Abstract
Resting-state functional connectivity magnetic resonance imaging is proving to be an essential tool for the characterization of functional networks in the brain. Two of the major networks that have been identified are the default mode network (DMN) and the task positive network (TPN). Although prior work indicates that these two networks are anti-correlated, the findings are controversial because the anti-correlations are often found only after the application of a pre-processing step, known as global signal regression, that can produce artifactual anti-correlations. In this paper, we show that, for subjects studied in an eyes-closed rest state, caffeine can significantly enhance the detection of anti-correlations between the DMN and TPN without the need for global signal regression. In line with these findings, we find that caffeine also leads to widespread decreases in connectivity and global signal amplitude. Using a recently introduced geometric model of global signal effects, we demonstrate that these decreases are consistent with the removal of an additive global signal confound. In contrast to the effects observed in the eyes-closed rest state, caffeine did not lead to significant changes in global functional connectivity in the eyes-open rest state.
Keywords: resting-state fMRI, global signal, functional connectivity, anti-correlation, default mode network, task positive network, caffeine
Introduction
In resting-state functional magnetic resonance imaging (fMRI), the strength of the connections between different brain regions is reflected by correlations between low-frequency fluctuations in the blood oxygenation level dependent (BOLD) signal. The spatial pattern of these resting-state correlations are then used to infer the existence of functional networks (Fox and Raichle, 2007). For example, in the study that pioneered resting-state fMRI, (Biswal et al., 1995) demonstrated the existence of correlated fluctuations in the motor cortex network. Since that initial finding, a number of additional functional networks have been identified, including the default mode network (DMN), which is comprised of brain regions that tend to show decreased activity during the performance of attention demanding tasks (Raichle et al., 2001). Resting-state fluctuations in the DMN have been shown to be anti-correlated with the resting-state signal found in another set of brain regions, referred to as the task positive network (TPN), that exhibit increased activity during the execution of an attention demanding task (Fox et al., 2005; Fransson, 2005a). The presence of these two anti-correlated networks is consistent with an intuitively appealing picture of the brain alternating between introspective and extrospective states.
Recently, the presence of negative correlations between the DMN and TPN has been called into question due to potential biases introduced by a key pre-processing step known as global signal regression, in which a global mean signal component is regressed out of all voxel time courses prior to the computation of correlations. In particular, (Murphy et al., 2009) argued that the anti-correlations reported by (Fox et al., 2005) were an artifact of global signal regression and that the DMN and TPN were positively correlated in the absence of this pre-processing step. Indeed, it can be shown mathematically that global signal regression causes the distribution of correlations to be centered about zero, thereby forcing the existence of negative correlations (Anderson et al., 2011; Fox et al., 2009; Murphy et al., 2009; Weissenbacher et al., 2009). However, anti-correlation between the DMN and TPN has been observed without the application of global signal regression (Chai et al., 2012; Chang and Glover, 2009; Fox et al., 2009; Fransson, 2005b). The spatial patterns of anti-correlation are less pronounced than those observed with global signal regression, but can be enhanced with the application of noise reduction methods utilizing regressors based on either physiological measures (Chang and Glover, 2009) or component-based analysis of the data (Behzadi et al., 2007; Chai et al., 2012). Finally, electrophysiological studies have demonstrated anti-correlations in the local field potential power measured in the cat homologues of the DMN and TPN (Popa et al., 2009).
As an alternate approach to determining whether anti-correlations exist without the confounding effects of global signal regression, we can consider whether there are brain states in which network anti-correlations are more visible. We have previously shown that caffeine significantly reduces resting-state BOLD functional connectivity in the motor cortex (Rack-Gomer et al., 2009). In this paper, we take the next step and demonstrate that caffeine causes both widespread reductions in functional connectivity and a decrease in the global signal amplitude. To gain further insight into these effects, we adopt the geometric framework of (He and Liu, 2012) to show that the decreases in global signal and connectivity are consistent with the removal of an additive global signal confound. We find that caffeine is in essence performing a type of “natural” global signal reduction, without the complications of the signal processing approaches. As a direct consequence of the reduction of this global signal component, caffeine enhances the visibility of the anti-correlation between the DMN and TPN. Finally, we examine how the global effects of caffeine depend on whether the subject has their eyes open or closed, motivated by prior work demonstrating that modulations of this condition can affect the resting-state signal (Bianciardi et al., 2009; McAvoy et al., 2008; Zou et al., 2009).
Methods
Experimental protocol
Ten healthy volunteers participated in this study after providing informed consent (4 males and 6 females, aged 24 to 33 years with an average age of 25.6 years). As prior work has shown that differences in dietary caffeine consumption may cause variability in the BOLD response (Jones et al., 2000; Reeves et al., 2002), we recruited caffeine-naive subjects who consumed less than 50 mg caffeine daily (as assessed over a two month period prior to the study).
A repeated measures design was used in our study, in which each subject participated in two imaging sessions: a caffeine session and a control session. The order of the two sessions was randomized in a double-blinded manner. For each session, the operator obtained a capsule that contained 200mg of either caffeine or cornstarch. The two imaging sessions were separated by at least two weeks. Each session consisted of a pre-dose and a post-dose imaging section, with each section lasting for about one hour. Upon completion of the pre-dose section, participants were taken out of the magnet and given the capsule. The subject was then placed back in the scanner, with the first functional scan of the post-dose section obtained approximately 40 minutes after capsule ingestion. This interval was chosen based on studies showing that the absorption of caffeine from the gastrointestinal tract reaches 99% about 45 min after ingestion, with a half-life ranging from 2.5 to 4.5 hours (Fredholm et al., 1999).
Each scan section consisted of (1) a high-resolution anatomical scan, (2) two baseline cerebral blood flow (CBF) scans using arterial spin labeling (ASL) (one eyes-closed and one eyes-open), and (3) two 5 minute resting-state scans (one eyes-closed and one eyes-open). Subjects were instructed to lie still in the scanner and not fall asleep during resting-state scans. The order of eyes-open and eyes-closed scans was randomized. During eyes-open resting-state scans, subjects were asked to maintain attention on a black square located at the center of a grey background. During eyes-closed resting-state scans, subjects were asked to imagine the black square. Field maps were also acquired to correct for magnetic field inhomogeneities.
Data acquisition
Imaging data were acquired on a 3 Tesla GE Discovery MR750 whole body system using an eight channel receiver coil. High resolution anatomical data were collected using a magnetization prepared 3D fast spoiled gradient (FSPGR) sequence (TI=600ms, TE=3.1ms, flip angle = 8 degrees, slice thickness = 1mm, FOV = 25.6cm, matrix size = 256×256×176).
Whole brain ASL data were acquired over twenty axial slices using optimized pseudo-continuous ASL (PCASL) (Jung et al., 2010; Shin et al., 2010) with 2D spiral readout (slice thickness = 4mm, slice gap = 1mm, FOV = 24cm, TE = 3.3ms, TR = 4.1s, matrix size = 64×64, tag duration = 1.9s, post labeling delay = 1.6s). In addition, a cerebrospinal fluid (CSF) reference scan (Chalela et al., 2000) and a minimum contrast scan (Wang et al., 2005) were acquired for use in CBF quantification. The CSF scan consisted of a single-echo, single repetition scan acquired at full relaxation and echo time equal to 9.1 ms, while the minimum contrast scan was acquired with TR = 2 s and TE = 11 ms. Both scans used the same in-plane parameters and slice coverage as the ASL scans.
Whole brain BOLD resting-state data were acquired over thirty axial slices using an echo planar imaging (EPI) sequence (flip angle = 70 degrees, slice thickness = 4mm, slice gap = 1mm, FOV = 24cm, TE = 30ms, TR = 1.8s, matrix size = 64×64). Both BOLD and ASL scans used the same in-plane parameters and the slice coverage of the ASL scans overlapped with the center 20 slices of the BOLD scans.
Field maps were acquired using a gradient recalled acquisition in steady state (GRASS) sequence (TE1 = 6.5ms, TE2 = 8.5ms), with the same in-plane parameters and slice coverage as the BOLD resting-state scans. The phase difference between the two echoes was then used for magnetic field inhomogeneity correction of BOLD and ASL data (Fessler et al., 2005; Jenkinson, 2003).
Cardiac pulse and respiratory effect data were monitored using a pulse oximeter (InVivo) and a respiratory effort transducer (BIOPAC), respectively. The pulse oximeter was placed on each subject’s index finger while the respiratory effort belt was placed around each subject’s abdomen. Physiological data were sampled at 40Hz using a multi-channel data acquisition board (National Instruments).
MR data pre-processing
AFNI and FSL were used for MRI data pre-processing (Cox, 1996; Smith et al., 2004; Woolrich et al., 2009). The high resolution anatomical data were skull stripped and segmentation was applied to estimate white matter (WM), grey matter (GM) and cerebral spinal fluid (CSF) partial volume fractions. In each scan section, the anatomical volume was aligned to the functional volume of the first resting-state run using AFNI. The anatomical volume in the post-dose scan section was then registered to the pre-dose volume, and the rotation and shift parameters obtained from this registration were applied to the post-dose functional images.
The first 6 TRs (10.8 seconds) of the BOLD data were discarded to allow magnetization to reach a steady state. A binary brain mask was created using the skull-stripped anatomical data. For each slice, the mask was eroded by two voxels along the border to eliminate voxels at the edge of the brain (Rack-Gomer and Liu, 2012). For each run, nuisance terms were removed from the resting-state BOLD time series through multiple linear regression. These nuisance regressors included: i) linear and quadratic trends, ii) six motion parameters estimated during image co-registration, iii) RETROICOR (Glover et al., 2000) and RVHRCOR (Chang and Glover, 2009) physiological noise terms calculated from the cardiac and respiratory signals, and iv) the mean BOLD signal calculated from WM and CSF regions, where these regions were defined using partial volume thresholds of 0.99 for each tissue type and erosion of two voxels in each direction to minimize partial voluming with gray matter. The corrected BOLD time series were then low pass filtered using a cut-off frequency of 0.08Hz. This cutoff frequency was chosen for consistency with previous functional connectivity studies (Biswal et al., 1997; Cordes et al., 2001; Fox et al., 2005).
Baseline CBF
For each subject, a mean ASL image was formed from the average difference of the control and tag images from the ASL resting-state scan data (Liu and Wong, 2005). This mean ASL image was then corrected for coil inhomogeneities using the minimum contrast image (Wang et al., 2005) and converted to physiological units (ml/(100 g-min)) of CBF using the CSF image as a reference signal (Chalela et al., 2000; Liau and Liu, 2009). Whole brain baseline CBF was then calculated by averaging CBF values across voxels with grey matter partial volume of larger than 0.8 (Jung et al., 2010).
Whole brain connectivity and amplitude metrics
To define ROIs for the assessment of whole brain changes in connectivity, we used Freesurfer to segment the cerebral cortex into functional regions (aparc+aseg.mgz) (Desikan et al., 2006). These ROIs were common across the pre-dose and post-dose scans, which were registered to each other. We discarded ROIs for which any subject had 5 or less voxels within a region, resulting in a total of 40 ROIs (20 in each hemisphere) that were common across subjects. Supp. Fig. 1 displays the ROIs from one representative subject. The correlation between the average BOLD time courses was calculated for each of the resulting 780 ROI pairs. The correlation values were then converted to z-scores using Fisher z-transformation.
To evaluate the effect of caffeine on connectivity, the change in z-score metric was calculated for each ROI pair by subtracting the pre-dose value from the post-dose one, with a positive change corresponding to a higher value in the post-dose section. We then used a repeated measures two-way analysis of variance (ANOVA) (Keppel and Wickens, 2004) to examine the effects of two factors on the change in z-score: (1) the effect of the caffeine/control session and (2) the effect of the ROI pair.
For each voxel, a percent change time series was obtained from the preprocessed MR time series by subtracting the mean value and then dividing the resulting difference by the mean value. A global mean signal was formed as the average of the percent change time series across all voxels within the brain, and the standard deviation of this mean signal was defined as the global signal amplitude. For each voxel, we also computed the standard deviation of the percent change time series and defined the mean BOLD signal amplitude for each run as the average of the standard deviation values across the brain. Two-tailed paired t-tests were used to compare post-dose and pre-dose amplitude values. The relation between the global signal amplitude and the mean BOLD amplitude across subjects and conditions was assessed with regression analysis.
Anti-correlation between the DMN and TPN
To assess the extent of anti-correlation between nodes within the DMN and TPN, we used previously determined seed coordinates to define ROIs within these networks. Coordinates in the DMN and TPN were obtained by converting the MNI coordinates in a previous paper (Van Dijk et al., 2010) to Talairach coordinates using a nonlinear MNI to Talairach conversion algorithm (Lacadie et al., 2008). The centers of the DMN and TPN regions are listed in Table 1. To convert the Talairach coordinates into individual subject space, a 12-parameter affine transformation matrix was first estimated by registering the pre-dose anatomical volume to the T1 template in AFNI (TT_avg152T1+tlrc). The matrix was then applied to warp the seed coordinates into the space of the pre-dose anatomical volume. Note that these ROIs were common to the pre-dose and post-dose data, which were registered to each other. Seed ROIs were then created using a sphere with a diameter of 12mm centered about each seed coordinate. The BOLD time courses within each ROI were averaged.
Table 1.
Definition of seed coordinates in the default mode network and task positive network
| Brain regions | Talairach Coordinates |
|---|---|
| The default mode network (DMN) | |
| posterior cingulate cortex (PCC) | (0, 51, 26) |
| lateral parietal | (48, 59, 35) (−47, 59, 31) |
| medial prefrontal cortex | (0, −46, −7) |
| hippocampal formation | (23, 23, −13) (−23 22 −15) |
| The task positive network (TPN) | |
| frontal eye field | (38, 2, 44) (−40, 1, 45) |
| intraparietal cortex | (24, 55, 47) (−23 54 48) |
| middle temporal area | (54, 60, 3) (−54, 58, 1) |
To provide a qualitative view of BOLD connectivity between the PCC and the rest of the brain across conditions, the average BOLD time series in the PCC was first correlated with every voxel in the brain for each subject and condition. The correlation values were converted to z-scores using the Fisher z-transformation. The z-score spatial map for each subject was then warped to Talairach space using the 12-parameter affine transformation matrix described in the last paragraph. For each condition, a two-sided one sample t-test on the z-scores across subjects was then performed for each voxel in the Talairach space. The resulting t-statistic maps indicate the extent to which the z-scores across the sample differ from zero
To quantitatively assess BOLD connectivity between nodes in the DMN and TPN, each ROI in the DMN was correlated with every ROI in the TPN. Since we defined 6 ROIs in the DMN and 6 ROIs in the TPN, 36 correlation values were obtained. The extent of anti-correlation between the DMN and TPN was quantified by counting the number of anti-correlated links at different threshold levels (-0.3 to 0 in steps of 0.05). For each threshold level, the number of links was defined as the number of correlation values that were below the specified threshold. To assess differences in the number of anti-correlated links between the pre-dose and post-dose sections, two-tailed paired t-tests were conducted.
Results
Baseline CBF
Baseline CBF values are shown in Table 2. A significant decrease (eyes-closed: t(9)=-9.31, p=6e-6; eyes-open: t(9)=-11.38, p=1e-6) in baseline CBF was found in the caffeine session, with an average decline of 23%. No significant change (eyes-closed: t(9)=-0.99, p=0.35; eyes-open: t(9)=-1.65, p=0.13) in the baseline CBF was found in the control session. This result is consistent with previous observations of caffeine-related CBF reductions by our group (Behzadi and Liu, 2006; Liau et al., 2008; Liu et al., 2004; Rack-Gomer et al., 2009).
Table 2.
Pre-dose and post-dose whole brain mean baseline CBF values
| Subject | Eyes-closed | Eyes-open | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Caffeine Session CBF ml/(100 g min) |
Control Session CBF ml/(100 g min) |
Caffeine Session CBF ml/(100 g min) |
Control Session CBF ml/(100 g min) |
|||||
|
|
||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 72.2 | 56.4 | 78.4 | 66.6 | 67.9 | 51.6 | 69.0 | 65.3 1 |
| 2 | 57.7 | 42.6 | 60.5 | 62.0 | 57.4 | 39.9 | 61.9 | 66.6 |
| 3 | 58.8 | 47.4 | 55.1 | 59.8 | 57.2 | 46.2 | 61.4 | 63.3 |
| 4 | 72.7 | 59.5 | 72.0 | 71.7 | 72.7 | 58.5 | 72.9 | 69.0 |
| 5 | 63.3 | 56.6 | 68.0 | 65.8 | 64.5 | 56.9 | 70.6 | 68.7 |
| 6 | 70.4 | 45.8 | 69.8 | 71.3 | 69.9 | 49.5 | 69.9 | 66.5 |
| 7 | 66.8 | 42.4 | 66.2 | 64.9 | 60.9 | 40.1 | 59.1 | 61.7 |
| 8 | 59.6 | 44.3 | 59.0 | 54.5 | 55.3 | 42.8 | 58.1 | 52.9 |
| 9 | 78.9 | 61.4 | 64.1 | 61.2 | 72.3 | 56.9 | 62.8 | 58.3 |
| 10 | 74.6 | 59.7 | 73.2 | 74.7 | 74.1 | 61.0 | 82.7 | 76.8 |
| Mean | 67.5 | 51.6 | 66.6 | 65.2 | 65.2 | 50.3 | 66.8 | 64.9 |
| SD | 7.4 | 7.8 | 7.1 | 6.2 | 7.1 | 7.9 | 7.6 | 6.5 |
| p-value | 6e-6 | 0.35 | 1e-6 | 0.13 | ||||
Mean values were computed across voxels with grey matter partial volume >0.8. Significance values were computed with two-tailed paired t tests.
Whole brain BOLD connectivity
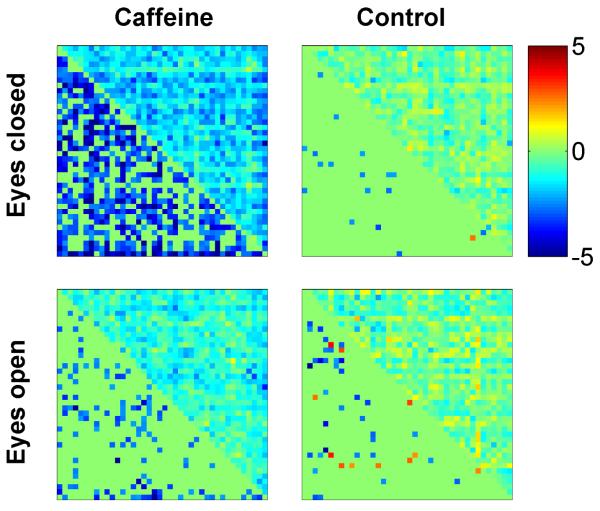
Fig. 1 shows the z-scores between all pairs of ROIs for the eyes-closed scans from a representative subject. The x and y axes correspond to the ROI indices (1-40), and each entry displays a z-score for one pair of ROIs. The z-scores in the post-dose caffeine data are visibly lower than those in the pre-dose caffeine data, whereas the pre-dose and post-dose control data do not exhibit a dose-related decrease in the z-scores. Fig. 2 summarizes the changes (post-dose minus pre-dose) in BOLD connectivity observed across the sample for both the caffeine and control sessions. Each connectivity matrix is divided into upper right and lower left triangles. An entry in the upper right triangle displays the mean change in z-score (post-dose minus pre-dose averaged across subjects) and the respective entry in the lower left triangle displays the corresponding t-statistic (entries with p>0.05 are set to zero). Blue colored entries in the lower left triangles indicate a significant decrease in BOLD connectivity, while red-colored entries indicate a significant increase. A qualitative examination of the plots reveals widespread decreases in connectivity in the eyes-closed caffeine data, a smaller number of connectivity decreases in the eyes-open caffeine data, and scattered decreases and increases in the control data. A quantitative assessment is provided by the results of the two-way repeated measures ANOVA summarized in Table 3. For the eyes-closed data, there was a significant effect of the caffeine/control factor (p=0.01), reflecting widespread changes in connectivity in the caffeine session that were not observed in the control session. The interaction term between the caffeine/control factor and the ROI pair factor was not significant (p = 0.23), indicating that the effect of the caffeine/control factor was largely independent of the ROI pair. Post-hoc two-tailed t-tests showed that in the caffeine session (eyes-closed condition), there was a significant decrease in the mean z-score across all ROI pairs (t(9)=−5.63, p=3e-4) reflecting decreased overall connectivity with caffeine, while no significant change in the mean z-score was found in the control session (t(9)=−0.69, p=0.51). In the eyes-open condition, the caffeine/control factor was not found to be significant (p = 0.13). As no significant effect was observed, post-hoc tests were not performed for the eyes-open data.
Figure 1.
Connectivity matrix for a representative subject showing z-scores between all pairs of ROIs. Each entry displays the z-score of one pair of ROIs, with the x and y axes corresponding to the ROI indices (1-40). In the caffeine session, the z-scores in the post-dose session are visibly lower than those in the pre-dose section. In the control session, the z-scores are comparable in both the pre-dose and post-dose sections. ROI labels (1-20 left hemisphere; 21-40 right hemisphere): anterior cingulate, middle frontal, cuneus, fusiform, inferior parietal, isthmus cingulate, lateral orbitofrontal, medial orbitofrontal, pars opercularis, post central, posterior cingulate, precentral, precuneus, rostral anterior cingulate, rostral middle frontal, superior frontal, superior parietal, superior temporal, supramarginal, insula.
Figure 2.
Mean BOLD connectivity changes across the sample. Each subplot is divided into an upper right triangle showing the change in z-scores and a lower left triangle showing the corresponding t-statistics (entries with p>0.05 are not filled in). A negative value (blue color) corresponds to a caffeine-related decrease in connectivity.
Table 3.
Repeated measures two-way ANOVA statistics using change in z-score as a metric
| eyes-closed | eyes-open | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Factor | F | dof | p | F | dof | p |
| Caffeine/Control | 10.45 | (1,9) | 0.01 | 1 2.70 | (1,9) | 0.13 |
| ROI pairs | 1.41 | (779,7011) | <1e-6 | 1.44 | (779,7011) | <1e-6 |
| Interaction | 1.04 | (779,7011) | 0.23 | 0.99 | (779,7011) | 0.60 |
Global signal and mean BOLD amplitudes
Fig. 3 displays the global signal amplitudes in both the caffeine and control sessions, with the solid green lines representing equality between the pre-dose and post-dose sections. In the eyes-closed condition, global signal amplitude was significantly reduced (t(9)=-4.31, p=0.002, 42% average reduction) in the caffeine session but not in the control session (t(9)=0.83, p=0.43). We also found significant global signal amplitude reduction (t(9)=-2.31, p=0.05, 28% average reduction) for the eyes-open data in the caffeine session but not in the control session (t(9)=0.65, p=0.54).
Figure 3.
Post-dose versus pre-dose global signal amplitudes for the caffeine and control sessions.
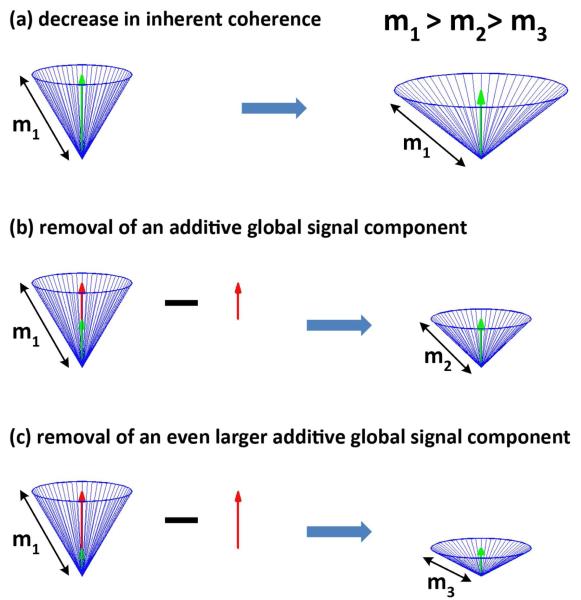
To better understand how the observed decreases in global signal amplitude are related to the widespread reductions in connectivity, it is helpful to consider a simple geometric picture of resting-state data that was recently introduced by our group (He and Liu, 2012). In the model, each resting-state time course is represented by a vector. We consider the simplified scenario in which all resting-state vectors are of the same amplitude (i.e. length denoted as m1) and are equally distributed around the vertical axis, as shown in Fig. 4a. The green vertical vector in Fig. 4a is the global signal, which is the mean of the resting-state vectors. An inherent decrease in the correlation of all resting-state vectors can be modeled as an increase in the angles between the resting-state vectors (right-hand side of Fig. 4b). This causes the amplitude of the global signal to decrease even though the lengths of the resting-state vectors remain constant.
Figure 4.
A simplified geometric model of resting-state data and the global signal. In (a), an overall decrease in the correlation can be modeled as an increase in the angles between the resting-state vectors (blue). This causes the amplitude of the global signal (green) to decrease even though the amplitudes of the resting-state vectors do not change. In contrast, as shown in panels (b) and (c), overall correlation can also be decreased by the removal of an additive global signal component (red arrow), leading to decreases in both the global signal amplitude and the amplitude of the resting-state vectors.
On the other hand, a decrease in connectivity could also be caused by the removal of an additive global signal component (depicted by the red arrow in Fig. 4b). The removal of this additive global signal component has three effects: (1) a decrease in the global signal amplitude; (2) a decrease in the amplitude of the resting-state vectors (i.e. m2 < m1); and (3) an increase in the angular spread of the cone, corresponding to a decrease in the correlation between the vectors. As the amplitude of the additive global signal grows (red arrow in Fig. 4c), its removal leads to an even greater decrease in the amplitude of the resting-state vectors (i.e. m3 < m2).
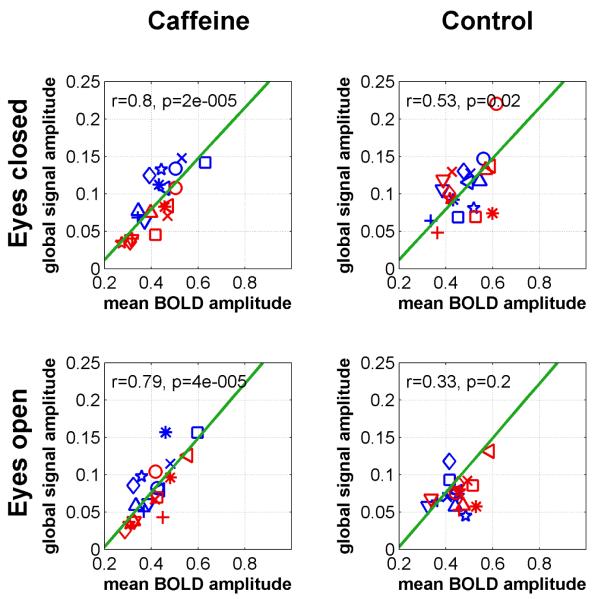
These geometric arguments suggest that if changes in connectivity are driven largely by the presence of an additive global signal component, then we should find a strong correlation between global signal amplitude and mean BOLD amplitude. In addition, we expect to find a strong correlation between any caffeine induced changes in global signal amplitude and the corresponding changes in mean BOLD amplitude. Fig. 5 plots the pre-dose and post-dose global signal amplitudes against the mean BOLD amplitudes for all subjects. Consistent with the findings of (He and Liu, 2012), significant linear relations were observed in both the caffeine (r=0.80, p=2e-5) and control (r=0.53, p=0.02) sessions in the eyes-closed condition. In the eyes-open condition, a significant linear relation was observed in the caffeine session (r=0.79, p=4e-5) but not in the control session (r=0.33, p=0.2). In the post-dose caffeine data there is a visible decrease in both the global signal and mean BOLD amplitudes as compared to their pre-dose values. To quantify this relation, Fig. 6 plots the change in the global signal amplitude against the change in the mean BOLD amplitude (eyes-closed: 16% average reduction, eyes-open: 7% average reduction) for the caffeine session data. Significant correlations were observed in both the eyes-closed (r=0.89, p=0.0006) and eyes-open (r=0.76, p=0.01) conditions. Taken together, Figs. 5 and 6 indicate that caffeine is removing an additive global signal component.
Figure 5.
Global signal amplitude versus mean BOLD amplitude. Significant correlations are observed under all conditions.
Figure 6.
Caffeine-related changes in the global signal amplitude versus corresponding changes in the mean BOLD amplitude. A significant correlation is observed in both the eyes-closed and eyes-open conditions.
In the eyes-closed condition, the caffeine-related reduction in the global signal amplitude and the mean BOLD amplitude are visibly larger than in the eyes-open condition. To quantify the difference, Supp. Fig. 2 compares the global signal and mean BOLD amplitudes in the eyes-open and eyes-closed conditions. Prior to the caffeine dose, both the global signal and mean BOLD amplitudes showed a trend towards having smaller values in the eyes-open versus eyes-closed condition (global signal amplitude: t(9)=−1.82, p=0.10; mean BOLD amplitude: t(9)=-1.99, p=0.08). After the intake of caffeine capsule, no significant difference was found between the eyes-open and eyes-closed conditions (global signal amplitude: t(9)=0.38, p=0.71; mean BOLD amplitude: t(9)=0.23, p=0.82). Supp. Fig. 3 compares the changes (post-dose minus pre-dose) in the global signal amplitude and mean BOLD amplitude in the eyes-open and eyes-closed conditions. There was a trend towards smaller changes in the eyes-open condition (global signal amplitude (t(9)=2,p=0.08; BOLD signal amplitude t(9)=1.67, p=0.13).
Anti-correlation between the DMN and TPN
Fig. 7 shows the whole brain voxel-wise correlations with the PCC seed ROI for three representative subjects using the eyes-closed caffeine session data. In addition to the standard pre-processing procedure as described in the methods section, we also generated correlation maps after regressing out the global signal. For each subject, correlation maps for the pre-dose and post-dose sections are displayed with and without global signal regression (GSR). Fig. 8 shows the group-level functional connectivity maps with and without GSR. Prior to the application of GSR, anti-correlations are visibly enhanced with the administration of caffeine. After GSR, anti-correlations are observed in both the pre-dose and post-dose data, as expected. To first order, the patterns of anti-correlation in the post-dose correlation maps without GSR are similar to those observed in both the pre-dose and post-dose maps after GSR. Supp. Figs. 4 and 5 display the corresponding connectivity maps using the eyes-open caffeine session data. In contrast to the eyes-closed condition, anti-correlations are visible in the pre-dose maps and are not visibly enhanced by the administration of caffeine.
Figure 7.
Connectivity maps from the caffeine session for three representative subjects obtained using a seed signal from the PCC seed ROI. Maps obtained with and without global signal regression are shown on the right-hand and left-hand sides, respectively. Prior to the application of GSR, anti-correlations in the eyes-closed condition are enhanced with caffeine.
Figure 8.
Group-level connectivity maps from the caffeine session (eyes-closed) with and without global signal regression are shown on the right-hand and left-hand sides, respectively.
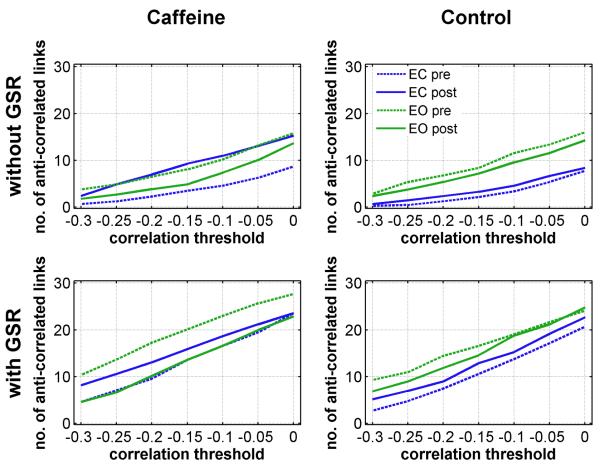
To quantify the effect of caffeine on the anti-correlations between the DMN and TPN, we compared the number of anti-correlated links at different threshold levels (group mean) across conditions. The top and bottoms rows in Fig. 9 display the number of anti-correlated links obtained without and with GSR, respectively. As shown in the upper left-hand plot, caffeine increased the number of anti-correlated links in the eyes-closed condition (dashed and solid blue lines), and the number of anti-correlated links after the caffeine dose was similar to the number of links found in both the pre-dose and post-dose eyes-open data. In the control session, there was not a pronounced shift in the number of anti-correlated links, and the number of links was less in the eyes-closed condition as compared to the eyes-open condition. As expected, the number of anti-correlated links was increased across all conditions with the application of GSR, although the effect was relatively larger for the eyes-closed data obtained without caffeine (i.e. compare dashed blue lines in bottom and top rows of the caffeine session (left column) and compare blue lines in bottom and top rows of the control session (right column)). Replotting the same results in a different format, the top and bottom rows in Supp. Fig. 6 display the number of anti-correlated links obtained in the eyes closed and eyes-open conditions, respectively. In this format, the increased number of anti-correlated links with (green) and without (blue) GSR is more evident. Table 4 summarizes two-tailed paired t-tests comparing the number of anti-correlated links in the pre-dose and post-dose sessions. In the eyes-closed condition (without GSR), a significant increase in the number of anti-correlated links was observed in the caffeine session at all threshold levels (t(9)>2.8, p<0.03). In the control session (eyes-closed without GSR), no significant difference was found. In the eyes-open condition, no significance difference (without GSR) was found for either the caffeine or control sessions.
Figure 9.
Mean number of anti-correlated links at different threshold levels. The first and second rows show the number of links obtained without and with global signal regression (GSR), respectively.
Table 4.
Two-tailed t-statistics comparing the number of anti-correlated links between the post-dose and pre-dose sections (i.e. post-dose minus pre-dose).
| Eyes-closed condition | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| without GSR | with GSR | |||||||
|
| ||||||||
| Caffeine | Control | Caffeine | Control | |||||
|
| ||||||||
| Threshold | t(9) | p-value | t(9) | p-value | t(9) | p-value | t(9) | p-value |
| 0 | 2.98 | 0.02 | 0.24 | 0.81 | 0.00 | 1.00 | 1.20 | 0.26 |
| −0.05 | 2.85 | 0.02 | 0.59 | 0.57 | 0.98 | 0.35 | 1.29 | 0.23 |
| −0.1 | 3.92 | 0.004 | 0.72 | 0.49 | 1.15 | 0.28 | 1.02 | 0.34 |
| −0.15 | 4.28 | 0.002 | 0.82 | 0.43 | 1.19 | 0.26 | 1.57 | 0.15 |
| −0.2 | 4.55 | 0.001 | 0.93 | 0.37 | 2.03 | 0.07 | 0.88 | 0.40 |
| −0.25 | 3.96 | 0.003 | 1.17 | 0.27 | 2.56 | 0.03 | 1.33 | 0.22 |
| −0.3 | 4.64 | 0.001 | 0.74 | 0.48 | 2.65 | 0.03 | 1.84 | 0.10 |
| Eyes-open condition | ||||||||
| 0 | −0.81 | 0.44 | −0.77 | 0.46 | −4.52 | 0.002 | 0.41 | 0.69 |
| −0.05 | −1.27 | 0.24 | −0.84 | 0.42 | −5.76 | 0.0003 | −0.38 | 0.71 |
| −0.1 | −1.36 | 0.21 | −1.07 | 0.31 | −3.80 | 0.004 | −0.18 | 0.86 |
| −0.15 | −1.55 | 0.16 | −0.68 | 0.52 | −3.34 | 0.009 | −1.18 | 0.27 |
| −0.2 | −1.58 | 0.15 | −0.94 | 0.37 | −4.27 | 0.002 | −1.75 | 0.11 |
| −0.25 | −1.45 | 0.18 | −1.06 | 0.32 | −5.25 | 0.0005 | −1.32 | 0.22 |
| −0.3 | −1.59 | 0.15 | −0.46 | 0.65 | −3.80 | 0.004 | −1.70 | 0.12 |
Discussion
We have shown that caffeine leads to widespread reductions in resting-state BOLD connectivity when subjects are studied in the eyes-closed state. These reductions in connectivity were accompanied by significant decreases in both the amplitude of the global signal and the mean amplitude of the BOLD signals. When viewed within the geometric framework of (He and Liu, 2012), these decreases suggest that caffeine reduces an additive global signal confound (e.g. Fig.s 4 through 6). With this reduction, anti-correlations between the DMN and TPN were significantly enhanced with the administration of caffeine.
In contrast to the eyes-closed findings, decreases in connectivity were not as widespread in the eyes-open rest state. This difference is consistent with the trends towards larger global signal and mean BOLD amplitudes in the eyes-closed versus the eyes-open condition, when these quantities are observed in the pre-dose state. These trends are roughly in line with prior findings of higher resting-state BOLD amplitudes in sensory regions for the eyes-closed versus eyes-open condition (McAvoy et al., 2008). After the administration of caffeine, the global signal and mean BOLD amplitudes in the eyes-closed and eyes-open states were not significantly different. A significant correlation was found between the global signal amplitude and mean BOLD amplitude for both the eyes-closed and eyes-open states, indicating the presence of an additive global confound in each condition. A possible interpretation of these observations is that the additive global confound is initially greater in the eyes-closed versus the eyes-open condition. After the caffeine-related reduction of this confound, the remaining global signal component becomes more similar between the two conditions.
As a consequence of the caffeine-induced reduction in the global signal, anti-correlations between the DMN and the TPN were visibly enhanced in the eyes-closed condition without the need for mathematical global signal regression. Because the initial identification of anti-correlations relied on the use of global signal regression, their existence has been actively debated in the literature (Fox et al., 2005; Fox et al., 2009; Murphy et al., 2009). The fact that anti-correlations are more easily detected after caffeine supports the argument that their existence is not simply a mathematical artifact of global signal regression. Although the anti-correlations are enhanced by caffeine, the degree of enhancement is less than the level achieved with global signal regression (Supp. Fig. 06). This is because caffeine reduces but does not completely eliminate the global signal. Thus, our findings also help to support some of the previously described drawbacks of global signal regression with regards to overestimating the strength and extent of underlying anti-correlations (Chai et al., 2012; Murphy et al., 2009). Although not applicable in all situations, it may be useful to consider the use of caffeine as a type of “natural” global signal regression for studies in which subjects have their eyes closed and the ability to characterize anti-correlations is of interest. In addition, caffeine usage should be carefully considered when interpreting the presence or absence of anti-correlations for subjects studied in the eyes-closed condition.
It should be noted that in our study the effect of caffeine on the detection of anti-correlations between the DMN and TPN was limited to the eyes-closed condition. In the eyes-open condition, anti-correlations were already detectable both prior to the administration of caffeine and without the use of global signal regression, and their detection was not further enhanced by caffeine. The increased presence of anti-correlations in the pre-dose eyes-open state is consistent with the trend towards smaller global signal amplitudes in the eyes-open state. Thus, use of the eyes-open rest state may be preferable for studies in which it is desirable to minimize the presence of the global signal.
To our knowledge, a direct comparison of the degree of anti-correlation observed in the eyes-open and eyes-closed conditions has not been explicitly addressed by prior studies. (Chai et al., 2012) found anti-correlations in the eyes-open condition after regressing out principal component regressors derived from white matter and CSF regions, similar to the results reported here using average regressors from these regions. However, (Jo et al., 2010) did not find evidence for anti-correlations between the DMN and TPN in the eyes-open fixation condition when using localized white matter regressors. (Chang and Glover, 2009) found evidence for anti-correlations in the eyes-closed state and demonstrated that they were enhanced by the application of physiological noise reduction methods. A confound in interpreting the prior studies is that the caffeine state of the subjects is not typically reported, so it is not known to what extent differences in the prior observations of anti-correlations between the DMN and TPN may have reflected systematic differences in caffeine usage prior to the scans.
With regards to the mechanisms through which caffeine can reduce an additive global signal confound, it is important to note that caffeine affects both the neural and vascular systems of the brain through its antagonism of adenosine receptors (Fredholm et al., 1999; Pelligrino et al., 2010). As shown here and in numerous prior studies, caffeine significantly reduces cerebral blood flow and there is some evidence to suggest that it also increases baseline oxygen metabolism (Griffeth and Buxton, 2011). When combined, these changes will tend to decrease baseline blood oxygenation and thus increase the BOLD response to neural input. However, caffeine also appears to tighten the coupling between changes in blood flow and oxygen metabolism, which decreases BOLD sensitivity to neural activity (Chen and Parrish, 2009; Griffeth and Buxton, 2011). These two opposing effects have been found to result in either no change or a slight increase in the BOLD response to stimulus (Chen and Parrish, 2009; Griffeth and Buxton, 2011; Laurienti et al., 2002; Liau et al., 2008; Mulderink et al., 2002). While it is difficult to extrapolate task-related results to the interpretation of resting-state data, these findings would tend to argue against changes in baseline blood flow and metabolism as being the primary mechanisms for decreases in the resting-state BOLD amplitude.
In a simultaneous electrophysiological and fMRI study of primate models, (Scholvinck et al., 2010) found widespread correlations between local field potential power fluctuations and resting-state BOLD signals, suggesting a significant neural component of the global signal. The potential for caffeine to reduce this global neural component is supported by prior studies showing that a 200mg dose reduces the power of resting electroencephalography (EEG) activity in the alpha, beta, and theta bands (Dimpfel et al., 1993; Siepmann and Kirch, 2002). In addition, the coherence of anterior cortex neural fluctuations in the alpha and theta bands is decreased by caffeine when compared to periods of caffeine abstinence (Reeves et al., 2002). Simultaneous EEG/fMRI recordings have shown that resting-state BOLD fluctuations are significantly correlated with EEG power fluctuations in the alpha band (de Munck et al., 2007; Goldman et al., 2002; Laufs et al., 2003a; Moosmann et al., 2003; Ritter et al., 2008), the beta band (Laufs et al., 2003b), and the theta band (Scheeringa et al., 2008). Further studies will be useful for directly assessing the relation between EEG measures and the global resting-state fMRI signal.
In conclusion, caffeine appears to reduce an additive global signal component in resting-sate fMRI data. This reduction is consistent with an overall decrease in functional connectivity and an enhanced detection of the anti-correlations between the DMN and TPN in the eyes-closed state. Although the available evidence suggests that caffeine’s reduction of the global confound reflects a decrease in the neural components of the global signal, further studies will be needed to more fully understand its effects.
Supplementary Material
Acknowledgements
This work was supported by NIH Grants R01NS051661, R21MH096495, and ONR MURI Award No. N00014-10-1-0072.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JS, Druzgal TJ, Lopez-Larson M, Jeong EK, Desai K, Yurgelun-Todd D. Network anticorrelations, global regression, and phase-shifted soft tissue correction. Hum Brain Mapp. 2011;32:919–934. doi: 10.1002/hbm.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Liu TT. Caffeine reduces the initial dip in the visual BOLD response at 3 T. Neuroimage. 2006;32:9–15. doi: 10.1016/j.neuroimage.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neuroimage. 2009;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo planar MRI. Magn. Reson. Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Chai XJ, Castanon AN, Ongur D, Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Parrish TB. Caffeine’s effects on cerebrovascular reactivity and coupling between cerebral blood flow and oxygen metabolism. Neuroimage. 2009;44:647–652. doi: 10.1016/j.neuroimage.2008.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- de Munck JC, Goncalves SI, Huijboom L, Kuijer JP, Pouwels PJ, Heethaar RM, Lopes da Silva FH. The hemodynamic response of the alpha rhythm: an EEG/fMRI study. Neuroimage. 2007;35:1142–1151. doi: 10.1016/j.neuroimage.2007.01.022. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dimpfel W, Schober F, Spuler M. The influence of caffeine on human EEG under resting conditions and during mental loads. Clin Investig. 1993;71:197–207. doi: 10.1007/BF00180102. [DOI] [PubMed] [Google Scholar]
- Fessler JA, Lee S, Olafsson VT, Shi HR, Noll DC. Toeplitz-based iterative image reconstruction for MRI with correction for magnetic field inhomogeneity. Ieee Transactions on Signal Processing. 2005;53:3393–3402. [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005a;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005b;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Goldman RI, Stern JM, Engel J, Jr., Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffeth VE, Buxton RB. A theoretical framework for estimating cerebral oxygen metabolism changes using the calibrated-BOLD method: modeling the effects of blood volume distribution, hematocrit, oxygen extraction fraction, and tissue signal properties on the BOLD signal. Neuroimage. 2011;58:198–212. doi: 10.1016/j.neuroimage.2011.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H, Liu TT. A geometric view of global signal confounds in resting-state functional MRI. Neuroimage. 2012;59:2339–2348. doi: 10.1016/j.neuroimage.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–582. doi: 10.1016/j.neuroimage.2010.04.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HE, Herning RI, Cadet JL, Griffiths RR. Caffeine withdrawal increases cerebral blood flow velocity and alters quantitative electroencephalography (EEG) activity. Psychopharmacology (Berl) 2000;147:371–377. doi: 10.1007/s002130050005. [DOI] [PubMed] [Google Scholar]
- Jung Y, Wong EC, Liu TT. Multiphase pseudocontinuous arterial spin labeling (MP-PCASL) for robust quantification of cerebral blood flow. Magn Reson Med. 2010;64:799–810. doi: 10.1002/mrm.22465. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: a Researcher’s Handbook. Pearson Prentice Hall; Upper Saddle River: 2004. [Google Scholar]
- Lacadie CM, Fulbright RK, Rajeevan N, Constable RT, Papademetris X. More accurate Talairach coordinates for neuroimaging using non-linear registration. NeuroImage. 2008;42:717–725. doi: 10.1016/j.neuroimage.2008.04.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Kleinschmidt A, Beyerle A, Eger E, Salek-Haddadi A, Preibisch C, Krakow K. EEG-correlated fMRI of human alpha activity. Neuroimage. 2003a;19:1463–1476. doi: 10.1016/s1053-8119(03)00286-6. [DOI] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America. 2003b;100:11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurienti PJ, Field AS, Burdette JH, Maldjian JA, Yen YF, Moody DM. Dietary caffeine consumption modulates fMRI measures. Neuroimage. 2002;17:751–757. [PubMed] [Google Scholar]
- Liau J, Liu TT. Inter-subject variability in hypercapnic normalization of the BOLD fMRI response. Neuroimage. 2009;45:420–430. doi: 10.1016/j.neuroimage.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau J, Perthen JE, Liu TT. Caffeine reduces the activation extent and contrast-to-noise ratio of the functional cerebral blood flow response but not the BOLD response. Neuroimage. 2008;42:296–305. doi: 10.1016/j.neuroimage.2008.04.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TT, Behzadi Y, Restom K, Uludag K, Lu K, Buracas GT, Dubowitz DJ, Buxton RB. Caffeine alters the temporal dynamics of the visual BOLD response. Neuroimage. 2004;23:1402–1413. doi: 10.1016/j.neuroimage.2004.07.061. [DOI] [PubMed] [Google Scholar]
- Liu TT, Wong EC. A signal processing model for arterial spin labeling functional MRI. Neuroimage. 2005;24:207–215. doi: 10.1016/j.neuroimage.2004.09.047. [DOI] [PubMed] [Google Scholar]
- McAvoy M, Larson-Prior L, Nolan TS, Vaishnavi SN, Raichle ME, d’Avossa G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J Neurophysiol. 2008;100:922–931. doi: 10.1152/jn.90426.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmann M, Ritter P, Krastel I, Brink A, Thees S, Blankenburg F, Taskin B, Obrig H, Villringer A. Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. Neuroimage. 2003;20:145–158. doi: 10.1016/s1053-8119(03)00344-6. [DOI] [PubMed] [Google Scholar]
- Mulderink TA, Gitelman DR, Mesulam MM, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 2002;15:37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino DA, Xu HL, Vetri F. Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis. 2010;20(Suppl 1):S51–62. doi: 10.3233/JAD-2010-091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Pare D. Contrasting Activity Profile of Two Distributed Cortical Networks as a Function of Attentional Demands. Journal of Neuroscience. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack-Gomer AL, Liau J, Liu TT. Caffeine reduces resting-state BOLD functional connectivity in the motor cortex. Neuroimage. 2009;46:56–63. doi: 10.1016/j.neuroimage.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves RR, Struve FA, Patrick G. Topographic quantitative EEG response to acute caffeine withdrawal: a comprehensive analysis of multiple quantitative variables. Clin Electroencephalogr. 2002;33:178–188. doi: 10.1177/155005940203300409. [DOI] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20585. DOI: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Bastiaansen MC, Petersson KM, Oostenveld R, Norris DG, Hagoort P. Frontal theta EEG activity correlates negatively with the default mode network in resting state. Int J Psychophysiol. 2008;67:242–251. doi: 10.1016/j.ijpsycho.2007.05.017. [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A. 2010;107:10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Jung Y, Shankaranarayanan A, Restom K, Guo J, Luh WM, Bandettini PA, Wong EC, Liu TT. Intl. Soc. Mag. Reson. Med. Stockholm; Sweden: 2010. Semi-Automated Correction of Phase Errors in Optimized Pseudo-Continuous Arterial Spin Labeling; p. 1744. [Google Scholar]
- Siepmann M, Kirch W. Effects of caffeine on topographic quantitative EEG. Neuropsychobiology. 2002;45:161–166. doi: 10.1159/000054958. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qiu M, Constable RT. In vivo method for correcting transmit/receive nonuniformities with phased array coils. Magn Reson Med. 2005;53:666–674. doi: 10.1002/mrm.20377. [DOI] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage. 2009;47:1408–1416. doi: 10.1016/j.neuroimage.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zou QH, Long XY, Zuo XN, Yan CG, Zhu CZ, Yang YH, Liu DQ, He Y, Zang YF. Functional Connectivity Between the Thalamus and Visual Cortex Under Eyes Closed and Eyes Open Conditions: A Resting-State fMRI Study. Human Brain Mapping. 2009;30:3066–3078. doi: 10.1002/hbm.20728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.