Abstract
Epithelial cell adhesion molecule (EpCAM) is a single-transmembrane protein, which is involved in numerous cellular processes including cell adhesion, proliferation, maintenance of stemness of embryonic cells and progenitors, migration and invasion. Activation of signal transduction by EpCAM is warranted by regulated intramembrane proteolysis and nuclear translocation of the intracellular domain EpICD. Here, we describe matrix metalloproteinase 7 (MMP7) as a target gene of EpCAM signalling viaEpICD nuclear translocation. EpCAM and MMP7 expression pattern and levels positively correlated in vitro and in vivo, and were strongly elevated in primary carcinomas of the head and neck area. Hence, MMP7 is a novel target of EpCAM signalling.
Keywords: epithelial cell adhesion molecule, epithelial adhesion molecule cell intracellular domain, matrix metalloprotease 7
Introduction
Epithelial cell adhesion molecule (EpCAM) is an integral transmembrane protein involved in homophilic cell adhesion (Litvinov et al. 1994) and signal transduction to induce cell proliferation (Munz et al. 2004, 2009; Osta et al. 2004). Human EpCAM signalling is triggered upon cell-to-cell contact and subsequent regulated intramembrane proteolysis, which includes sequential cleavages by tumour necrosis factor alpha–converting enzyme (TACE) in the extracellular domain, generating the shed ectodomain EpEX, and by a gamma-secretase complex incorporating presenilin-2 (PS2) (Denzel et al. 2009; Maetzel et al. 2009). Cleavage(s) by the gamma-secretase complex result(s) in the formation and release of the signalling intracellular domain termed EpICD, which translocates into the nucleus in complex with members of the Wnt signalling (FHL2, β-catenin, Lef-1) and contacts DNA to regulate gene transcription in cancer and stem cells (Maetzel et al. 2009; Lu et al. 2010; Chaves-Perez et al. 2012). Target genes of EpCAM signalling in cancer comprise most notably genes involved in the regulation of the cell cycle (Maaser & Borlak 2008), with the proto-oncogenes c-Myc and cyclin D1 representing the most prominent members (Munz et al. 2004; Chaves-Perez et al. 2012). In embryonic stem cells, EpICD is recruited to promoters of genes associated with stem cell rather than differentiated features such as c-Myc, Oct-4, Sox2 and Nanog (Lu et al. 2010). Although expressed in various simple epithelia, EpCAM is expressed more strongly in carcinomas and even more so in tumour-initiating cells (also termed cancer stem cells) (Balzar et al. 1999; Marhaba et al. 2008; Gires et al. 2009; Gires 2012, 2011). Regulatory elements of the epcam gene promoter are responsive to Tcf4, a downstream effector of the Wnt pathway (Yamashita et al. 2007). Owing to the frequent deregulation of the Wnt pathway in cancer cells and to its functions in progenitor cells (Reya & Clevers 2005), a concomitant up-regulation of EpCAM in these cell types may possibly be mediated by the Wnt pathway. Depending on the cell type, the microenvironment it resides in and the availability of interaction partners and activating proteases, a cell will have the aptitude either to trigger proliferation via regulated intramembrane proteolysis of EpCAM and activation or repression of target genes, or to induce cell adhesion via homophilic interactions of EpCAM molecules (Gires #b2012, 2011).
Another reported major function of EpCAM refers to the migratory and invasive phenotype of breast carcinoma cell lines. Strong EpCAM overexpression was associated with enhanced invasion of cell lines into extracellular matrix (Osta et al. 2004). More recent studies on upstream regulators disclosed that binding of the tumour suppressor p53 to promoter elements of the epcam gene silenced EpCAM expression and decreased the invasive potential of breast carcinoma cells. Inversely, inhibition of p53 expression increased both EpCAM expression and invasion, a phenotype that was reversed upon transcriptional repression of EpCAM with siRNA treatment (Sankpal et al. 2009). Downstream effectors of EpCAM with respect to migration and invasion are so far unexplored. In this respect, we have analysed the genes regulated upon repression of EpCAM and selected genes involved in invasion and matrix degradation. We describe in more detail matrix metalloproteinase 7 (MMP7) as a novel target gene of EpCAM signalling. MMP7 is a target of EpCAM, which is induced at the transcriptional level upon nuclear translocation of EpICD. Expression patterns and levels of EpCAM and MMP7 correlated closely in vivo in tumours suggesting that there is biological relevance of this association during malignancy.
Materials and methods
Cell lines, plasmids and cell counting
Human embryonic kidney HEK293 cells, HeLa cervix and FaDu hypopharynx carcinoma cells were purchased from ATCC. Transfectants were generated upon magnet-assisted transfection (MaTra; Iba, Göttingen, Germany). EpICD was cloned into the expression vector pCAG141 to generate pCAG-EpICD. Empty pCAG served as a vector control in each experiment. EpICD was fused to a mutated ligand-binding domain of the human oestrogen receptor (ERT, kind gift of Prof. Dr. Georg Bornkamm) and cloned into pCAG141. Induction of nuclear translocation of EpICD-ERT was accomplished with 100 nM 4-hydroxytamoxifen (Sigma, Munich, Germany). HEK293 transfectants, HeLa and FaDu wild-type cells were cultured in DMEM with 10% foetal calf serum (FCS). Selection of stable transfectants of HeLa cells expressing EpICD-ERT was achieved with standard DMEM medium supplemented with 1 μg/ml puromycin (Sigma). Cell numbers were assessed at different time points upon trypan blue exclusion assay.
Flow cytometry
Cells were stained with an anti-EpCAM antibody (HO.3, kind gift from Dr. H. Lindhofer, Germany) diluted 1:50 in PBS-3%-FCS for 10 min at RT, washed three times in PBS-3%-FCS and stained with an fluorescein isothiocyanate–conjugated secondary antibody. For control stainings, the primary antibody was omitted. Measurement of cell surface expression of EpCAM was performed in a FACSCalibur device (BD Pharmingen, Heidelberg, Germany).
Semi-quantitative RT-PCR
Total RNA from cell lines was isolated using the High Pure RNA Isolation Kit (Qiagen RNeasy, HIlden, Germany), and cDNA was generated using the reverse transcription system (Promega, Madison, US) according to the manufacturer's instructions. Semi-quantitative PCR analysis for the expression of epcam, mmp7 and gapdh (95 °C for 30 s, annealing for 30 s, 72 °C for 30 s) was conducted with specific primers: GAPDH 258 bp: FW: 5′-tgtcgctgttgaagtcagaggaga-3′, BW: 5′-agaacatcatccctgcctctactg-3′; MMP7 (311 bp): FW: 5′-gagtgccagatgttgcagaa-3′, BW: 5′-gtgagcatctcctccgagac-3′; EpCAM (777 bp): 5′-actgtcatttgctcaaagctggctgcc-3′, BW: 5′-tgcattgagttccctatgcatctc-3′.
siRNA treatment
FaDu carcinoma cells (3 × 105) were transiently transfected using the MaTra system (Iba) with 100 nM of a control siRNA or the EpCAM-specific siRNA.
Control siRNA: 5′-UCGUCCGUAUCAUUUC AAU-3′
EpCAM siRNA: 5′-UGCCAGUGUACUUCAGU UG-3′
EpCAM siRNA binding site corresponds to nucleotides coding for amino acid sequence 47QCTSVG52 (N- to C-terminus) within the extracellular domain of EpCAM. EpICD-YFP mRNA is not a target for EpCAM siRNA, and hence, EpICD-YFP expression remained unaffected.
Human materials, immunohistochemistry, immunocytochemistry and immunofluorescence stainings
Samples were obtained from cases of HNSCC and from normal mucosas based on institutional ethics approval by the ethics committee of the local medical faculty (Ethikkommision der Medizinischen Fakultät der Ludwig-Maximilians-Universität München) and written informed consent. Samples originated from the hypopharynx, tonsils and the vallecula with a status range T3-T4, N0-N2b, M0 and G3, according to UICC TNM staging (O'Sullivan & Shah 2003). Frozen sections of 4 μm thickness were cut on Superfrost plus slides and subjected to immunohistochemical analysis using the avidin–biotin–PO complex method (Vectastain; Vector laboratories, Burlingame, CA, USA) according to the manufacturer′s protocol. In brief, sections were fixed for 5 min at room temperature (RT) in acetone, followed by incubation with fresh 3.5% paraformaldehyde for an additional 5 min at RT. Endogenous peroxidase activity was blocked using 0.03% H2O2 in PBS for 10 min, followed by overnight incubation at 4°C with EpCAM VU1D9-specific (Cell Signaling Technology, Danvers, MA, USA) (Spizzo et al. 2011), MMP7-specific (Millipore, Billerica, MA, USA) (Fang et al. 2010) and Ki67-specific (Dako Diagnostica, Hamburg, Germany) antibodies. Thereafter, sequential incubations with biotinylated anti-mouse secondary antibody for EpCAM- and Ki67-antibody, respectively, and biotinylated anti-rabbit secondary antibody for MMP7 antibody were applied followed by a peroxidase labelled avidin–biotin complex (Vector laboratories). Amino-ethyl-carbazole (AEC) substrate was used for the detection of antigen/antibody complexes, generating a red-brown staining. Counterstaining was achieved with haematoxylin (blue). Negative controls were conducted simultaneously using mouse or rabbit isotype control antibody (cell signalling). All samples were judged independently and in a blinded fashion by two scientists.
HeLa transfectants were analysed in a fluorescence laser scanning system (TCS-SP2 scanning system and DM IRB inverted microscope; Leica, Solms, Germany). For EpICD-ERT detection, cells were fixed according to a protocol described previously (Brock et al. 1999) and stained with an EpICD-specific antibody and Alexa 647-labelled secondary antibody, followed by Hoechst labelling of nuclear DNA (Sigma).
Quantitative real-time PCR (qPCR)
Total RNA from transiently transfected cells was isolated using the RNeasy kit (Qiagen). First-strand cDNA was synthesized using 1 μg of total RNA (DNase-treated) in a 20 μl reverse transcriptase reaction mixture (QuantiTect Rev. Transcription Kit; Qiagen). qPCR was performed on a LightCycler 480 II PCR system (Roche Applied Science, Indianapolis, IN, USA) using LightCycler probes master mix (Roche Applied Science). TaqMan gene expression assays for EpCAM and MMP7 were purchased as predesigned gene expression assays (Applied Biosystems, Life Technologies, Foster City, CA, US). Primers and TaqMan probe for the endogenous control HPRT1 were designed according to sequences from the nucleotide sequence database NCBI using ABI Prism Primer Express Software 2.0.0 (Applied Biosystems): HPRT1 FW: 5′-gctttccttggtcaggcagta-3′, BW: 5′-gtctggcttatatccaacacttcgt-3′, probe: 5′-gtctggcttatatccaacacttcgt-3′. All probes were spanning an exon–exon boundary. Primers and probes were applied in a final concentration of 800 and 150 nM respectively. Expression levels were normalized to the endogenous control HPRT1 according to the equation 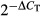 , where ΔCT was defined as CT gene of interest – CTHPRT1.
, where ΔCT was defined as CT gene of interest – CTHPRT1.
Luciferase reporter assay
FaDu hypopharynx carcinoma cells (3 × 105 cells per well) were transfected with 1.5 μg wild-type or mutated MMP7 reporter constructs (Brabletz et al. 1999) (kind gift of Prof. Dr. Thomas Brabletz) and EpCAM-specific or control siRNA (100 nM). Luciferase activity was determined after 24 h using commercially available assay systems (Biothema, Handen, Sweden) and normalized for CMV-β-galactosidase activity of a co-transfected plasmid (0.1 μg per well).
Enzyme-linked immunosorbent assay (ELISA)
Cells were plated and optionally treated with EpCAM or control siRNA. Two days after plating, cells were collected and the ELISA was performed according to the instructions of R&D Quantikine, Human MMP7 ELISA Kit manual (R&D Systems, Minneapolis, MN, USA).
Gene expression analysis
HNSCC line FaDu was transiently transfected with siRNA oligonucleotides representing control siRNA and EpCAM siRNAs. Suppression of EpCAM expression was assessed upon RT-PCR, qRT-PCR and FACS using specific antibodies. EpCAM suppression at the cell surface was commonly in a range of 40–50% compared with control siRNA. After 24–48 h, cells were harvested and total RNA extracted using the RNeasy kit (Qiagen). RNA purity was assessed in a bioanalysis, and RIN values were 10. RNA from control and EpCAM siRNA–transfected cells were then subjected to a whole-genome Agilent array for the determination of transcriptional regulation associated with EpCAM suppression. The cut-off was set to greater than two-fold up- and down-regulation. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE37257 (Edgar et al. 2002) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE37257).
Statistics
The statistical distribution of the different data sets was determined using the Kolmogorov–Smirnov test. All data sets followed a Gaussian distribution. Therefore, Student's t-test was applied to calculate the statistical significance of differences between treatment groups. P-values below 0.05 were considered significant (*P < 0.05; **P < 0.01; ***P < 0.001). Bars and error bars in the histograms represent mean values ± SD of at least three independent experiments.
Results
MMP7 expression is regulated by EpCAM
FaDu hypopharynx carcinoma cells were transiently transfected with a control siRNA or an EpCAM-specific siRNA. After 2 days, expression of EpCAM was assessed upon flow cytometry with antibodies, which specifically bind within the EGF-repeat in the extracellular domain of EpCAM (Balzar et al. 1999). Compared to control siRNA, EpCAM siRNA induced a mean 43% reduction in EpCAM expression at the cell surface (Figure 1a). Reduction in EpCAM expression upon siRNA treatment was confirmed by RT-PCR and qPCR (Figure 1c,d). EpCAM down-regulation resulted in diminished cell proliferation assessed upon cell counting. The observed 43% decrease in cell surface expression of EpCAM translated in cell numbers diminished by 35% (Figure 1b). This is a confirmation of earlier findings on the reduced proliferation of breast and colon cancer cell lines following siRNA-mediated knock-down of EpCAM (Munz et al. 2004; Osta et al. 2004; Maetzel et al. 2009), which validates the settings of EpCAM knock-down used in this study. Equal amounts of total RNA (RIN score = 10) from control and EpCAM siRNA–treated FaDu cells were next subjected to a single, explorative cDNA microarray analysis with the aim of identifying potential target genes of the signal transduction cascade of EpCAM. Results of the cDNA array are deposited in the form of an NCBI GEO file GSE37257. All genes, which were up- or down-regulated by at least twofold (greater than two-fold; 716 genes up-regulated, 552 genes down-regulated), were then subjected to a Panther classification software analysis (http://www.pantherdb.org/). Special emphasis was put on the regulation of genes associated with signalling pathways. Angiogenesis, Alzheimer′s disease-presenilin pathway, Wnt signalling pathway, inflammation and cadherin signalling pathways were most strongly affected by EpCAM knock-down (Figure S1a). Messenger RNA levels of selected genes differently regulated following partial knock-down of EpCAM included the co-regulated genes for secreted frizzled-related protein 5, protocadherin 10, dickkopf homologue 2, MMP24, SPARC, Myst3, ErbB4, MMP12, WISP2, MMP7, Nanos1 and WNT9A, and the counter-regulated genes for MMP11, fibronectin 1, DACT3, WNT2B, WNT5A, APC2, Spondin 1 and cateninα2. All genes except for protocadherin 10, Myst3, ErbB4 and WNT2B were validated in RT-PCR assays (Figure S1b). As several matrix metalloproteases were regulated after EpCAM knock-down and EpCAM is known to correlate with invasion, we chose this protein family for further in-depth analysis. MMP7 is a soluble factor, which, unlike other family members, is preferentially expressed in epithelial cells (Wilson et al. 1995) and is a target of the Wnt signalling pathway (Brabletz et al. 1999). As EpCAM also displays highest expression in epithelia and signals in cooperation with components of the Wnt signalling pathway, we chose to proceed with the analysis of MMP7. SiRNA-mediated knock-down of EpCAM resulted in a comparable 50% down-regulation of MMP7 mRNA levels in RT-PCR and qPCR (Figure 1c,d, respectively).
Figure 1.
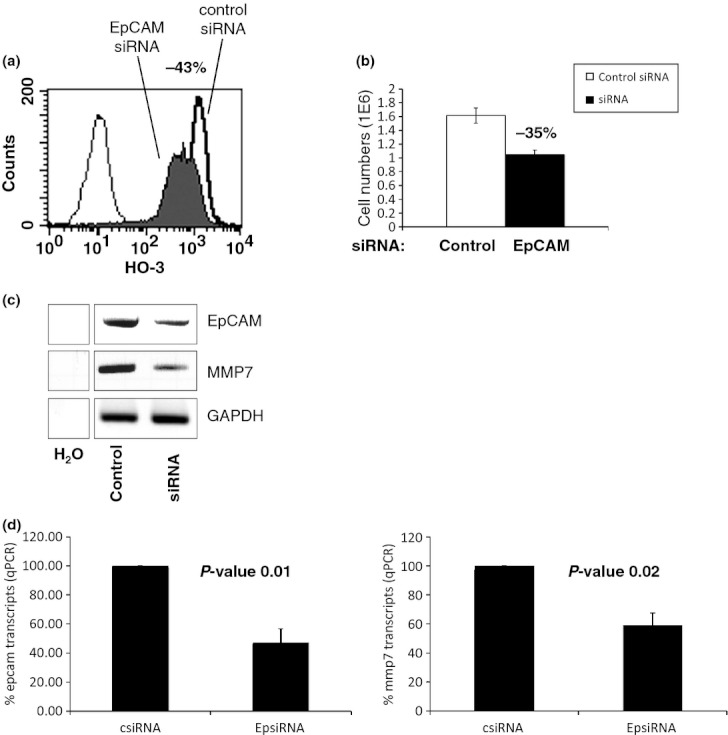
MMP7 expression correlates with EpCAM. (a) FaDu hypopharynx carcinoma cells were transiently transfected with a control siRNA or an EpCAM-specific siRNA. Expression levels of EpCAM were assessed upon flow cytometry with specific antibodies (HO-3) in both samples. Control transfectants are represented with a solid line and EpCAM siRNA transfectants with a solid histogram. EpCAM siRNA induced a mean 43% reduction in EpCAM expression at the cell membrane. Control staining is displayed as a thin-lined graph. (b) Numbers of viable FaDu cells after transfection with control or EpCAM siRNA were determined in a standard trypan blue exclusion assay after 2 days. Shown are the mean and standard deviations of three independent experiments. Cell numbers were reduced by 35% in average after transfection of EpCAM-specific siRNA. Open graph represents mean values of FaDu cells transfected with control siRNA and solid graph EpCAM siRNA. (c) Levels of EpCAM, MMP7 and GAPDH mRNAs were determined upon RT-PCR in FaDu transfectants. Two days after transfection of control or EpCAM siRNA, cells were harvested, mRNA was isolated, and EpCAM, MMP7 and GAPDH levels were assessed with specific primer pairs. Shown are representative results from three independent experiments. H2O served as a negative control. (d) Same as in (c) except mRNA measurement was conducted via quantitative PCR. Shown are% EpCAM (left panel) and MMP7 (right panel) mRNA expression levels from two independent experiments. P-values are indicated for each subfigure.
EpCAM and MMP7 expression in vivo in head and neck squamous cell carcinomas
Co-regulation of mmp7 and epcam mRNA levels suggested a similar expression pattern and level of both proteins in tissues in vivo. Normal and hyperplastic mucosas, and carcinomas of the hypopharynx were analysed upon immuno-histochemistry. Consecutive serial sections were stained with antibodies specific to EpCAM and MMP7. EpCAM was primarily detected in the first suprabasal membrane layers of normal mucosas and showed a slightly increased expression in successional suprabasal layers in hyperplastic mucosas, with an increasing proportion of positive cells (Figure 2). Overexpression of both EpCAM and MMP7 and increased expression coverage throughout tissue were observed in carcinomas as compared with normal mucosa. Furthermore, MMP7 expression positively correlated with the patterns of EpCAM in the great majority of cases (Figure 2). This tight correlation was further seen in single tumour cells detached from the bulk of malignant cells, which commonly displayed equal staining levels for EpCAM and MMP7 (see Figure 2, lower panel, cells marked with asterisk).
Figure 2.
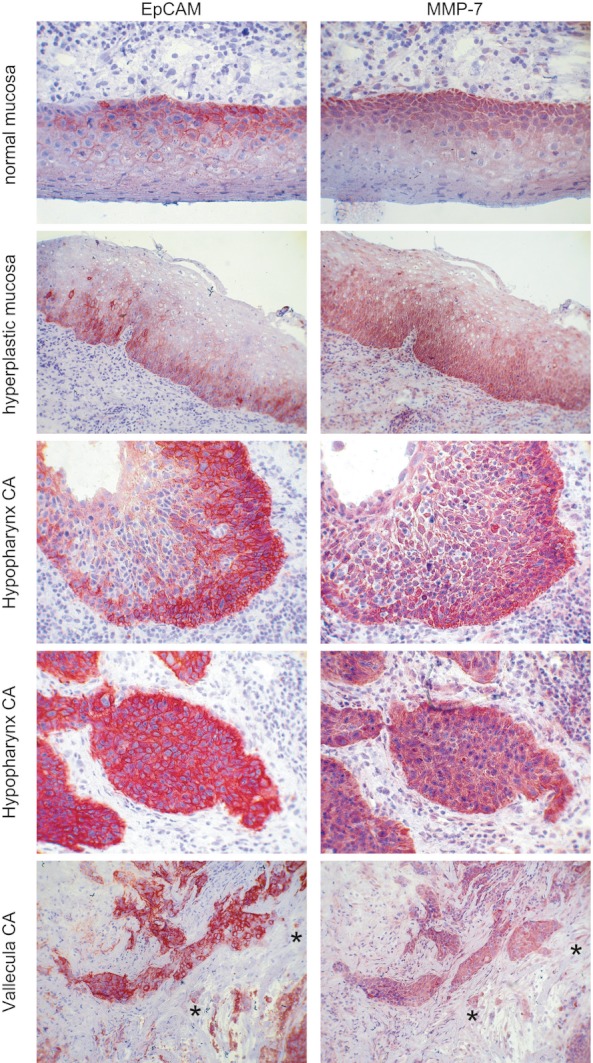
EpCAM and MMP7 expression correlates in vivo in primary human samples. Serial cryosections of normal and dysplastic mucosa, and head and neck carcinoma samples were stained with EpCAM- or MMP7-specific antibodies (red staining). Shown are representative examples. EpCAM and MMP7 pattern and expression levels tightly correlated, even at the single-cell levels (exemplified with *).
However, in distinct cases, EpCAM and MMP7 staining patterns were non-coincident or even oppositely correlated. Representative examples of coincident, non-coincident and opposing staining patterns of EpCAM and MMP7 are depicted in Figure 3. To quantify the correlation between EpCAM and MMP7 expression patterns, areas of tumour cells and of epithelial cells in normal mucosas were compared in consecutive sections. Both expression pattern and intensity were addressed. In HNSCC, EpCAM and MMP7 expression coincided in 66% of cases, were non-coincident in 27% and opposing in 7% of samples (Figure 4a). In 86% of samples staining intensities were similar, while 14% of samples were characterized by opposing staining intensities. In normal mucosa, only 50% of samples showed coincident staining patterns of EpCAM and MMP7, while 40% were non-coincident and 10% opposing. Comparably to the pattern seen in tumours, EpCAM and MMP7 expression intensities were similar in 90% and opposing in 10% of cases (Figure 4b).
Figure 3.
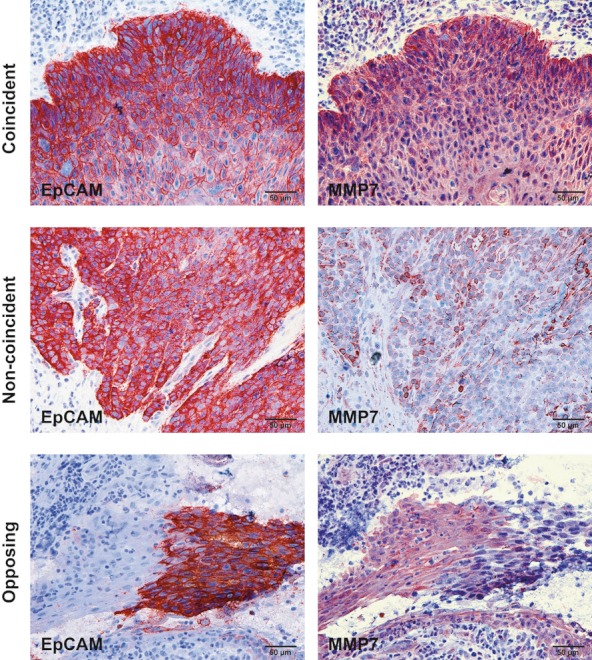
Coincident, non-coincident and opposing expression patterns of EpCAM and MMP7. Serial cryosections of normal and dysplastic mucosa, and head and neck carcinoma samples were stained with EpCAM- or MMP7-specific antibodies (red staining). Shown are representative examples of coincident, non-coincident and opposing expression patterns of EpCAM and MMP7.
Figure 4.
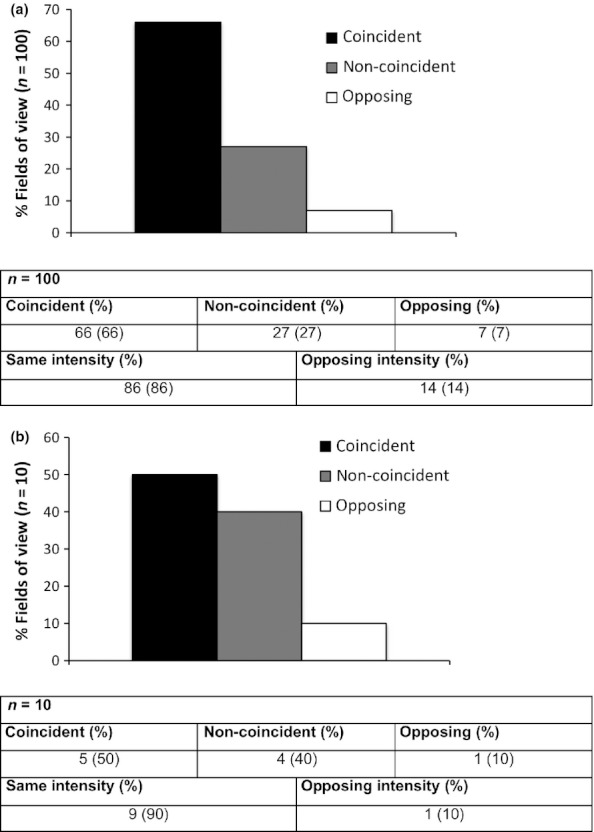
EpCAM and MMP7 expression correlates in vivo in primary human samples. Serial cryosections of normal (n = 10) and head and neck carcinoma samples (n = 20) were stained with EpCAM- or MMP7-specific antibodies. Fields of view (FOV) of normal mucosa (one FOV/sample) and tumours (five FOV/sample) were assessed with respect to staining patterns and intensity of EpCAM and MMP7. Results are given as percentages of FOV with coincident, non-coincident or opposing patterns for tumours (a) and normal mucosas (b) Similarities in staining intensities are given in each table.
mmp7 regulation depends on EpICD
To discriminate mmp7 regulation associated with EpCAM signalling from concomitant effects related to additional functions of EpCAM such as cell adhesion, the signalling moiety EpICD was co-transfected with EpCAM-specific siRNA. mmp7 mRNA levels were reconstituted to standard levels after treatment of cells with EpCAM-specific siRNA and simultaneous overexpression of EpICD (Figure 5a), confirming that mmp7 is a target gene of EpCAM signalling. As a positive control, expression levels of c-myc, which is a reported transcriptional target of EpCAM (Maetzel et al. 2009), were assessed in parallel. Like mmp7, c-myc expression was diminished after the treatment of FaDu cells with EpCAM siRNA and reconstituted upon EpICD overexpression (Figure S2). For the case of the target gene wisp-2 (Wnt-1-inducible signalling pathway protein 2), complementation of FaDu cells with EpICD was not sufficient to restore initial mRNA levels (Figure 5b), suggesting that wisp-2 down-regulation following EpCAM knock-down is not a direct signalling effect of EpCAM. Complementation of mmp7 mRNA levels upon co-expression of EpICD was corroborated using quantitative PCR approaches (Figure 5c).
Figure 5.
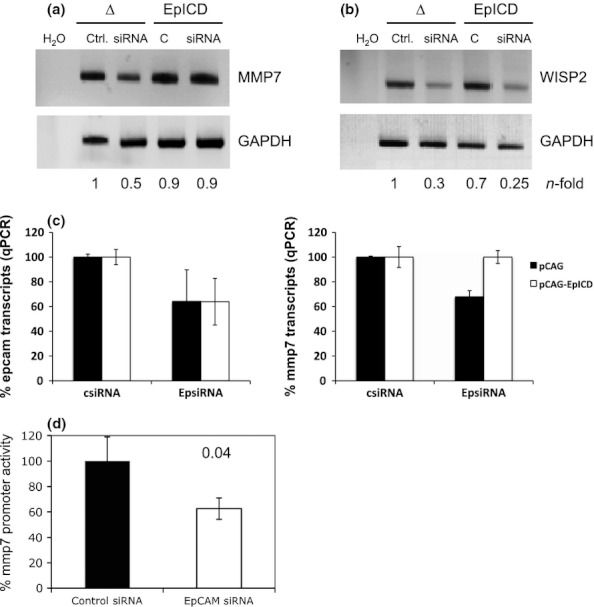
MMP7 is a signalling target of EpCAM. (a) FaDu cells were transiently transfected with control or EpCAM siRNA to regulate EpCAM expression. Simultaneously, cells were co-transfected with a control pCAG or pCAG-EpICD plasmid. After 2 days, cells were harvested, mRNA was isolated, and MMP7 and GAPDH levels were assessed with specific primer pairs. Shown are representative results from three independent experiments. H2O served as a negative control. (a) Same as in (a) except primer pairs specific to WISP2 have been used. (c) Same as in (a) except mRNA measurement was conducted via quantitative PCR using HPRT1 as endogenous control. Shown are% epcam (left panel) and mmp7 (right panel) mRNA expression levels from two independent experiments. (d) FaDu cells were transiently transfected with control or EpCAM siRNA to regulate EpCAM expression. Simultaneously, cells were co-transfected with luciferase reporter plasmid for the MMP7 promoter region. As a control, luciferase reporter plasmid for the MMP7 promoter region, which carried mutated Tcf consensus sequences, was transfected in parallel. Promoter activation was calculated as the ratio of non-mutated vs. mutated reporter, and values after transfection of control siRNA were set to 100%. Shown are mean values and standard deviations of three independent experiments performed in duplicates. P-values are indicated for each subfigure.
Regulation of the mmp7 promoter by EpCAM was further addressed in luciferase reporter assays. Transcription of the mmp7 gene is dependent on Wnt signalling, β-catenin and Lef-1 consensus sites (Brabletz et al. 1999). Therefore, the mmp7 promoter and a variant, which carries mutations in the described Tcf binding sites (Brabletz et al. 1999), were used to drive the expression of the luciferase gene. FaDu cells were transiently transfected with reporter plasmids together with control or EpCAM specific siRNA. After 2 days, activation of the mmp7 promoter was measured as the activity of luciferase as a surrogate marker and with luciferin as a substrate. mmp7 promoter activity is given as the ratio of wild-type and mutated reporter constructs to assess Tcf-specific EpCAM-mediated effects on mmp7 activation. Suppression of EpCAM expression with specific siRNAs resulted in a 40% reduction in mmp7 promoter activity, which was in accordance with knock-down efficiencies of EpCAM siRNAs (Figure 5d).
Nuclear translocation of EpICD is essential for mmp7 transcription
As complementation of EpCAM with the signalling moiety EpICD was instrumental with respect to the regulation of mmp7 transcription, we next analysed the dependency of mmp7 regulation upon nuclear translocation of EpICD. HeLa cervix carcinoma cells were stably transfected with a fusion protein consisting of EpICD and the oestrogen receptor ligand-binding domain termed EpICD-ERT. As a control, cells were transfected with ERT only. Nuclear translocation of EpICD-ERT is controlled upon the addition of 4-hydroxytamoxifen (4-OHT) to the culture medium. In the absence of 4-OHT, EpICD-ERT is retained in the cytoplasm of cells, while it translocates into the nucleus after the addition of 4-OHT (Figure 6a). Nuclear translocation of EpICD-ERT was associated with the induction of described target genes of EpCAM such as c-myc (Figure 6b). In this cellular background, levels of mmp7 mRNA were analysed in a time course in the presence or absence of 4-OHT in the culture medium. Two hours after the induction of EpICD nuclear translocation, levels of mmp7 mRNA were enhanced, which was most consistent after 4 h (Figure 6c). Hence, nuclear translocation of EpICD induced mmp7 transcription.
Figure 6.
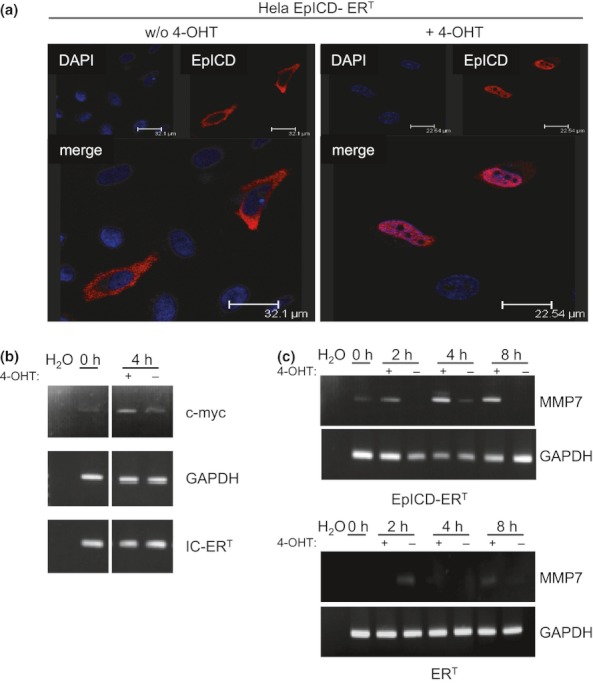
Nuclear translocation of EpICD is essential for transcriptional induction of MMP7. (a) HeLa cells transiently transfected with EpICD-ERT were cultured in the presence or absence of 100 nM 4-hydroxytamoxifen (4-OHT). EpICD-ERT was stained with EpICD-specific antibodies (red) and the localization assessed using confocal laser scanning microscopy. Nuclei were stained with DAPI (blue). Shown are representative images of three independent experiments. (b) At the indicated time points (0 and 4 h post-treatment), mRNAs from non-induced and induced cells were isolated, and the expression of c-myc, GAPDH and IC-ERT was assessed upon RT-PCR with specific primer pairs. Shown are representative results of three independent experiments. H2O served as a negative control. (c) Transient HeLa transfectants expressing ERT or EpICD-ERT were treated with 4-OHT (+) or with solvent only (−), for the indicated time periods (0, 2, 4 and 8 h). mRNA levels of MMP7 and GAPDH were assessed upon RT-PCR with specific primer pairs. Shown are representative results of three independent experiments. H2O served as a negative control.
Release of soluble MMP7 correlates with EpCAM expression
In the next experiments, the potential association of EpCAM expression with levels of soluble MMP7 was assessed in an ELISA. Three cell lines with differing levels of EpCAM expression were tested for the presence of MMP7 in the cell culture supernatants. FaDu (hypopharynx carcinoma), HeLa (cervix carcinoma) and HEK293 (adenovirus-immortalized embryonic kidney cells) were first assessed for the expression of EpCAM at the cell surface using immunostaining and flow cytometry. FaDu cells displayed highest amounts of plasma membrane-associated EpCAM, while HeLa displayed minute amounts, and HEK293 cells revealed negative for EpCAM (Figure 7a). Mean fluorescence intensities of EpCAM expression are summarized in the graph in the left lower panel of Figure 7a. Amounts of MMP7 in the supernatants of these three cell lines positively correlated with EpCAM levels at the cell surface (Figure 7a, lower right panel). As this correlation might reflect a concomitant expression rather than a causal relation, FaDu cells were transiently transfected with EpCAM-specific siRNA to address changes in MMP7 secretion. The efficiency of EpCAM knock-down was measured by immunostaining and flow cytometry and revealed to be 60% on average (Figure 7b, left panel). As a result of EpCAM knock-down, MMP7 levels in the supernatant of EpCAM siRNA vs. control siRNA were decreased by 80% on average (Figure 7b, right panel).
Figure 7.
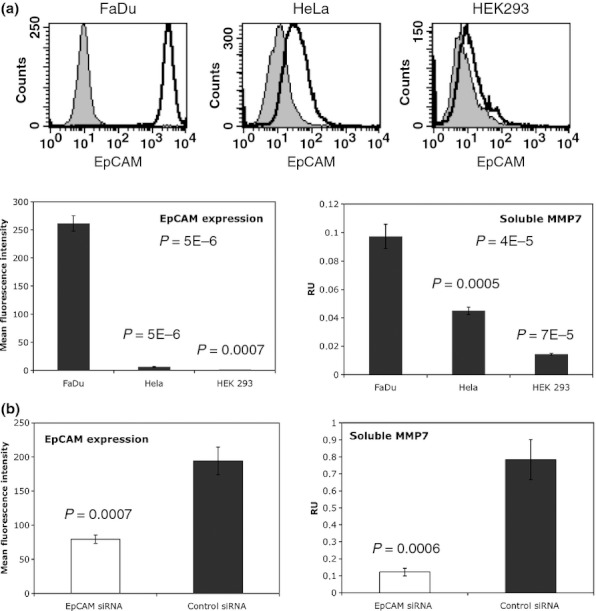
Levels of soluble MMP7 positively correlate with EpCAM expression. (a) Representative histograms of EpCAM FACS stainings in FaDu, HeLa and HEK293 cells are shown. Left panel: Expression of EpCAM was assessed upon flow cytometry with specific antibodies on FaDu, HeLa and HEK293 cells. Shown are mean fluorescence intensity ratios of EpCAM staining vs. control staining with standard deviations. Right panel: Levels of MMP7 in supernatants of FaDu, HeLa and HEK293 cells were measured with an ELISA kit. Soluble MMP7 levels are given as mean relative units (RU) with standard deviations of three independent experiments performed in duplicates. (b) Left panel: FaDu cells were transiently transfected with control siRNA or EpCAM siRNA. After 2 days, EpCAM expression at the cell surface was assessed upon flow cytometry with specific antibodies. Shown are mean fluorescence intensity ratios of EpCAMvs. control stainings with standard deviations from three independent experiments. Right panel: Supernatants from siRNA-treated FaDu cells were collected after 2 days, and levels of soluble MMP7 were measured with an ELISA kit. Soluble MMP7 levels are given as mean relative units (RU) with standard deviations of three independent experiments performed in duplicates. P-values are indicated for each subfigure.
EpCAM and MMP7 expression correlates with areas of proliferation
EpCAM reportedly induces cell proliferation and fosters cell cycle progression via the control of key regulators of the cell cycle including cyclins (Chaves-Perez et al. 2012), and consequently, knock-down of EpCAM results in loss of proliferation (Munz et al. 2004; Osta et al. 2004; Maetzel et al. 2009). As EpCAM and MMP7 expression might relate to areas of proliferation and tissue remodelling, the expression of the proliferation marker Ki67 was assessed in primary samples of head and neck carcinomas. EpCAM, MMP7 and Ki67 displayed strongly overlapping expression patterns in vivo (Figure S3a). Next, FaDu carcinoma cells were transiently transfected with control or EpCAM siRNA and co-transfected with empty vector or an EpICD expression vector. Knock-down of EpCAM in the presence of empty vector resulted in a substantial decrease in Ki67 expression, while ectopic expression of EpICD rescued Ki67 expression after EpCAM knock-down (Figure S3b). Ki67 expression was diminished approximately 80% upon knock-down of EpCAM, and EpICD fully restored Ki67 expression (Figure S3c).
Discussion
The capacity of EpCAM to transduce proliferation-inducing signals from the plasma membrane to the nucleus based on regulated intramembrane proteolysis has shed new light on EpCAM as a mediator of proliferation, stem cell features, migration and invasion, and several other cellular processes (Munz et al. 2009; Gires 2011, 2012). Preferential localization of EpICD in the nucleus, where it deploys its oncogenic signalling capacities (Denzel et al. 2009), was observed in colon carcinomas (Maetzel et al. 2009), was associated with a more aggressive phenotype in thyroid tumours (Ralhan et al. 2010a,b) and appears to be a common feature of carcinomas in general (Ralhan et al. 2010a,b). Here, EpCAM expression was modulated by the use of siRNA, and an explorative whole-genome array was conducted. Molecular pathways that were most strongly affected upon EpCAM knock-down were angiogenesis, the presenilin pathway, the Wnt pathway, chemokine-mediated inflammation and the cadherin signalling pathway. These findings are in line with earlier publications, because EpCAM has been connected to the presenilin, Wnt and cadherin pathways (Litvinov et al. 1997; Yamashita et al. 2007; Maaser & Borlak 2008; Maetzel et al. 2009). As EpCAM signalling involves components of the Wnt pathway, we concentrated on known targets of Wnt such as matrix metalloproteinase 7 (Brabletz et al. 1999; Crawford et al. 1999) for the subsequent analysis of EpCAM target genes. We show a tight correlation between EpCAM and MMP7 expression in transformed epithelia of the head and neck area. Commonly, EpCAM and MMP7 were most strongly expressed at the edge of tumour areas (see Figure 2 middle panels). This overexpression at the tumour edge further correlated with proliferative areas as monitored by Ki67 staining. These ‘leading edges’ of tumours are the site of most prominent tissue remodelling, and hence, overexpression of proteases involved in extracellular matrix (ECM) degradation appears consequential. In primary tissues, unlike cell lines tested, a small proportion of samples displayed opposing expression pattern of EpCAM and MMP7. It is at the moment unclear how this apparently mutually exclusive expression of both molecules is regulated. Obviously, mmp7 gene regulation is not confined to EpCAM but strongly relies on the Wnt signalling pathway (Brabletz et al. 1999), and other signalling pathways and inducers/repressors might dominate over EpCAM. Future work should, in this respect, aim at understanding the molecular basis for this differential activation of MMP7. Using an ELISA for the detection of the MMP7 pro-enzyme and active MMP7, we could demonstrate a tight correlation between expression of endogenous EpCAM and levels of MMP7 in cell culture supernatants. Because MMP7 is first secreted as an inactive pro-enzyme, whose activity is tightly regulated, future work will aim at the understanding of the subsequent regulation of MMP7 activity based on EpCAM expression levels.
MMP7 substrates can be classified into ECM proteins and non-ECM proteins. MMP7 has a broad spectrum of ECM substrates such as collagen, vitronectin, proteoglycans, elastin and fibronectin (Ii et al. 2006). Degradation of the ECM by MMPs is a key process in tumour progression, tissue remodelling, invasion and metastasis formation (Shiomi & Okada 2003). Accordingly, MMP7 is overexpressed in numerous tumours including colon, pancreas, breast, lung, prostate, oesophagus, and head and neck [reviewed in (Ii et al. 2006)] as is EpCAM. Additionally, MMP7 is active in proteolytic shedding of ectodomains and, by doing so, regulates the biological activities of membrane proteins such as E-cadherin, insulin-like growth factor–binding proteins and epidermal growth factor. Shedding of the ectodomain of E-cadherin fosters invasion and migration (Noe et al. 2001), while induction of shed EGF results in the activation of the ErbB4 receptor, organogenesis and proliferation (Yu et al. 2002). EpCAM signalling-dependent induction of MMP7 can thus be seen as a novel target, which, upon further activation to a fully functional protease, has the ability to regulate tumour cell adhesion, migration and invasion as seen in breast carcinoma cells (Litvinov et al. 1994, 1997; Osta et al. 2004) and, indirectly, cell proliferation, all known features of EpCAM (Munz et al. 2004; Osta et al. 2004; Maaser & Borlak 2008; Maetzel et al. 2009). Induction of MMP7 by EpCAM depends on its signalling abilities, rather than its cell adhesive functions, and requires nuclear translocation of the signal-transducing moiety EpICD. Hence, MMP7 is a signalling target of EpCAM, which could potentially be involved in EpCAM-dependent tissue remodelling and tumour spreading as was reported for breast carcinomas (Osta et al. 2004; Sankpal et al. 2009).
Funding
Part of this work was funded by the Deutsche Krebshilfe (Grant 109080 to OG) and the Austrian Science Fond FWF (Grant J3201 to PM).
Declaration of interest
The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1. (a) Panther analysis of EpCAM-regulated genes. Genes with a leastwise two-fold regulation after EpCAM knock-down were subjected to a cluster analysis using the Panther software according to signalling pathways. Numbers of genes in the given pathways are displayed. (b) Selected genes, which were either co-regulated with or counter-regulated to EpCAM are listed. Where indicated (X), RT-PCR was established for the given gene and regulation pattern confirmed in independent siRNA-transfected FaDu cell samples.
Figure S2. EpICD regulates c-myc expression. FaDu cells were transiently transfected with control or EpCAM siRNA and co-transfected with empty vector or an expression plasmid for EpICD. After 32 h, cells were lysed and c-myc expression assessed with specific antibodies in an SDS-PAGE. Shown are the representative results from two independent experiments.
Figure S3. EpCAM and EpICD regulate Ki67 expression. (a) Consecutive sections of head and neck squamous cell carcinomas were stained with EpCAM, MMP7-, and Ki67-specific antibodies. Representative results are shown. (b) FaDu head and neck carcinoma cells were transiently transfected with control or EpCAM-specific siRNA and co-transfected with either empty vector or an expression plasmid for EpICD. Thereafter, cells were stained for Ki67 expression, Shown are representative results from two independent experiments. (c) Ki67-positive cells were counted and displayed as mean arbitrary units with standard deviations from two independent experiments, where control siRNA and empty vector treated cells were set to one.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Balzar M, Winter MJ, de Boer CJ, Litvinov SV. The biology of the 17-1A antigen (Ep-CAM) J. Mol. Med. 1999;77:699–712. doi: 10.1007/s001099900038. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Dag S, Hlubek F, Kirchner T. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am. J. Pathol. 1999;155:1033–1038. doi: 10.1016/s0002-9440(10)65204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock R, Hamelers IH, Jovin TM. Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry. 1999;35:353–362. doi: 10.1002/(sici)1097-0320(19990401)35:4<353::aid-cyto8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Chaves-Perez A, Mack B, Maetzel D, et al. EpCAM regulates cell cycle progression via control of cyclin D1 expression. Oncogene. 2012 doi: 10.1038/onc.2012.75. doi: 10.1038/onc.2012.75. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–2891. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- Denzel S, Maetzel D, Mack B, Eggert C, Barr G, Gires O. Initial activation of EpCAM cleavage via cell-to-cell contact. BMC Cancer. 2009;9:402. doi: 10.1186/1471-2407-9-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang YJ, Lu ZH, Wang F, et al. Prognostic impact of ERbeta and MMP7 expression on overall survival in colon cancer. Tumour Biol. 2010;31:651–658. doi: 10.1007/s13277-010-0082-0. [DOI] [PubMed] [Google Scholar]
- Gires O. Lessons from common markers of tumor-initiating cells in solid cancers. Cell. Mol. Life Sci. 2011;68:4009–4022. doi: 10.1007/s00018-011-0772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gires O. EpCAM in hepatocytes and their progenitors. J. Hepatol. 2012;56:490–492. doi: 10.1016/j.jhep.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Gires O, Klein CA, Baeuerle PA. On the abundance of EpCAM on cancer stem cells. Nat. Rev. Cancer. 2009;9:143. doi: 10.1038/nrc2499-c1. author reply 143. [DOI] [PubMed] [Google Scholar]
- Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp. Bio.l Med. (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- Litvinov SV, Velders MP, Bakker HA, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell-cell adhesion molecule. J. Cell Biol. 1994;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov SV, Balzar M, Winter MJ, et al. Epithelial cell adhesion molecule (Ep-CAM) modulates cell-cell interactions mediated by classic cadherins. J. Cell Biol. 1997;139:1337–1348. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TY, Lu RM, Liao MY, et al. Epithelial cell adhesion molecule regulation is associated with the maintenance of the undifferentiated phenotype of human embryonic stem cells. J. Biol. Chem. 2010;285:8719–8732. doi: 10.1074/jbc.M109.077081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaser K, Borlak J. A genome-wide expression analysis identifies a network of EpCAM-induced cell cycle regulators. Br. J. Cancer. 2008;99:1635–1643. doi: 10.1038/sj.bjc.6604725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maetzel D, Denzel S, Mack B, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009;11:162–171. doi: 10.1038/ncb1824. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler MW, Zoeller M. CD44 and EpCAM: cancer-initiating cell markers. Curr. Mol. Med. 2008;8:784–804. doi: 10.2174/156652408786733667. [DOI] [PubMed] [Google Scholar]
- Munz M, Kieu C, Mack B, Schmitt B, Zeidler R, Gires O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene. 2004;23:5748–5758. doi: 10.1038/sj.onc.1207610. [DOI] [PubMed] [Google Scholar]
- Munz M, Baeuerle PA, Gires O. The emerging role of EpCAM in cancer and stem cell signaling. Cancer Res. 2009;69:5627–5629. doi: 10.1158/0008-5472.CAN-09-0654. [DOI] [PubMed] [Google Scholar]
- Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J. Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- Osta WA, Chen Y, Mikhitarian K, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004;64:5818–5824. doi: 10.1158/0008-5472.CAN-04-0754. [DOI] [PubMed] [Google Scholar]
- O'Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin. Surg. Oncol. 2003;21:30–42. doi: 10.1002/ssu.10019. [DOI] [PubMed] [Google Scholar]
- Ralhan R, Cao J, Lim T, Macmillan C, Freeman JL, Walfish PG. EpCAM nuclear localization identifies aggressive thyroid cancer and is a marker for poor prognosis. BMC Cancer. 2010a;10:331. doi: 10.1186/1471-2407-10-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralhan R, He HC, So AK, et al. Nuclear and cytoplasmic accumulation of Ep-ICD is frequently detected in human epithelial cancers. PLoS ONE. 2010b;5:e14130. doi: 10.1371/journal.pone.0014130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Sankpal NV, Willman MW, Fleming TP, Mayfield JD, Gillanders WE. Transcriptional repression of epithelial cell adhesion molecule Contributes to p53 control of breast cancer invasion. Cancer Res. 2009;69:753–757. doi: 10.1158/0008-5472.CAN-08-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi T, Okada Y. MT1-MMP and MMP-7 in invasion and metastasis of human cancers. Cancer Metastasis Rev. 2003;22:145–152. doi: 10.1023/a:1023039230052. [DOI] [PubMed] [Google Scholar]
- Spizzo G, Fong D, Wurm M, et al. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J. Clin. Pathol. 2011;64:415–420. doi: 10.1136/jcp.2011.090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Mol. Biol. Cell. 1995;6:851–869. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Budhu A, Forgues M, Wang XW. Activation of hepatic stem cell marker EpCAM by Wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007;67:10831–10839. doi: 10.1158/0008-5472.CAN-07-0908. [DOI] [PubMed] [Google Scholar]
- Yu WH, Woessner JF, Jr, McNeish JD, Stamenkovic I. CD44 anchors the assembly of matrilysin/MMP-7 with heparin-binding epidermal growth factor precursor and ErbB4 and regulates female reproductive organ remodeling. Genes Dev. 2002;16:307–323. doi: 10.1101/gad.925702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


