Abstract
The microtubule-associated protein τ is a family of six isoforms that becomes abnormally hyperphosphorylated and accumulates in the form of paired helical filaments (PHF) in the brains of patients with Alzheimer's disease (AD) and patients with several other tauopathies. Here, we show that the abnormally hyperphosphorylated τ from AD brain cytosol (AD P-τ) self-aggregates into PHF-like structures on incubation at pH 6.9 under reducing conditions at 35°C during 90 min. In vitro dephosphorylation, but not deglycosylation, of AD P-τ inhibits its self-association into PHF. Furthermore, hyperphosphorylation induces self-assembly of each of the six τ isoforms into tangles of PHF and straight filaments, and the microtubule binding domains/repeats region in the absence of the rest of the molecule can also self-assemble into PHF. Thus, it appears that τ self-assembles by association of the microtubule binding domains/repeats and that the abnormal hyperphosphorylation promotes the self-assembly of τ into tangles of PHF and straight filaments by neutralizing the inhibitory basic charges of the flanking regions.
Alzheimer's disease (AD) has polyetiology. In less than 5% of the cases, the disease cosegregates almost completely with one or more specific mutations in the amyloid precursor protein, presenilin-1 or presenilin-2 genes (for review, see ref. 1), and in over 95% of the cases, the exact cause is not yet known. Independent of the etiology, AD is characterized histopathologically by the presence of numerous neurons with neurofibrillary tangles of paired helical filaments (PHF) and straight filaments (SF) and extracellular deposits of amyloid β as the major component of senile (neuritic) plaques in the brain. Although the exact nature of a direct relationship, if any, between these two hallmark lesions of AD is presently not understood, the presence of neurofibrillary degeneration appears to be required for the clinical expression of the disease, i.e., dementia (2, 3). Microtubule-associated protein τ, which is primarily expressed in neurons, is abnormally hyperphosphorylated in AD brain and, in this altered form, is the major protein subunit of PHF/SF (4–8). The neurofibrillary tangles are also glycosylated (9), glycated (10), and have lipid peroxide adducts (11). The abnormal hyperphosphorylation of τ apparently precedes both its polymerization into filaments (12) and the oxidative stress response (13). Furthermore, phosphorylated, but not native, τ protein assembles into ≈2-nm and ≈10-nm filaments following reaction with the lipid peroxidation product, 4-hydroxy-2-nonenal (14). Neurofibrillary pathology similar to that in AD is also a key histopathological brain lesion in several other dementias, commonly referred to as tauopathies (for review, see ref. 15). The recent discovery of the cosegregation of specific mutations in the τ gene with disease in inherited cases of frontotemporal dementia with Parkinsonism linked to chromosome 17 (FTDP-17) has confirmed that certain abnormalities in the τ protein can be a primary cause of neurodegeneration and dementia in the affected individuals (16, 17).
Human brain τ is a family of six proteins derived from a single gene by alternative mRNA splicing (18, 19). The proteins differ in whether they contain three (τ3L, τ3S, or τ3) or four (τ4L, τ4S, or τ4) tubulin binding domains (repeats, R) of 31 or 32 amino acids each near the C-terminal and two (τ3L, τ4L), one (τ3S, τ4S), or no (τ3, τ4) inserts of 29 amino acids each in the N-terminal portion of the molecule; the two amino-terminal inserts, 1 and 2, are coded by exon 2 and exon 3, respectively. All of the six τ isoforms are present in a hyperphosphorylated state in PHF from AD brain (4–8, 20). The level of τ in AD brain is ≈4–8-fold higher than in age-matched normal brains, and this increase is in the form of abnormally hyperphosphorylated protein (21). In AD brain, abnormally hyperphosphorylated τ is present both as cytosolic protein (6, 22) and as polymerized into PHF (4, 5, 7, 8). Unlike normal τ, which contains two to three phosphate groups, the cytosolic hyperphosphorylated τ from AD brain (AD P-τ) contains 5 to 9 mol of phosphate per mol of the protein (22).
In vitro assembly of τ into SF and PHF-like structures has been achieved under different conditions, such as urea treatment for 60 h, incubations with unsaturated free fatty acids, tRNA, heparin or polyglutamic acid, employing a τ fragment, τ concentrations up to 12 mg/ml, and incubation times up to several days (23–33). However, none of these conditions used for τ assembly is consistent with the presence in PHF of all six τ isoforms abnormally hyperphosphorylated as entire or nearly entire protein molecules. Here, we show (i) that AD P-τ assembles into tangles of PHF/SF filaments and that phosphorylation is essential for its self-assembly, and (ii) that upon hyperphosphorylation, each of the six τ isoforms readily self-assembles into tangles of PHF/SF.
Materials and Methods
Isolation of Recombinant Human τ Isoforms and Their Deletion Mutants, AD P-τ and Brain Normal-τ.
The constructs encoding different human brain τ isoforms were subcloned, and recombinant proteins were purified as described (34) except that the perchloric acid extraction was avoided. The phosphocellulose-purified τ was further purified on a Sephacyl 300 column at 4°C.
Constructs of τ fragments were generated, expressed in Escherichia coli, and purified as described (35, 36). As judged by SDS/PAGE, all six isoforms as well as the τ fragments were practically pure (see Fig. 2b). The purity of fragments τ266–391 and τ297–391 was shown previously (36).
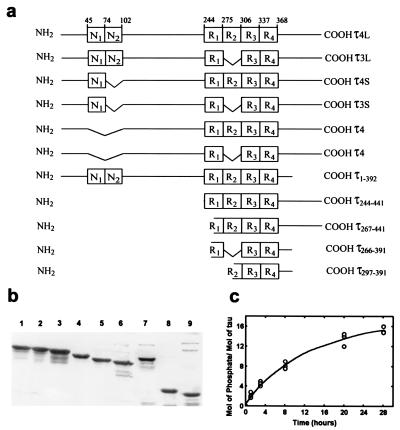
Figure 2.
Schematic of human τ isoforms and τ fragments, SDS/PAGE, and phosphorylation. (a) The amino acid residue numbers are according to τ441, (τ4L). (b) Coomassie blue-stained patterns of the SDS/PAGE (10% gel) of human τ isoforms and fragments shown in a, 4 μg/lane, except constructs τ266–391 and τ297–391, which were shown previously (36). Lanes 1, τ4L; 2, τ3L; 3, τ4S; 4, τ3S; 5, τ4; 6, τ3; 7, τ4L1–392; 8, τ4L244–441; and 9, τ4L267–441. (c) Time course of incorporation of [32P]. τ was hyperphosphorylated as described under Materials and Methods. Similar incorporation was obtained with any of the six τ isoforms. The curve shows phosphorylation of τ4L.
AD P-τ and normal τ were purified from frozen human brains (obtained within 6 h postmortem) as described previously (22). Briefly, for AD P-τ, a 27,000 to 200,000 × g pellet fraction from AD cerebral cortex was extracted in 8 M urea, dialyzed first against 50 mM Tris, pH 7.0, and then against three changes of PC buffer (25 mM Mes, pH 6.4, containing 0.5 mM MgCl2/1 mM DTT/0.1 mM EDTA), followed by phosphocellulose column chromatography. The presence of τ was detected by Western blots developed with Tau-1 antibody with or without previous dephosphorylation with alkaline phosphatase (AP) of the proteins on the membrane. The AD P-τ eluted at a salt concentration of ≈0.2 M NaCl. The τ peak was brought to 2 M NaCl concentration by adding solid salt, and it was loaded onto a phenyl Sepharose high-performance column (Amersham Pharmacia) (0.2-ml bed volume/g of starting material). τ was detected in the unbound fraction. This fraction was concentrated with ≈20 kDa of polyethylene glycol powder. Once concentrated, the sample was dialyzed against 5 mM Mes buffer, pH 6.8, containing 0.05 mM EGTA, lyophilized, and kept at −75°C until used.
In Vitro Hyperphosphorylation of τ.
Hyperphosphorylation of recombinant τ was performed by using 100,000 × g brain extract from a 20-day-old rat as the source of protein kinase as described previously (37). The reaction was carried out at 35°C in 60 mM Hepes, pH 7.4/8 mM MgCl2/5 mM EGTA/2 mM ATP/2 mM DTT/20 nM calyculin A/1 mM 4-[2-aminoethylamino]-benzenesulfonyl fluoride (AEBSF, a serine protease inhibitor) and from 0.1 to 1 mg/ml τ protein and 1 μl of brain extract per 20 μl of the incubation mixture. After 2 and 8 h of incubation, NaF (17 mM) and ATP (2 mM), respectively, were added. The mol 32P/mol τ was calculated by using [γ-32P]ATP of a known specific activity and, as control, the brain extract without exogenous τ.
Self-Assembly of τ.
The self-assembly of AD P-τ was studied incubating 0.4 mg/ml of the protein without treatment, treated with AP or with endoglycosidase F/N-glycosidase F in 100 mM Mes buffer, pH 6.9, containing 2 mM EGTA/0.5 mM MgCl2/1 mM AEBSF/2 mM DTT/20 nM calyculin A/17 mM NaF. Following incubation for 90 min at 35°C, 10 μl of the incubated sample was applied on a 300-mesh carbon-coated grid and negatively stained with 2% phosphotungstic acid as described previously (38). The self-assembly of all recombinant τs (0.02–0.333 mg/ml) and τ constructs (0.5 mg/ml) was performed under the same conditions of AD P-τ self-assembly and analyzed by negative stain electron microscopy (NSEM). The self-assembly of AD P-τ, recombinant τs, and constructs could be detected as early as after 60 min of incubation. The self-assembly of τ promoted by hyperphosphorylation was studied as above during the phosphorylation reaction taking 10-μl aliquots of the reaction mixture at different incubation times.
Deglycosylation and Dephosphorylation of AD P-τ and Protein and τ Assays.
AD P-τ was deglycosylated with 4 units/ml endoglycosidase F/N-glycosidase F (Boehringer Mannheim) as described (9). Deglycosylation was detected by Western blots (4 μg/lane) with lectin Galanthus nivalas agglutinin (GNA, detects terminally linked mannose) and peanut agglutinin (PNA, detects galactose-β(1–3)-N-acetylgalactosamine) according to the manufacturer's (Boehringer Mannheim) instructions. The dephosphorylation of AD P-τ with AP was carried out as described (39). Protein concentration was estimated by the method of Bensadoun and Weinstein (40). Sample preparation and immunoblots were carried out as described previously (41). The levels of recombinant τ isoforms and fragments and AD P-τ were determined by the radioimmuno-slot-blot method of Khatoon et al. (21). Because mAb Tau-1 recognizes τ only when it is not phosphorylated at Ser-195/198/199/202, to detect AD P-τ, the blots were pretreated with AP, 196 units/ml in 0.1 M Tris, pH 8.0/1 mM phenylmethylsulfonyl fluoride for 15 h before incubation with the primary antibody.
Results
AD P-τ Self-Polymerizes into Tangles of PHF/SF Filaments.
The intraneuronal concentration of τ, which is primarily expressed in neurons, is at least 100 μg/ml in normal brain, and this value is severalfold increased in AD brain because of the accumulation of the abnormally hyperphosphorylated τ (see ref. 21). To test whether human τ is able to self-polymerize, 0.4 mg of AD P-τ/ml (Fig. 1f) was incubated in 100 mM Mes buffer, pH 6.9, containing 2 mM EGTA/1 mM MgCl2/20 mM NaF/1 mM AEBSF for 90 min at 35°C, and examined on carbon-coated grids by NSEM. AD P-τ self-assembled into tangles of PHF mixed with SF (Fig. 1a). The dimensions of these PHF were very similar to those of AD PHF: a wide part of ≈20 nm, which narrowed to ≈10 nm at every ≈80 nm. Within the bundles of PHF, some 4-nm protofilaments and SF of ≈15 nm, similar to the SF in AD, were also observed. The AD P-τ also self-assembled into PHF/SF at pH 6.7, 7.0, and 7.5 (data not shown).
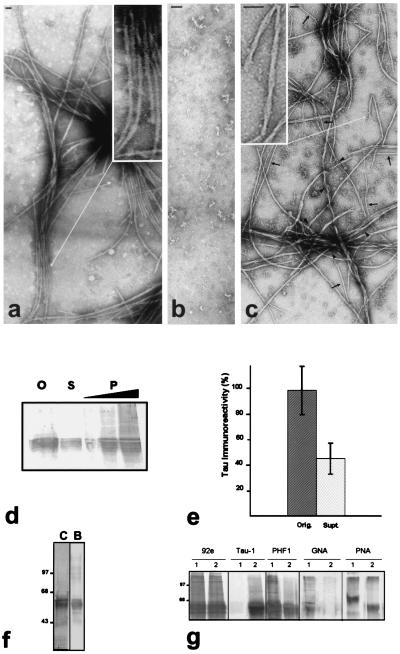
Figure 1.
In vitro polymerization of AD P-τ into tangles of PHF/SF and the effects of dephosphorylation and deglycosylation. AD P-τ, 0.4 mg/ml, without treatment (a), dephosphorylated by AP (b), or deglycosylated by endoglycosidase F/N-glycosidase F (c), was incubated for 90 min, and the products of the assembly were examined by NSEM. Dephosphorylation, but not deglycosylation, completely abolished AD P-τ polymerization. Bar represents 50 nm. (Insets) PHF at higher magnifications. Arrows label examples of 10–15-nm (straight) and 4-nm (arrowhead) filaments. (d) AD P-τ, 0.4 mg/ml, was incubated as above to induce assembly, and the aggregated protein was separated from the nonaggregated protein by centrifugation at 35°C and 100,000 × g for 15 min. The pellet (P) was resuspended to its original volume, and equivalent samples of the original mixture (O), the supernatant (S), and the pellet (1, 2, and 4×) were analyzed by Western blots by using Tau-1 antibody and dephosphorylation of the proteins on the blot with AP. (e) The amount (mean ± SD of 4 values) of AD P-τ present in the original and the supernatant fractions was quantitated by scanning the immunoblots. (f) SDS/PAGE (10% gel) of AD P-τ and blot of a lane from the same gel developed with Tau-1 antibody after dephosphorylation. One strip (8 μg of protein/lane) was stained with Coomassie blue (C), and another strip (2 μg of protein/lane) was developed with Tau-1 antibody after dephosphorylation of the proteins on the membrane (B). (g) For in vitro dephosphorylation and deglycosylation of AD P-τ, aliquots of AD P-τ were treated with (2) or without (1) the addition of AP to dephosphorylate (panels labeled 92e, Tau-1, and PHF1) or endoglycosidase F/N-glycosidase F to deglycosylate (panels labeled GNA and PNA) the proteins as described. The immunoblots were developed with 92e (dilution 1/5,000) to detect the total amount of τ, Tau-1 (1/50,000) to detect dephosphorylated τ, and PHF1 (1/250) to detect phosphorylated τ. The increase in Tau-1 staining and decrease in PHF1 staining show dephosphorylation of AD P-τ. The immunoblots were developed with lectin GNA or PNA to detect glycosylation. Decrease in the staining with the lectins shows deglycosylation of AD P-τ by the glycosidase.
To determine the proportion of τ that self-assembles into these filamentous structures, a sample of AD P-τ was incubated as above to induce assembly, and the aggregated protein was separated from the nonaggregated protein by centrifugation at 100,000 × g for 15 min at 35°C. The amount of AD P-τ detected in the soluble fraction by quantitative scanning of the blot represented ≈50% of the total AD P-τ in the incubation system (Fig. 1e). The amount of τ detected in the soluble fraction plus the insoluble fraction did not account for the original amount of τ present in the system because a considerable amount of the aggregated protein could not be monomerized by SDS, it partly did not enter the gel, and it partly was seen as a smear (Fig. 1d). The presence of a smear in SDS/PAGE is very characteristic of AD PHF preparations (42). When the soluble fraction of AD P-τ was overloaded in the gel, no significant smear was detected. These findings suggest that the interaction involved in the self-assembly of AD P-τ in vitro is similar to that of the τ in PHF.
Unlike normal τ, AD P-τ is glycosylated (9). We studied the effect of hyperphosphorylation (5, 6) and glycosylation (9) on the self-assembly of AD P-τ into PHF/SF. AD P-τ was either dephosphorylated with AP or deglycosylated with endoglycosidase F/N-glycosidase F as described previously (9, 39), and the dephosphorylated/deglycosylated protein was incubated to induce self-assembly as above. The dephosphorylation of ADP-τ was confirmed by Western blots developed with phosphorylation-dependent specific antibodies against dephosphorylated (Tau-1) τ and phosphorylated (PHF-1) τ (Fig. 1g); deglycosylation of ADP-τ was confirmed by Western blots developed for lectin GNA, which detects terminally linked mannose, and PNA, which detects galactose-β(1–3)-N-acetylgalactosamine (Fig. 1g). No filaments could be assembled from the dephosphorylated AD-P-τ (Fig. 1b), whereas deglycosylated AD-P-τ was able to self-aggregate into PHF/SF (Fig. 1c). The deglycosylated AD P-τ, however, differed from the untreated protein in having a decreased tendency to form tangles and an increased tendency of the 4-nm protofilaments to dissociate from PHF/SF. Previous studies have shown that deglycosylation of AD PHF untwists them, forming sheets of protofilaments (9).
Hyperphosphorylation Promotes Self-Assembly of τ into PHF and SF.
Our above studies by using ADP-τ suggested that the abnormal hyperphosphorylation might be sufficient to induce self-assembly of τ into PHF/SF. To confirm the role of the abnormal hyperphosphorylation in the self-assembly of τ, we generated all six human τ isoforms as recombinant proteins in E. coli, in vitro hyperphosphorylated each purified recombinant τ with the protein kinases present in the normal brain extract, and studied the self-assembly of these τs. The τ constructs used and purity of the recombinant τs are shown in Fig. 2 a and b.
Phosphorylation of each of the six τ isoforms with rat brain extract resulted in ≈12–15 mol of phosphates per mol of the protein during ≈24 h (Fig. 2c). This in vitro hyperphosphorylation promoted both the self-assembly of each of the six τ isoforms and the lateral association of filaments into tangles (Fig. 3) The in vitro formed tangles were up to several micrometers in length and contained both PHF and SF (Fig. 3a). The filaments formed from the in vitro phosphorylated τs were (i) straight ≈2.5–4-nm filaments, which in many cases established lateral interactions forming bundles of PHF-like filaments, with a wide region of ≈8 nm, a narrow region of ≈4 nm and a twist length of 40–50 nm, admixed with SF of about 10 nm in diameter (Fig. 3c); (ii) PHF-like filaments, with a wide region of ≈18 nm, a narrow region of 6–10 nm, and a twist every 75–95 nm (Fig. 3 d and e); (iii) SF of about 15 nm in diameter (Fig. 3g); and (iv) occasional PHF- like filaments that were wider and had a longer periodicity (Fig. 3f) than AD PHF.
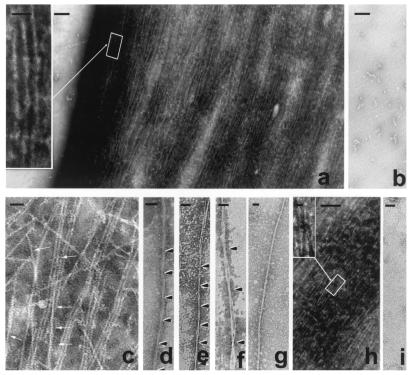
Figure 3.
The formation of tangles of PHF-like and SF from in vitro hyperphosphorylated τ. τ, 0.5 mg/ml, was incubated with rat brain extract in the presence of ATP to induce hyperphosphorylation of τ (a) or incubated with nonhydrolyzable ATP, AMP–PNP, as a control (b). τ hyperphosphorylation (≈12–15 mol of phosphate per mol of protein) induced its self-polymerization into straight and PHF-like (Inset) filaments (a), irrespective of the τ isoform used for the phosphorylation assay. The magnification bars in a and b represent 500 nm and, in the Inset, 50 nm. Intertwining 4-nm filaments generate small PHF-like structures (arrows) as follows: τ4 (c); PHF, τ3L (d); τ protofilaments forming PHF, τ4S (e); τ protofilaments forming bigger PHF, τ4S (f); ≈15-nm straight filament, τ3 (g); mixture of all six τ isoforms hyperphosphorylated (h), and Inset shows a PHF from the tangle formed; and control sample incubated without ATP, τ4S (i). Bars represent 40 nm in c–g and i, 200 nm in h, and 25 nm in Inset.
The self-assembly of τ was found to depend on the degree of phosphorylation. No filaments were observed by NSEM when less than 10 mol of phosphate per mol of protein were incorporated in τ 3L. When the phosphorylation reached a plateau (about 15 mol of phosphate per mol of protein) further incubations of the phosphorylated τ resulted in the increment of the tangles of filaments. It seems that with the incubation time, the 4-nm protofilaments laterally associate into PHF/SF and tangle (data not shown).
In AD, a mixture of the six τ isoforms is found in PHF. When a mixture of the six isoforms (0.1 mg/ml each) was hyperphosphorylated in vitro to a stoichiometry of ≈15 mol of phosphate per mol of protein, the most abundant structure that could be found was tangles of PHF admixed with SF of ≈8- and ≈18-nm width (Fig. 3h). Incubation of τ with rat brain extract in the absence of ATP (Fig. 3i) or in the presence of a nonhydrolyzable analogue of ATP (AMP–PNP; Fig. 3b) used as controls did not yield any filaments. These studies confirmed that hyperphosphorylation induced self-assembly of τ into PHF/SF.
Microtubule Binding Domain of τ Is Able to Self-Polymerize, and the Flanking Regions Are Inhibitory.
Because τ seems to self-assemble by hydrophobic interactions and microtubule binding repeats R2 and R3 in the 4 R τs and R3 in 3 R τs have β structure, our above studies led us to a hypothesis that the basic charges in the regions flanking to the repeats are inhibitory and that the abnormal hyperphosphorylation neutralizes these inhibitory regions. We therefore examined whether self-assembly into PHF can be achieved from the microtubule binding domains, i.e., repeat regions alone, and whether flanking regions have any inhibitory effect. To study self-assembly, 0.5 mg/ml τ constructs τ266–391 and τ297–391 (see Fig. 2a) were individually incubated in self-assembly conditions and examined by NSEM. Both constructs were able to self-assemble into PHF-like structures (Fig. 4 d and e) and 2.5–4-nm protofilaments (data not shown). However, neither τ constructs τ244–441 and τ267–441, which only contained the carboxyl-terminal half of the molecule, nor τ1–392, which lacked the carboxyl-terminal 49 amino acid residues, self-assembled into filaments (data not shown).
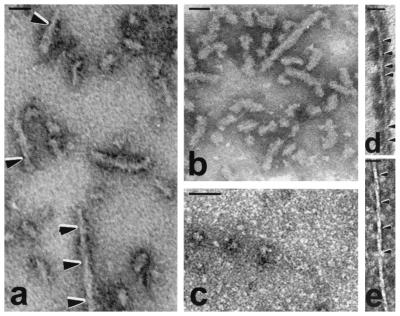
Figure 4.
Self-assembly of whole τ and of microtubule binding region. Polymerization of τ4L, 0.04 mg/ml (a), and the association of τ4L with normal brain τ (b) is shown; similar PHF with fuzzy coat were obtained when τ4L was coassembled with τ3L, τ3S, τ3, τ4S, or τ4 (not shown). Acid and heat treatment of τ4L (0.04 mg/ml) inhibited the self-assembly of τ (c). τ constructs τ266–391 and τ297–391 containing the microtubule binding region (0.5 mg/ml) were able to polymerize into short PHF-like filaments, with twists every ≈40 nm (d) or every ≈80 nm (e); similar but several twists longer than those formed from τ4L. Bar represents 50 nm.
Although the incubation of each of the six recombinant τ isoforms with rat brain extract in the absence of ATP as the phosphorylation control in the present study (see above) had failed to reveal any formation of filaments, we investigated their self-assembly by incubating of 0.02 to 0.333 mg/ml of each τ individually in self-assembly conditions. We found that τ4L self-assembled into short PHF (Fig. 4a). These PHF had a wide region of ≈17 nm, a narrow region of 7 nm, and a twist every 70–90 nm. The structures formed were short (≈2–3 twists) and grew longer (≈4–5 twists) with increase in incubation time from 1 h to 24 h examined (figure not shown). Rare or no filaments were detected using the other five τ isoforms. In the case of τ3L, only occasional PHF-like structures were formed (data not shown). These findings suggest that the inhibition of the formation of PHF seeds in the phosphorylation control experiment (see above) was probably due to interaction of τ4L with one or more proteins in the brain extract.
The effect of the addition of the other isoforms to τ4L was examined by incubating τ4L with each one of the other τ isoforms or with normal human brain τ. The association of τ4L with other τs also resulted in the formation of PHF-like structures. However, although these PHF remained short, they had a larger diameter and appeared heavily decorated with a fuzzy coat around the filaments (Fig. 4b). The width of the filaments was increased from 16.6 ± 2.3 nm to 19.7 ± 2.1 nm. These results suggested that the longest human τ isoform, τ4L, is able to self-assemble into PHF-like seed structures.
Previous studies had failed to self-assemble τ under conditions where the concentration of the τ used was physiological or no other cofactors were required. In these cases, τ had been purified by different methods and usually making use of its acid and/or heat stability (23–25, 27, 29). To address this discrepancy between our results and those reported previously, we investigated the effect of exposing τ to low pH and heat; τ isolated by these treatments is known to be biologically active in promoting microtubule assembly (e.g., see refs. 39 and 43). One aliquot of τ4L was made pH 2.7 with HCl and heated in boiling water for 5 min (5). After heating, the sample was cooled down and adjusted to pH 6.9 with the addition of NaOH. The treated and untreated samples were incubated to promote the self-assembly. The untreated τ was able to polymerize (Fig. 4a), whereas the one acid and heat-treated lost the ability to polymerize into PHF-like structures (Fig. 4c). Acid (pH 2.7) treatment of AD P-τ also inhibited its ability to self-assemble into filaments (data not shown). These findings suggest that the conformation of τ is altered with acid and heat treatment in a way that the ability to self-assemble is lost. Like the hyperphosphorylated τ (see above), the ability of τ4L to polymerize into PHF-like structures does not seem to be modulated by the sulfhydryl linking of cysteine in the molecule because these structures were also seen when 2 mM β-mercaptoethanol was added to the incubation system (figure not shown).
Discussion
Neurofibrillary degeneration is pivotally involved in the pathogenesis of AD and other tauopathies. In every tauopathy known to date, the neurofibrillary changes are made up of abnormally hyperphosphorylated τ (for review, see ref. 15). Ever since the discovery in 1986 of the abnormal hyperphosphorylation of τ in AD brain and the subsequent aberrant τ as the major protein subunit of PHF/SF (5, 6), understanding the role of the abnormal hyperphosphorylation of τ in neurofibrillary degeneration has been a major goal of research on the biology of AD and related tauopathies. The present study shows (i) the AD P-τ can self-assemble into tangles of PHF/SF and that this assembly is abolished by dephosphorylation but not by deglycosylation; (ii) as in AD P-τ, the hyperphosphorylation induces the self-assembly of all six human brain τ isoforms into tangles of PHF/SF under physiological conditions of protein concentration, ionic strength, pH, temperature, reducing conditions, and the absence of any cofactor; and (iii) of all of the six isoforms, only τ4L and τ constructs containing the microtubule binding domains τ266-391 and τ297-391 in unphosphorylated state can self-assemble into short PHF.
Of the six human brain τ isoforms, only τ4L could self-assemble into short PHF (≈2–3 twist length) without hyperphosphorylation. The self-assembly of τ induced by its hyperphosphorylation appeared different from that of unphosphorylated τ4L into PHF. No protofilaments could be detected in these PHF seeds, and no SF/protofilaments were observed in these preparations. Coassembly of τ4L with any one of the remaining five τ isoforms did not elongate and resulted only in a fuzzy coat on these filaments. In contrast, hyperphosphorylation induced self-assembly of all six human brain τ isoforms into PHF/SF, which were several microns in length, and like AD PHF (see ref. 38), they were made from ≈2.5–4-nm protofilaments. Furthermore, similar to AD neurofibrillary tangles (see refs. 44 and 45), the tangles formed in vitro from the hyperphosphorylated τ contained (in addition to PHF) filaments of different morphologies (i.e., ≈2.5, 4, 10, and 15 nm of SF) and twisted ribbons and sheets. These findings, plus the fact that AD PHF contains all six τ isoforms and in the abnormally hyperphosphorylated state, suggest that PHF/SF seen in AD are in all likelihood predominantly products of self-assembly of τ induced by its hyperphosphorylation. This is further supported by the finding that AD P-τ can self-assemble and that this capacity is lost upon dephosphorylation. Given the high affinity of τ (Kd ≈100 nM) to microtubules (46) and more than 10-fold excess of tubulin than τ that exists in the brain, practically all τ in neurons is probably bound to microtubules. In normal neuron, τ is seen bound only to microtubules and not self-aggregated into filaments. The contribution of the self-assembly of unphosphorylated τ4L into PHF seeds is thus less likely, but it cannot be ruled out. Certain intronic mutations 5′ to exon 10 in τ gene in some inherited cases of FTDP-17 have been reported to selectively result in overexpression of 4Rτs (17). In such cases, τ4L on hyperphosphorylation might lead to acceleration of assembly into PHF and their lateral association into neurofibrillary tangles.
τ is an unusual protein that has long stretches of charged (positively and negatively) regions that are not conducive for intermolecular hydrophobic association (47). Of the four microtubule binding repeats in τ, the predicted amino acids having β-structure are concentrated in R2 and R3 (33) and can self-assemble into filaments in vitro; R2 and R3 have also been shown to coassemble with heparin into PHF (48). It is likely that the way the charged regions are located, the rest of the molecule has an inhibitory effect upon the self-polymerization of τ. Of all of the τ isoforms, this inhibitory effect seems to be the least in τ4L. The N-terminal inserts are highly acidic, and the presence of these inserts markedly neutralizes the basic charge of τ. For instance, the theoretical isoelectric points of τ4, τ4S, and τ4L, respectively, are 9.46, 8.96, and 8.24. The presence of the extra repeat, the R2, and the two N-terminal inserts probably promotes the intermolecular hydrophobic interaction in τ4L sufficiently to result in its self-assembly into PHF, and hyperphosphorylation further enhances this process. The abnormal hyperphosphorylation that occurs in AD and other tauopathies neutralizes the basic inhibitory charge of τ. Most of the sites at which τ is hyperphosphorylated flank the microtubule binding domains (see refs. 49 and 50). Neutralization of basic charge by hyperphosphorylation in these flanking regions probably neutralizes their inhibitory effect and allows τ to self-assemble into filaments. However, the nature of the neutralization by the two N-terminal inserts and that by the abnormal hyperphosphorylation is most likely different, as evidenced by the formation of filaments with different morphologies.
The types of filaments that resulted from the self-assembly of hyperphosphorylated τs suggest that PHF are most probably formed from ≈2.5–4-nm protofilaments. These protofilaments, which were coiled structures, might form by lateral association either SF of ≈8–15 nm (from 2–6 protofilaments) or sheets (from n protofilaments), or, because of the tension of the coils, intertwine individually (PHF with half dimensions) or in pairs (regular size PHF) to form PHF. PHF of ≈16 nm in width (which narrowed to ≈8 nm) and of half these dimensions observed from hyperphosphorylated τs in the present study are similar to those described from AD brain (44, 45).
The in vitro assembly of PHF from various τ isoforms by the addition of polyanionic cofactors such as heparin, heparan sulfate (28, 30), tRNA (29), or polyglutamate (51) reported previously might have involved the neutralization of the basic charge of τs by these reagents. However, unlike the self-assembly induced by the hyperphosphorylation of τ in the present study, the coassembly of τ with polyanions is very slow, and neither lateral association of filaments into tangles nor presence of any protofilaments has been reported. Interestingly, unlike the present study and in AD and other tauopathies in which τ in PHF is always abnormally hyperphosphorylated, phosphorylation had been reported to inhibit the coassembly of τ with polyanions into PHF (52). Thus, it seems that the PHF in AD and other tauopathies are not a product of coassembly of τ with polyanions.
In the normal neuron, τ is seen mostly as bound to microtubules and not as self-aggregated into filaments. In AD, FTDP-17, and other tauopathies, τ is released from microtubules most likely upon abnormal hyperphosphorylation, resulting in their disassembly. On the one hand, the released hyperphosphorylated τ, which is resistant to proteolysis (53), sequesters the normal τ, causing inhibition of assembly and the disassembly of microtubules (39, 54). On the other hand, τ self-assembles because of its hyperphosphorylation into PHF/SF. τ self-assembles probably by intermolecular hydrophobic interaction (55) and through its microtubule binding repeat R3 (in the case of 3Rτs) and R2 and R3 (in the case of 4R τs), but only when the rest of the molecule (i.e., amino-terminal and carboxyl-terminal regions flanking the repeats, which are inhibitory) are neutralized. In AD and other tauopathies, these inhibitory regions are neutralized by abnormal hyperphosphorylation. Thus, the abnormal hyperphosphorylation of τ is critically involved in neurofibrillary degeneration in AD and other tauopathies. Inhibition of these processes might arrest AD, FTDP-17, and related tauopathies.
Acknowledgments
We thank Drs. M. Goedert and L.I. Binder for τ plasmids and antibody Tau-1, respectively; Qiongli Wu for her technical assistance in the purification of the recombinant τs; Fred Connell for helping with electron microscopy; and Ms. Janet Biegelson and Ms. Sonia Warren for secretarial assistance. Autopsied brain specimens were provided by the Brain Tissue Resource Center (Public Health Service Grant MH/NS 31862), McLean Hospital, Belmont, MA, and by New York State Institute for Basic Research Tissue Bank (Dr. P. Kozlowski). These studies were supported in part by the New York State Office of Mental Retardation and Developmental Disabilities and by National Institutes of Health Grants TW00507, AG05892, AG08076, and NS18105.
Abbreviations
- AD
Alzheimer's disease
- AD P-τ
Alzheimer's disease abnormally hyperphosphorylated τ
- AP
alkaline phosphatase
- NSEM
negative stain electron microscopy
- PHF
paired helical filaments
- SF
straight filaments
- AEBSF
4-[2-aminoethylamino]-benzenesulfonyl fluoride
- GNA
Galanthus nivalas agglutinin
- PNA
peanut agglutinin
References
- 1.Finch C, Tanzi R E. Science. 1997;278:407–411. doi: 10.1126/science.278.5337.407. [DOI] [PubMed] [Google Scholar]
- 2.Tomlinson B E, Blessed G, Roth M J. Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 3.Arigada P A, Growdon J H, Hedley-White E T, Hyman B T. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 4.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaidi M S, Wisniewski H M. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 5.Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski H M, Binder L I. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal K, Grundke-Iqbal I, Zaidi T, Merz P A, Wen G Y, Shaikh S S, Wisniewski H M, Alafuzoff I, Winblad B. Lancet. 1986;2:421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal K, Grundke-Iqbal I, Smith A J, George L, Tung Y-C, Zaidi T. Proc Natl Acad Sci USA. 1989;86:5646–5650. doi: 10.1073/pnas.86.14.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee M Y, Balin B J, Otvos L, Trojanowski J Q. Science. 1991;251:675–678. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 9.Wang J-Z, Grundke-Iqbal I, Iqbal K. Nat Med. 1996;2:871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 10.Ledesma M D, Bonay P, Colaco C, Avila J. J Biol Chem. 1994;261:21614–21619. [PubMed] [Google Scholar]
- 11.Yan S-D, Chen X, Schmidt A-M, Brett J, Godman G, Zou Y-S, Scott C W, Caputo C, Frappier T, Smith M A, et al. Proc Natl Acad Sci USA. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski H M. Brain Res. 1989;477:90–99. doi: 10.1016/0006-8993(89)91396-6. [DOI] [PubMed] [Google Scholar]
- 13.Takeda A, Smith M A, Avila J, Nunomura A, Siedlak S L, Zhu X, Perry G, Sayre L M. J Neurochem. 2000;75:1234–1241. doi: 10.1046/j.1471-4159.2000.0751234.x. [DOI] [PubMed] [Google Scholar]
- 14.Perez M, Cuadros R, Smith M A, Perry G, Avila J. FEBS Lett. 2000;486:270–274. doi: 10.1016/s0014-5793(00)02323-1. [DOI] [PubMed] [Google Scholar]
- 15.Tolnay M, Probst A. Neuropathol Appl Neurobiol. 1999;25:171–187. doi: 10.1046/j.1365-2990.1999.00182.x. [DOI] [PubMed] [Google Scholar]
- 16.Hutton M, Lendon C L, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chackraverty S, Isaacs A, Grover A, et al. Nature (London) 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 17.Spillantini M G, Murrell J R, Goedert M, Farlow M R, Klug A, Ghetti B. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Himmler A, Drechsel D, Kirschner M W, Martin D W., Jr Mol Cell Biol. 1989;9:1381–1388. doi: 10.1128/mcb.9.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goedert M, Spillantini M G, Jakes R, Rutherford D, Crowther R A. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 20.Goedert M, Spillantini M G, Cairns N J, Crowther R A. Neuron. 1992;8:159–168. doi: 10.1016/0896-6273(92)90117-v. [DOI] [PubMed] [Google Scholar]
- 21.Khatoon S, Grundke-Iqbal I, Iqbal K. J Neurochem. 1992;59:750–753. doi: 10.1111/j.1471-4159.1992.tb09432.x. [DOI] [PubMed] [Google Scholar]
- 22.Köpke E, Tung Y-C, Shaikh S, Alonso A C, Iqbal K, Grundke-Iqbal I. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 23.Montejo de Garcini E, Serrano L, Avila J. Biochem Biophys Res Commun. 1986;141:790–796. doi: 10.1016/s0006-291x(86)80242-x. [DOI] [PubMed] [Google Scholar]
- 24.Crowther R A, Olesen O F, Jakes R, Goedert M. FEBS Lett. 1992;309:199–202. doi: 10.1016/0014-5793(92)81094-3. [DOI] [PubMed] [Google Scholar]
- 25.Wille H, Drewes G, Biernat J, Mandelkow E-M, Mandelkow E. J Cell Biol. 1992;118:573–584. doi: 10.1083/jcb.118.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schweers O, Mandelkow E-M, Biernat J, Mandelkow E. Proc Natl Acad Sci USA. 1995;92:8463–8467. doi: 10.1073/pnas.92.18.8463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson D M, Binder L I. J Biol Chem. 1995;270:24306–24314. doi: 10.1074/jbc.270.41.24306. [DOI] [PubMed] [Google Scholar]
- 28.Perez M, Valpuesta J M, Medina M, Montejo de Garcini E, Avila J. J Neurochem. 1996;67:1183–1190. doi: 10.1046/j.1471-4159.1996.67031183.x. [DOI] [PubMed] [Google Scholar]
- 29.Kampers T, Friedhoff P, Biernat J, Mandelkow E-M, Mandelkow E. FEBS Lett. 1996;399:344–349. doi: 10.1016/s0014-5793(96)01386-5. [DOI] [PubMed] [Google Scholar]
- 30.Goedert M, Jakes R, Spillantini M G, Hasegawa M, Smith M J, Crowther R A. Nature (London) 1996;383:550–553. doi: 10.1038/383550a0. [DOI] [PubMed] [Google Scholar]
- 31.Yanagawa H, Chung S H, Ogawa Y, Sato K, Shibata-Seki T, Masai J, Ishiguro K. Biochemistry. 1998;37:1979–1988. doi: 10.1021/bi9724265. [DOI] [PubMed] [Google Scholar]
- 32.Friedhoff P, von Bergen M, Mandelkow E-M, Mandelkow E. Biochim Biophys Acta. 2000;1502:122–132. doi: 10.1016/s0925-4439(00)00038-7. [DOI] [PubMed] [Google Scholar]
- 33.von Bergen M, Friedhoff P, Bienart J, Heberle J, Mandelkow E M, Mandelkow E. Proc Natl Acad Sci USA. 2000;97:5129–5134. doi: 10.1073/pnas.97.10.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh T J, Haque N, Grundke-Iqbal I, Iqbal K. FEBS Lett. 1995;358:267–272. doi: 10.1016/0014-5793(94)01445-7. [DOI] [PubMed] [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novak M, Kabat J, Wischik C M. EMBO J. 1993;12:365–370. doi: 10.1002/j.1460-2075.1993.tb05665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng-Fischöfer Q, Biernat J, Mandelkow E M, Illenberger S, Godemann R L, Mandelkow E. Eur J Biochem. 1998;252:542–552. doi: 10.1046/j.1432-1327.1998.2520542.x. [DOI] [PubMed] [Google Scholar]
- 38.Wisniewski H M, Merz P A, Iqbal K. J Neuropathol Exp Neurol. 1984;43:643–656. doi: 10.1097/00005072-198411000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Alonso A C, Zaidi T, Grundke-Iqbal I, Iqbal K. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bensadoun A, Weinstein D. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 41.Grundke-Iqbal I, Iqbal K, Tung Y-C, Wang G P, Wisniewski H M. Acta Neuropathol. 1984;62:259–267. doi: 10.1007/BF00687607. [DOI] [PubMed] [Google Scholar]
- 42.Iqbal K, Zaidi T, Thompson C H, Merz P A, Wisniewski H M. Acta Neuropathol. 1984;62:167–177. doi: 10.1007/BF00691849. [DOI] [PubMed] [Google Scholar]
- 43.Friedhoff P, von Gergen M, Mandelkow E M, Davies P, Mandelkow E. Proc Natl Acad Sci USA. 1998;95:15712–15717. doi: 10.1073/pnas.95.26.15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruben G C, Iqbal K, Wisniewski H M, Johnson J E, Jr, Grundke-Iqbal I. Brain Res. 1993;602:164–179. doi: 10.1016/0006-8993(92)91092-s. [DOI] [PubMed] [Google Scholar]
- 45.Ruben G C, Iqbal K, Grundke-Iqbal I, Johnson J E., Jr Brain Res. 1993;602:1–13. doi: 10.1016/0006-8993(93)90234-e. [DOI] [PubMed] [Google Scholar]
- 46.Goode B L, Denis P E, Panda D, Radeke M J, Miller H P, Wilson L, Feinstein S C. Mol Biol Cell. 1997;8:353–365. doi: 10.1091/mbc.8.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruben G C, Iqbal K, Grundke-Iqbal I, Wisniewski H M, Ciardelli T L, Johnson J E., Jr J Biol Chem. 1991;266:22019–22027. [PubMed] [Google Scholar]
- 48.Arrasate M, Perez M, Armas-Portela R, Avila J. FEBS Lett. 1999;446:199–202. doi: 10.1016/s0014-5793(99)00210-0. [DOI] [PubMed] [Google Scholar]
- 49.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal K, Grundke-Iqbal I. Neurobiol Aging. 1995;16:365–380. [Google Scholar]
- 51.Kampers T, Pangalos H, Geerts H, Wiech H, Mandelkow E. FEBS Lett. 1999;451:39–44. doi: 10.1016/s0014-5793(99)00522-0. [DOI] [PubMed] [Google Scholar]
- 52.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow E-M. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 53.Wang J-Z, Gong C-X, Zaidi T, Grundke-Iqbal I, Iqbal K. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 54.Alonso A C, Grundke-Iqbal I, Iqbal K. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 55.Ruben G C, Ciardelli T L, Grundke-Iqbal I, Iqbal K. Synapse. 1997;27:208–229. doi: 10.1002/(SICI)1098-2396(199711)27:3<208::AID-SYN7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]