAD+ and NADH) and NADP (including NADP+ and NADPH) may be among the fundamental common mediators of various biological processes, including energy metabolism, antioxidation, and the generation of oxidative stress (Ying, 2008). The present results showed that, consistent with the responses of hGSH/hGSSGh and ASA/DHA, Cd caused a consumption of reduced NADPH, but a significant increase in NADP+, as evidenced by the observed decrease in NADPH/NADP+ (Supplementary Table S2 at JXB online). Meanwhile, the NADH/NAD+ ratio was also decreased, which was caused mainly by the increases in the NAD+ content, consistent with previous findings (Iturbe-Ormaetxe et al., 1998). In contrast, pre-treatment with either SA or haemin could block these tendencies, both of which were significantly reversed when ZnPPIX was added as well. It was also observed that pre-treatment with ZnPPIX alone led to a significantly decreased NAD(P)H/NAD(P)+ ratio.
Since it was possible that the observed re-establishment of redox homeostasis was caused by the modulation of ROS, the distribution of ROS in the root apexes was investigated. Supplementary Fig. S4 at JXB online shows that the ROS level increased 2.76-fold following treatment with 50 µM Cd. Conversely, pre-treatment with SA or haemin inhibited ROS formation, which was blocked by the combination with ZnPPIX pre-treatment. It was also observed that SA applied alone produced an increase in ROS distribution compared with the control sample.
Gene expression analysis of GSH/hGSH and ASA pools
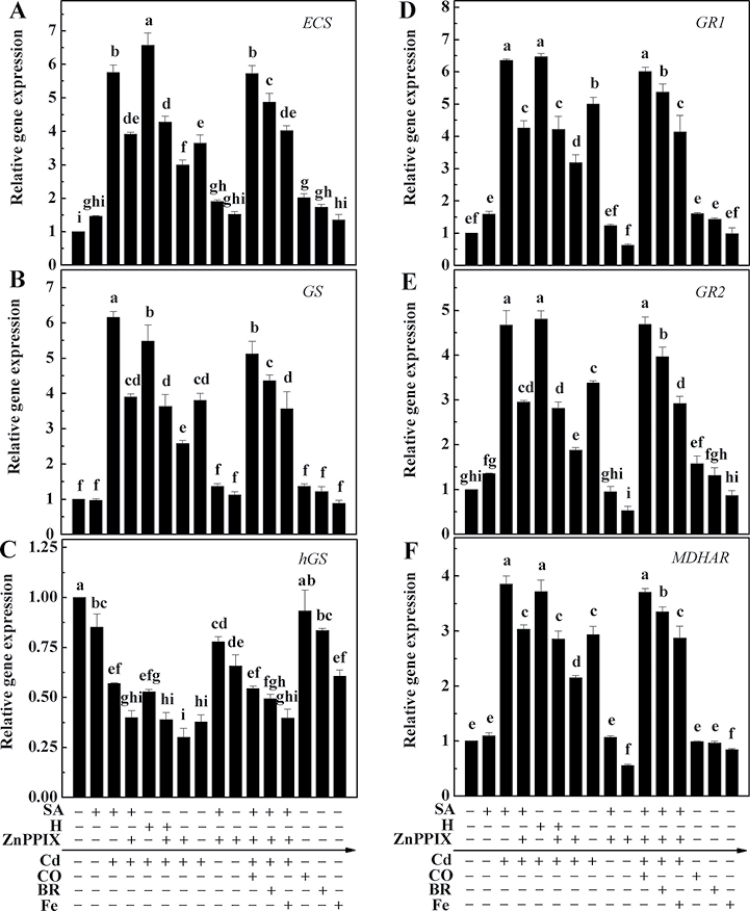
As there were changes in the pools of GSH/hGSH and ASA after Cd exposure, the expression of genes involved in their synthesis and/or metabolism, namely ECS, GS, hGS, GR1, GR2, and MDHAR, was analysed. Cd produced a clear induction of ECS and GS transcripts, which were strengthened by the pre-treatment with SA or haemin, and further blocked by simultaneously added ZnPPIX (Fig. 5A, 5B). Changes in GR1, GR2, and MDHAR exhibited similar tendencies (Fig. 5D–F). Upon Cd exposure, however, the expression of hGS declined significantly, compared with Cd-free control samples. Moreover, in the presence of SA or haemin, hGS expression was elevated, and this was clearly blocked by the addition of ZnPPIX (Fig. 5C). Further results confirmed different restoration effects of CO (in particular) and BR on the ZnPPIX-induced inhibition of the expression of above genes, which were consistent with the partial reversal of oxidative damage and Cd toxicity (Figs 1, 2; Supplementary Fig. S3 at JXB online). However, the combination of Fe, as well as the CO, BR, or Fe alone treatments brought about weaker responses. Significant effects in the down-regulation of hGS and MDHAR transcripts were also observed when ZnPPIX was applied alone.
Fig. 5.
Effects of salicylic acid (SA), ZnPPIX, haemin (H), CO, bilirubin (BR), and Fe (II) citrate (Fe) on gene expression in the root tissues of alfalfa seedling upon Cd stress. Five-day-old seedlings were pre-treated or not with 10 µM SA, 20 µM haemin (H), 100 µM ZnPPIX alone, or the combination treatments for 12h, and then exposed to 50 µM CdCl2, 50% saturated aqueous CO solution (CO), 20 µM bilirubin (BR), 20 µM Fe (II) citrate (Fe), or the combination treatments for another 24h. Then, the transcript levels of ECS (A), GS (B), hGS (C), GR1 (D), GR2 (E), and MDHAR (F) were analysed by real-time RT-PCR. Expression levels of genes are presented relative to the control samples, normalized against expression of two internal reference genes in each sample. Date are the means ±SE of at least three independent experiments. Within each set of experiments, bars with different letters are significantly different at P < 0.05 according to Duncan’s multiple range test.
Discussion
The beneficial effect of HO-1 on SA-induced alleviation of oxidative stress and Cd toxicity caused by Cd stress
This study confirms that SA arrested Cd-induced oxidative stress and toxicity in alfalfa seedling roots (Figs 1, 2; Supplementary Fig. S3 at JXB online), which is in agreement with the observations that SA alleviates Cd toxicity in barley (Metwally et al., 2003), cucumber (Shi and Zhu, 2008), pea (Popova et al., 2009), and rice seedlings (Mishra and Choudhuri, 1999; Guo et al., 2007), but contrasts with the negative role of SA observed in Arabidopsis (Zawoznik et al., 2007).
Further data support a linear signal transduction cascade involving up-regulation of MsHO1 downstream of the SA responses. First, exogenous application of haemin, an HO-1 inducer, confers a similar cytoprotective role to SA in the alleviation of oxidative stress and Cd toxicity (Figs 1, 2; Supplementary Fig. S3 at JXB online). Treatment with SA and haemin trigger MsHO1 gene expression, at the translational, transcriptional, and enzymatic levels (Fig. 3). In animals, ample evidence has illustrated that HO-1 is highly induced by a variety of agents or stimuli causing oxidative stress, such as H2O2, GSH sdepletors, UV irradiation, and hyperoxia (Ryter et al., 2002). Following these stimuli, the induction of HO activity by de novo enzyme synthesis is normally associated with an increase in HO-1 mRNA and corresponding protein levels. The maximal induction of MsHO1 expression conferred by 10 µM SA (Fig. 3) also matches its cytoprotective performance in the alleviation of Cd-induced overproduction of TBARS (Fig. 1). The present findings are consistent with those reported by Grosser et al. (2003) and Oberle et al. (2002), that HO-1 amplifies the therapeutic effects of certain stimuli in animals, such as aspirin and pentaerythrityl trinitrate (PETN), a long-acting NO donor.
Subsequent experiments showed that the potent HO-1 inhibitor ZnPPIX (Supplementary Fig. S2 at JXB online) could block responses of SA and haemin in the induction of MSHO1 gene expression (Fig. 3), alleviating overproduction of TBARS (Fig. 1) and oxidative stress (Supplementary Fig. S3 at JXB online), as well as lowering the Cd toxicity (Fig. 2). These observations confirmed that the SA- and haemin-induced cytoprotective effects were MsHO1 specific.
In fact, the above cytoprotective activities of HO-1 might be due to the catalytic products of its enzymatic reactions (Noriega et al., 2004; Shekhawat and Verma, 2010). For example, previous work (Han et al., 2008) showed that exposure to Cd induced the production of endogenous CO in alfalfa seedling roots, consistent with the changes in HO-1 gene expression, and that CO pre-treatment decreased the Cd-dependent oxidative stress, mainly via the modulation of enzymes associated with GSH metabolism. In addition, BV exhibits antioxidant and cytoprotective effects that may enhance the HO-1 responses in animals and plants (Piantadosi 2002; Dulak and Józkowicz, 2003; Noriega et al., 2004; Matsumoto et al., 2006). Interestingly, in the experimental conditions used here, it was also observed that the increase in the content of TBARS conferred by ZnPPIX plus SA or haemin followed by exposure to Cd could be differentially reversed when CO or BR was added (Fig. 1). Similar cytoprotective responses were observed in the histochemical staining for the detection of peroxidation of lipids and injury of plasma membrane integrity in root apexes (Supplementary Fig. S3 at JXB online). The addition of CO (in particular) and BR could differentially reverse the seedling root growth inhibition and Cd accumulation in SA plus ZnPPIX-pre-treated alfalfa plants (Fig. 2). However, treatment with Fe2+ failed to alleviate the inhibition of seedling root growth. These various pieces of pharmacological evidence therefore support the idea that SA and up-regulation of MsHO1 might be on a linear signalling pathway in the process of the alleviation of oxidative stress and Cd toxicity.
Redox state homeostasis is involved in HO-1-mediated responses
In plants, HO-1/CO is associated with antioxidant processes when subjected to various abiotic stresses, including salinity stress (Xie et al., 2011), UV-B radiation (Yannarelli et al., 2006), and Cd toxicity (Noriega et al., 2004; Han et al., 2008). Exposure to Cd induced a reduction in the amounts of GSH and ASC, as well as the activities of catalase (CAT), GSH reductase (GR), and POD (Rodríguez-Serrano et al., 2006). In this report, it was further shown that the up-regulation of MsHO1 driven by SA pre-treatment is an early event in the stimulation of antioxidative enzyme expression, which simultaneously alleviated Cd-induced lipid peroxidation and toxicity. Subsequent data support the establishment of redox homeostasis downstream of MsHO1-mediated responses. The growth of alfalfa plants pre-treated with SA and haemin clearly increased when compared with samples treated with Cd alone (Fig. 2A), and they suffered considerably less Cd-induced oxidative injury (Fig. 1; Supplementary Fig. S3 at JXB online). Further experiments confirmed that this was due to induced activation of the antioxidant detoxifying enzymes APX, SOD, and POD, including total or isozymic activities, or the corresponding transcripts (Fig. 4). These increased enzymatic activities resulted in partial prevention of oxidative injury to membranes (Fig. 1) in root tissues. These effects were confirmed by the histochemical staining for the detection of peroxidation of lipids and injury of plasma membrane integrity (Supplementary Fig. S3) as well as the ROS distribution (Supplementary Fig. S4) in root apexes. Moreover, the protective roles of SA and haemin in the activation of antioxidant detoxifying enzymes and the distribution of ROS were suppressed differentially by the potent HO-1 inhibitor ZnPPIX. These data further support the hypothesis that MsHO1 up-regulation may mediate SA-induced antioxidant behaviours as well as the alleviation of Cd toxicity (Fig. 2).
In higher plants, it is well known that Cd toxicity is mediated by oxidative stress, and that Cd not only inhibits plant growth, but also affects GSH and ASA metabolism. Redox buffering in the apoplasts protects the plasmalemma from oxidation (Foyer et al., 2001). It is known that reduced GSH levels play a central role in protecting plants from environmental stresses, including oxidative stress or toxicity from exposure to certain heavy metals (Xiang and Oliver, 1998; Sharma and Dietz, 2009). For example, in a comparison of 10 pea genotypes showing differing Cd sensitivity, the GSH level and the GSH/GSSG ratio were inversely linked to Cd sensitivity (Metwally et al., 2005). In alfalfa and soybean plants, a GSH homologue, hGSH, is also abundantly present instead of, or in addition to, GSH (Matamoros et al., 1999). As expected, the quantification of GSH and hGSH pools by HPLC methods (Table 1) showed that hGSH was significantly more abundant than GSH in alfalfa seedling roots under normal growth conditions. It was also suggested that GSH and hGSH play a major role in plant development and plant adaptation to biotic and abiotic stresses (Baldacci-Cresp et al., 2012). Most importantly, many of the roles ascribed to GSH are also performed by hGSH, particularly the control of the cellular redox status and ROS scavenging (Dalton et al., 1986). In this study, a relationship between hGSSGh accumulation and the amount of oxidative stress has been demonstrated, as the serious oxidation of hGSH (Table 1) was coincident with the accumulation of H2O2 (Supplementary Fig. S4 at JXB online) as well as the decreased APX1/2 transcripts and corresponding activity (Fig. 4A, 4E) in Cd-treated alfalfa seedling roots. Previously, it was shown that the enhancement of reduced GSH concentrations and high GSH/GSSG ratios might provide some explanation for the cytoprotective role of CO in mediating Cd-induced oxidative stress in alfalfa root tissues (Han et al., 2008). Similar responses of hGSH/hGSSGh and ASA homeostasis to SA and haemin were observed (Table 1). In contrast, both SA- and haemin-induced restoration of hGSH/hGSSGh and ASA/DHA was obviously blocked by the addition of ZnPPIX (Table 1), in agreement with the reversed effects on the inhibition of lipid peroxidation (Fig 1; Supplementary Fig. S3), the enhancement of antioxidant enzyme expression (Fig. 4), and the decreased ROS distribution (Supplementary Fig. S4). Changes in the expression of the genes involved in GSH, hGSH, and ASA synthesis and/or metabolism, ECS, GS, hGS, GR1, GR2, and MDHAR (Fig. 5), were correlated with the parameters of GSH/hGSH and ASA pools (Table 1). These results may provide an explanation for the cytoprotective role of MsHO1 in mediating SA-induced alleviation of Cd toxicity and corresponding oxidative stress in plant tissues.
Although a significant increase in reduced GSH content was observed in Cd-treated samples, it was also noticed that the total level of GSH, hGSH, and hGSSGh was lower in Cd-treated seedlings in comparison with the control sample (Table 1). The observed phenomenon of enhanced reduced GSH content could be partially explained by the induction of GSH synthesis genes (ECS and GS) in Cd-stressed seedlings (Fig. 5A, 5B). In view of the fact that the major glutathione pool obtained from alfalfa seedling roots was hGSH, the obvious down-regulation of hGS transcripts (Fig. 5C), the important synthetic gene responsible for the synthesis of the hGSH pool, could be used to explain the decrease in total levels of GSH, hGSH, and hGSSh, which might be the result of the increase in synthesis of phytochelatins (PCs) in Cd-stressed plants (Zhu et al., 1999).
hGSH/hGSSGh and ASA/DHA are also regarded as important redox couples through their interaction with NAD(P)H/NAD(P)+, providing the conditions to support mitochondrial oxidative phosphorylation, generation of ATP, and hence key anabolic activities (May et al., 1998). It has also been suggested that tolerance to metal toxicity is more dependent on the availability of reduced cell metabolites, such as NAD(P)H, than on the antioxidant enzyme capacity of plant tissues (León et al., 2002). It is further suggested that the higher NAD(P)H/NAD(P)+ ratios conferred by SA and haemin pre-treatment (Supplementary Table S2 at JXB online) could favour the functionality of the ascorbate–glutathione cycle, and this is confirmed by the inducible responses of APX1/2, GR1/2, and MDHAR expression and APX activities (Figs 4, 5). The reversal response of the NAD(P)H/NAD(P)+ ratio triggered by the HO-1 inhibitor ZnPPIX further confirms that the SA- and haemin-induced cytoprotective effects are HO-1 specific.
In conclusion, the data for the first time showed the up-regulation of MsHO1 involving the SA-induced amelioration of Cd-induced toxicity and oxidative stress in the root tissues of alfalfa seedlings, and provide additional information on important aspects of SA signalling functions in plants. The significant alteration of antioxidant enzyme expression, the hGSH/hGSSGh, ASA/DHA, and NAD(P)H/NAD(P)+ ratios observed here, and their previously demonstrated induction by SA (Mishra and Choudhuri, 1999; Metwally et al., 2003; Shi and Zhu, 2008; Clemente et al., 2012), confirm the involvment of MsHO1 in SA-induced cytoprotection against Cd toxicity and its part in these interrelated events.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Effects of SA and ZnPPIX on the chlorophyll content of alfalfa seedling leaves upon Cd stress.
Figure S2. Effects of ZnPPIX on the activity of purified MsHO1 protein.
Figure S3. Effects of salicylic acid (SA), ZnPPIX, and haemin (H) pre-treatment on CdCl2-induced lipid peroxidation (A) and the loss of plasma membrane integrity (B) in the root tips of alfalfa (Medicago sativa).
Figure S4. Confocal images of ROS production in root tips of alfalfa (Medicago sativa).
Table S1. The sequences of primers for real-time RT-PCR.
Table S2. Reduced and oxidized nicotinamide (NADH and NAD+, NADPH and NADP+), and the ratio of NADH/NAD+ and NADPH/NADP+ in alfalfa seedling roots.
Supplementary Material
Acknowledgements
This work was supported by a grant obtained from the National Natural Science Foundation of China (grant no. 30971711) and the Fundamental Research Funds for the Central Universities (grant no. KYZ200905).
References
- Baldacci-Cresp F, Chang C, Maucourt M, et al. (Homo)glutathione deficiency impairs root-knot nematode development in Medicago truncatula . PLoS Pathogens. 2012;8:e1002471. doi: 10.1371/journal.ppat.1002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Analytical Biochemistry 44 276–287 [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72 248–254 [DOI] [PubMed] [Google Scholar]
- Brune A, Dietz KJ. 1995. A comparative analysis of element composition of barley roots and leaves under cadmium-, molybdenum-, nickel- and zinc-stress. Journal of Plant Nutrition 18 853–868 [Google Scholar]
- Brunetti P, Zanella L, Proia A, De Paolis A, Falasca G, Altamura MM, Sanità di Toppi L, Costantino P, Cardarelli M. 2011. Cadmium tolerance and phytochelatin content of Arabidopsis seedlings over-expressing the phytochelatin synthase gene AtPCS1. Journal of Experimental Botany 62 5509–5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Geng B, Xu S, Xuan W, Nie L, Shen W, Liang Y, Guan R. 2011. BnHO1, a haem oxygenase-1 gene from Brassica napus, is required for salinity and osmotic stress-induced lateral root formation Journal of Experimental Botany 62 4675–4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Ricigliano JW, Klessig DF. 1993. Purification and characterization of a soluble salicylic acid-binding protein from tobacco. Proceedings of the National Academy of Sciences, USA 90 9533–9537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente MR, Bustos-Sanmamed P, Loscos J, James EK, Pérez-Rontomé C, Navascués J, Gay M, Becana M. 2012. Thiol synthetases of legumes: immunogold localization and differential gene regulation by phytohormones. Journal of Experimental Botany 63 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho MH, Brunet J, Bazin J, Kranner I, d’ Arcy-Lameta A, Zuily-Fodil Y, Contour-Ansel D. 2010. Homoglutathione synthetase and glutathione synthetase in drought-stressed cowpea leaves: expression patterns and accumulation of low-molecular-weight thiols. Journal of Plant Physiology 167 480–487 [DOI] [PubMed] [Google Scholar]
- Dalton DA, Russell SA, Hanus FJ, Pascoe GA, Evans HJ. 1986. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proceedings of the National Academy of Sciences, USA 83 3811–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, et al. 1994. A central role of salicylic acid in plant disease resistance. Science 266 1247–1250 [DOI] [PubMed] [Google Scholar]
- Dulak J, Józkowicz A. 2003. Carbon monoxide—a ‘new’ gaseous modulator of gene expression. Acta Biochimica Polonica 50 31–47 [PubMed] [Google Scholar]
- Durner J, Shah J, Klessig DF. 1997. Salicylic acid and disease resistance in plants. Trends in Plant Science 7 266–274 [Google Scholar]
- Dutilleul C, Garmier M, Noctor G, Mathieu C, Chétrit P, Foyer CH, de Paepe R. 2003. Leaf mitochondria modulate whole cell redox homeostasis, set antioxidant capacity, and determine stress resistance through altered signaling and diurnal regulation. The Plant Cell 15 1212–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Theodoulou FL, Delrot S. 2001. The functions of inter- and intracellular glutathione transport systems in plants. Trends in Plant Science 6 486–492 [DOI] [PubMed] [Google Scholar]
- Fu GQ, Xu S, Xie YJ, Han B, Nie L, Shen WB, Wang R. 2011. Molecular cloning, characterization, and expression of an alfalfa (Medicago sativa L.) heme oxygenase-1 gene, MsHO1, which is pro-oxidants-regulated. Plant Physiology and Biochemistry 49 792–799 [DOI] [PubMed] [Google Scholar]
- Gibson KD, Neuberger A, Tait GH. 1963. Studies on the biosynthesis of porphyrin and bacteriochlorophyll by Rhodopseudomonas spheroides. 4. S-Adenosylmethionine–magnesium protoporphyrin methyltransferase Biochemical Journal 88 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosser N, Abate A, Oberle S, Vreman HJ, Dennery PA, Becker JC, Pohle T, Seidman DS, Schröder H. 2003. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin Biochemical and Biophysical Research Communications 308 956–960 [DOI] [PubMed] [Google Scholar]
- Guo B, Liang YC, Zhu YG, Zhao FJ. 2007. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress Environmental Pollution 147 743–749 [DOI] [PubMed] [Google Scholar]
- Han Y, Zhang J, Chen XY, Gao ZZ, Xuan W, Xu S, Ding X, She WB. 2008. Carbon monoxide alleviates cadmium-induced oxidative damage by modulating glutathione metabolism in the roots of Medicago sativa New Phytologist 177 155–166 [DOI] [PubMed] [Google Scholar]
- Herschbach C, Pilch B, Tausz M, Rennenberg H, Grill D. 2002. Metabolism of reduced and inorganic sulphur in pea cotyledons and distribution into developing seedlings New Phytologist 153 73–80 [Google Scholar]
- Huang BK, Xu S, Xuan W, Li M, Cao ZY, Liu KL, Ling TF, Shen WB. 2006. Carbon monoxide alleviates salt-induced oxidative damage in wheat seedling leaves Journal of Integrative Plant Biology 48 249–254 [Google Scholar]
- Iturbe-Ormaetxe I, Escuredo PR, Arrese-Igor C, Becana M. 1998. Oxidative damage in pea plants exposed to water deficit or paraquat Plant Physiology 116 173–181 [Google Scholar]
- Janda T, Szalai G, Tari I, Páldi E. 1999. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L) plants Planta 208 175–180 [Google Scholar]
- Krantev A, Yordanova R, Janda T, Szalai G, Popova L. 2008. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants Journal of Plant Physiology 165 920–931 [DOI] [PubMed] [Google Scholar]
- Lang D, Reuter S, Buzescu T, August C, Heidenreich S. 2005. Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation International Immunology 17 155–165 [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. 1983. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts Biochemical Journal 210 899–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León AM, Palma JM, Corpas FJ, Gómez M, Romero-Puertas MC, Chatterjee D, Mateos RM, Del Río LA, Sandalio LM. 2002. Antioxidative enzymes in cultivars of pepper plants with different sensitivity of cadmium Plant Physiology and Biochemistry 40 813–820 [Google Scholar]
- Leshem Y, Seri L, Levine A. 2007. Induction of phosphatidylinositol 3-kinase-mediated endocytosis by salt stress leads to intracellular production of reactive oxygen species and salt tolerance The Plant Journal 51 185–197 [DOI] [PubMed] [Google Scholar]
- Liu K, Xu S, Xuan W, et al. 2007. Carbon monoxide counteracts the inhibition of seed germination and alleviates oxidative damage caused by salt stress in Oryza sativa Plant Science 172 544–555 [Google Scholar]
- Matamoros MA, Moran JF, Iturbe-Ormaetxe I, Rubio MC, Becana M. 1999. Glutathione and homoglutathione synthesis in legume root nodules Plant Physiology 121 879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. 2006. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction Molecular and Cellular Biochemistry 291 21–28 [DOI] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, van Montagu M, Inzé D. 1998. Glutathione homeostasis in plants: implications for environmental sensing and plant development Journal of Experimental Botany 49 649–667 [Google Scholar]
- Mazel A, Leshem Y, Tiwari BS, Levine A. 2004. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7(AtRabG3e) Plant Physiology 134 118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally A, Finkemeier I, Georgi M, Dietz KJ. 2003. Salicylic acid alleviates the cadmium toxicity in barley seedlings Plant Physiology 132 272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwally A, Safronova VI, Belimov AA, Dietz K. 2005. Genotypic variation of the response to cadmium toxicity in Pisum sativum L Journal of Experimental Botany 56 167–178 [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Brach T, Marty L, Kreye S, Rouhier N, Jacquot JP, Hell R. 2007. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer The Plant Journal 52 973–986 [DOI] [PubMed] [Google Scholar]
- Mishra A, Choudhuri MA. 1999. Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice Biologia Plantarum 42 409–415 [Google Scholar]
- Muramoto T, Tsurui N, Terry MJ, Yokota A, Kohchi T. 2002. Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis Plant Physiology 130 1958–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts Plant and Cell Physiology 22 867–880 [Google Scholar]
- Noctor G, De Paepe R, Foyer CH. 2007. Mitochondrial redox biology and homeostasis in plants Trends in Plant Science 12 125–134 [DOI] [PubMed] [Google Scholar]
- Noctor G, Gómez L, Vanacker H, Foyer CH. 2002. Interactions between biosynthesis, compartmentation, and transport in the control of glutathione homeostasis and signalling Journal of Experimental Botany 53 1283–1304 [DOI] [PubMed] [Google Scholar]
- Noriega GO, Balestrasse KB, Batlle A, Tomaro ML. 2004. Heme oxygenase exerts a protective role against oxidative stress in soybean leaves Biochemical and Biophysical Research Communications 323 1003–1008 [DOI] [PubMed] [Google Scholar]
- Oberle S, Abate A, Grosser N, Vreman HJ, Dennery PA, Schneider HT, Stalleicken D, Schröder H. 2002. Heme oxygenase-1 inductiom may explain the antioxidant profile of pentaerythrityl trinitrate Biochemical and Biophysical Research Communications 290 1539–1544 [DOI] [PubMed] [Google Scholar]
- Ortega-Villasante C, Hernández LE, Rellán-Álvarez R, Del Campo FF, Carpena-Ruiz RO. 2007. Rapid alterration of cellular redox homeostasis upon exposure to cadmium and mercury in alfalfa seedlings New Phytologist 176 96–107 [DOI] [PubMed] [Google Scholar]
- Ortega-Villasante C, Rellán-Álvarez R, Del Campo FF, Carpena-Ruiz RO, Hernández LE. 2005. Cellular damage induced by cadmium and mercury in Medicago sativa Journal of Experimental Botany 56 2239–2251 [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Otterbein SL, Ifedigbo E, Liu F, Morse DE, Fearns C, Ulevitch RJ, Knickelbein R, Flavell RA, Choi AM. 2003. MKK3 mitogen-activated protein kinase pathway mediates carbon monoxide-induced protection against oxidant-induced lung injury American Journal of Pathology 163 2555–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA. 2002. Biological chemistry of carbon monoxide Antioxidants and Redox Signaling 4 259–270 [DOI] [PubMed] [Google Scholar]
- Pompella A, Maellaro E, Casini AF, Comporti M. 1987. Histochemical detection of lipid peroxidation in the liver of bromobenzene-poisoned mice American Journal of Pathology 129 295–301 [PMC free article] [PubMed] [Google Scholar]
- Popova LP, Maslenkova LT, Yordanova RY, Ivanova AP, Krantev AP, Szalai G, Janda T. 2009. Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings Plant Physiology and Biochemistry 47 224–231 [DOI] [PubMed] [Google Scholar]
- Queval G, Noctor G. 2007. A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: application to redox profiling during Arabidopsis rosette development Analytical Biochemistry 363 58–69 [DOI] [PubMed] [Google Scholar]
- Raskin I. 1992. Role of salicylic acid in plants Annual Review of Plant Physiology and Plant Molecular Biology 43 439–463 [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Pazmiňo DM, Testillano PS, Risueňo MC, Del Río LA, Sandalio LM. 2009. Cellular response of pea plants to cadmium toxicity: cross talk between reactive oxygen species, nitric oxide, and calcium Plant Physiology 150 229–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA, Sandalio LM. 2006. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo Plant, Cell and Environment 29 1532–1544 [DOI] [PubMed] [Google Scholar]
- Ryter SW, Otterbein LE, Morse D, Choi AM. 2002. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance Molecular and Cellular Biochemistry 234 / 235 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SS, Dietz KJ. 2009. The relationship between metal toxicity and cellular redox imbalance Trends in Plant Science 14 43–50 [DOI] [PubMed] [Google Scholar]
- Shekhawat GS, Verma K. 2010. Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence Journal of Experimental Botany 61 2255–2270 [DOI] [PubMed] [Google Scholar]
- Shen WB, Xu LL, Ye MB. 1999. New progress in plant disease resistance induced by salicylic acid Progress in Biochemistry and Biophysics 26 237–240 [Google Scholar]
- Shi QH, Zhu Zh J. 2008. Effects of exogenous salicylic acid on manganese toxicity, element contents and antioxidative system in cucumber Environmental and Experimental Botany 63 317–326 [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multipleinternal control genes Genome Biology 3, research0034. 1–0034.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GD, Pichersky E. 2007. Nicotinamidase participates in the salvage pathway of NAD biosynthesis in Arabidopsis The Plant Journal 49 1020–1029 [DOI] [PubMed] [Google Scholar]
- Wu M, Huang J, Xu S, Ling T, Xie Y, Shen W. 2011. Haem oxygenase delays programmed cell death in wheat aleurone layers by modulation of hydrogen peroxide metabolism Journal of Experimental Botany 62 235–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C, Oliver DJ. 1998. Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis The Plant Cell 10 1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Ling T, Han Y, et al. 2008. Carbon monoxide enhances salt tolerance by nitric oxide-mediated maintenance of ion homeostasis and up-regulation of antioxidant defense in wheat seedling root Plant, Cell and Environment 31 1864–1881 [DOI] [PubMed] [Google Scholar]
- Xie YJ, Xu S, Han B, Wu MZ, Yuan XX, Han Y, Gu Q, Xu DQ, Yang Q, Shen WB. 2011. Evidence of Arabidopsis salt acclimation induced by up-regulation of HY1 and the regulatory role of RbohD-derived reactive oxygen species synthesis The Plant Journal 66 280–292 [DOI] [PubMed] [Google Scholar]
- Xie Y, Xu D, Cui W, Shen W. 2012. Mutation of Arabidopsis HY1 causes UV-C hypersensitivity by impairing carotenoid and flavonoid biosynthesis and the down-regulation of antioxidant defence Journal of Experimental Botany 63 (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Chen Z. 1999. Salicylic acid induces rapid inhibition of mitochondrial electron transport and oxidative phosphorylation in tobacco cells Plant Physiology 120 217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, Ye MB, Shen WB. 2008. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process Plant Physiology 148 881–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. 2001. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots Plant Physiology 125 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Qi M, Mei C. 2004. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress The Plant Journal 40 909–919 [DOI] [PubMed] [Google Scholar]
- Yannarelli GG, Noriega GO, Batlle A, Tomaro ML. 2006. Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species Planta 224 1154–1162 [DOI] [PubMed] [Google Scholar]
- Ying W. 2008. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences Antioxidants and Redox Signaling 10 179–206 [DOI] [PubMed] [Google Scholar]
- Zawoznik MS, Groppa MD, Tomaro ML, Benavides MP. 2007. Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana Plant Science 173 190–197 [Google Scholar]
- Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N. 1999. Overexpression of glutathione synthetase in Indian Mustard enhances cadmium accumulation and tolerance Plant Physiology 119 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.