Summary
Age-related loss of muscle mass and strength (sarcopenia) leads to a decline in physical function and frailty in the elderly. Among the many proposed underlying causes of sarcopenia, mitochondrial dysfunction is inherent in a variety of aged tissues. The intent of this study was to examine the effect of aging on key groups of regulatory proteins involved in mitochondrial biogenesis and how this relates to physical performance in two groups of sedentary elderly participants, classified as high- and low-functioning based on the Short Physical Performance Battery test. Muscle mass was decreased by 38% and 30% in low-functioning elderly (LFE) participants when compared to young and high-functioning elderly (HFE) participants, respectively, and positively correlated to physical performance. Mitochondrial respiration in permeabilized muscle fibers was reduced (41%) in the LFE group when compared to the young, and this was associated with a 30% decline in COX activity. Levels of key metabolic regulators, SIRT3 and PGC-1α were significantly reduced (50%) in both groups of elderly participants when compared to young. Similarly, the fusion protein OPA1 was lower in muscle from elderly subjects, however no changes were detected in Mfn2, Drp1 or Fis1 among the groups. In contrast, protein import machinery (PIM) components Tom22 and cHsp70 were increased in the LFE group when compared to the young. This study suggests that aging in skeletal muscle is associated with impaired mitochondrial function and altered biogenesis pathways, and that this may contribute to muscle atrophy and the decline in muscle performance observed in the elderly population.
Keywords: aging, sarcopenia, mitochondria, skeletal muscle, PGC-1α
Introduction
The aging process is associated with a progressive decline in muscle mass and is referred to as sarcopenia. The sarcopenic phenotype is characterized by lower muscle strength, increased muscle fatigability, and reduced endurance capacity that may contribute to increased frailty and a greater risk of falls and other disabilities (Hurley, 1995). While a number of factors have been associated with age-related sarcopenia, the primary mechanisms contributing to this process are still uncertain.
A decline in mitochondrial function is a common phenomenon that has been readily observed in several different types of aged tissues and is particularly prominent in highly oxidative tissues such as skeletal muscle, in both animals and humans (Lanza & Nair, 2010). The mitochondrial theory of aging is based on the premise that cumulative damage caused by the production of free radicals can alter mitochondrial DNA (mtDNA; e.g. point mutations and deletions; Hiona & Leeuwenburgh, 2008). Defects in mtDNA can lead to a decline in mtDNA abundance and a reduced number of genes encoding mitochondrial proteins. This results in the improper assembly of the electron transport chain (ETC), as well as components of oxidative phosphorylation (Short et al., 2005). Lower mitochondrial protein synthesis rates, disturbances in mitochondrial enzyme activities, and lower oxidative capacity and ATP synthesis have all been reported in aged tissue (Rooyackers et al., 1996; Short et al., 2005). Furthermore, damage to mtDNA, lipids, and proteins by free radicals can activate cell death pathways, inducing the release of mitochondrial proteins such as cytochrome c into the cytosol, and the fragmentation of DNA that has been linked to muscle fiber atrophy and reduced physical function with aging (Marzetti et al., 2011). A role for mitochondrial dysfunction in aging is evidenced by mtDNA mutator mice that express a proofreading deficient mitochondrial DNA polymerase gamma (Polg; Kujoth et al., 2005). These mutant mice have increased mtDNA mutations and display age-related phenotypes, including severe sarcopenia which is likely due to defects in the assembly of functional ETC holoenzyme complexes (Hiona et al., 2010). Similar findings of increased mtDNA mutagenesis and reduced mitochondrial enzyme activities have been reported in human skeletal muscle with age (Hiona & Leeuwenburgh, 2008). Although the impact of these somatic mutations and deletions on organismal survival has not been completely established, these studies implicate a role for mitochondria in the aging process.
Mitochondrial biogenesis is an intricate process tightly controlled by key mitochondrial regulators. The proper assembly and functioning of mitochondria is achieved largely by nuclear-encoded proteins. Thus, a coordinated effort by both the nuclear and mitochondrial genomes is required to maintain mitochondrial integrity. In recent years, the importance of coactivators and transcription factors has been highlighted. In particular, the peroxisome proliferator-activated receptor γ coactivator α (PGC-1α) has gained wide acceptance as being the major regulator of mitochondrial content and oxidative metabolism in several tissues, including skeletal muscle. Overexpression of PGC-1α in skeletal muscle induces a fast- to slow- fiber type transition and increases mitochondrial content and oxidative metabolism through its activation of target genes involved in substrate metabolism (Handschin & Spiegelman, 2006). Furthermore, PGC-1α is upregulated in both animals and humans during acute and chronic contractile activity (Handschin & Spiegelman, 2006). Previous studies have reported reduced levels of PGC-1α in aged muscle (Baker et al., 2006; Safdar et al., 2010), as well as in conditions of muscle wasting (Sacheck et al., 2007; Sandri et al., 2006). Interestingly, overexpression of PGC-1α preserves mitochondrial function and muscle integrity in these conditions, partly through the stimulation of mitochondrial biogenesis and the attenuation of cell death pathways (Sandri et al., 2006; Wenz et al., 2009).
In addition to mitochondrial biogenesis regulatory proteins, the significance of mitochondrial fusion and fission proteins has recently been highlighted in aging. These proteins regulate mitochondrial morphology and function, and protect the integrity of the organelle by facilitating the mixing of intracellular metabolites and mtDNA. Fusion and fission proteins also segregate damaged and energy deficient organelles through autophagy (Seo et al., 2010). Defects in mitochondrial fusion and fission proteins have dramatic consequences on mitochondrial function. For example, mutations in the fusion proteins Mfn2 and OPA1 cause two neurodegenerative diseases, Charcot-Marie-Tooth type 2A and dominant optic atrophy, respectively (Seo et al., 2010). Muscle-specific Mfn1 and Mfn2 knockouts results in mtDNA depletion and the accumulation of organelles with a high level of mutant mtDNA (Chen et al., 2010). Consequently, these animals have severe mitochondrial dysfunction and muscle atrophy. In human skeletal muscle, the mitochondrial fusion protein Mfn2, a downstream target of PGC-1α and estrogen-related receptor alpha (ERRα), is upregulated with acute exercise and downregulated with obesity, insulin resistance, and aging (Seo et al., 2010). Furthermore, mitochondrial morphology factors have also been linked to another group of proteins known as the mitochondrial protein import machinery (PIM; Seo et al., 2010). Collectively, the primary task of this machinery is the import and targeting of all nuclear-encoded mitochondrial proteins to subcompartments of the organelle where they are assembled into a functional reticulum. Similar to the aforementioned groups of proteins, the PIM are also sensitive to changes in muscular activity, altering the import rate of proteins into mitochondrial subcompartments and contributing to mitochondrial biogenesis (Takahashi et al., 1998). While the effect of aging on the protein import pathway was recently demonstrated in animals (Joseph et al., 2010), not much is known regarding this translocation system in humans.
Numerous studies have linked mitochondrial dysfunction with reduced skeletal muscle function during aging (Hiona & Leeuwenburgh, 2008). The extent of this association however, remains under debate since some studies have revealed no effect of age on muscle mitochondrial properties (Barrientos et al., 1996; Rasmussen et al., 2003). In part, some of the divergent results may be due to differences in physical activity status that may influence mitochondrial enzyme activity levels and the extent of muscle mass loss that is experienced with age (Barrientos et al., 1996; Safdar et al., 2010). Recently, Safdar et al. (2010) reported that mitochondrial function was preserved in active older individuals compared to age-matched sedentary individuals and that this was partly due to differences in antioxidant capacity, inflammatory markers, and mitochondrial oxidative capacity between the two groups. Hence, these data suggest the potential of a physically active lifestyle in attenuating the decline in mitochondrial function previously reported with age and highlight the role of physical activity in human aging research.
The primary purpose of this study was to investigate skeletal muscle changes in cellular and molecular mechanisms involved in mitochondrial biogenesis in two distinct groups of sedentary elderly subjects, classified as high- and low-functioning on the basis of physical performance measures. While these subjects differed in their overall functionality, their physical activity levels were similar. Thus, we specifically sought to assess whether high- and low-functionality in the elderly was associated with distinct mitochondrial protein expression profiles in skeletal muscle irrespective of physical activity status. We hypothesized that the extent of mitochondrial decline with aging would be correlated with physical function parameters, with the highest mitochondrial decay being observed in low-functioning elderly (LFE) individuals when compared to high-functioning elderly (HFE) individuals. Firstly, our findings provide support for mitochondrial dysfunction contributing to functional decline in muscle with age. Additionally, our data suggest that the extent of mitochondrial impairment correlates to physical function in the elderly.
Results
Participant Characteristics
The main participant characteristics are provided in Table 1. A total number of 35 individuals participated in the study, including 23 older and 12 young men and women. No significant differences were observed in gender, height, weight, and body mass index (BMI) among the three groups. However, elderly participants had higher amounts of intermuscular adipose tissue (IMAT) compared to young participants (P < 0.05). Older participants in the LFE group were on average 6 years older than in the HFE group (P < 0.05), and appropriate statistical analyses (ANCOVA) were used to account for this difference. The thigh muscle mass was 38% and 30% lower (P < 0.05) in the LFE group when compared to the young and HFE group, respectively. The greater atrophy observed in muscle from the LFE group was not due to age or gender differences between the two elderly populations. Additionally, muscle size was positively correlated with the SPPB score in HFE and LFE individuals (r=0.48, P < 0.05; data not shown). The SPPB test has been extensively validated and is predictive of future mobility disability risk and mortality in the elderly (Guralnik et al., 1994). The two elderly groups did not significantly differ in terms of the number of conditions they exhibited (Buford et al., 2012).
Table 1.
Participant Characteristics
| Characteristic | Young (Y) | High-Functioning Elderly (HFE) | Low-Functioning Elderly (LFE) | P-value for group difference |
|---|---|---|---|---|
| Sample Size (n) | 12 | 12 | 11 | |
| Age (y) | 23±1 | 75±1* | 81±1*† | <0.05 |
| Females, % (n) | 33(4) | 33(4) | 45(5) | 0.789 |
| Weight (kg) | 73±3 | 82±5 | 78±4 | 0.299 |
| Height (cm) | 173±3 | 170±3 | 167±3 | 0.211 |
| BMI (kg/m2) | 24±1 | 28±1 | 28±1 | 0.09 |
| Muscle size (cm3) | 527±29 | 466±39 | 325±34*† | <0.05 |
| Subcutaneous fat (cm3) | 361.6±62 | 291.8±34 | 348.9±61 | 0.6 |
| IMAT (cm3) | 88.57±5 | 119.3±11* | 121.4±8* | <0.05 |
| SPPB Total Score | N/A | 11.42±0.2 | 6.091±0.5† | <0.001 |
Values are reported as means ± SE. Young (Y), High-Functioning Elderly (HFE), and Low-Functioning Elderly (LFE). HFE determined by a score of ≥ 11 and LFE ≤ 7 on the Short Physical Performance Battery (SPPB) test. Young participants did not perform the SPPB. BMI, Body Mass Index; IMAT, Intermuscular adipose tissue. N/A, not applicable.
P < 0.05 vs. young;
P < 0.05 LFE vs. HFE.
Muscle fiber cross sectional area (CSA) and fiber type differences
Type II fibers in LFE subjects displayed a reduced CSA when compared to young participants (Table 2; P < 0.05). No difference in type II fiber CSA was detected between HFE and young subjects. In addition, neither the number nor the CSA of type I fibers differed among the groups (Table 2).
Table 2.
Fiber type composition and area in vastus lateralis from young and high- and low-functioning elderly participants
| Variable | Young (Y) | High Functioning (HFE) | Low Functioning (LFE) |
|---|---|---|---|
| Type I (%) | 55±3 | 45±7 | 56±6 |
| Type II (%) | 45±3 | 55±7 | 44±6 |
| Type I area (μm2) | 6759±843 | 8626±1153 | 6713±961.6 |
| Type II area (μm2) | 6578±777 | 7614±1662 | 4335±592.3* |
Values are reported as means ± SE.
P < 0.05 vs. young.
Effect of age on mitochondrial function in skeletal muscle
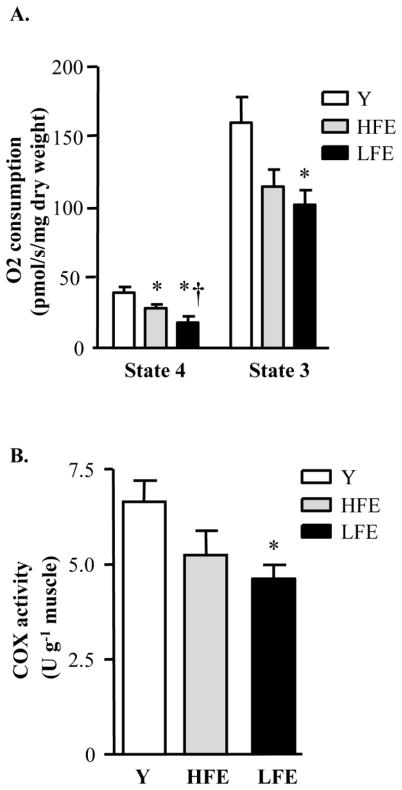
To assess mitochondrial bioenergetics and function, oxygen (O2) consumption and cytochrome c oxidase (COX) activity were measured in permeabilized fibers. Basal rates of pyruvate-malate-induced state 4 respiration were reduced in the elderly groups when compared to young. Moreover, LFE individuals displayed significantly lower state 4 respiration relative to the HFE group (Fig. 1A; P < 0.05), a difference that could not be attributed to age or gender. ADP-stimulated respiration (state 3) was 41% lower in the LFE subjects compared to young participants (P < 0.05). Although not statistically significant, there was a strong trend for a decrease in state 3 respiration between HFE subjects and young individuals (P=0.06). COX activity in whole muscle homogenates, used as an indicator of mitochondrial function (Adhihetty et al., 2009; Safdar et al., 2010), was reduced by 30% in LFE subjects relative to the young group (Fig. 1B; P < 0.05). No difference in COX activity was observed between HFE and young participants. The decrease in mitochondrial content does not entirely account for the decline in state 3 respiration rates observed in the LFE group suggesting that mitochondrial bioenergetics is compromised in skeletal muscle from LFE individuals compared to young and that there is a trend for mitochondrial function decline in the HFE group.
Fig. 1.
Mitochondrial bioenergetics in muscle from young and functionally-distinct elderly individuals. (A) Respiration in permeabilized muscle fibers from young (Y), high-functioning elderly (HFE) and low-functioning elderly (LFE) subjects was measured with pyruvate/malate (5 mM/2 mM) in basal respiratory state 4 or ADP-stimulated (2 mM) respiratory state 3 and expressed as pmol/s/mg of dry weight. Values are means ± SE; n = 5–9 per group.*P < 0.05 vs. young; †P < 0.05 vs. HFE (B) Cytochrome c oxidase (COX) activity, expressed as unit per gram of tissue. Values are means ± SE; n = 5–9 per group.*P < 0.05 vs. young.
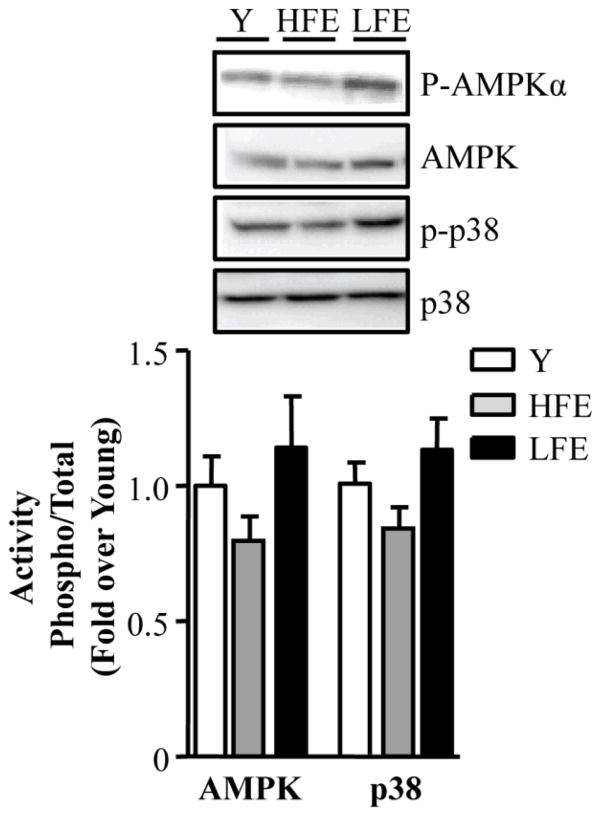
Age-related changes in AMPKα and p38 signaling pathways
To determine whether upstream mitochondrial signaling pathways were altered in young and elderly participants, levels of two key signaling molecules, 5′ AMP-activated protein kinase (AMPKα) and p38 mitogen-activated protein kinase (MAPK) were assessed. No significant difference in either parameter was detected between groups (Fig 2).
Fig. 2.
Effect of age on cellular signaling molecules. Representative Western blots of phosphorylated 5′ AMP-activated protein kinase (P-AMPKα), total AMPK (AMPK), phosphorylated p38 mitogen-activated protein kinase (p-p38) and total p38 (p38). Summary of experiments of AMPK and p38 activation are shown below as the phosphorylated form of the kinase over the total content and expressed as a fold of young. Values are means ± SE; n = 11–12 per group.
Age-related changes in transcriptional and metabolic regulators in aging muscle
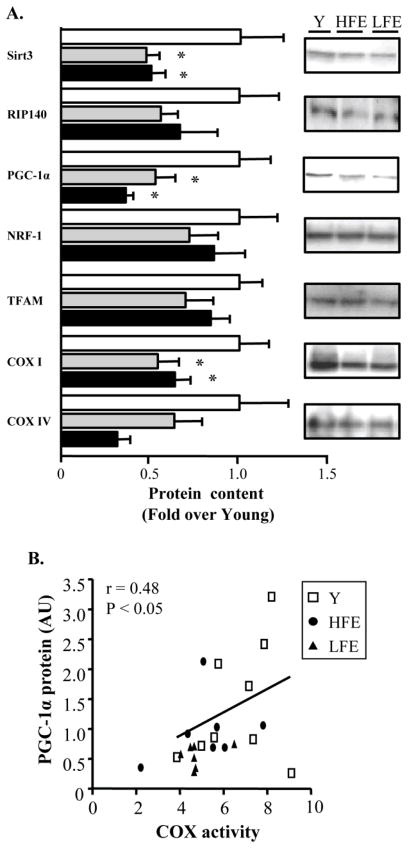
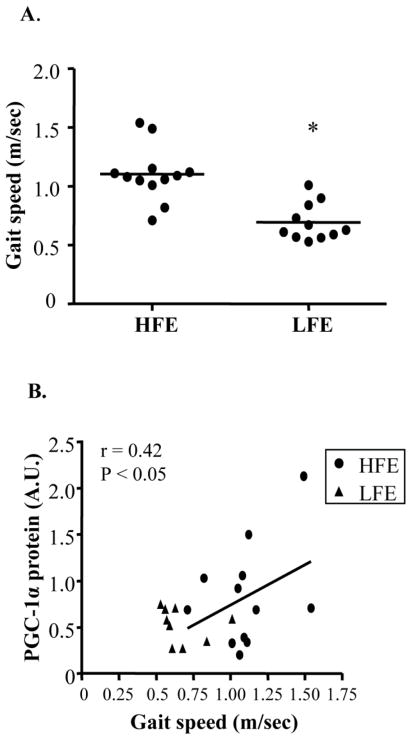
The induction of mitochondrial gene expression and biogenesis is largely controlled by the coordinated actions of various transcriptional and metabolic regulators such as PGC-1α and Sirtuin 3 (SIRT3). Levels of both these proteins were reduced by ~50% in elderly participants compared to young (Fig. 3A; P < 0.05). Furthermore, PGC-1α protein levels were positively correlated with COX activity in all groups (Fig. 3B; r=0.48, P < 0.05). In contrast, levels of the cofactor receptor-interacting proteins 140 (RIP140), a repressor of PGC-1α target genes (Powelka et al., 2006), were not different with age or between LFE and HFE participants. Similarly, levels of the PGC-1α target proteins nuclear respiratory factor 1 (NRF-1) and mitochondrial transcription factor A (Tfam) did not differ across groups. However, abundance of the mtDNA-encoded subunit COX I was lower with aging (P < 0.05). Finally, no significant changes in the nuclear-encoded COX IV protein were observed. Interestingly, decreased mitochondrial function and content in the LFE subjects was associated with reduced functional capacity as indicated by gait speed on the 4-m walk test (Fig. 4A; P < 0.05). Furthermore, a positive correlation was determined between PGC-1α and the 4-m walk test (Fig. 4B; r=0.42, P < 0.05).
Fig. 3.
Age-related changes in key metabolic and transcriptional regulators. (A) Western blot analysis of SIRT3 = sirtuin 3; RIP140 (nrip1) = nuclear receptor-interacting protein 140; PGC-1α =peroxisome proliferator activated receptor gamma (PPARγ) Coactivator 1-alpha; NRF-1 = nuclear respiratory factor 1; Tfam = mitochondrial transcription factor A; COX I and IV = cytochrome c oxidase subunit I and IV. A summary of repeated experiments (left) along with the corresponding protein blot (right). All values were normalized to actin and expressed as a fold of young. Values are means ± SE; n = 11–12 per group. *P < 0.05 vs. young. (B) Relationship between COX activity and PGC-1α in young and elderly individuals. n = 7–9 per group. r = Pearson correlation coefficient.
Fig. 4.
Association between PGC-1α and physical function in elderly subjects. (A) Gait speed in HFE and LFE individuals with each point representing an individual. n = 11–12 per group. *P < 0.05 vs. HFE. (B) Relationship between gait speed and PGC-1α protein in HFE and LFE subjects. n = 9–12 per group. r = Pearson correlation coefficient.
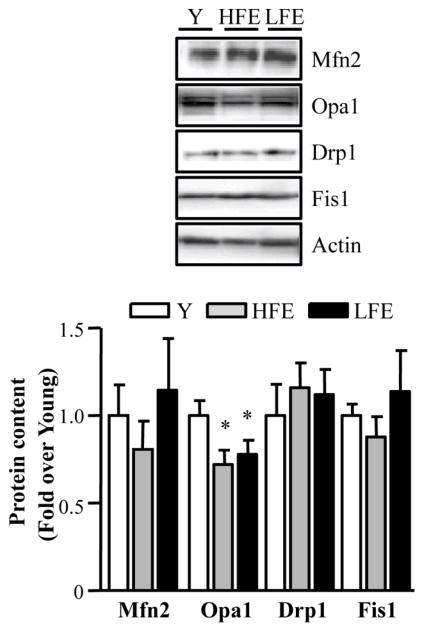
Effect of age on mitochondrial fusion and fission pathways
While no changes were observed in Mfn2 protein levels among the groups, a significant decline in the fusion protein OPA1 was detected in elderly participants compared to young (Fig. 5; P < 0.05). Finally, no differences in the levels of the mitochondrial fission proteins Drp1 and Fis1 were observed (Fig. 5).
Fig. 5.
Mitochondrial fusion and fission proteins in young and elderly subjects. Western blots of fusion Mfn2 (mitofusin 2) and Opa1 (optic Atrophy Type I), and fission proteins Drp1 (dynamin-related protein 1) and Fis1 (fission 1). Two bands were detected for Opa1 representing the long and short isoforms. Both bands were quantified for total Opa1 protein. Summary of repeated experiments is shown below. All values were normalized to actin and expressed as a fold of young. Values are means ± SE; n = 11–12 per group. *P < 0.05 vs. young.
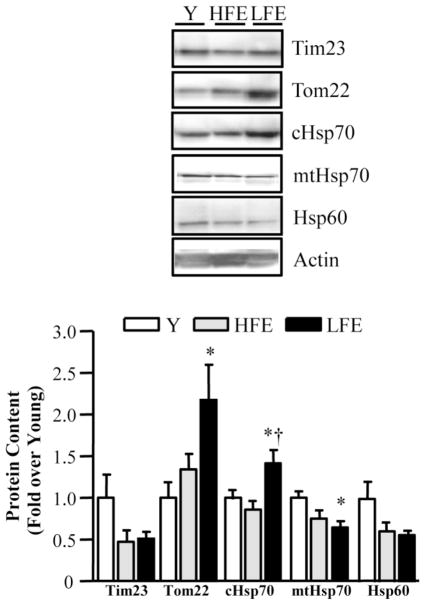
Effect of age on mitochondrial protein import components
The PIM consists of two primary assembly complexes known as the translocases of the outer membrane (TOM complex) and the translocases of the inner membrane (TIM complex). TOM22, as well as cytosolic heat shock protein 70 (cHSP70) were upregulated in skeletal muscle from LFE participants compared to young (Fig. 6; P < 0.05). cHsp70 was also higher in the LFE group when compared to the HFE group (P<0.05) and independent of age and gender. In contrast, levels of mitochondrial Hsp70 (mtHsp70) were reduced with aging (P < 0.05) in the LFE compared to the young group. A trend toward an age-related decrease in Hsp60 (P=0.06) protein levels was also evident.
Fig. 6.
Alterations in mitochondrial protein import machinery (PIM) components. Western blot analysis of PIM with a graphical representation depicted below. Tim23 = translocase of the inner membrane protein 23; Tom22 = translocase of the outer membrane protein 22; cHsp70 = cytosolic heat shock protein 70; mtHsp70 = mitochondrial heat shock protein 70; Hsp60 = heat shock protein 60. All values were normalized to actin and expressed as a fold of young. Values are means ± SE; n = 11–12 per group. *P < 0.05 vs. young; †P < 0.05 vs. HFE.
Discussion
Aerobic capacity declines with age due to a loss in skeletal muscle mass and a lower muscle oxidative capacity (Rooyackers et al., 1996; Short et al., 2005). The molecular and cellular disturbances responsible for the decline in oxidative capacity with progressive age are currently unknown. While mitochondria have been implicated, the extent of mitochondrial decline with aging and how this relates to poorer muscle quality and function remain controversial. Thus, the present study was designed to investigate age-related changes in skeletal muscle mitochondrial function and to determine whether mitochondrial properties differ in elderly sedentary individuals with low physical performance relative to high physical performance.
Consistent with previous studies, our results show that muscle mass declines with age and that this is likely attributed to fiber atrophy that preferentially occurs in type 2 fibers (Lexell & Taylor, 1991). There are also reports that aging leads to a greater proportion of hybrid fibers that cannot strictly be classified as type I or type IIA but rather co-express both MHC I and IIA isoforms (referred to as type IIC fibers). While the mechanisms responsible for these hybrid fibers are not well understood, a dysregulation in the expression of MHC isoforms by nuclei within individual fibers has been postulated (Andersen, 2003). These hybrid fibers are most accurately assessed using electrophoresis of MHC isoforms in single fibers that allow examination of a longer section of the muscle fiber. Therefore the number of fibers detected co-expressing MHC I and MHC IIA is drastically reduced by histological methods where analysis is limited to a small cross-sectional area of the fiber. Thus, although we were not able to accurately estimate the percentage of these hybrid fibers with the methods used in this study we intend to include this unique fiber population in future studies with similar subject populations.
Reductions in the quantity of muscle in elderly individuals were also associated with mitochondrial decay as demonstrated by reduced bioenergetics and the content of mitochondria in muscle. Interestingly, these changes were most prominent in LFE individuals. Previous reports have shown that skeletal muscle from elderly individuals and particularly those with poor physical function are more prone to oxidative damage when compared to elderly individuals with higher physical performance. This is evidenced by higher levels of 8-hydroxy 2-deoxyguanosine (8-OHdG) and protein carbonylation, as well as altered redox homeostasis and lower antioxidant capacity (Parise et al., 2005; Safdar et al., 2010). Additionally, poor physical performance in the elderly is associated with elevated levels of chronic inflammatory markers including interleukin 6 (IL-6) and nuclear factor kappa B (NF-κB; (Buford et al., 2010; Cesari et al., 2004). This hypothesis is supported by the greater decline observed in mitochondrial respiration, as well as COX activity in the LFE individuals that may contribute to higher rates of free radical formation and muscle damage. Moreover, recent data obtained from our group in the same population of elderly subjects demonstrate a strong correlation between apoptotic signaling proteins and reduced muscle mass and function further implicating increased oxidative stress as a possible contributor of muscle aging (Marzetti et al. 2012). Collectively these reports point to an association between oxidative damage, mitochondrial function, and muscle mass loss in aging skeletal muscle, particularly in individuals with poor physical function. The specific molecular details however, regarding this relationship are still unclear and require further investigation.
It is well established that PGC-1α is an important regulator of mitochondrial mass and function in skeletal muscle (Handschin & Spiegelman, 2006). Furthermore, declines in PGC-1α levels precede the loss of muscle mass in aging (Baker et al., 2006), as well as in a variety of muscle wasting conditions including fasting and denervation (Sacheck et al., 2007; Sandri et al., 2006). In these models of atrophy, muscle wasting was prevented by the ectopic expression of PGC-1α (Sacheck et al., 2007; Sandri et al., 2006). In aged mice, PGC-1α overexpression was also associated with improved whole body health compared with age-matched wild-type animals (Wenz et al., 2009). In our study, levels of PGC-1α were reduced in both high- and low-functioning elderly individuals. In addition, PGC-1α levels were positively correlated with COX activity, as well as gait speed in elderly participants suggesting that this protein may be an important predictor in the degree of physical function decline linked to mitochondrial dysfunction. This is supported by previous data illustrating a strong relationship between COX activity and muscle strength in the elderly (Safdar et al. 2010). Such an assumption is in line with previous evidence showing that PGC-1α mRNA levels are significantly correlated with oxidative capacity and training status in healthy subjects (Garnier et al., 2005). As discussed above, PGC-1α levels increase in response to physical activity and can be used as a marker of mitochondrial biogenesis in muscle. This is also evidenced in active elderly individuals where levels of this protein were preserved and similar to young individuals when compared to sedentary individuals (Safdar et al., 2010). The finding that this protein was equally reduced in both HFE and LFE individuals when compared to young subjects would suggest that both elderly groups had similar physical activity lifestyles.
Our study also demonstrates that sirtuin 3 (SIRT3) levels are affected with aging. Lanza et al. (2008) reported similar findings in human skeletal muscle from individuals with a sedentary proteomic profile. Human SIRT3, is a mitochondrial NAD+ -dependent deacetylase that has been associated with longevity in humans (Rose et al., 2003). Perhaps a clue linking SIRT3 to aging is supported by recent studies demonstrating that SIRT3 in muscle promotes the expression of PGC-1α (Palacios et al., 2009) and vice versa (Kong et al., 2010). In addition, overexpression of SIRT3 or PGC-1α in cultured myotubes suppresses levels of ROS (Kong et al., 2010). This data is in agreement with the finding that SIRT3 similarly to PGC-1α, also has the ability to reduce oxidative damage by influencing key antioxidant defense systems (Someya et al., 2010). Intriguingly, a PGC-1α/Tfam complex was recently identified in association with mtDNA nucleotides suggesting that PGC-1α translocates to mitochondria where it may play a role in retrograde signaling between the nucleus and mitochondria (Aquilano et al., 2010). Although the exact molecular understanding has yet to be fully elucidated, it appears that age-related disturbances in PGC-1α and SIRT3 levels may increase oxidative damage and mutations to DNA, compromising mitochondrial function and contributing to muscle atrophy observed with aging.
Age-related sarcopenia has also been associated with altered mitochondrial morphology, as illustrated by the appearance of enlarged and depolarized mitochondria with aberrant mitochondrial cristae formation (Navratil et al., 2008). OPA1 is present in the mitochondrial inner membrane and is important for mitochondrial fusion and cristae morphology (Arnoult et al., 2005). Interestingly, giant mitochondria in cultured myoblasts display reduced levels of OPA1 and are associated with impaired fusion capacity (Navratil et al., 2008). These findings imply that aberrant mitochondria may not restore their function by fusing and exchanging their contents with functional organelles, leading to compromised bioenergetics. Noteworthy, our results indicate that OPA1 levels but not Mfn2 are reduced in aged human muscle suggesting perhaps an additional pathway leading to mitochondrial dysfunction in late life. Moreover, repression of OPA1 can also result in mitochondrial fragmentation through greater fission. These alterations are accompanied by decreased cell growth and oxygen consumption, as well as the induction of apoptosis (Arnoult et al., 2005; Chen et al., 2005). The latter occurs primarily through the remodeling of cristae junctions that control the release of cytochrome c. Interestingly, mitochondrial morphology proteins interact with members of the Bcl-2 family of cell death proteins (Brooks et al., 2007), however the significance of such interactions is yet unclear. Thus, dysregulation in fusion and/or fission events resulting in improper mixing of intracellular metabolites and mtDNA may compromise the integrity of the mitochondrial genome by favoring the accumulation of mtDNA mutations. This leads to enhanced oxidative damage, triggering the release of pro-apoptotic proteins and irreversible cell death pathways. Altogether these data suggest the importance of maintaining proper mitochondrial dynamics through fusion and fission proteins and the consequences of aberrant mitochondrial morphology for cellular health.
With regard to the PIM, results from the present study are consistent with previous findings in animals (Joseph et al., 2010). Indeed, in muscle from aged rats levels of the outer membrane receptor protein Tom22 are increased relative to their younger counterparts. In the same animals, these changes resulted in higher rates of precursor protein import and assembly into mitochondrial complexes (Joseph et al., 2010). Tom22 is crucial for the import and assembly of Tom40, a protein that forms the TOM complex, the main entry gate into mitochondria (Humphries et al., 2005). The upregulation of Tom22 in aged muscle is likely a compensatory response by the cell to increase the import and assembly of nuclear-encoded proteins into mitochondria and maintain oxidative capacity in the face of impaired synthesis of mtDNA-encoded components. This phenomenon has also been observed in other conditions of compromised bioenergetics including mtDNA depletion and mitochondrial disease (Joseph et al., 2004). A direct interaction between components of the PIM and mitochondrial morphology, as well as apoptosis proteins has also been reported (Seo et al., 2010) implying that mitochondrial regulatory pathways might not be functionally independent and that their roles in mitochondrial biogenesis are multifaceted. This study is the first to show the adaptability of the PIM components in muscle from elderly individuals and highlights the potential of this previously unexplored area of mitochondrial biology in aging research.
In summary, results from the present study indicate that mitochondrial regulation and function are reduced in aged human skeletal muscle and this appears to be related to the level of physical performance in the elderly. Despite correcting for age and gender, LFE individuals displayed greater declines in mitochondrial bioenergetics and muscle mass loss than HFE individuals. This finding supports our hypothesis of an association between the extent of skeletal muscle mitochondrial decline and physical function status with aging. However, it is important to note that mitochondrial dysfunction was also observed in HFE participants, which implicate aging as a primary contributor to this process. Moreover, the finding that key regulatory proteins including Sirt3, PGC-1α, and COX I are reduced to the same extent in both elderly groups would suggest that non-mitochondrial factors may be involved in declines in muscle function and potentially muscle mass losses with age. Additionally, while we wanted to control for differences in physical activity levels, the possibility of variations in recreational activity levels between the groups cannot be completely ruled out. Despite these limitations, this study highlights the importance of mitochondria in aging skeletal muscle and the complex interplay among various regulatory pathways such as mitochondrial dynamics and mitochondrial protein import in muscle. The data from this study suggests that mitochondrial dysregulation may contribute towards skeletal muscle decline with age and that functionality in the elderly is correlated with mitochondrial parameters irrespective of physical activity. Collectively, these data provide insight for the therapeutic potential of mitochondrially-targeted interventions/strategies in the treatment of sarcopenia and the improvement of physical function in the elderly population.
Experimental Procedures
Participants
A total of thirty-five participants including 12 young (8 male and 4 female) and 23 older subjects (14 male and 9 female) were included in this study. These participants were part of a subset from a larger study protocol assessing sarcopenia in aging. Elderly participants were further divided into high-functioning (HF) and low-functioning (LF) based on their performance on the Short Physical Performance Battery (SPPB) test that includes a standing balance, a 4-m walk course and a chair stand test (Guralnik et al., 1994). Participants with a SPPB score of ≥ 11 were classified as HF, while participants with a score of ≤ 7 were classified as LF. Participants in all groups were sedentary and exercised less than 20 min/week in the 2 months prior to the study. Participants were informed of the procedures involved prior to enrolment in the study and gave their written informed consent, as approved by the University of Florida Institutional Review Board. Additional exclusion criteria were as follows; history of smoking in the prior 12 months, congestive heart failure NYHA Class III or IV, previous stroke with upper and/or lower extremity involvement within prior 6 months, peripheral vascular disease Fontaine Class III/IV, history of life-threatening cardiac arrhythmias, active treatment for cancer or history of cancer in prior 3 years, severe neurological disorders including Parkinson’s disease, renal disease requiring dialysis, lung disease requiring steroids, lower extremity amputation, severe osteoarthritis that interferes with physical function, complicated diabetes, inflammatory diseases (e.g., active rheumatoid arthritis, vasculitis, autoimmune disorders, and inflammatory bowel disease), life-threatening illnesses with an estimated life expectancy of less than 1 year.
Muscle biopsy
Skeletal muscle samples were obtained under local anesthesia from the vastus lateralis muscle using a percutaneous needle biopsy (Buford et al., 2010). Muscles were immediately processed for respiration measurements and histochemistry. Remaining tissue was immediately frozen and stored at −80°C.
Preparation of permeabilized muscle fibers
Permeabilized fibers were prepared as previously described (Kuznetsov et al., 2008). Briefly, muscle samples were placed in ice-cold Buffer X containing 60 mM K-MES, 35 mM KCl, 7.23 mM K2EGTA, 2.77 CaK2EGTA, 20 mM imidazole, 0.5 mM DTT, 20 mM taurine, 5.7 mM ATP, 15 mM PCr, and 6.56 mM MgCl2·6 H2O (pH 7.1, 295 mosmol/kgH2O) and connective tissue removed. Muscle pieces were cut into small bundles (~8–10 mg) and gently separated into single fibers. Myofibers were permeabilized in Buffer X with saponin (50 μg/ml) and incubated on a rotator for 30 min at 4 °C. Permeabilized fibers were washed in ice-cold Buffer Z containing 110 mM K-MES, 35 mM KCl, 1 mM EGTA, 5 mM K2HPO4, and 3 mM MgCl2·6 H2O, 0.05 mM pyruvate, and 0.02 mM malate with 0.5 mg/ml BSA (pH 7.1, 295 mosmol/kgH2O) on a rotator at 4 °C for 45 min. Fiber bundles were washed in Buffer Z containing 5 mM pyrophosphate to deplete fibers of endogenous nucleotides.
Mitochondrial respiration in permeabilized muscle fibers
Respiration was measured using an Oroboros O2K Oxygraph (Inssbruck, Austria) according to methods previously described (Kuznetsov et al., 2008). Once the O2 flux rate was stable, permeabilized muscle fibers were weighed and added to the chamber consisting of Buffer Z containing 20 mM creatine. The O2 flux rate was measured following the addition of a substrate and inhibitor protocol which consisted of 5 mM pyruvate and 2 mM malate, 2 mM ADP, 10 μM cytochrome c, 10 mM succinate, 2 μg/ml oligomycin and 0.5μM carbonylcyanide p-trifluoromethoxyphenylhydrazone. Respiratory control ratios calculated as state 3/state 4 indicated well-coupled mitochondria in all groups measured (mean RCR = 5.0±0.48). At the end of the experiment, fiber bundles were removed and dried overnight.
Cytochrome c oxidase (COX) activity
Enzyme activity was measured spectrophotometrically in whole muscle homogenates by measuring the maximal rate of oxidation of fully reduced cytochrome c (Adhihetty et al., 2009).
Immunohistochemistry for fiber typing and CSA
Serial sections from samples were prepared as previously described (McClung et al., 2007). Briefly, sections were exposed to primary antibodies specific to dystrophin (Thermo Scientific, Rockford, IL), MHC type I (Development Studies Hybridoma Bank, Iowa City, IA) and MHC type IIa (Developmental Studies Hybridoma Bank, Iowa City, IA). Sections were incubated with rhodamine red anti-rabbit secondary antibody, Alexa Fluor 350 goat anti-mouse secondary antibody and Alexa Fluor 488 goat anti-mouse secondary antibody (Molecular Probes, Eugene, OR). Sections were washed, mounted and sealed with a cover slip and viewed under an inverted fluorescence microscope (Carl Zeiss Axiovert 200). Images were acquired at 10X and analyzed for myofiber cross-sectional area (μm2) using Scion Image software.
Immunoblotting
Whole muscle homogenates were prepared as previously described (Adhihetty et al., 2009). Proteins were separated on 8–15% polyacrylamide gels and transferred to nitrocellulose membranes. Blots were blocked for 1 h in StartingBlock T20 (Thermo Scientific) and probed with the appropriate primary antibody (1:200 for Tom40; 1:500 for pAMPKα, T-AMPKα, p-p38, T-p38, Tim23, PGC-1α, NRF-1, SIRT3, RIP140, Mfn2, Opa1, Drp1; 1:1,000 for Tfam, mtHsp70, cHsp70, Hsp60, Tom22, Fis1; 1:5,000 for actin). Following, blots were incubated with anti-rabbit secondary antibodies at a dilution of 1:1,000 (PGC-1α, Mfn2, Fis1), 1:1,500 (p-AMPK, T-AMPK, p-p38, T-p38, SIRT3) or 1:2,500 (RIP140, Tfam, NRF-1), anti-mouse secondary at a dilution of 1:1,000 (Opa1, Drp1, Tim23, Tom22, mtHsp70, cHsp70, Hsp60) or 1:10,000 (actin). Blots were washed, exposed to the DuoLux chemiluminescence kit, and proteins visualized using a ChemiDoc XRS imager from Biorad. A description of antibodies used in this study can be found in Table 3 of the Supporting Information. Immunoblotting of actin or Ponceau S staining was used to normalize for the amount of protein loaded. Bands were quantified using Biorad Image Lab software.
Statistical Analysis
Data were initially analyzed for normal distribution and homogeneity of variance. For continuous variables, descriptive statistics were evaluated using a one-way analysis of variance (ANOVA; Table 1). The Tukey’s post-hoc test was applied to identify individual group differences. The chi-square test was used to evaluate categorical descriptive variables. Primary outcomes were evaluated using an ANOVA with Tukey follow-up. Where differences were observed between HF and LF groups (muscle size, state 4 respiration, cHsp70 protein), a follow-up one-way analysis of covariance (ANCOVA) was performed to adjust for potential confounders, e.g. age and gender. Measures of functional status obtained from elderly subjects were analyzed using an independent sample t-test. Pearson’s correlation coefficient (r) was performed to assess associations between PGC-1α and mitochondrial content, as well as gait speed. Differences were considered statistically significant if P < 0.05. Data are presented as mean ± standard error (SE).
Supplementary Material
Acknowledgments
This study was supported by the University of Florida (UF), Claude D. Pepper Older Americans Independence Center (OAIC). The OAIC is funded by a grant from the NIH/NIA (1P30AG028740).
We are grateful to the OAIC Recruitment Core at UF, as well as the technical assistance of L.A. Gilbert and L. J. Myers. We would like to thank all subjects for their participation in this study.
Footnotes
Author Contributions
A.-M. J. and C.L. were responsible for all aspects of the study. P. J. A. performed experiments and assisted with the manuscript write-up. J. M. A. and B. D. S. performed muscle biopsies. T. W. B., L.M.N., H. A. L., S. E. W., T. M. M., E. M., and M. P. contributed to the design of the original study, sample preparation, and the manuscript.
Table 3: Description of commercially available antibodies used in the study.
References
- Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1alpha on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol. 2009;297:C217–C225. doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- Andersen JL. Muscle fibre type adaptation in the elderly human muscle. Scand J Med Sci Sports. 2003;13:40–47. doi: 10.1034/j.1600-0838.2003.00299.x. [DOI] [PubMed] [Google Scholar]
- Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005;280:35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Betik AC, Krause DJ, Hepple RT. No decline in skeletal muscle oxidative capacity with aging in long-term calorically restricted rats: effects are independent of mitochondrial DNA integrity. J Gerontol A Biol Sci Med Sci. 2006;61:675–684. doi: 10.1093/gerona/61.7.675. [DOI] [PubMed] [Google Scholar]
- Barrientos A, Casademont J, Rotig A, Miro O, Urbano-Marquez A, Rustin P, Cardellach F. Absence of relationship between the level of electron transport chain activities and aging in human skeletal muscle. Biochem Biophys Res Commun. 1996;229:536–539. doi: 10.1006/bbrc.1996.1839. [DOI] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci. 2010;65:532–537. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, Leeuwenburgh C, Manini TM. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012;47:38–44. doi: 10.1016/j.exger.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Pahor M, Bartali B, Cherubini A, Penninx BW, Williams GR, Atkinson H, Martin A, Guralnik JM, Ferrucci L. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79:289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier A, Fortin D, Zoll J, N’Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005;19:43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- Hiona A, Leeuwenburgh C. The role of mitochondrial DNA mutations in aging and sarcopenia: implications for the mitochondrial vicious cycle theory of aging. Exp Gerontol. 2008;43:24–33. doi: 10.1016/j.exger.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiona A, Sanz A, Kujoth GC, Pamplona R, Seo AY, Hofer T, Someya S, Miyakawa T, Nakayama C, Samhan-Arias AK, Servais S, Barger JL, Portero-Otin M, Tanokura M, Prolla TA, Leeuwenburgh C. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS One. 2010;5:e11468. doi: 10.1371/journal.pone.0011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries AD, Streimann IC, Stojanovski D, Johnston AJ, Yano M, Hoogenraad NJ, Ryan MT. Dissection of the mitochondrial import and assembly pathway for human Tom40. J Biol Chem. 2005;280:11535–11543. doi: 10.1074/jbc.M413816200. [DOI] [PubMed] [Google Scholar]
- Hurley BF. Age, gender, and muscular strength. J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):41–44. doi: 10.1093/gerona/50a.special_issue.41. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Ljubicic V, Adhihetty PJ, Hood DA. Biogenesis of the mitochondrial Tom40 channel in skeletal muscle from aged animals and its adaptability to chronic contractile activity. Am J Physiol Cell Physiol. 2010;298:C1308–C1314. doi: 10.1152/ajpcell.00644.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph AM, Rungi AA, Robinson BH, Hood DA. Compensatory responses of protein import and transcription factor expression in mitochondrial DNA defects. Am J Physiol Cell Physiol. 2004;286:C867–C875. doi: 10.1152/ajpcell.00191.2003. [DOI] [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van RH, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Nair KS. Mitochondrial function as a determinant of life span. Pflugers Arch. 2010;459:277–289. doi: 10.1007/s00424-009-0724-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57 doi: 10.2337/db08-0349. f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lexell J, Taylor CC. Variability in muscle fibre areas in whole human quadriceps muscle: effects of increasing age. J Anat. 1991;174:239–249. [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in Skeletal Myocytes: A Potential Target for Interventions against Sarcopenia and Physical Frailty - A Mini-Review. Gerontology. 2011;58:99–106. doi: 10.1159/000330064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzetti E, Lees HA, Manini TM, buford TW, Aranda JM, Jr, Calvani R, Capani G, Marsiske M, Lott DJ, Vandenborne K, Bernabei R, Pahor M, Leeuwenburgh C, Wohlgemuth SE. Skeletal muscle apoptotic signaling predicts thigh muscle volume and gait sped in community-dwelling older persons: an exploratory study. PLoS One. 2012;7:e32829. doi: 10.1371/journal.pone.0032829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585:203–215. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratil M, Terman A, Arriaga EA. Giant mitochondria do not fuse and exchange their contents with normal mitochondria. Exp Cell Res. 2008;314:164–172. doi: 10.1016/j.yexcr.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, III, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise G, Brose AN, Tarnopolsky MA. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp Gerontol. 2005;40:173–180. doi: 10.1016/j.exger.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Powelka AM, Seth A, Virbasius JV, Kiskinis E, Nicoloro SM, Guilherme A, Tang X, Straubhaar J, Cherniack AD, Parker MG, Czech MP. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen UF, Krustrup P, Kjaer M, Rasmussen HN. Human skeletal muscle mitochondrial metabolism in youth and senescence: no signs of functional changes in ATP formation and mitochondrial oxidative capacity. Pflugers Arch. 2003;446:270–278. doi: 10.1007/s00424-003-1022-2. [DOI] [PubMed] [Google Scholar]
- Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De BG. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, deBeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;5:e10778. doi: 10.1371/journal.pone.0010778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123:2533–2542. doi: 10.1242/jcs.070490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, Nair KS. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Chesley A, Freyssenet D, Hood DA. Contractile activity-induced adaptations in the mitochondrial protein import system. Am J Physiol. 1998;274:C1380–C1387. doi: 10.1152/ajpcell.1998.274.5.C1380. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.