Abstract
Utilizing postmortem data (Breese, et al., 2000), we hypothesized that the densities of high-affinity neuronal α4β2 nicotinic acetylcholine receptors (nAChRs) in the brain exist in a continuum from highest to lowest as follows: smokers without schizophrenia > smokers with schizophrenia > nonsmokers without schizophrenia > nonsmokers with schizophrenia. Application of the Kruskal-Wallis Test (Stata, 2003) to the postmortem data (Breese, et al., 2000) confirmed the hypothesized order in the cortex and the hippocampus and attained significance in the caudate and the thalamus.
Positron emission tomography (PET) was performed for 60 minutes at 6 hours after the intravenous administration of 444 megabequerels [MBq] (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA), a radiotracer for high-affinity neuronal α4β2 nAChRs, as a bolus plus continuous infusion to 10 adults (7 men and 3 women) (6 smokers including 5 with paranoid schizophrenia and 4 nonsmokers) ranging in age from 22 to 56 years (mean 40.1, standard deviation 13.6). The thalamic nondisplaceable binding potential (BPND) was 1.32 ± 0.19 (mean ± standard deviation) for healthy control nonsmokers; 0.50 ± 0.19 for smokers with paranoid schizophrenia; and 0.51 for the single smoker without paranoid schizophrenia. The thalamic BPNDs of nonsmokers were significantly higher than those of smokers who smoked cigarettes a few hours before the scans (P = 0.0105) (StataCorp, 2003), which was likely due to occupancy of nAChRs by inhaled nicotine in smokers. Further research is needed to rule out the effects of confounding variables.
Keywords: nicotinic acetylcholine receptor, Kruskal-Wallis H Statistic, rank sum tests
INTRODUCTION
Schizophrenia is a devastating disorder presenting in adolescence or young adulthood and afflicting approximately one percent of the population (American Psychiatric Association, 2000). During adolescence and young adulthood, people with schizophrenia typically develop the positive symptoms of schizophrenia, including hallucinations, perceptions of stimuli absent from the environment, and delusions, fixed false beliefs, as well as the negative symptoms of schizophrenia, including loss of interest in people, lack of motivation, and inattention. Thus, approximately 3,070,066 Americans suffer from schizophrenia (United States Census Bureau, Population Division, 2010). Paranoid delusions that people are plotting harm characterize paranoid schizophrenia, the most common form of schizophrenia. A panoply of influences, including genetic, obstetric, traumatic, chemical, toxic, infectious, stressful, and environmental items, likely play a role in the pathogenesis of schizophrenia (Tandon et al., 2008), a disorder associated with alterations in the brain development during the prenatal period (Lafargue and Brasic, 2000). Extant treatments for schizophrenia provide limited benefits. People with schizophrenia experience marked social, educational, and occupational problems. Both positive and negative symptoms prevent many people with schizophrenia from holding permanent jobs. Therefore, quantitative biological estimates of physiologic parameters in schizophrenia are needed to provide tools for novel therapeutic agents.
Dysfunction of the nicotinic system likely characterizes a subset of individuals with schizophrenia. Information about the density and the distribution of nAChRs in people with schizophrenia has been limited to the study of specimens from autopsy. Breese and colleagues (2000) measured the concentration of radioligands for high-affinity α4β2 nAChRs in the caudate, the cortex, the hippocampus, and the thalamus of smokers and nonsmokers with and without schizophrenia (Table I). Utilizing the ranksum procedure (Stata, 2003), the two-sample Wilcoxon rank-sum (Mann-Whitney) test without correction for multiple comparisons, we computed the probability of the difference in the concentrations of high-affinity α4β2 nAChRs in the caudate, the cortex, the hippocampus, and the thalamus of (A) smokers and nonsmokers and (B) people with schizophrenia and people without schizophrenia (Stata, 2003) (Table II). Smokers significantly differed from nonsmokers in the concentration of radioligands for high-affinity α4β2 nAChRs in the cortex (P = 0.0320) without correction for multiple comparisons (Table II). There were trends for significant differences between smokers and nonsmokers in the concentration of radioligands for high-affinity α4β2 nAChRs in the thalamus (P=0.0780) and the caudate (P=0.0814) without correction for multiple comparisons (Table II). People with schizophrenia did not differ significantly from people without schizophrenia in any of the regions (Stata, 2003). Utilizing the data about binding to high-affinity α4β2 nAChRs in the caudate, the cortex, the hippocampus, and the thalamus of human autopsy specimens (Tables I and II), we hypothesized that the densities of high-affinity neuronal α4β2 nAChRs exist on a continuum, from highest to lowest, as follows: smokers without schizophrenia > smokers with schizophrenia > nonsmokers without schizophrenia > nonsmokers with schizophrenia. We then tested the hypothesis that the binding in the four groups in the autopsy data (Breese et al., 2000) was from the same population utilizing the kwallis procedure, the Kruskal-Wallis Test (Stata, 2003) (Table III). In each of the four regions the rank sums of the binding of the nicotinic receptors followed the hypothesized order (Stata, 2003). In particular, in the caudate and the thalamus the results were significantly different without employing a Bonferroni correction for multiple comparisons (Stata, 2003) (Table III). The changes of death and autopsy likely distorted the data drawn from postmortem specimens. These results from autopsy specimens (Table I) cannot be generalized to living populations. We seek to (1) identify biological subgroups of people with schizophrenia who may be helped by nicotine and related compounds and (2) provide the means to document the effects of therapies on the underlying deficits in neuronal high-affinity α4β2 nAChRs of the affected portions of the brains of individuals with schizophrenia and related conditions (Brašić et al., 2004, 2009, 2010b; Zhou et al., 2001, 2002a, 2004).
TABLE I.
Densities of high-affinity neuronal α4β2 nicotinic acetylcholine receptors (nAChRs) in femtomoles/mg protein [mean ± standard deviation (sample size)] in volumes of interest (VOIs) of human autopsy specimens (Breese et al., 2000)
| Group | Caudatea | Cortexa | Hippocampusb | Thalamusb |
|---|---|---|---|---|
| Control smokers | 21.1 ± 12.6 (31) | 36.6 ± 23.5 (34) | 18.9 ± 11.3 (27) | 50.9 ± 17.8 (19) |
| Smokers with schizophrenia | 13.9 ± 8.1 (20) | 32.8 ± 20.3 (35) | 16.2 ± 8.2 (17) | 45.5 ± 14.7 (12) |
| Control nonsmokers | 12.4 ± 6.3 (16) | 23.0 ± 11.8 (16) | 11.8 ± 4.3 (12) | 33.3 ± 13.4 (11) |
| Nonsmokers with schizophrenia | 13.8 ± 6.3 (5) | 18.4 ± 3.5 (6) | 14.7 ± 8.4 (5) | 53.2 ± 21.3 (5) |
[3H]epibatidine nicotinic acetylcholine receptor (nAChR) cortical binding (Breese et al., 2000).
[3H]nicotine nicotinic acetylcholine receptor (nAChR) cortical binding (Breese et al., 2000).
TABLE II.
Probabilitiesa by the two-sample Wilcoxon rank-sum (Mann-Whitney) test that there are significant differences in the densities of high-affinity neuronal α4β2 nicotinic acetylcholine receptors (nAChRs) in the volumes of interest (VOIs) of human autopsy specimens (Breese et al., 2000) between groups (Stata, 2003)
| Group | Caudateb | Cortexb | Hippocampusc | Thalamusc |
|---|---|---|---|---|
| Smoker versus nonsmoker | 0.0814 | 0.0320 | 0.1112 | 0.0780 |
| People with schizophrenia versus people without schizophrenia | 0.1582 | 0.8156 | 0.9880 | 0.4516 |
without Bonferroni and other corrections for multiple comparisons.
[3H]epibatidine nicotinic acetylcholine receptor (nAChR) cortical binding (Breese et al., 2000).
[3H]nicotine nicotinic acetylcholine receptor (nAChR) cortical binding (Breese et al., 2000).
TABLE III.
Application of the Kruskal-Wallis Test to the hypothesis that the densities of high-affinity neuronal α4β2 nicotinic acetylcholine receptors (nAChRs) in the volumes of interest (VOIs) of human autopsy specimens (Breese et al., 2000) are from the same population (Stata, 2003)
| Group | Caudatea | Cortexa | Hippocampusb | Thalamusb |
|---|---|---|---|---|
| Chi-squared statistic with tiesc | 7.860 | 4.933 | 3.117 | 8.108 |
| Probabilityd | 0.0490 | 0.1768 | 0.3739 | 0.0438 |
[3H]epibatidine nicotinic acetylcholine receptor (nAChR) cortical binding (Breese et al., 2000).
[3H]nicotine nicotinic acetylcholine receptor (nAChR) cortical binding (Breese et al., 2000).
for the rank sums that the groups are ordered as follows: smokers without schizophrenia > smokers with schizophrenia > nonsmokers without schizophrenia > nonsmokers with schizophrenia (Stata, 2003).
without Bonferroni and other corrections for multiple comparisons.
We used 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) (Horti et al., 2010), an analog of 5-[123I]iodo-3-(2(S)-azetidinylmethoxy)pyridine (5-[123I]IA), the radiotracer employed in our pilot study (Brašić et al., 2009), to bind to the neuronal high-affinity α4β2 nAChRs for the current study to obtain the greater resolution and the higher specificity of positron emission tomography (PET) (Leung, 2006) utilizing high resolution research tomography (HRRT) (Sossi et al., 2005). 2-[18F]FA has demonstrated the potential for much wider clinical use than 5-[123I]IA. Whole-body dosimetry of 2-[18F]FA in healthy adults demonstrated the greatest radiation uptake in the urinary bladder (Kimes et al., 2003), followed by the liver and the kidneys. The mean effective dose equivalent of 2-[18F]FA was 0.0278 mSv/MBq (0.103 rem/mCi), an acceptable radiation dosimetry of 2-[18F]FA (Obrzut et al., 2005). PET with 2-[18F]FA has shown that half the α4β2 neuronal nAChRs in the brains of humans were occupied after smoking only one or two mouthfuls of cigarette smoke and that plasma nicotine levels of 0.87 ng/mL were associated with occupation of half the α4β2 neuronal nAChRs in the brains of humans (Brody et al., 2006). Furthermore, people with Parkinson’s disease demonstrated reductions of 2-[18F]FA binding potential in the midbrain, the pons, the anterior cingulate cortex, the frontoparietal cortex, and the cerebellum (Meyer et al., 2009). Additionally, PET with 2-[18F]FA showed decreased nondisplaceable binding potentials (BPNDs) in the regions commonly affected in Alzheimer’s disease (Kendziorra et al., 2011). We also sought to develop a comprehensive assessment battery for the identification and the characterization of the behavioral symptoms and signs and the psychiatric diagnoses and movement disorders present in people with paranoid schizophrenia, nicotine dependence, and other neuropsychiatric disorders. We share our thorough rating protocols on our unique populations of smokers with paranoid schizophrenia with our colleagues to facilitate their evaluation of people with possible neuropsychiatric disorders (Brašić et al., 2009, 2010b).
Since the absolute density of nAChRs cannot be measured from a single scan, we evaluated the binding potential (BPND) of nAChRs after one scan on each member of a study population of nonsmokers and smokers. We demonstrated that 5-[123I]IA, a novel potent radioligand for neuronal high-affinity α4β2 nAChRs, provided a means to evaluate the binding and the distribution of neuronal high-affinity α4β2 nAChRs in the living human brain of healthy adults (Brašić et al., 2009). High plasma nicotine level was significantly associated with low 5-[123I]IA binding in the caudate head, the cerebellum, the cortex, the fusiform gyrus, the hippocampus, the parahippocampus, the pons, the putamen, and the thalamus (Brašić et al., 2009). These findings confirm that 5-[123I]IA competed with nicotine to occupy nAChRs (Brašić et al., 2009). We concluded that 5-[123I]IA is a safe, well-tolerated, and effective pharmacologic agent for human subjects to estimate neuronal high-affinity α4β2 nAChR in the living human brain (Brašić et al., 2009).
If a brain imaging procedure can determine alterations in the density and distributions of neuronal high-affinity α4β2 nAChRs in the living human brain of people with paranoid schizophrenia who do and do not smoke cigarettes, then those who likely will benefit from the administration of nicotine and nicotinic agonists can likely be identified. Thus, this procedure will likely provide a tool to identify the subclass of people with paranoid schizophrenia who may benefit from nicotinic agents. Additionally, the results of this study will likely identify a subclass of people with paranoid schizophrenia who will not benefit from nicotinic agonists. Thus, they can then be spared the adverse effects of fruitless administrations of nicotine and nicotinic agonists and other related therapeutic interventions. The results of this study will likely also benefit people with nicotine dependence and other neuropsychiatric disorders.
MATERIALS AND METHODS
Participants
Four healthy nonsmoking normal control participants and six cigarette smokers, including five with paranoid schizophrenia according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR ) (American Psychiatric Association, 2000; First et al., 1997), were recruited through advertisements in Baltimore, Maryland (Table IV). The smokers were not abstinent from smoking. They smoked the morning of the studies a few hours before the scans.
TABLE IV.
Demographic and clinical characteristics of ten human participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | Spanish/Hispanic/Latinoa | Mutliraciala | Smokera | Schizophreniaa | Ed | Et | LPE | P |
|---|---|---|---|---|---|---|---|---|---|
| 22 | 1 | . | . | 0 | 0 | . | 4 | . | G |
| 23 | 1 | . | 0 | 0 | 0 | 6 | 4 | . | H |
| 23 | 0 | . | . | 0 | 0 | 3 | 3 | . | G |
| 31 | 0 | 0 | 0 | 0 | 0 | . | 1 | . | H |
| 45 | 1 | 0 | 0 | 1 | 0 | 2 | 4 | 4 | H |
| 47 | 1 | 0 | 0 | 1 | 1 | 3 | 2 | 4 | H |
| 49 | 1 | 0 | 0 | 1 | 1 | 2 | 1 | 1 | H |
| 51 | 1 | 0 | 0 | 1 | 1 | 4 | 1 | 1 | H |
| 53 | 1 | 0 | 1 | 1 | 1 | 2 | 2,4 | 3 | H |
| 56 | 0 | 0 | 0 | 1 | 1 | 4 | 1 | 4 | H |
Ed = Education: 2 = Grade 7 to 12 (without graduating high school), 3 = Graduate high school or high school equivalent, 4 = Part college, 6 = Graduated 4-year college (First et al., 1997, p. 7); Et = Ethnicity: 1 = African American, Black, or Negro; 2 = Alaska Native, Aleut, American Indian, Eskimo, or Native American; 3 = Asian, Asian Indian, Chamorro, Chinese, Filipino, Guamanian, Japanese, Korean, Native Hawaiian, Pacific Islander, Samoan, or Vietnamese; 4 = Caucasian, European, or White (Brašić, 2003a); LPE = Lateral Preferences Examination (Brasic and Wong, 2011; Denckla, 1985): 1 = all items right; 3 = eye right, foot mixed, hand right; 4 = eye left, hand and foot right; P = PET scanner; G = General Electric Advance; H = high resolution research tomograph; Period (.) = missing data.
0 = absent, 1 = present.
Neuropsychiatric evaluation
All subjects underwent a comprehensive battery including the medical, neurological, and psychiatric histories, physical examination, and laboratory assessments (Brasic and Wong, 2011).
Self-rating forms
All participants were asked to complete a battery of self-rating forms covering several disorders common in adults with schizophrenia and related neuropsychiatric disorders.
Attention-Deficit/Hyperactivity Disorder (ADHD)
All participants completed the Wender-Utah Rating Scale (WURS), a 61-item instrument for retrospective recall by adults of childhood with sections about behavioral (42 items), medical (7 items), and school problems (12 items) (Ward et al., 1993; Wender, 1995; Wender et al., 2001). Of the 61 items, 25 items are utilized to diagnose Attention-Deficit/Hyperactivity Disorder (ADHD) (American Psychiatric Association, 2000) in childhood. The ranges of possible scores for the sections are as follows: behavioral problems (BP) (0, 172), medical problems (MP) (0, 28), school problems (SP) (0, 48), and ADHD (0, 100) (Ward et al., 1993). Since scores on the 25 items associated with ADHD of 46 or higher identify 86% of adults with ADHD (Ward et al., 1993), we diagnosed ADHD if a person has a score of 46 or higher on the ADHD items of the WURS (Ward et al., 1993) (Table V).
Table V.
Subjective ratings of attention, obsessions, and compulsions of participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]Fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | BP | MP | SP | ADHD | CGISADD | CC | UMMOCI | CGISOCD | NIMHGOCS |
|---|---|---|---|---|---|---|---|---|---|---|
| Range of scores | (0, 1) | (0, 172) | (0, 28) | (0, 48) | (0, 100) | (0, 6) | (0, 54) | (0, 60) | (0, 6) | (1, 15) |
| 22 | 1 | . | . | . | . | . | . | . | . | . |
| 23 | 1 | . | . | . | . | . | . | . | . | . |
| 23 | 0 | . | . | . | . | . | . | . | . | . |
| 31 | 0 | . | . | . | . | . | . | . | . | . |
| 45 | 1 | 4 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 0 |
| 47 | 1 | 32 | 1 | 6 | 22 | 1 | 2 | 4 | 3 | 7 |
| 49 | 1 | 72 | 4 | 10 | 57 | 5 | 23 | 22 | 6 | 5 |
| 51 | 1 | 15 | 4 | 8 | 10 | 0 | 1 | 6 | 1 | 3 |
| 53 | 1 | 34 | 1 | 9 | 17 | 1 | 23 | 24 | 1 | 1 |
| 56 | 0 | 20 | 0 | 8 | 8 | 0 | 29 | 14 | 0 | 0 |
Wender Utah Rating Scale (WURS) (Ward et al., 1993): BP = Behavioral problems, MP = Medical problems, SP = School problems, ADHD = Attention-Deficit/Hyperactivity Disorder; CGISADD = Clinical Global Impression Scale for Attention Deficit Disorder (Leckman et al., 1988); CC = Compulsion Checklist (Marks, 1986, page 49); UMMOCI = University of Miami Modified Obsessive-Compulsive Inventory (Dominguez et al., 1989); CGISOCD = Clinical Global Impression Scale for Obsessive-Compulsive Disorder (Leckman et al., 1988); NIMHGOCS = National Institute of Mental Health Global Obsessive-Compulsive Scale (Pato and Pato, 1991); Period (.) = missing data.
0 = absent; 1 = present.
Compulsions
The Compulsion Checklist (CC) is a self-rating form listing 18 activities with a range of possible scores from 0 to 54 (Marks, 1986, page 49). The University of Miami Modified Obsessive-Compulsive (O-C) Inventory (UMMOCI) (Dominguez et al., 1989, page 216) is a self-rated twenty-item tool with scores ranging from 0 to 60 (Table V).
Nicotine dependence
The Fagerström Test for Nicotine Dependence (FTND) (Balfour and Fagerström, 1996) is a self-rating form with a range of possible scores from 0 to 10 (Balfour and Fagerström, 1996). Rustin (2000) suggests scoring as follows: 7 to 10 points = highly dependent; 4 to 6 points = moderately dependent; less than 4 points = minimally dependent (Table VI).
Table VI.
Psychiatric diagnoses of people who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | CSa | CCaIa | CPa | LADa | LCaDa | LCoDa | GAFSC | GAFSH | FTND | TDQ |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range of scores | (1,0) | (1,0) | (1,0) | (1,0) | (1,0) | (1,0) | (1,0) | (1, 100) | (1, 100) | (1, 10) | (1, 10) |
| 22 | 1 | . | . | . | . | . | . | . | . | . | . |
| 23 | 1 | . | . | . | . | . | . | . | . | . | . |
| 23 | 0 | . | . | . | . | . | . | . | . | . | . |
| 31 | 0 | . | . | . | . | . | . | . | . | . | . |
| 45 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 72 | 73 | 7 | 1 |
| 47 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 43 | 45 | 8 | 6 |
| 49 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 28 | 29 | 4 | 7 |
| 51 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 53 | 56 | 7 | 8 |
| 53 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 46 | 47 | 6 | 7 |
| 56 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 51 | 51 | 10 | 6 |
CS = Current Schizophrenia, Paranoid Type (First et al., 1997); CCaI = Current Cannabis Intoxication (First et al., 1997); CP = Current Post-Traumatic Stress Disorder (First et al., 1997); LAD = Lifetime Alcohol Dependence (First et al., 1997); LCaD = Lifetime Cannabis Dependence (First et al., 1997); LCoD = Lifetime Cocaine Dependence (First et al., 1997); GAFSC = Global Assessment of Functioning Scale Current Rating (First et al., 1997); GAFSH = Global Assessment of Functioning Scale Highest Past Year Rating (First et al., 1997); FTND = Fagerström Test for Nicotine Dependence (Balfour and Fagerström, 1996); TDQ = Tobacco Dependence Questionnaire (Kawakami et al., 1999); Period (.) = missing data.
0 = absent; 1 = present.
The Tobacco Dependence Questionnaire (TDQ) is a ten-item self-rating form with a range of possible scores from 0 to 10 (Kawakami et al., 1999). Kawakami and colleagues (1999) report that a score of greater than or equal to 6 is the cutoff for a score of nicotine dependence according to the criteria of the text revision of the fourth edition of the Diagnostic and statistical manual of mental disorders (American Psychiatric Association, 1994) with 72.4 % sensitivity and 55.2 % specificity (Table VI).
Examiner rated instruments
Global functioning
The Clinical Global Impressions (CGI) is a tool to monitor the functioning of a patient before, during, and after drug studies. The CGI assesses the Severity of Illness (SI) on a scale with a range of (1, 7) and the Global Improvement (GI) on a scale with a range of (1, 7). The CGI also expresses in a matrix entitled the Efficacy Index (EI) with a range of (1, 16) the combined assessments of (A) the Side Effects (SE) on a scale with a range of (1, 4) and (B) the Therapeutic Effect (TE) on a scale with a range of (1, 4) (Guy, 1976) (Table VII).
Table VII.
Objective characteristics of examiner-rated assessments of the global functioning of participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | CGISI | CGIGI | CGIEI | CGIRGE | MMSE | SSC |
|---|---|---|---|---|---|---|---|
| Range of scores | (1, 0) | (1, 7) | (1, 7) | (1, 16) | (1, 7) | (0, 30) | (0, 55) |
| 22 | 1 | . | . | . | . | . | . |
| 23 | 1 | . | . | . | . | . | . |
| 23 | 0 | . | . | . | . | . | . |
| 31 | 0 | . | . | . | . | . | . |
| 45 | 1 | 1 | 4 | 13 | 4 | 28 | 0 |
| 47 | 1 | . | . | . | . | 29 | 0 |
| 49 | 1 | 6 | 4 | 13 | 4 | 28 | 0 |
| 51 | 1 | 6 | 4 | 13 | 4 | 29 | 0 |
| 53 | 1 | 6 | 4 | 13 | 4 | 24 | 4 |
| 56 | 0 | 4 | 4 | 13 | 4 | 25 | 0 |
Clinical Global Impressions: CGISI = Severity of Illness, CGIGI = Global Improvement, CGIEI = Efficacy Index (Guy, 1976); CGIRGE = Clinical Global Improvement Rater Global Evaluation (Pato and Pato, 1991); MMSE = Mini-Mental State Examination (Blacker, 2009; Folstein et al., 1975; Marder, 1995); SSC = Serotonin Syndrome Checklist (Brasić et al., 1998); Period (.) = missing data.
0 = absent; 1 = present.
The Clinical Global Improvement Rater Global Evaluation (CGIRGE) evaluates the therapeutic effect of an intervention before, during, and after the therapy on a scale with a range of (1, 7) (Pato and Pato, 1991) (Table VII).
The Mini-Mental State Examination (MMSE) (Blacker, 2009; Folstein et al., 1975; Marder, 1995) is a 19-item procedure to screen the current cognitive function of the patient producing scores in the range of 0 to 30. A score of 30 indicates normal functioning. A score of 20 or less is indicative of dementia (Folstein et al., 1975). Scores of 20 to 24 indicate mild dementia (Blacker, 2009) (Table VII).
The Serotonin Syndrome Checklist (SSC) is an 11-item procedure to score traits characteristic of the serotonin syndrome producing scores ranging from 0 to 55 (Brasić et al., 1998). The likelihood of a serotonin syndrome increases with higher scores (Table VII).
Psychiatric diagnoses
All smokers, including all participants with schizophrenia, were administered the Structured Clinical Interview for DSM-IV Axis I Disorders---Clinician Version (SCID-CV), (First et al., 1997) is a structured interview to utilize the criteria of the Diagnostic and statistical manual of mental disorder (American Psychiatric Association, 1994, 2000) to diagnose both current and lifetime conditions including mood disorders, psychoses, abuse and dependence for both alcohol and other substances, anxiety disorders, somatoform disorders, eating disorders, and adjustment disorders. The SCID-CV also provides the Global Assessment of Functioning Scale (GAFS) with scores ranging from 0 to 100 for the current (C) rating and for the highest (H) rating in the past year (Blacker, 2009; First et al., 1997; Marder, 1995) (Table VI). All participants with schizophrenia met the criteria for a current diagnosis of Schizophrenia, Paranoid Type (First et al., 1997) (Table VI). Although the four nonsmoking healthy controls did not undergo this assessment with the SCID-CV (First et al., 1997), there was no evidence of mental disorders during the medical history and the assessment procedures.
The Brief Psychiatric Rating Scale Anchors (BPRSA) (Brašić et al., 2009, 2010b; Marder, 1995; McMahon et al., 2002; Overall & Gorham, 1962; Woerner et al., 1988) is a 20-item tool to assess the presence of symptoms and signs of mental illness with a score ranging from 20 to 140 (Marder, 1995; McMahon et al., 2002; Overall & Gorham, 1962; Woerner et al., 1988).
The Scale for the Assessment of Negative Symptoms (SANS) and the Scale for the Assessment of Positive Symptoms (SAPS) are instruments to be completed by a trained rater after a semi-structured interview of the participant to characterize the symptoms of schizophrenia. The SANS is a 24-item semi-structured interview to provide ratings of affective flattening or blunting ranging 0 to 35, alogia ranging 0 to 25, avolition-apathy ranging 0 to 20, anhedonia – asociality ranging 0 to 25, and attention ranging 0 to 15 (Andreasen, 1984a; Blacker, 2009; Marder, 1995). The Scale for the Assessment of Positive Symptoms (SAPS) is a 35-item semi-structured interview to provide ratings of hallucinations ranging 0 to 35, delusions ranging 0 to 65, bizarre behavior ranging 0 to 25, positive formal thought disorder ranging 0 to 45, and inappropriate affect ranging 0 to 5 (Andreasen, 1984b; Blacker, 2009; Marder, 1995).
Attention-Deficit/Hyperactivity Disorder (ADHD)
The Clinical Global Impression Scale for Attention Deficit Disorder (CGISADD) is a single-item scale to rate the symptoms of inattention, impulsivity, and hyperactivity of the individual utilizing all sources of information with a range of (0, 6) (Leckman et al., 1988) (Table V).
Obsessive-Compulsive Disorder (OCD)
The Clinical Global Impression Scale for Obsessive-Compulsive Disorder (CGISOCD) is a single-item scale to rate the obsessions and compulsions of the individual utilizing all sources of information with a range of (0, 6) (Leckman et al., 1988) (Table V).
The National Institute of Mental Health Global Obsessive-Compulsive Scale (NIMHGOCS) is a single-item tool to score the obsessions and compulsions of a person on the range (1, 15) (Pato and Pato, 1991) (Table V).
The Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) is a 21-item protocol with five items to rate obsessions yielding an obsession score ranging from 0 to 20 and five items to rate compulsions yielding a compulsion score ranging from 0 to 20 (Goodman et al., 1989a,b).
Lateral Preferences Examination (LPE)
To conduct the Lateral Preferences Examination (LPE), the examiner asks the participant to perform simple tasks using the preferred eye, hand, and foot. The results are scored 1 if all items are right, 2 if all items are left, 3 if items are mixed with some items right and some items left, and 4 if the eye alone is different from the hand and the foot (Brasic and Wong, 2011; Denckla, 1985) (Table IV).
Movement assessment procedures
General dyskinesias
The Abnormal Involuntary Movement Scale (AIMS) (Brasic, 2003b; Brasic et al., 2010a,b; National Institute of Mental Health, 1988) is a twelve-item scale rating a person at rest and while performing several motor tasks. The possible scores range from 0 to 40 (Table VIII).
Table VIII.
Objective general dyskinesia characteristics of participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | AIMS | F | NT | UE | LE | EB | TSRS |
|---|---|---|---|---|---|---|---|---|
| Range of scores | (0, 40) | (0, 96) | (0, 48) | (0, 48) | (0, 48) | (0, 24) | (0, 1000) | |
| 22 | 1 | . | . | . | . | . | . | . |
| 23 | 1 | . | . | . | . | . | . | . |
| 23 | 0 | . | . | . | . | . | . | . |
| 31 | 0 | . | . | . | . | . | . | . |
| 45 | 1 | 0 | 16 | 8 | 8 | 8 | 4 | 1 |
| 47 | 1 | 3 | 20 | 8 | 10 | 8 | 4 | 39 |
| 49 | 1 | 2 | 20 | 9 | 9 | 16 | 7 | 74 |
| 51 | 1 | 4 | 20 | 8 | 8 | 8 | 4 | 25 |
| 53 | 1 | 4 | 21 | 9 | 8 | 9 | 4 | 11 |
| 56 | 0 | 2 | 16 | 8 | 9 | 8 | 4 | 0 |
AIMS = Abnormal Involuntary Movement Scale (Brasic, 2003b; Brasic et al., 2010a; National Institute of Mental Health,1988); the Rating Scale for Tardive Dyskinesia (RSTD) (Simpson et al., 1979) Face score = F, Neck and trunk score = NT, Upper extremities score = UE, Lower extremities score = LE, Entire body score = EB; TSRS = Timed Stereotypies Rating Scale (Brasic, 2003b; Brasic et al., 2010a; Campbell, 1985); Period (.) = missing data.
0 = absent; 1 = present.
The Movement Disorder Checklist (MCC) is a 23-item tool to score the presence or absence of characteristics of movement disorders to be classified by means of algorithms (Brasic, 2003b; Brasic et al., 2010a).
The Rating Scale for Tardive Dyskinesia (RSTD) (Simpson et al., 1979) is a procedure to rate dyskinesias in the face (F) with 16 items ranging from 16 to 96, in the neck and the trunk (NT) with 8 items ranging from 8 to 48, in the upper extremities (UE) with 8 items ranging from 8 to 48, in the lower extremities (LE) with 8 items ranging from 8 to 48, and in the entire body (EB) with 4 items ranging from 4 to 24 (Simpson et al. 1979) (Table VIII).
The Timed Stereotypies Rating Scale (TSRS) is a 50-item procedure to score the occurrence of 50 stereotypies during 20 ten-second intervals of a ten-minute observation period producing scores ranging from 0 to 1000 (Brasic, 2003b; Brasic et al., 2010a,b; Campbell, 1985) (Table VIII).
Akathisia
The Rating Scale for Acute Drug-induced Akathisia (RSADIA) is a tool to rate akathisia objectively (O) with seven items ranging 0 to 21, subjectively (S) with three items ranging from 0 to 9, and globally (G) with one item ranging from 0 to 3 (Sachdev, 1994) (Table IX).
Table IX.
Objective akathisia and tic characteristics of participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | RSADIA | RSDIA | CGISTS | ||||
|---|---|---|---|---|---|---|---|---|
| . | . | O | S | GR | O | S | GCAA | |
| Range of scores | (0, 1) | (0, 21) | (0, 9) | (0, 3) | (0, 3) | (0, 6) | (0, 5) | (0, 6) |
| 22 | 1 | . | . | . | . | . | . | . |
| 23 | 1 | . | . | . | . | . | . | . |
| 23 | 0 | . | . | . | . | . | . | . |
| 31 | 0 | . | . | . | . | . | . | . |
| 45 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | . |
| 47 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 |
| 49 | 1 | 3 | 3 | 2 | 2 | 4 | 3 | 1 |
| 51 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 53 | 1 | 0 | 1 | 1 | 0 | 2 | 1 | 0 |
| 56 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 |
RSADIA = Rating Scale for Acute Drug-induced akathisia (Sachdev, 1994); RSDIA = Rating Scale for Drug-induced Akathisia (Barnes, 1989); O = Objective (Barnes, 1989; Sachdev, 1994); S = Subjective (Barnes, 1989; Sachdev, 1994); GR = Global Rating (Sachdev, 1994); GCAA = Global Clinical Assessment of Akathisia (Barnes, 1989); CGISTS = Clinical Global Impression Scale for Tourette Syndrome (Leckman et al., 1978); Period (.) = missing data.
0 = absent; 1 = present.
The Rating Scale for Drug-induced Akathisia (RSDIA) is an instrument to rate akathisia objectively (O) with one item ranging from 0 to 3, subjectively (S) with two items ranging from 0 to 6, and globally (G) with one item ranging from 0 to 5 (Barnes, 1989) (Table IX).
Gilles de la Tourette syndrome (TS)
Since tics may result from neuroleptic treatment for schizophrenia, the smoking participants, including all the participants with schizophrenia, were assessed for the signs and symptoms of Gilles de la Tourette syndrome (TS). The Clinical Global Impression Scale for Tourette Syndrome (CGISTS) is a single-item scale to rate the tics of the individual utilizing all sources of information with a range of (0, 6) (Leckman et al., 1988) (Table IX).
The Yale Global Tic Severity Scale (YGTSS) is an instrument incorporating an interview with the patient and the family and an examination of the patient to rate for the preceding week motor tics ranging from 0 to 25, phonic tics ranging from 0 to 25, and impairment ranging from 0 to 50 (Leckman et al., 1989).
Positron emission tomography (PET) procedure
Equivalence of bolus injection and of bolus injection followed by continuous infusion
The procedure was first performed on the initial two healthy adult volunteer participants to establish the feasibility of the protocol for the participants with paranoid schizophrenia. The first sets of studies on healthy nonsmoking adults with arterial blood sampling were performed on a GE Advance PET Scanner. Two healthy young nonsmoking adults, one man and one woman, underwent two administrations of 2-[18F]FA separated by a week followed by scans on a GE Advance Scanner. For the first protocol a radial arterial line was utilized to obtain samples of plasma throughout the scans to construct time-activity curves corrected for decay of radioactivity and metabolites.
The woman, a 23-year-old nonsmoker, underwent two procedures on a GE Advance Scanner with 2-[18F]FA. For the first study with arterial blood sampling she received a bolus injection of 274.17 MBq (7.41 mCi) 2-[18F]FA followed by scans on the GE Advance Scanner on from 0–120, 155–230, and 235–350 minutes after the radiotracer injection. A week later without arterial blood sampling she received the intravenous administration of a bolus injection followed by the continuous infusion of 415.51 MBq (11.23 mCi) 2-[18F]FA in 20 mL in a 20 mL syringe with the injection of 11 mL over the first minute and 0.025 mL per minute for 2 to 100 minutes after the commencement of the intravenous infusion with another scan on the GE Advance Scanner. The radioactivity was administered with a bolus:infusion ratio of 390:330. Scans were obtained for 5 twenty-minute frames beginning 272 minutes after the beginning of the administration of the radiotracer. The regional nondisplaceable binding potential (BPND) values (Innis et al., 2007) were calculated using the right occipital lobe as the reference region as mean radioactivity of regions (mA) over mean radioactivity of the reference region (mARef) minus one [BPND = (mA/mARef) −1]. Then, regional BPNDs of the bolus plus continuous infusion experiment were compared to regional BPNDs of the bolus only experiment completed one week earlier. For the bolus only experiment, BPND was calculated as follows:
| (Equation 1) |
where mDV stands for mean of the distribution volume mDVRef stands for mean of the distribution volume for the reference region, the occipital lobe (Innis et al., 2007).
Time-activity curves were obtained for the first two of the healthy normal control participants who underwent arterial blood sampling. A column-switch high performance liquid chromatography (HPLC) method was employed to analyze the plasma of the arterial blood samples (Hilton et al., 2000). Equilibrium was attained in the striatum and the occipital lobe, the reference structure, at 5 to 6 hours after the administration of 2-[18F]FA. Since the evaluations of the first two healthy adults established the equivalence of (A) a simplified reference tissue method for a bolus-plus-continuous-infusion scan without arterial blood sampling and (B) mathematical modeling with arterial input functions after a bolus scan, the (A) simplified reference tissue method for a bolus-plus-continuous-infusion scan without arterial blood sampling was utilized for all following scans. All subsequent scans without an arterial line were performed on a high resolution research tomograph (HRRT).
All subjects underwent the construction of a thermoplastic face mask with openings for the eyes, nose, and mouth to position the head for all scans. Each healthy control participant underwent an MRI of the brain without contrast with a sequence of acquisitions (Brašić et al., 2009, Table II, page 346) for co-registration with the PET scan.
Six cigarette smokers, five with schizophrenia and one without schizophrenia, and four healthy adult volunteer control participants underwent PET brain scans for 60 minutes at approximately six hours following the intravenous injection of a bolus followed by an infusion of approximately 444 MBq (12 mCi) 2-[18F]FA. Approximately 444 MBq (12 mCi) 2-[18F]FAwere administered intravenously to each participant over five hours (Tables X and XI). Four separate PET acquisitions were obtained for 60 minutes at five to six hours after the radiotracer injection. Images were collected using a 128 x 128 matrix. Images were reconstructed on a 64 x 64 matrix with pixel size = 3.56 mm and slice thickness = 3.56 mm. The PET studies were obtained to coincide with a transverse plane at the anterior commissure-posterior commissure (AC-PC) line. The angle of the initial transverse plan to the AC-PC plane was determined by the MRI co-registered with the PET images. The images were corrected for decay and metabolites. Co-registration of the MRI and PET studies was performed parallel to the AC-PC plane. Polygonal VOIs were drawn on MRI slices to generate time-activity curves on the PET slices.
Table X.
Nondisplaceable binding potentials (BPNDs) in volumes of interest (VOIs) of participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | 2-[18F]FA | Th | F | T | P | O | C | Fu |
|---|---|---|---|---|---|---|---|---|---|
| 22 | 1 | 413.29 (11.17) | 1.57 | 0.05 | 0.00 | 0.00 | 0.07 | 0.12 | 0.00 |
| 23 | 1 | 679.32 (18.36) | 1.24 | 0.08 | −0.16 | −0.10 | −0.17 | −0.09 | −0.14 |
| 23 | 0 | 415.51 (11.23) | 1.12 | 0.08 | −0.16 | −0.14 | −0.23 | −0.07 | −0.15 |
| 31 | 0 | 697.08 (18.84) | 1.36 | 0.04 | −0.09 | −0.04 | −0.08 | −0.02 | −0.10 |
| 45 | 1 | 421.80 (11.40) | 0.51 | 0.10 | 0.02 | 0.14 | 0.038 | 0.11 | 0.03 |
| 47 | 1 | 444.00 (12.00) | 0.28 | 0.22 | 0.05 | 0.22 | 0.053 | 0.14 | 0.048 |
| 49 | 1 | 425.13 (11.49) | 0.37 | 0.19 | 0.15 | 0.23 | 0.17 | 0.29 | 0.13 |
| 51 | 1 | 433.27 (11.71) | 0.69 | −0.15 | −0.19 | −0.13 | −0.13 | −0.17 | −0.15 |
| 53 | 1 | 448.44 (12.12) | 0.70 | 0.20 | 0.08 | 0.24 | 0.10 | 0.15 | 0.06 |
| 56 | 0 | 435.12 (11.76) | 0.48 | 0.07 | −0.04 | 0.08 | −0.05 | 0.04 | −0.01 |
2-[18F]FA = Dose of 2-[18F]FA in MBq (mCi); Th = Thalamus; F = Frontal cortex; T = Temporal cortex; P = Parietal cortex; O = Occipital cortex; C = Cingulate cortex; Fu = Fusiform gyrus.
0 = absent, 1 = present.
Table XI.
Nondisplaceable binding potentials (BPNDs) in in volumes of interest (VOIs) of participants who underwent positron emission tomography (PET) of the brain following the intravenous administration of 444 MBq (12 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA)
| Age in years | Male sexa | 2-[18F]FA | H | I | P | C | G | Pa |
|---|---|---|---|---|---|---|---|---|
| 22 | 1 | 413.29 (11.17) | 0.07 | 0.08 | 0.16 | 0.13 | 0.09 | 0.02 |
| 23 | 1 | 679.32 (18.36) | −0.15 | −0.06 | −0.04 | −0.16 | −0.02 | −0.18 |
| 23 | 0 | 415.51 (11.23) | −0.15 | −0.06 | 0.02 | 0.017 | −0.02 | −0.13 |
| 31 | 0 | 697.08 (18.84) | 0.07 | 0.02 | 0.06 | −0.08 | 0.03 | −0.06 |
| 45 | 1 | 421.80 (11.40) | −0.01 | 0.10 | 0.22 | 0.04 | 0.15 | −0.06 |
| 47 | 1 | 444.00 (12.00) | −0.06 | 0.15 | 0.14 | 0.00 | 0.12 | 0.00 |
| 49 | 1 | 425.13 (11.49) | 0.09 | 0.27 | 0.29 | 0.06 | 0.28 | 0.06 |
| 51 | 1 | 433.27 (11.71) | −0.15 | −0.14 | 0.03 | −0.25 | −0.01 | −0.26 |
| 53 | 1 | 448.44 (12.12) | −0.03 | 0.19 | 0.28 | −0.08 | 0.29 | −0.05 |
| 56 | 0 | 435.12 (11.76) | −0.11 | 0.02 | 0.18 | −0.12 | 0.06 | −0.14 |
2-[18F]FA = Dose of 2-[18F]FA in MBq (mCi); H = Hippocampus; I = Insula; P = Putamen; C = Caudate nucleus; G = Globus pallidus; Pa = Parahippocampus.
0 = absent, 1 = present.
For all subjects, the maximal uptake of 2-[18F]FA occurred in the thalamus. There was minimal uptake in the other regions of the brain. The counts from the PET scans to estimate the uptake in the volumes of interest and the counts from the occipital cortex as the reference tissue were utilized for further analysis. The counts generated by the PET camera fit the radioactivity measurements of arterial blood sampling with correction for metabolites and decay.
The bilateral outlines of the cortex and selected structures (the cingulate, the frontal, the parietal, the temporal, and the occipital cortices, the caudate nucleus, the fusiform gyrus, the globus pallidus, the hippocampus, the insula, the parahippocampus, the putamen, and the thalamus) of a standard brain model were aligned in size and orientation to the PET radioactivity images in three dimensions (3D). The program for this purpose displayed six images of transverse, coronal, or sagittal view, one view at a time together with outline plots of the studied structures. The program utilized interactive displacement linearly or rotationally or enlargement or shrinkage of the outlined plots as a whole, a half (e.g., left side), or a quarter (e.g., left lower quarter) at selected magnitudes (e.g., 1, 3, and 7 mm for linear displacement) to align the outlined plots to the corresponding outlines given by PET images. After the alignment of the cortical outlines, the outlines of the basal structures were aligned separately. The view, displacement type, and displacement magnitude of each trial were stored in files (displacement histories) separately for spatial alignments of the cortex and the basal structures. The standard VOIs were transferred to individual PET spaces. In the process, individual grid points (i.e., the centers of the voxels) of the PET image volumes were displaced in two ways and averaged, being weighted for the distances from the two outline sets. The voxel-occipital cortex radioactivity volumes were obtained at each frame around 24 hours post injection (typically three 20 minute frames or two 30 minute frames) and averaged across frames.
VOIs drawn on the PET scans for the cerebellum, the occipital cortex, the pons, the striatum, and the thalamus were characterized for expression in visual representations. The transformed VOIs were applied to the averaged ratio volumes to obtain regional ratios, in addition to obtaining the radioactivity in the occipital cortex for each frame. The magnetic resonance (MR) volumes of the individual subjects were spatially normalized to a standard brain using programs of the Statistical Parametric Mapping 99 (SPM’99) (http://www.fil.ion.ucl.ac.uk/spm/software/spm99/) package (Ashburner and Friston, 1997; Ashburner et al., 1997). The PET volumes of these subjects were transferred to the standard brain space in the same manner as the MR volumes, after transfer to their MR space (Haut et al., 2000a,b). The individual PET volumes were smoothed using a Gaussian kernel (13 mm full-width at half-maximum). Standard VOIs were applied to spatially normalized PET volumes to obtain regional radioactivity at six hours post injection. The voxel-occipital cortical ratio volumes were generated by dividing voxel radioactivity by the mean occipital cortical radioactivity in the individual subjects.
To investigate the tracer kinetic properties of 2-[18F]FA in the living human brain during dynamic PET scans, we utilized compartmental modeling (Zhou et al., 2001). We employed a three-compartmental, five-parameter model to represent the PET kinetics of 2-[18F]FA, a reversibly binding radioligand (Zhou et al., 2001). Mathematical modeling of reversibly binding radioligands yielded the results on Tables X and XI (Zhou et al., 2001, 2002a, b, 2003).
RESULTS
The central methodology of this study was the examination of the nondisplaceable binding potentials (BPND) (Innis et al., 2007) and distribution of neuronal high-affinity α4β2 nAChRs in the brains of healthy people who do not smoke cigarettes, i.e., normal control subjects, and smokers with and without paranoid schizophrenia using positron emission tomography (PET) imaging with 2-[18F]FA, a safe and efficacious agent to visualize neuronal high affinity α4β2 nAChRs in humans (Schildan et al., 2007; Sorger et al., 2007). The research was conducted on a high resolution research tomograph (HRRT) (Siemens, Knoxville, Tennessee), a scanner with a resolution approaching 2 mm (Sossi et al., 2005). During this preliminary study, four healthy adult volunteers and six adults who smoked cigarettes (five with and one without paranoid schizophrenia) underwent PET imaging of the brain 5 to 6 hours after the intravenous administration of a bolus followed by a continuous infusion over 6 hours of approximately 444 MBq (12 mCi) 2-[18F]FA. The uptake of 2-[18F]FA in the nonsmokers was twice the uptake of the smokers who had just smoked cigarettes a few hours before the scans (Figure 1). These findings are consistent with our finding less uptake of 5-[123I]IA in the thalami of people with greater plasma nicotine levels (Brašić et al., 2009).
Fig. 1.
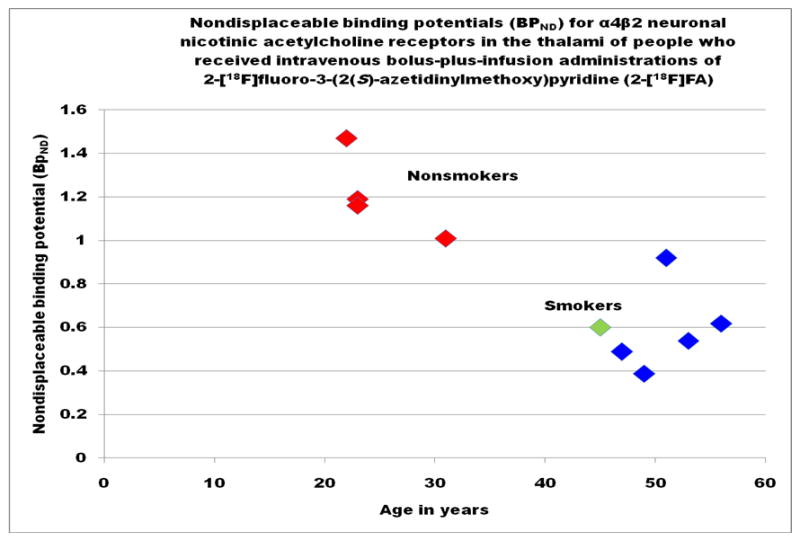
The nondisplaceable binding potential (BPND) (Innis et al., 2007) in the thalamus attained at equilibrium approximately 5 hours after the injection of an intravenous adminstration of 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) into ten human participants. The nondisplaceable binding potential (BPND) is represented on the ordinate (y-axis) while the age of the participant in years is represented on the abscissa (x-axis). Healthy nonsmokers without paranoid schizophrenia, normal controls, are represented by red diamonds. The single smoker without paranoid schizophrenia is represented by a green diamond. The smokers with paranoid schizophrenia are represented by dark blue diamonds.
Justification for modeling without arterial blood sampling
Figure 2 represents the images on a GE Advance Scanner in the first two hours after the intravenous administration of 413.29 MBq (11.17 mCi) 2-[18F]FA to a healthy 22-year-old man who did not smoke cigarettes demonstrating (A) nonspecific uptake in the third through fifth hours and (B) specific receptor binding at equilibrium (Innis et al., 2007). Prominent binding in the thalamus was present at equilibrium. Since 2-[18F]FA attained equilibrium at approximately 5 hours after administration, specific uptake primarily in the thalamus occurs in the later image (right panel of Figure 2).
Fig. 2.
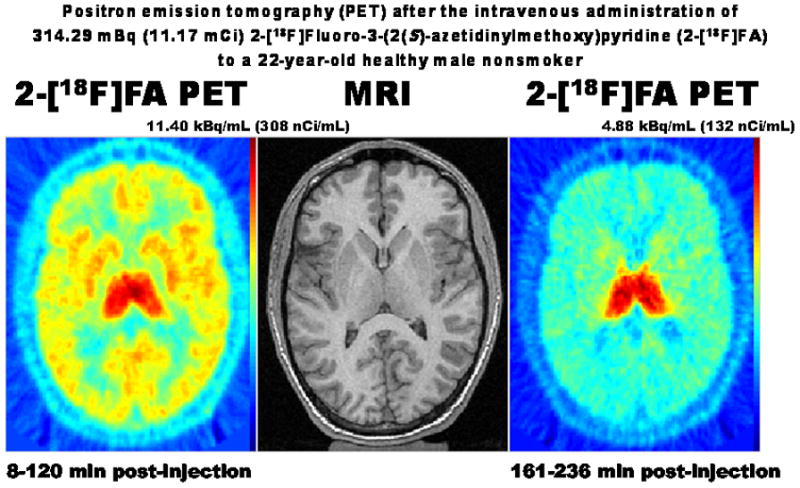
Transverse sections of the brain through the level of the thalamus and striatum of a healthy nonsmoking 22-year-old adult man. The center image shows magnetic resonance imaging (MRI) while the left and right panels show averaged positron emission tomography (PET) images from 8 to 120 min (left panel) and from 161 to 236 min (right panel) after the intravenous administration of 314.29 kBq (11.17 mCi) 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) as a bolus followed by continuous infusion. Marked uptake in the thalamus is evident in both left and right images. These images demonstrate the use of 2-[18F]FA PET to estimate the density and the distribution of the high-affinity α4β2 nicotinic acetylcholine receptors (nAChRs) in the living human brain. During the first two hours of the scan nonspecific uptake occurs in the cortex, the thalamus, and some deep nuclei of the brain (left panel). Since 2-[18F]FA attains equilibrium at approximately 5 hours after administration, specific uptake primarily in the thalamus occurs in the later image (right panel).
The plasma time-activity curve for the first woman, corrected for radiotracer decay and metabolites, demonstrated rapid uptake of radioactivity to a peak of 0.143 MBq/mL (3.867 μCi/mL) at 19 seconds after the injection of a bolus of 274.17 MBq (7.41 mCi) 2-[18F]FA with rapid decay. The plasma time-activity curve (TAC) after the administration of a bolus followed by an infusion corrected for radiotracer decay and metabolites demonstrated a radioactivity concentration of 3.7 kBq/mL (0.1 μCi/mL) for 10 to 100 minutes after the commencement of the radiotracer infusion. The TACs appeared stable for cortical regions. The thalamic TAC reached plateau at 322 min from the initiation of the infusion. Then the plasma radioactivity decayed to 0.998 kBq/mL (0.054 μCi/mL) at 350 minutes after the commencement of the radiotracer infusion. The graph of the binding potentials obtained after the intravenous bolus injection of 7274.17 MBq (7.41 mCi) 2-[18F]FA (on the ordinate, y-axis) as a function of the binding potentials obtained after the bolus-plus-infusion scheme for the 23-year-old female nonsmoker approach identity (Figure 3) to demonstrate the equivalence of the two protocols, i.e., bolus plus continuous infusion and bolus only without continuous infusion. Thus, we conclude that the administration of 2-[18F]FA by a bolus followed by a continuous infusion for 360 minutes is an adequate means to estimate the nondisplaceable binding potential (BPND) (Innis et al., 2007) without arterial blood sampling.
Fig. 3.
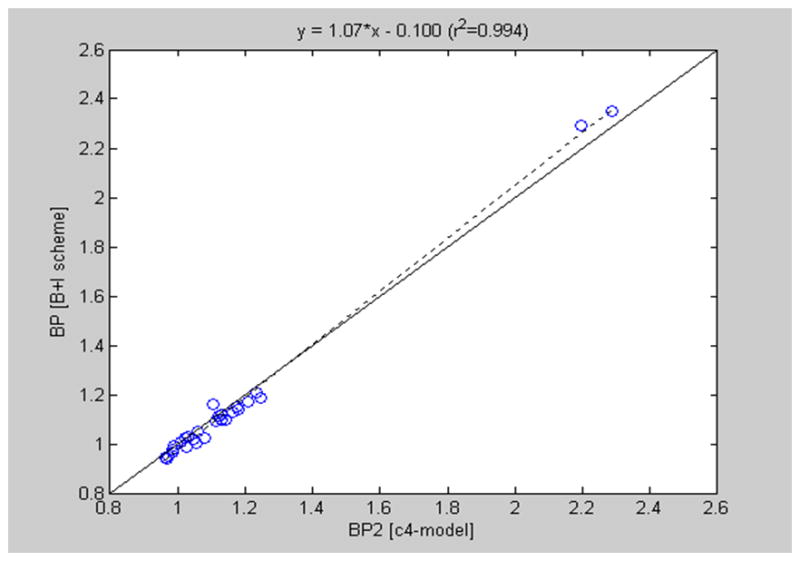
A healthy nonsmoking 23-year-old woman underwent positron emission imaging on a GE Advance Scanner after the intravenous administration of 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) as a 274.71 MBq (7.41 mCi) bolus injection (abscissa, x-axis) and a week later as an 415.51 MBq (11.23) mCi bolus plus infusion (ordinate, y-axis). The regional nondisplaceable binding potential (BPND) (Innis et al., 2007) of a four-compartment model of each scheme is presented as a scatter plot (y = 1.07*x − 0.100, r2=0.994). The line of identity (solid line) and regression line (dotted line) are shown.
On the basis of the results of protocols of the two healthy adults who do not smoke cigarettes on a GE Advance scanner following the intravenous bolus injection of 2-[18F]FA and following the intravenous bolus injection followed by the continuous infusion of 2-[18F]FA with a bolus:infusion ratio of 390:330, we concluded that equilibrium is attained after 300 to 36 minutes so that a sixty-minute scan 300 to 360 minutes after the start of a bolus plus infusion achieves equilibrium in the thalamus. We concluded that a simplified reference tissue method without arterial blood sampling is suitable for modeling the results of a sixty-minute scan 360 minutes following the infusion of a bolus following by a continuous infusion of 2-[18F]FA utilizing the occipital cortex as the reference region (Kendziorra et al., 2011).
Estimation of the nondisplaceable binding potential (BPND) and the distribution of neuronal high-affinity α4 β 2 nAChRs
Nondisplaceable binding potentials (BPNDs) (Innis et al., 2007) of participants were estimated for the thalamus, the fusiform gyrus, and the frontal, temporal, parietal, occipital, and cingulate cortices in Table X and for the caudate nucleus, the globus pallidus, the hippocampus, the insula, the parahippocampus, and the putamen in Table XI. The BPNDs for the cingulate, frontal, temporal, parietal, and occipital cortices, the fusiform gyrus (Table X), the hippocampus, the insula, the putamen, the caudate nucleus, the globus pallidus, and the parahippocampus (Table XI) display values hovering around zero for all participants indicating minimal uptake (Tables X and XI). Since all participants exhibited minimal radiotracer uptake in the frontal, temporal, parietal, occipital, and cingulate cortices, the fusiform gyrus (Table X), the hippocampus, the insula, the putamen, the caudate nucleus, the globus pallidus, and the parahippocampus (Table XI), no further analysis was appropriate for those regions. 2-[18F]FA displays limited value to evaluate the density and the distribution of high-affinity α4β2 nAChRs in the frontal, temporal, parietal, occipital, and cingulate cortices, the fusiform gyrus (Table X), the hippocampus, the insula, the putamen, the caudate nucleus, the globus pallidus, and the parahippocampus (Table XI). On the other hand, the BPND in the thalamus attained equilibrium approximately 5 hours after the injection of the radiotracer (Table X). These values are plotted on Figure 1.
The small sample sizes (six experimental subjects and four control subjects) required careful analytical statistics (Brašić et al., 2003b). Table IV summarizes the demographic and the clinical characteristics of all participants. Figure 1 represents a plot of the nondisplaceable binding potential (BPND) (Innis et al., 2007) in the thalamus attained at equilibrium approximately 5 hours after the injection of an intravenous adminstration of 2-[18F]FA to the ten human participants. The nondisplaceable binding potential (BPND) (Innis et al., 2007) is represented on the ordinate (y-axis) while the age of the participant in years is represented on the abscissa (x-axis). Healthy nonsmokers without paranoid schizophrenia, normal controls, are represented by red dots. The single smoker without paranoid schizophrenia is represented by a green dot. The smokers with paranoid schizophrenia are represented by dark blue dots. The nonsmokers without paranoid schizophrenia, aged 22 to 31, exhibit BPNDs in the thalamus approximately twice those of the smokers aged 45 to 56 who smoked cigarettes a few hours before the scans. The BPNDs in the thalamus of the single smoker without paranoid schizophrenia aged 45 and the five smokers with paranoid schizophrenia aged 47 to 56 were equivalent (Figure 1).
The participants in this study comprise two well-demarcated groups, nonsmokers aged 22 to 31 and smokers aged 45 to 56. The distributions of the BPNDs in the thalamus in these samples are likely not normally distributed. Therefore, nonparametric analysis is appropriate for this data. Application of the Wilcoxon Signed-Rank Test demonstrated a significant difference in the BPNDs in the thalamus of the two study groups. Thus, we have demonstrated that the 2-[18F]FA BPNDs in the thalamus was significantly higher for nonsmokers without paranoid schizophrenia aged 22 to 31 than for smokers aged 45 to 56 with and without paranoid schizophrenia who smoked cigarettes a few hours before scans (P = 0.0105) (StataCorp, 2003). The BPNDs of the single smoker without paranoid schizophrenia and the smokers with paranoid schizophrenia were comparable (Figure 1).
Psychiatric diagnoses
One participant with paranoid schizophrenia met the criteria for a current diagnosis of Cannabis Intoxication and another participant with paranoid schizophrenia met the criteria for a current diagnosis of Post-Traumatic Stress Disorder (First et al., 1997) (Table VI). Additionally, among the participants with paranoid schizophrenia, lifetime diagnoses of Alcohol Dependence and Cannabis Dependence were met by one, Alcohol Dependence and Cocaine Dependence by a second, and Cannabis Dependence and Cocaine Dependence by a third (First et al., 1997) (Table VI). All of the participants with paranoid schizophrenia scored under 60 on the Axis V Global Assessment of Functioning Scale for both Current Rating and Highest Rating Past Year to confirm the serious impairments in people with paranoid schizophrenia (Blacker, 2009; First et al., 1997) (Table VI). All the participants with paranoid schizophrenia also scored in multiple items on the Brief Psychiatric Rating Scale Anchors (BPRSA) (Brašić et al., 2009, 2010b; Marder, 1995; McMahon et al., 2002; Overall & Gorham, 1962; Woerner et al., 1988), the SANS (Andreasen, 1984a; Blacker, 2009; Marder, 1995), and the SAPS (Andreasen, 1984b; Blacker, 2009; Marder, 1995) to confirm the presence of serious symptoms of mental illness.
We observe that all smokers in the study exceeded the cutoff score (6) for nicotine dependence by means of the FTND (Balfour and Fagerström, 1996; Kawakami et al., 1999) (Table VI). All but one smoker exceeded the cutoff score (4) for nicotine dependence by means of the TDQ (Kawakami et al., 1999) (Table VI). These results suggest that all the smokers truly had nicotine dependence (Balfour and Fagerström, 1996; Kawakami et al., 1999) (Table VI).
Table V summarizes the results of participant-rated and examiner-rated rating scales for adult Attention-Deficit/Hyperactivity Disorder (Ward et al., 1993; Wender et al., 2001), compulsions (Marks, 1986, page 49) and obsessions (Dominguez et al., 1989, page 216). We have summarized the results of examiner-rated rating scales for Attention-Deficit/Hyperactivity Disorder (Leckman et al., 1978) and Obsessive-Compulsive Disorder (Goodman et al., 1989a,b; Leckman et al., 1978; Pato and Pato, 1991) in Table V, global functioning (American Psychiatric Association, 2000; Brasić et al., 1998; First et al., 1997; Guy, 1976; Folstein et al., 1975; Marder, 1995; Pato and Pato, 1991) in Tables VI and VII, general dyskinesias (Brasic, 2003b; Brasic et al., 2010a; Campbell, 1985; National Institute of Mental Health, 1988; Simpson et al., 1979) in Table VIII, and akathisia and tics (Barnes, 1989; Leckman et al., 1988; Sachdev, 1994) in Table IX. We note that only one participant, a man with paranoid schizophrenia and cannabis intoxication (Table VI), exceeded the cutoff score (46) for ADHD on the ADHD items of the WURS (Ward et al., 1993) and scored 5 Severe on the Clinical Global Impression Scale for Attention Deficit Disorder (CGISADD) (Leckman et al., 1988) (Table V). His inattentiveness likely represented an adverse effect of his intoxication. The participants with paranoid schizophrenia exhibited a range of obsessions and compulsions (Table V). The administration of the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) (Goodman et al., 1989a,b) to the 47-year-old man with paranoid schizophrenia likely yielded spurious results because the participant reported his delusions as obsessions and compulsions so the administration of this scale was omitted. Unreliable results are likely to be obtained when the Y-BOCS is administered to people with schizophrenia and other psychoses.
All participants with paranoid schizophrenia demonstrated evidence of illness on the CGISI (Table VII). None of the participants demonstrated scores indicative of dementia on the MMSE (20 or less) (Blacker, 2009; Folstein et al., 1975); however, one participant with paranoid schizophrenia scored 24 suggesting mild dementia, a finding consistent with paranoid schizophrenia (Table VII). None of the participants demonstrated scores high enough to merit the diagnosis of the serotonin syndrome on the SSC (Brasić et al., 1998) (Table VII).
Movement disorders
Each of the participants with paranoid schizophrenia demonstrated some characteristics of movement disorders on the Movement Disorders Checklist (Brasic, 2003b; Brasic et al., 2010a); however, none unequivocally fulfilled the algorithms for specific movement disorders (Brasic et al., 2010a). Each of the participants with paranoid schizophrenia some demonstrated evidence consistent with tardive dyskinesia on the Abnormal Involuntary Movement Scale (AIMS) (Brasic, 2003b; Brasic et al., 2010a; National Institute of Mental Health, 1988) and the Rating Scale for Tardive Dyskinesia (RSTD) (Simpson et al., 1979) (Table VIII). All the male participants with paranoid schizophrenia demonstrated evidence of stereotypies on the Timed Stereotypies Rating Scale (TSRS) (Brasic, 2003b; Brasic et al., 2010a; Campbell, 1985). (Table VIII). Most of the participants with paranoid schizophrenia demonstrated at least minimal evidence of akathisia (Barnes, 1989; Sachdev, 1994) (Table IX). Most of the participants with paranoid schizophrenia scored at most “1 Borderline” for tic symptoms on the Clinical Global Impression Scale for Tourette Syndrome (CGISTS) (Leckman et al., 1988) consistent with minimal symptoms (Table IX).
DISCUSSION
Utilizing postmortem data (Breese, et al., 2000), we hypothesized that the densities of high-affinity neuronal α4β2 nAChRs exist in a continuum from highest to lowest as follows: smokers without schizophrenia > smokers with schizophrenia > nonsmokers without schizophrenia > nonsmokers with schizophrenia. Based on the postmortem data the hypothesized continuum of the densities of high-affinity neuronal α4β2 nAChRs is present in the caudate, the cortex, the hippocampus, and the thalamus (Tables I, II, and III) (Stata, 2003).
Positron emission tomography (PET) was performed for 60 minutes at six hours after the intravenous administration of 444 MBq (12 mCi) 2-[18F]FA as a bolus plus continuous infusion to 10 adults (7 men and 3 women) (6 smokers including 5 with paranoid schizophrenia and 4 nonsmokers) ranging in age from 22 to 56 years (mean 40.1, standard deviation 13.6). Six cigarette smokers, five with and one without paranoid schizophrenia, and four healthy adult volunteer control participants underwent PET brain scans for 60 minutes at approximately five hours following the intravenous injection of a bolus followed by a continuous infusion of approximately 444 MBq (12 mCi) 2-[18F]FA. The first two healthy adults underwent both bolus and bolus plus infusion scans on a GE Advance Scanner to establish the accuracy of modeling of the bolus plus infusion scan after attaining equilibrium. All other scans were performed on the HRRT scanner. Equilibrium was attained approximately five hours after the start of an intravenous administration 2-[18F]FA. The absolute density (Bmax) of nAChRs cannot be measured with a single scan for each participant. As a surrogate measure of the absolute density (Bmax) of nAChRs, the nondisplaceable binding potential (BPND) was utilized for this study.
Although the small sample size and potential confounding variables limited our test of the hypothesis, we demonstrated that BPNDs of 2-[18F]FA in the thalami of the healthy nonsmokers aged 22 to 31 years were significantly higher than those of smokers aged 45 to 56 who smoked cigarettes a few hours before the scans, indicating a higher density of unoccupied nAChRs in nonsmokers than in smokers, in contrast to the postmortem findings where there was a trend (P = 0.0780) (Stata, 2003) (Table II) for smokers to have a greater density of unoccupied nAChRs than nonsmokers (Breese, et al., 2000). Since the smokers had smoked cigarettes shortly before their scans, many nAChRs were occupied by nonradioactive nicotine from cigarettes consumed before the scans. The thalamus attained significant nondisplaceable binding at equilibrium after approximately five hours of radiotracer administration. In this small sample of participants the BPND of 2-[18F]FA did not differentiate smokers from nonsmokers outside the thalamus. Thus, while a significant difference in the densities of high-affinity neuronal α4β2 nAChRs in the cortex was present between smokers and nonsmokers on autopsy specimens (Breese et al., 2000), we did not replicate that finding in our current population of living human participants due to multiple confounding influences.
The striking contrast between the BPNDs in the thalamus of the smokers and the nonsmokers has several possible explanations.
The neuronal high-affinity α4β2 nAChRs of the smokers likely were saturated with nicotine from cigarette smoking at the time of the administration of 2-[18F]FA. The smokers smoked cigarettes the morning of the scan so the nicotinic receptors were apparently already occupied by nicotine at the time of the administration of 2-[18F]FA. Therefore, few of the neuronal high-affinity α4β2 nAChRs were likely unoccupied at equilibrium in the smokers. Thus, the reduced BPNDs in smokers on Figure 1 likely reflect the many neuronal high-affinity α4β2 nAChRs already occupied by exogenous nonradioactive nicotine from cigarettes are not represented on the graph. Thus, the 2-[18F]FA scans of all the smokers likely represent scans after the administration through cigarettes of a blocking agent, nicotine. These results are consistent with the reported finding that after smoking to satiety, 2.8 cigarettes, smokers exhibit a 50% reduction in radioactivity from the abstinent baseline state (Brody et al., 2006). These findings were confirmed by other investigators who noted 67% receptor occupancy with 5-[123I]IA in smokers who smoked to satiety after 5 days abstinence (Esterlis et al., 2010).
The density of the neuronal high-affinity α4β2 nAChRs in the thalamus likely decline with increasing age. Additionally, there may be a marked reduction in the density of neuronal high-affinity α4β2 nAChRs throughout the brain proportional to age. This apparent decrement of density of neuronal high-affinity α4β2 nAChRs is likely similar to the decrement of density of dopamine D2 and serotonin receptors with age (Wong et al., 1984, 1988). The nature of reductions of 2-[18F]FA BPND with age merits investigation with populations of healthy adults as well as adults with paranoid schizophrenia and other diseases.
The data substantiate our hypothesis that a deficiency of the neuronal high-affinity α4β2 nAChRs may characterize a biologically distinct class of people with paranoid schizophrenia. A class of people with paranoid schizophrenia likely has a reduced BPND of the α4β2 nAChRs in the thalamus and other VOIs. Some people with paranoid schizophrenia have fewer nAChRs than people without schizophrenia.
Other issues influence the density of neuronal high-affinity α4β2 nAChRs. Gender, socioeconomic status, education, environmental exposures, and other influences likely affect the density and the distribution of nAChRs in the brain. There are likely differences between men and women in the density of neuronal high-affinity α4β2 nAChRs. Thus, as with dopamine receptors, the densities of neuronal high-affinity α4β2 nAChRs are likely higher in the periovulatory and luteal phases and lower in the follicular phase of the menstrual cycle (Wong et al., 1988). Additionally research on the BPNDs of 2-[18F]FA in women in the various phases of the menstrual cycle is needed. Further investigation to indentify the BPNDs of 2-[18F]FA in men and women throughout the lifespan is needed.
A combination of the above explanations and other unknown influences hold.
Further investigation is needed to confirm the participation of each of these hypotheses. The nondisplaceable binding potential (BPNDs) (Innis et al., 2007) for the frontal, temporal, parietal, occipital, and cingulate cortices, and the fusiform gyrus (Table X), and for the hippocampus, the insula, the putamen, the caudate nucleus, the globus pallidus, and the parahippocampus (Table XI) are all approximately zero. Thus, there is minimal radiotracer uptake at equilibrium in for the frontal, temporal, parietal, occipital, and cingulate cortices, and the fusiform gyrus (Table X), and for the hippocampus, the insula, the putamen, the caudate nucleus, the globus pallidus, and the parahippocampus (Table XI). We conclude that 2-[18F]FA has limited potential to estimate the density and the distribution of the neuronal high-affinity α4β2 nAChRs in the frontal, temporal, parietal, occipital, and cingulate cortices, and the fusiform gyrus (Table X), and for the hippocampus, the insula, the putamen, the caudate nucleus, the globus pallidus, and the parahippocampus (Table XI) in the brains of humans. Future research to estimate the density and the distribution of 2-[18F]FA in the cortex and white matter may benefit from the use of other agents (Horti and Wong, 2009).
The occurrence of reductions in vesicular acetylcholine transporters and neuronal high-affinity α4β2 nAChRs in the brains of rats treated with the cholinergic immunolesioning agent 192 IgG-saporin (SAP) (Quinlivan et al., 2007) suggests that reductions in neuronal high-affinity α4β2 nAChRs may occur along with reductions in vesicular acetylcholine transporters in people with paranoid schizophrenia. Future research to characterize possible deficits of vesicular acetylcholine transporters in people with paranoid schizophrenia is needed.
If a brain imaging procedure can determine alterations in the density and distributions of neuronal high-affinity α4β2 nAChRs in the living human brain of people with paranoid schizophrenia who do and do not smoke cigarettes, then those who likely will benefit from the administration of nicotine and nicotinic agonists can likely be identified. Although this study cannot differentiate alterations of brains of people with paranoid schizophrenia who do and do not smoke cigarettes, we present the current data on the special population of people with paranoid schizophrenia who smoke cigarettes to share with colleagues who may utilize the data for clinical, research, and administrative purposes.
Limitations
An optimal experimental design includes the utilization of exactly the same protocol for all participants. The healthy nonsmokers who participated in this study did not undergo the extensive psychiatric and neuropsychiatric evaluations so large portions of data are absent (Tables IV through IX).
A further limitation of this pilot study is the failure to match by age and sex the smokers with paranoid schizophrenia and the nonsmokers without paranoid schizophrenia. Thus, all the nonsmokers aged in their twenties and thirties have high nondisplaceable binding potentials (BPND) in the thalamus while the smokers with and without paranoid schizophrenia aged in their forties and fifties have low nondisplaceable binding potentials in the thalamus. While this may represent blocking of nAChRs by nicotine in the smokers, there is also possibly an inverse relationship between (BPND) and age (Fig. 1). Further research with age- and sex-matched controls for the experimental groups is mandatory to resolve this conundrum.
Limited uptake of 2-[18F]FA in the brains of our subjects may have resulted from our use of HPLC (Hilton et al., 2000). Future studies may benefit from the utilization of a one-step solid-phase extraction of nonmetabolized 2-[18F]FA (Shumway et al., 2007).
Another limitation is the small sample size. Pilot studies of paranoid schizophrenia are often hindered by limited knowledge of the study variables. This study must be replicated in other geographic regions to confirm the results. The design and analysis of experiments with small sample sizes requires specific consideration (Brašić et al., 2003c).
Ethical aspects must be addressed in future research. Participants with paranoid schizophrenia may lack the cognitive capacity to understand proposed research studies. Surrogates of people with paranoid schizophrenia may agree to the participation of their wards with paranoid schizophrenia because of desperation to seek assistance for their offspring. Research scientists may be so eager to evaluate investigations of paranoid schizophrenia that they may lack the objectivity to fully recognize the possible adverse effects of study protocols. Future studies of paranoid schizophrenia require objective evaluation by persons without an interest in the outcome (Chun et al., 2002).
We demonstrated that BPNDs in the thalamus of the healthy nonsmokers aged 22 to 31 years are significantly higher than those of smokers aged 45 to 56 who smoked cigarettes a few hours before the scans. This likely represents a blocking of the nAChRs by the exogenous nicotine from smoked cigarettes for the smokers. Although the densities of high-affinity neuronal α4β2 nAChRs exist in a continuum from highest to lowest as follows: smokers without schizophrenia > smokers with schizophrenia > nonsmokers without schizophrenia > nonsmokers with schizophrenia for in the caudate, the cortex, the hippocampus, and the thalamus for the data of autopsy specimens (Breese et al., 2000, Stata, 2003) (Table III), this relationship was not confirmed in this study probably due to the small samples and to confounding influences. 2-[18F]FA is a promising tool to estimate the density and the distribution of the neuronal high-affinity a4b2 nAChRs in the brain to characterize the pathophysiological mechanisms of paranoid schizophrenia as well as other neurological and psychiatric disorders.
[2-[18F]FA PET likely represents be a safe, effective technique to estimate the integrity of the nicotinic system in paranoid schizophrenia (Brašić et al., 2010b). Further studies to investigate the validity and reliability of PET following the intravenous injection of 2-[18F]FA to adults, adolescents, and children with paranoid schizophrenia and other neuropsychiatric diseases are needed. Therapeutic interventions for paranoid schizophrenia and other neuropsychiatric conditions may be monitored by serial performance of 2-[18F]FA PET before, during, and after clinical trials. Further research is needed to clarify the pathognomonic abnormalities in movement and chemistry in paranoid schizophrenia (Lafargue and Brasic, 2000; Wong and Brašić, 2001, 2005; Wong et al., 2003, 2007, 2009, 2011). Future research must include demographic data about study populations to identify racial and ethnic variations (Brašić, 2003a, 2004).
Acknowledgments
This research was sponsored by the Brain and Behavior Research Foundation (NARSAD) (JRB), the Essel Foundation (JRB), and the National Institutes of Health (NIH) by Public Health Service Grant DA09482 (DFW), and the National Center for Research Resources of the NIH M01RR00052 (Clinical Research Unit). Dr. Brašić is a member of the Medical Advisory Board of the Tourette Syndrome Association of Greater Washington, Silver Spring, Maryland.
Footnotes
Earlier versions of this manuscript were presented at the 3rd Annual Rett Syndrome Symposium, Rett Syndrome Research Foundation (www.rsrf.org), Baltimore, Maryland, June 17-19, 2002 (Chun et al., 2002; Zhou et al., 2002a); the 4th Annual Rett Syndrome Symposium, Baltimore, Maryland, June 23-25, 2003 (Brašić et al., 2003c), the 5th Annual Rett Syndrome Symposium, June 28-30, 2004, Baltimore, Maryland (Brašić et al., 2004, Zhou et al., 2004), and the 57th Annual Meeting of the Society of Nuclear Medicine in Salt Lake City, Utah, on June 5 to 9, 2010 (Brašić et al., 2010b).
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. (DSM-IV) [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision (DSM-IV-TR ) [Google Scholar]
- Andreasen NC. The Schedule for the Assessment of Negative Symptoms (SANS) Iowa City, Iowa: The University of Iowa; 1984a. [Google Scholar]
- Andreasen NC. The Schedule for the Assessment of Positive Symptoms (SAPS) Iowa City, Iowa: The University of Iowa; 1984b. [Google Scholar]
- Ashburner J, Friston K. Multimodal image coregistration and partitioning -- a unified framework. NeuroImage. 1997;6:209–217. doi: 10.1006/nimg.1997.0290. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Neelin P, Collins DL, Evans A, Friston K. Incorporating prior knowledge into jmage registration. NeuroImage. 1997;6:344–352. doi: 10.1006/nimg.1997.0299. [DOI] [PubMed] [Google Scholar]
- Balfour DJK, Fagerström KO. Pharmacology of nicotine and its therapeutic use in smoking cessation and neurodegenerative disorders. Pharmacol Ther. 1996;72:51–81. doi: 10.1016/s0163-7258(96)00099-x. [DOI] [PubMed] [Google Scholar]
- Barnes TRE. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154:672–676. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Blacker D. Psychiatric rating scales. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9. Vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 1032–1059. [Google Scholar]
- Brašić JR. Documentation of demographic data. Psychol Rep. 2003a;93:151–152. doi: 10.2466/pr0.2003.93.1.151. [DOI] [PubMed] [Google Scholar]
- Brašić JR. Treatment of movement disorders in autism spectrum disorders. In: Hollander E, editor. Autism Spectrum Disorders. Volume 24 of the Medical Psychiatry Series. New York: Marcel Dekker, Inc; 2003b. pp. 273–346. [Google Scholar]
- Brašić JR. Documentation of ethnicity. Psychol Rep. 2004;95:859–861. doi: 10.2466/pr0.95.3.859-861. [DOI] [PubMed] [Google Scholar]
- Brasić JR, Barnett JY, Sheitman BB, Lafargue RT, Kowalik S, Kaplan D, Tsaltas MO, Ahmad R, Nadrich RH, Mendonça MF. Behavioral effects of clomipramine on prepubertal boys with autistic disorder and severe mental retardation. CNS Spectrums: The International Journal of Neuropsychiatric Medicine. 1998;3(10):39–46. [Google Scholar]
- Brasic JR, Bronson B, Chun TT. Tardive Dyskinesia. eMedicine from WebMD. 2010a Updated January 21, 2010. Available at: http://emedicine.medscape.com/article/1151826-overview.
- Brasic J, Cascella N, Hussain B, Bisuna B, Kumar A, Raymont V, Guevara M, Horti A, Wong D. PET experience with 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]FA) in the living human brain of smokers and nonsmokers. J Nucl Med. 2010b;51 (Supplement 2):1828. [Google Scholar]
- Brašić JR, Rohde CA, Maris MA, Wong DF. Sample size determination for studies of Rett syndrome and other rare conditions. 4th Annual Rett Syndrome Symposium, Rett Syndrome Research Foundation; Baltimore, Maryland. June 23–25 2003; 2003. p. 46. www.rsrf.org. [Google Scholar]
- Brasic JR, Wong DF. PET scanning in autism spectrum disorders. eMedicine from WebMD. 2011 Updated June 7, 2011. http://www.emedicine.com/neuro/topic440.htm.
- Brašić JR, Zhou Y, Musachio JL, Hilton J, Fan H, Crabb A, Endres CJ, Reinhardt MJ, Dogan AS, Alexander M, Rousset O, Maris MA, Galecki J, Nandi A, Wong DF. Single photon emission computed tomography (SPECT) experience with (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5-[123I]IA) in the living human brain of smokers and nonsmokers. Synapse. 2009;63:339–358. doi: 10.1002/syn.20611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brašić JR, Zhou Y, Musachio J, Hilton J, Fan H, Crabb A, Endres CJ, Reinhardt MJ, Dogan AS, Alexander M, Rousset O, Maris MA, Kuwabara H, Wong DF. Neuropsychiatric assessment of (S)-5-[123I]iodo-3-(2-azetidinylmethoxy)pyridine (5IA). 5th Annual Rett Syndrome Symposium; June 28–30, 2004; Inn at the Colonnade, Baltimore, Maryland. Cincinnati, Ohio: Rett Syndrome Research Foundation (RSRF); 2004. p. 44. www.rsrf.org. [Google Scholar]
- Breese CR, Lee MJ, Adams CE, Sullivan B, Logel J, Gillen KM, Marks MJ, Collins AC, Leonard S. Abnormal regulation of high affinity nicotinic receptors in subjects with schizophrenia. Neuropsychopharmacology. 2000;23:351–364. doi: 10.1016/S0893-133X(00)00121-4. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, Jou J, Allen V, Tiongson E, Chefer SI, Koren AO, Mukhin AG. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Arch Gen Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. The Timed Stereotypies Rating Scale. Psychopharmacol Bull. 1985;21:1082. [Google Scholar]
- Chun TT, Brašić JR, Morgan RH. Is research on human being with Rett syndrome ethical?; 3rd Annual Rett Syndrome Symposium, Rett Syndrome Research Foundation; Baltimore, Maryland. June 17–19, 2002; 2002. p. 30. ( www.rsrf.org) [Google Scholar]
- Denckla MB. Revised neurological examination for subtle signs (1985) Psychopharmacol Bull. 1985;21(4):773–800. [PubMed] [Google Scholar]
- Dominguez RA, Jacobson AF, de la Gandara J, Goldstein BJ, Steinbock RM. Drug response asssessed by the Modified Maudsley Obsessive–Compulsive Inventory. Psychopharmacol Bull. 1989;25(2):215–218. [PubMed] [Google Scholar]
- Dursun SM, Reveley MA. Differential effects of transdermal nicotine on microstructured analyses of tics in Tourette’s syndrome: an open study. Psychol Med. 1997;27:483–487. doi: 10.1017/s0033291796003984. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Cosgrove KP, Batis JC, Bois F, Stiklus SM, Perkins E, Seibyl JP, Carson RE, Staley JK. Quantification of smoking-induced occupancy of β2-nicotinic acetylcholine receptors: estimation of nondisplaceable binding. J Nucl Med. 2010;51:1226–1233. doi: 10.2967/jnumed.109.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders---Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. II. Validity. Arch Gen Psychiatry. 1989a;46:1012–1016. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989b;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Guy W. DHEW Publ. No. (ADM) 76-338. Washington, DC: United States Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration; 1976. ECDEU assessment manual for psychopharmacology, revised, 1976; pp. 218–222.pp. 534–537. [Google Scholar]
- Haut MW, Kuwabara H, Leach S, Arias RG. Neural activation during performance of number-letter sequencing. Appl Neuropsychol. 2000a;7:237–242. doi: 10.1207/S15324826AN0704_5. [DOI] [PubMed] [Google Scholar]
- Haut MW, Kuwabara H, Leach S, Ty Callahan T. Age-related changes in neural activation during working memory performance. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2000b;7(2):119–129. [Google Scholar]
- Hilton J, Yokoi F, Dannals RF, Ravert HT, Szabo Z, Wong DF. Column-switching HPLC for the analysis of plasma in PET imaging studies. Nucl Med Biol. 2000;27:627–630. doi: 10.1016/s0969-8051(00)00125-6. [DOI] [PubMed] [Google Scholar]
- Horti AG, Gao Y, Kuwabara H, Dannals RF. Development of radioligands with optimized imaging properties for quantification of nicotinic acetylcholine receptors by positron emission tomography. Life Sci. 2010;86:575–584. doi: 10.1016/j.lfs.2009.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horti AG, Wong DF. Clinical perspective and recent development of PET radioligands for imaging cerebral nicotinic acetylcholine receptors. PET Clin. 2009;4:89–100. doi: 10.1016/j.cpet.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Kawakami N, Takatsuka N, Inaba S, Shimizu H. Developmental [sic] of screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addict Behav. 1999;24(2):155–166. doi: 10.1016/s0306-4603(98)00127-0. [DOI] [PubMed] [Google Scholar]
- Kendziorra K, Wolf H, Meyer PM, Barthel H, Hesse S, Becker GA, Luthardt J, Schildan A, Patt M, Sorger D, Seese A, Gertz H-J, Sabri O. Decreased cerebral α4β2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer's disease assessed with positron emission tomography. Eur J Nucl Med Mol Imaging. 2011;38:515–525. doi: 10.1007/s00259-010-1644-5. [DOI] [PubMed] [Google Scholar]
- Kimes AS, Horti AG, London ED, Chefer SI, Contoreggi C, Ernst M, Friello P, Koren AO, Kurian V, Matochik JA, Pavlova O, Vaupel DB, Mukhin AG. 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB J. 2003;17:1331–1333. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- Lafargue T, Brasic J. Neurodevelopmental hypothesis of schizophrenia: a central sensory disturbance. Med Hypotheses. 2000;55:314–318. doi: 10.1054/mehy.2000.1059. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, Cohen DJ. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–573. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Towbin KE, Ort SI, Cohen DJ. Clinical assessment of tic disorder severity. In: Cohen DJ, Bruun RD, Leckman JF, editors. Tourette's syndrome and tic disorders. New York: Wiley; 1988. pp. 55–78. [Google Scholar]
- Leonard S, Breese C, Adams C, Benhammou K, Gault J, Stevens K, Lee M, Adler L, Olincy A, Ross R, Freedman R. Smoking and schizophrenia: abnormal nicotinic receptor expression. Eur J Pharmacol. 2000;393:237–242. doi: 10.1016/s0014-2999(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Leung K. Molecular Imaging and Contrast Agent Database (MICAD) [Internet] Bethesda (MD): National Center for Biotechnology Information (US); 2006. 2-[18F]Fluoro-3-[2(S)-2-azetidinylmethoxy]pyridine; pp. 2004–2010. [Google Scholar]
- Marder SR. Psychiatric rating scales. In: Kaplan HI, Sadock BJ, editors. Comprehensive Textbook of Psychiatry/VI. 6. Vol. 1. Baltimore: Williams & Wilkins; 1995. pp. 619–635. [Google Scholar]
- Marks IM. Behavioural Psychotherapy : Maudsley Pocket Book of Clinical Management. Bristol: Wright; 1986. p. 49. [Google Scholar]
- McMahon RP, Kelly DL, Kreyenbuhl J, Kirkpatrick B, Love RC, Conley RR. Novel factor-based symptom scores in treatment resistant schizophrenia: implications for clinical trials. Neuropsychopharmacology. 2002;26:537–545. doi: 10.1016/S0893-133X(01)00387-6. [DOI] [PubMed] [Google Scholar]
- Meyer PM, Strecker K, Kendziorra K, Becker G, Hesse S, Woelpl D, Hensel A, Patt M, Sorger D, Wegner F, Lobsien D, Barthel H, Brust P, Gertz HJ, Sabri O, Schwarz J. Reduced alpha4beta2*-nicotinic acetylcholine receptor binding and its relationship to mild cognitive and depressive symptoms in Parkinson disease. Arch Gen Psychiatry. 2009;66:866–877. doi: 10.1001/archgenpsychiatry.2009.106. [DOI] [PubMed] [Google Scholar]
- National Institute of Mental Health, Alcohol Drug Abuse, and Mental Health Administration, Public Health Service, Department of Health, Education, and Welfare. Abnormal Involuntary Movement Scale (AIMS) Psychopharmacol Bull. 1988;24(4):781–783. [PubMed] [Google Scholar]
- Obrzut SL, Koren AO, Mandelkern MA, Brody AL, Hoh CK, London ED. Whole-body radiation dosimetry of 2-[18F]Fluoro-A-85380 in human PET imaging studies. Nucl Med Biol. 2005;32:869–874. doi: 10.1016/j.nucmedbio.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- Pato MT, Pato CN. Psychometrics in obsessive-compulsive disorder. In: Zohar J, Insel T, Rasmussen S, editors. The psychobiology of obsessive-compulsive disorder. New York: Springer; 1991. pp. 87–88. [Google Scholar]
- Quinlivan M, Chalon S, Vergote J, Henderson J, Katsifis A, Kassiou M, Guilloteau D. Decreased vesicular acetylcholine transporter and α4β2 nicotinic receptor density in the rat brain following 192 IgG-saporin immunolesioning. Neurosci Lett. 2007;415:97–101. doi: 10.1016/j.neulet.2006.08.065. [DOI] [PubMed] [Google Scholar]
- Rustin TA. Assessing nicotine dependence. American Family Physician; Aug 1, 2000. http://www.aafp.org/afp/20000801/579.html. [PubMed] [Google Scholar]
- Sachdev P. A rating scale for acute drug-induced akathisia: development, reliability, and validity. Biol Psychiatry. 1994;35:263–271. doi: 10.1016/0006-3223(94)91257-2. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Silver AA, Shytle RD, Philipp MK, Cahill DW, Fogelson HM, McConville BJ. Nicotine for the treatment of Tourette’s syndrome. Pharmacol Ther. 1997;74:21–25. doi: 10.1016/s0163-7258(96)00199-4. [DOI] [PubMed] [Google Scholar]
- Schildan A, Patt M, Sabri O. Synthesis procedure for routine production of 2-[18F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[18F]F-A-85380) Appl Radiat Isot. 2007;65:1244–1248. doi: 10.1016/j.apradiso.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Shumway DA, Pavlova OA, Mukhin AG. A simplified method for the measurement of nonmetabolized 2-[18F]F-A-85380 in blood plasma using solid-phase extraction. Nucl Med Biol. 2007;34:221–228. doi: 10.1016/j.nucmedbio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Lee JH, Zoubok B, Gardos G. A rating scale for tardive dyskinesia. Psychopharmacology (Berl) 1979;64:171–179. doi: 10.1007/BF00496058. [DOI] [PubMed] [Google Scholar]
- Sorger D, Becker GA, Patt M, Schildan A, Grossmann U, Schliebs R, Seese A, Kendziorra K, Kluge M, Brust P, Mukhin AG, Sabri O. Measurement of the α4β2* nicotinic acetylcholine receptor ligand 2-[18F]fluoro-A-85380 and its metabolites in human blood during PET investigation: a methodological study. Nucl Med Biol. 2007;34:331–342. doi: 10.1016/j.nucmedbio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Sossi V, de Jong HWAM, Barker WC, Bloomfield P, Burbar Z, Camborde ML, Comtat C, Eriksson LA, Houle S, Keator D, Knoss C, Krais R, Lammertsma AA, Rahmim A, Sibomana M, Teras M, Thompson CJ, Trebossen R, Votaw J, Walker M, Wienhard K, Wong DF. The second generation HRRT - a multi-centre scanner performance investigation. 2005 IEEE Nuclear Science Symposium Conference Record; 2005. pp. 2195–2199. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 8.0. College Station, TX: Stata Corporation; 2003. [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, "just the facts" what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau, Population Division. National and State Population Estimates. Annual Population Estimates 2000 to 2009. 2010 (NST-EST2009-01) http://www.census.gov/popest/states/NST-ann-est.html.
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: an aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 2003;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wender PH. Attention-Deficit Hyperactivity Disorder in Adults. New York, NY: Oxford University Press; 1995. [Google Scholar]
- Wender PH, Wolf LE, Wasserstein J. Adults with ADHD: an overview. Ann N Y Acad Sci. 2001;931:1–16. [PubMed] [Google Scholar]
- Woerner MG, Mannuzza S, Kane JM. Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull. 1988;24(1):112–117. [PubMed] [Google Scholar]
- Wong DF, Brašić JR. In vivo imaging of neurotransmitter systems in neuropsychiatry. Clin Neurosci Res. 2001;1 (1–2):35–45. [Google Scholar]
- Wong DF, Brašić JR. Progress in the neuropsychiatry of schizophrenia. Psychiatr Times. 2005;22(3):57, 58, 60. [Google Scholar]
- Wong DF, Brašić JR, Cascella N. Neuroreceptor imaging of schizophrenia. In: Shenton ME, Turetsky BI, editors. Understanding neuropsychiatric disorders: insights from neuroimaging. Cambridge, UK: Cambridge University Press; 2011. pp. 78–87. [Google Scholar]
- Wong DF, Broussolle EP, Wand G, Villemagne V, Dannals RF, Links JM, Zacur HA, Harris J, Naidu S, Braestrup C, Wagner HN, Jr, Gjedde A. In vivo measurement of dopamine receptors in human brain by positron emission tomography. Age and sex differences. Ann N Y Acad Sci. 1988;515:203–214. doi: 10.1111/j.1749-6632.1988.tb32986.x. [DOI] [PubMed] [Google Scholar]
- Wong DF, Gründer G, Brašić JR. Brain imaging research: does the science serve clinical practice? Int Rev Psychiatry. 2007;19:541–558. doi: 10.1080/09540260701564849. [DOI] [PubMed] [Google Scholar]
- Wong DF, Gründer G, Cascella NG, Brašić JR. Molecular brain imaging in schizophrenia. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. 9. Chapter 12.9 in Volume 1. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 1519–1530. [Google Scholar]
- Wong DF, Maini A, Rousset OG, Brašić JR. Positron emission tomography--a tool for identifying the effects of alcohol dependence on the brain. Alcohol Res Health. 2003;27:161–173. [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Wagner HN, Jr, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH, Petronis JD, Folstein MF, Toung JKY, Burns HD, Kuhar MJ. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226 (4681):1393–1396. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- Wüllner U, Gündisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schütz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J. Smoking upregulates α4β2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430:34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Brasic JR, Fan H, Maini A, Wong DF. Kinetic analysis and parametric imaging of [123I](S)-5-iodo-3-(2-azetidinylmethoxy)pyridine (5IA) for subjects with Rett syndrome. 3rd Annual Rett Syndrome Symposium; June 17–19, 2002; Inn at The Colonnade, Baltimore, Maryland. Cincinnati, Ohio: Rett Syndrome Research Foundation; 2002a. pp. 31–32. 2002. [Google Scholar]
- Zhou Y, Brašić JR, Musachio JL, Crabb AH, Fan H, Hilton J, Wong DF. Quantification of α4β2 neuronal nicotinic acetylcholine receptor binding in humans with dynamic single photon emission computed tomography (SPECT). 5th Annual Rett Syndrome Symposium; June 28–30, 2004; Inn at the Colonnade, Baltimore, Maryland. Cincinnati, Ohio: Rett Syndrome Research Foundation (RSRF); 2004. p. 45. www.rsrf.org. [Google Scholar]
- Zhou Y, Brasic JR, Musachio JL, Zukin SR, Kuwabara H, Crabb AH, Endres CJ, Hilton J, Fan H, Wong DF. Human [123I]5-A-85380 dynamic SPECT studies in normals: kinetic analysis and parametric imaging. Nuclear Science Symposium Conference Record, 2001 Institute of Electrical and Electronics Engineers (IEEE), Incorporated. 2001;3:1335–1340. [Google Scholar]
- Zhou Y, Endres CJ, Brašić JR, Huang S-C, Wong DF. Linear regression with spatial constraint to generate paramet9ric images of ligand-receptor dynamic PET studies with a simplified reference tissue model. NeuroImage. 2003;18:975–989. doi: 10.1016/s1053-8119(03)00017-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Endres CJ, Dogan AS, Brasic JR, Wong DF, Huang S-C. A new linear paramet ric imaging algorithm derived from a simplified reference tissue model for ligand-receptor dynamic PET studies. In: Senda M, Kimura Y, Herscovitch P, editors. Brain Imaging Using PET. Amsterdam: Academic Press; 2002b. pp. 33–39. [Google Scholar]


