Abstract
Phototropins and phytochromes are the major photosensory receptors in plants and they regulate distinct photomorphogenic responses. The molecular mechanisms underlying functional interactions of phototropins and phytochromes remain largely unclear. We show that the tomato (Lycopersicon esculentum) phytochrome A deficient mutant fri lacks phototropic curvature to low fluence blue light, indicating requirement for phytochrome A for expression of phototropic response. The hp1 mutant that exhibits hypersensitive responses to blue light and red light reverses the impairment of second-positive phototropic response in tomato in phytochrome A-deficient background. Physiological analyses indicate that HP1 functions as a negative regulator of phototropic signal transduction pathway, which is removed via action of phytochrome A. The loss of HP1 gene product in frihp1 double mutant allows the unhindered operation of phototropic signal transduction chain, obviating the need for the phytochrome action. Our results also indicate that the role of phytochrome in regulating phototropism is restricted to low fluence blue light only, and at high fluence blue light, the phytochrome A-deficient fri mutant shows the normal phototropic response.
Plants use multiple photoreceptors to perceive changes in quality and quantity of light and to regulate growth and development. These photoreceptors sense UV-B, blue/UV-A, and red/far-red regions of the light spectrum. Blue light regulates a variety of physiological processes in plants and also in animals. Studies in higher plants have led to the identification of several types of blue light photoreceptors, all of which are flavin-containing proteins. Cryptochromes, which are related to the DNA repair enzyme DNA photolyase, function as the blue light photoreceptors for circadian rhythms and other light-regulated responses in plants, insects, and mammals (Lin and Shalitin, 2003). Plant phototropins, which are blue light-regulated protein kinases, control mechanical processes like phototropism and chloroplast movement in plants (Briggs and Christie, 2002). The red/far-red absorbing photoreceptors, phytochromes, which also have protein kinase activity, regulate a range of developmental processes, including seed germination, shade avoidance, and transition to flowering (Sharma, 2001; Quail, 2002).
The analysis of relationship between blue light intensity and phototropic curvature in several plant species has shown that phototropic responses can typically be classified as two types of positive phototropism based on their dependence on stimulus intensity, duration, and reciprocity; pulse-induced phototropism or “first-positive curvature,” and time-dependent phototropism or “second-positive curvature” (Iino, 1990; Liscum, 2002). Biochemical and genetic evidences indicate that phototropic responses in plants are regulated by phototropins, which consists of two members, phot1 and phot2. Phototropins characteristically have a Ser/Thr protein kinase domain in the C-terminal end and two specialized domains designated LOV domains in the N-terminal end (Briggs et al., 2001; Briggs and Christie, 2002). On exposure to blue light in the presence of ATP, phototropins show strong autophosphorylation (Sakai et al., 2001; Salomon et al., 2003).
The functional role of these two photoreceptors has been gradually understood by genetic and biochemical analysis of Arabidopsis mutants. The seedlings of phot1 mutant still show curvature in response to high-fluence unidirectional blue light; however, phototropic curvatures are significantly reduced in phot1phot2 double mutant seedlings (Sakai et al., 2001). The analysis of phot2 mutants have shown that it is required for the movement of chloroplasts to the anticlinal side of cell walls for avoidance of high light intensities by self shading (Kagawa et al., 2001). The phot1phot2 double mutants are defective in the chloroplast accumulation to the periclinal cell walls that maximizes light interception under weak light, signifying the role of these photoreceptors in chloroplast movement. Additionally, these two photoreceptors redundantly regulate blue light-activated stomatal opening (Kinoshita et al., 2001). Though both photoreceptors regulate several photoresponses, the studies using fluence dependence of photoresponses revealed that the phototropins mediate responses at different fluence levels, whereas phot1 mediates responses to low fluence blue light (<1 μmol m–2 s–1), and phot2 acts at much higher fluence of blue light (>1 μmol m–2 s–1; Liscum, 2002).
The information on the downstream components involved in phototropin signaling is, at the moment, limited. Although several positive and negative regulatory components have been identified for phytochrome and cryptochrome, the numbers of loci identified regulating phototropic signal transduction are rather few. Only one mutant locus, nph3, has been identified that specifically disrupts phototropism under low fluence blue light (Liscum and Briggs, 1996; Motchoulski and Liscum, 1999) without affecting blue light-mediated autophosphorylation of phot1. Another mutant locus, rpt2, reduces phototropism in partially de-etiolated seedlings at high fluence white light. The RPT2 gene has sequence and structural homologies to the NPH3 gene (Sakai et al., 2000), and these genes presumably function in early phototropic signaling and likely act as modular scaffold proteins to recruit or activate components of the phototropic transduction chains, including the photoreceptors phot1 and phot2 (Motchoulski and Liscum, 1999).
Although only UV-A/blue light is effective in inducing phototropism, in several species, a red light preirradiation-activating phytochrome significantly enhances the subsequent phototropic responsiveness (Janoudi and Poff, 1992; Liu and Iino, 1996; Parks et al., 1996; Liscum and Stowe-Evans, 2000). The examination of phototropism in Arabidopsis mutants deficient in specific phytochrome species revealed that phytochrome functions as a regulator of phototropic enhancement. In particular, phyA acts as enhancer under low fluence conditions, whereas phyB acts as enhancer at high fluence conditions (Janoudi et al., 1997a, 1997b). The red preirradiation of seedlings enhances the amplitude of subsequent blue light-mediated phototropism and also reduces the time threshold needed to elicit phototropic response. The studies using phytochrome mutants revealed that phyA and phyB together regulate the time threshold of phototropism. Only scanty information is available about the interaction between phototropins and phytochromes in regulation of phototropism. Based on the analysis of nph4/arf7 mutants, it is proposed that a phytochrome-mediated increase in auxin responsiveness may be the likely mechanism for phototropic enhancement (Stowe-Evans et al., 1998, 2001; Liscum, 2002). The role of other blue light-absorbing photoreceptor-cryptochrome in regulation of phototropism appears to be limited to its effect on growth and development (Lascève et al., 1999; Whippo and Hangarter, 2003).
In species other than Arabidopsis, there is limited information available about the biochemical and genetic regulation of phototropism. Though tomato (Lycopersicon esculentum) has a range of phytochrome-deficient mutants that have been well characterized at physiological and genetic levels, their role in phototropism has not been examined. The most studied aurea (au) mutant is defective in phytochrome chromophore synthesis and is deficient in bulk pool of phyA (Sharma et al., 1993; Terry and Kendrick, 1996). The mutants lacking specific phytochrome species such as phyA-specific fri mutant (van Tuinen et al., 1995b), phyB1-specific tri mutant (van Tuinen et al., 1995a), and phyB2-specific mutants (Kerckhoffs et al., 1999) have been isolated and characterized. Tomato also has mutants in phytochrome signal pathway like high pigment1 (hp1) mutant, which shows exaggerated phytochrome-mediated responses (Peters et al., 1992, 1998). The nonallelic high pigment mutant hp2, which also shows similar phenotype, is encoded by a gene homologous to DET1 of Arabidopsis (Mustilli et al., 1999).
The analysis of phototropism in tomato mutants can complement and extend the observations hitherto obtained only with Arabidopsis, particularly the role of different phytochrome species and interaction of blue light-mediated phototropism with an element located in signal transduction pathway of phytochrome. Here, we report experiments showing that the deficiency of phyA nearly abolishes phototropism under low fluence blue light. We also demonstrate a novel role for HP1, where it appears to act as a negative regulator of phot1-triggered signal transduction chain, revealing likely mode of participation of phytochrome in regulation of phototropism enhancement.
RESULTS
Loss of Second-Positive Curvature in Phytochrome A-Deficient Seedlings Is Restored by Mutation at hp1 Locus
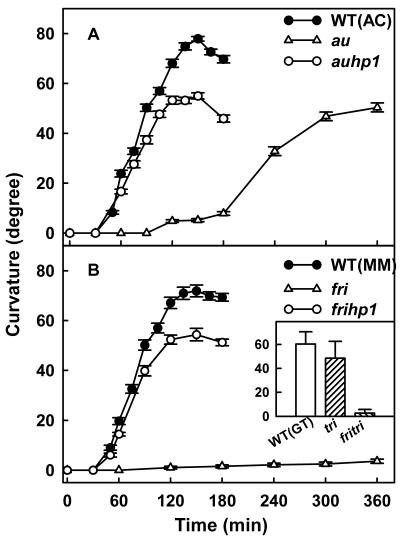
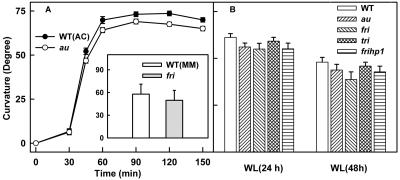
To study how phytochromes are involved in regulation of phot1 function, we compared the phototropism in au mutant of tomato that is deficient in all phytochrome species with that of wild type (Fig. 1A). When exposed to continuous low fluence blue light, wild-type seedlings showed a lag phase of about 45 min and reached a maximum curvature by 2 h. Compared with wild type, the time-course profile of curvature was quite different in the au mutant. The au mutant exhibited delayed onset of phototropism with a prolonged lag phase of nearly 1.5 h and later sluggishly developed phototropic curvature. Even after 6 h of blue light exposure, the extent of curvature in au mutant was less than that observed for wild type at 2 h. Interestingly, the auhp1 double mutant showed phototropic curvature nearly comparable with the wild type, showing virtually total reversal of au effect.
Figure 1.
Time course of induction of phototropic curvature in wild-type and mutant seedlings exposed to continuous blue light. At the time intervals indicated, the seedlings were removed and angle of curvature was measured. For au and fri seedlings, the blue light exposure was continued for 6 h. A, Wild-type, au, and auhp1 mutants. B, Wild-type, fri, and frihp1 mutants. The inset shows the comparison of the mean curvature value (±sd) of wild-type, tri, and fritri mutants after 3 h of continuous blue light exposure.
Because the au mutant is likely deficient in all phytochrome species, the role of specific phytochromes in phototropism was examined by using the phyA-deficient fri mutant and the phyB1-defective tri mutant (Fig. 1B). In comparison with the au mutant, the fri mutant showed no phototropic curvature, even after a prolonged blue light exposure of 6 h. Interestingly, for this mutant as well, the frihp1 double mutant showed a time course of phototropic curvature nearly similar to wild type, showing that the hp1 mutation reversed the impairment of phototropism caused by fri mutation. Because the above results showed a role for phyA, we also examined a role for other phytochrome species by using tri mutant deficient in phyB1. Contrastingly, the tri seedlings showed phototropism comparable with wild type, whereas the fritri double mutant was nonphototropic, like the fri mutant (Fig. 1B, inset). The above results indicated that phyA-deficient seedlings are severely impaired in second-positive phototropism.
Mutation at hp1 Locus Reduces the Time Threshold Needed to Elicit Second-Positive Curvature
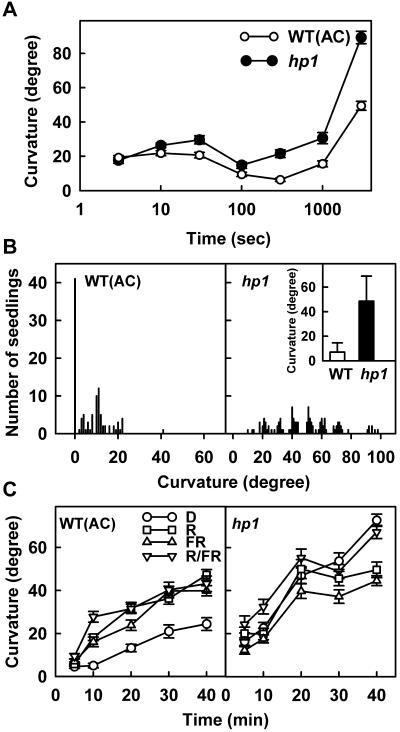
The second-positive curvature of plants has a distinctive feature in that it requires a continuous blue light irradiation for a minimum duration, which distinguishes it from first-positive curvature that can be induced by a short duration pulse of blue light. Because the hp1 mutation can overcome the phyA deficiency in au and fri seedlings, the influence of this mutation on the fluence response curve for phototropic curvature was determined. Dark-grown seedlings were exposed to a fixed fluence of 0.1 μmol m–2 s–1 for varying duration, and curvature was determined after 2 h. Figure 2A shows that the amplitude of first-positive curvature was much higher in the hp1 mutant compared with the wild type. Additionally, the fluence response profiles for hp1 mutant differed considerably from the wild type for the time threshold required to elicit second-positive curvature. Although a zone of indifferent phototropic responsivity between 100 and 1,000 s distinctly separated the first-positive curvature from second-positive curvature in the wild-type seedlings, the hp1 mutant seedlings initiated second-positive curvature without an intervening zone of indifferent phototropic responsivity. A comparison of phototropic curvature after a 10-min pulse of blue light clearly highlights this difference between the wild-type and hp1 mutants. On 10 min of blue light exposure, the hp1 mutant showed about 47° curvature, whereas the wild type showed only a 7° curvature (Fig. 2B). Examination of the time threshold in wild-type and hp1 seedlings for second-positive curvature showed that at least a 10-min continuous blue light exposure is needed to elicit the curvature in the wild type (Fig. 2B), whereas in the hp1 mutant, the time threshold is reduced to just 5 min (Fig. 2A). Preirradiation with a red, far-red, or red/far-red light 90 min before the onset of blue light exposure reduced the time threshold to 5 min in the wild-type seedlings. On the other hand, similar preirradiation of the hp1 seedlings with red, far-red, or red/far-red exposure had no effect on time threshold, and the values were nearly the same as the nonpreirradiated control (Fig. 2C).
Figure 2.
A, Dose-response curve for wild-type and hp1 mutant seedlings. Dark-grown seedlings were exposed to a single pulse of blue light of varying duration and were returned to darkness. The curvatures were recorded 2 h after the beginning of light exposure. B, Frequency distribution histogram of time threshold for second-positive curvature of the wild-type mutant with the hp1 mutant. The seedlings were exposed to blue light for 10 min and were returned to darkness. Curvature was measured 2 h after the beginning of light exposure. The inset shows the comparison of the mean curvature value (±sd) of the wild-type mutant with the hp1 mutant. C, Time threshold comparison of wild-type seedlings with the hp1 mutant. Dark-grown seedlings were exposed to blue light for the duration indicated on abscissa or were exposed to 5 h red, far-red, or red light followed by far-red light 90 min before the onset of blue light exposure. Curvature was measured 90 min after the beginning of blue light exposure.
Phytochrome Deficiency also Reduces Single-Pulse-Induced Phototropism
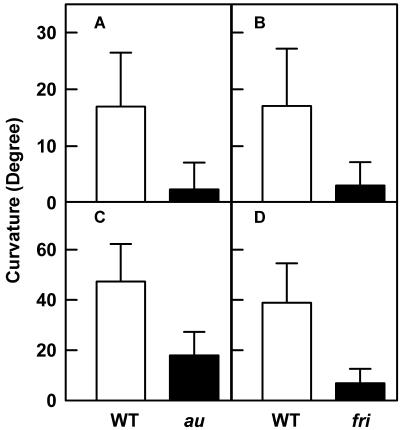
Because au and fri seedlings showed impaired second-positive curvature, we examined whether phyA deficiency also similarly impaired first-positive curvature. The responsiveness of wild-type and mutant seedlings to the unidirectional blue light was examined by exposing seedling to short pulse(s) of blue light to induce first-positive curvature. A single pulse of blue light induced curvature of 17° in wild-type Ailsa Craig (AC) seedlings, whereas in the au mutant, the response was highly reduced with curvature of only 2.4° (Fig. 3A). The first-positive curvature was also extremely reduced in the fri mutant, and it showed only a 3° curvature toward the blue light (Fig. 3B). The responsivity of wild-type and mutant seedlings to multiple pulses of blue light that amplify first-positive curvature (Steintiz and Poff, 1986) exhibited patterns similar to single-pulse treatment. The multiple blue light pulses stimulated first-positive curvature by 2.7-fold to about 47° curvature in wild type (AC), whereas it stimulated curvature in the au mutant 5-fold from 3° to 18° (Fig. 3C). However, the fri mutant was nearly deficient in the first-positive curvature and, even after treatment with multiple pulses, showed only a stimulation of curvature from 3° to 7° compared with wild type Moneymaker (MM) where curvature increased from 17° to 39° (Fig. 3D).
Figure 3.
Comparison of first-positive curvature of au (A and C) and fri mutants (B and D) with their respective wild-type backgrounds. Dark-grown seedlings were exposed to a single pulse of blue light for 10 s and were returned to darkness (A and B). Alternately, the dark-grown seedlings were exposed to five 10-s pulses of blue light separated by 10-min dark intervals (C and D). Curvature was measured 2 h after the light pulse. The data are represented as mean curvature value (±sd) of wild type and mutant.
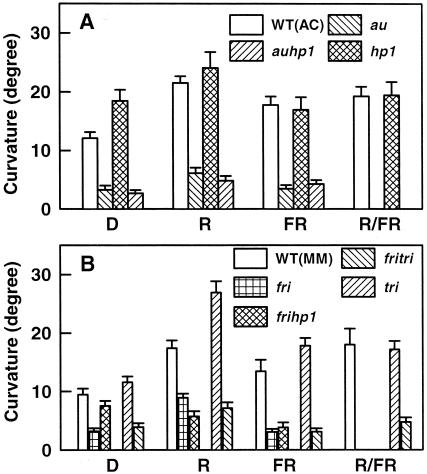
These results highlight that fact that phyA deficiency impairs the phototropic responsivity of the seedlings to unidirectional blue light. In view of this, we compared the responsivity of tomato and its mutant seedlings with phytochrome-mediated enhancement of first-positive curvature. Figure 4A shows that a prior exposure of red or far-red light enhanced first-positive curvature in the au mutant, but compared with wild type, enhancement was drastically impaired. Even without a preirradiation, the hp1 mutant showed higher curvature than that of the wild type. However, the auhp1 mutant exhibited first-positive curvature similar to the au mutant. Figure 4B shows that the fri mutant displayed extremely reduced first-positive curvature. Interestingly, whereas the curvature of dark-grown seedlings of the auhp1 mutant was similar to the au mutant, the frihp1 seedlings showed higher curvature than the fri mutant. Moreover, a red preirradiation enhanced curvature in the fri mutant. Although the tri seedlings showed curvature similar to wild type, the fritri mutant seedlings exhibited curvatures similar to the fri mutant.
Figure 4.
Comparison of the enhancement of first-positive curvature by phytochrome in wild-type and phyA- or phyB1-deficient mutant seedlings. Dark-grown (D) seedlings were pretreated to 5 min of red or 5 min of far-red or red light followed by far-red light and were returned to darkness for 90 min before exposure to a blue light pulse. Thereafter, the seedlings were exposed to a single pulse of blue light of a 10-s duration and were returned to darkness. Curvature was measured 90 min after the beginning of blue light exposure. A, Wild type, au, hp1, and auhp1. B, Wild type, fri, tri, frihp1, and fritri.
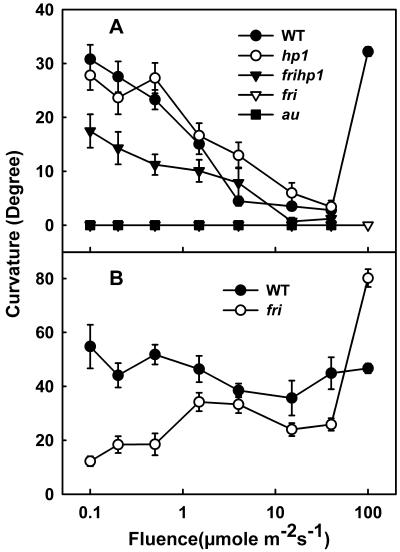
Phytochrome A-Deficient Mutant Shows Normal Phototropism at Higher Fluences of the Blue Light
The above results indicate that phyA deficiency in the fri mutant impaired first- and second-positive responses initiated by blue light. It is now believed that the very high fluence blue light of prolonged duration can trigger phototropic responses via combined action of phot1 and phot2. The phototropic curvature was examined in the etiolated seedlings exposed to varying fluences of blue light for 2 h (Fig. 5A). The phototropic curvature of wild-type seedlings showed a dependence to light fluence, and the magnitude of curvature decreased with increasing fluence. A similar pattern was also observed for the hp1 and frihp1 double mutants. Contrastingly, au and fri seedlings did not show any phototropic curvature for the entire range of fluence used (0.1–40 μmol m–2 s–1). To examine whether the fri seedling respond to a higher fluence of light, wild-type and fri seedlings were exposed to 100 μmol m–2 s–1 blue light for 2 h. Interestingly, at this intensity, the curvature reappeared in wild-type seedlings, but the fri seedlings were still deficient in phototropism. The exposure of wild-type and fri seedlings to varying fluences of blue light for a prolonged duration of 12 h showed a contrastingly different pattern. Although the wild-type seedlings showed nearly the same curvature irrespective of fluence used, the magnitude of curvature in the fri seedlings increased with increase in fluence and was maximal at 100 μmol m–2 s–1 irradiation (Fig. 5B).
Figure 5.
Hypocotyl phototropism in etiolated tomato wild-type, au, fri, hp1, and frihp1 mutant seedlings. The curvatures of 3-d-old etiolated seedlings were measured after 2 h (A) or 12 h (B) of onset of blue light exposure at the fluence rates indicated.
It is plausible that the loss of phototropic curvature in the seedlings deficient in phyA could have arisen due to reduction or loss of differential growth mediated by auxin. However, the examination of geotropic responsivity of these seedlings ruled out the above possibility. On horizontally orientating dark-grown seedlings, the kinetics of onset and increase of gravitropic curvature in wild-type seedlings were similar to that observed in au (Fig. 6A) and fri (Fig. 6A, inset) seedlings. The loss of phyA-mediated phototropic curvature appeared to be the feature of etiolated seedlings. The de-etiolation of seedlings under continuous white light for 24 h recovered phototropism in fri and au mutant seedlings similar to wild type, indicating that phyA deficiency does not block phototropic curvature after de-etiolation (Fig. 6B).
Figure 6.
A, Time course of geotropic curvature in wild-type and au mutant seedlings. At the time intervals indicated, the seedlings were removed and the angle of curvature was measured. For fri seedlings shown in the inset, the curvature was measured after 2 h of geotropic stimulation. B, Phototropism in wild-type, au, fri, tri, and frihp1 mutant seedlings. The 2-d-old etiolated seedlings were grown for 24 or 48 h under continuous white light and were returned to darkness for 2 h. Thereafter, the seedlings were stimulated with unidirectional white light for 8 h and curvatures were determined.
The Blue Light-Mediated Autophosphorylation and Chloroplast Movement Are Normal in Tomato Mutants
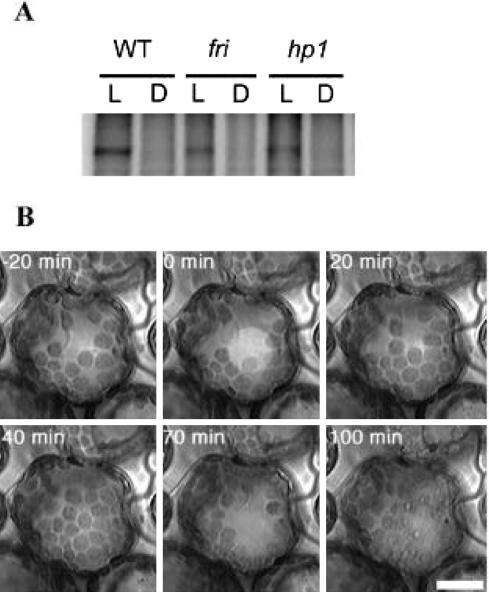
The possibility that the phyA deficiency in fri and au mutant seedlings might have affected the activity of phototropin molecule per se was examined by studying blue light-mediated phosphorylation of proteins in crude homogenates of tomato and its mutant seedlings (Short and Briggs, 1994). It has been shown that blue light-mediated appearance of a phosphorylated band of approximately 120 kD Mr in etiolated seedlings likely reflects the phosphorylation of phot1, as Arabidopsis mutants deficient in phot1 lack the phosphorylation (Liscum and Briggs, 1995). The tomato seedlings appeared to be normal with respect to blue light-induced phosphorylation. Although no phosphorylation could be detected in dark-grown seedlings, blue light-irradiated wild-type, as well as hp1 and fri mutant seedlings, showed phosphorylation of a 121-kD protein band, which has a molecular mass within the range of the molecular mass reported for phot1 (Fig. 7A).
Figure 7.
A, Blue light-mediated phosphorylation in tomato hypocotyls of wild-type, fri, and hp1 mutant seedlings. The autoradiograph shows the in vitro blue light-dependent phosphorylation of soluble protein fractions prepared from tomato seedlings. The samples were irradiated with white light at a total fluence of 2,000 μmol m–2 s–1. B, Blue light-induced chloroplast relocation movement in a cell of cotyledon of wild-type seedling. The cell of cotyledon adapted under weak white light conditions (5 μmol m–2 s–1) was observed under red light. The cell was irradiated with weak blue light (3.7 μmol m–2 s–1) for 40 min (0–40 min) and then with strong blue light (56 μmol m–2 s–1) for 30 min (40–70 min). After irradiation, the cell was continuously observed without blue light irradiation (70–100 min).
It is now known that the phot1 and phot2 control also control other mechanical responses such as movement of chloroplasts in plant cells in response to ambient light intensity. The possibility that the phytochrome deficiency impaired the process of chloroplast movement was examined by studying chloroplast accumulation and avoidance in tomato and its mutant seedlings. Figure 7B shows the accumulation as well as avoidance reaction of chloroplasts in the cotyledons of wild-type (AC) tomato seedlings under weak blue light and the strong blue light. Comparison of blue light-mediated accumulation of chloroplasts at low fluence light intensity and avoidance of chloroplast at high light intensity in au, fri, and frihp1 mutants with wild type revealed that chloroplast movement in above mutants was similar to that of wild type.
DISCUSSION
Phytochrome A Is Required for Expression of First- and Second-Positive Curvature in Tomato Seedlings
Plant photoreceptors though independently affect many developmental responses; there is a growing body of evidence that the effect of individual photoreceptors can also be modulated by other photoreceptors (Mohr, 1994). For instance, the phototropic movement of etiolated seedlings is directly controlled by phototropins and not by phytochromes. However, a pretreatment with red light enhances the phototropic response to a subsequent unilateral blue-light treatment. In this case, phytochrome acts as an amplifier of a phototropin-regulated response. Conversely, phytochrome-mediated responses can also be amplified by other photoreceptors such as cryptochromes. The examination of mutants deficient in different photoreceptors and downstream signal transduction pathways has significantly improved our understanding about the nature of the interactions among photoreceptors for several photoresponses. In case of phototropism, it has been highlighted that phyA is the major effecter of phot1-dependent phototropism in Arabidopsis, and its action may arise via its influence on auxin sensitivity or action on members of Aux/indole-3-acetic acid (IAA) family (Stowe-Evans et al., 2001; Liscum, 2002).
In conformity with earlier observations, the results of this study demonstrate that in etiolated tomato seedlings, in addition to blue light photoreceptor, the activation of phytochrome is also necessary for the full expression of low fluence blue light-mediated phototropism. The marked reduction of first-positive and second-positive curvature in the au mutant of tomato, which by virtue of chromophore deficiency possess all phytochrome species at a reduced level, provides a good evidence for an active participation of at least one species of phytochrome in blue light-mediated phototropism in tomato. Among the phytochrome species present in tomato (Alba et al., 2000), the results obtained using tomato mutants lacking phyA and phyB1 favors a likely participation of phyA in this response. The phototropic responsiveness is lost specifically only in phyA-deficient fri or double mutant fritri seedlings, but phyB1-deficient tri seedlings show normal phototropic response.
Among first- and second-positive phototropic curvatures, the deficiency of phyA affects second-positive curvature most severely with near loss of phototropic curvature at low fluence blue light. Comparatively, fri and au mutant seedlings retain some remnants of first-positive curvature, as evident by presence of extremely weak curvature in response to single pulse of blue light. We reasoned that the above reduction in first-positive curvature results from a reduction in the active pool of phytochrome in mutants. In such a case exposure of seedlings to multiple blue light pulses interspersed with dark intervals, a treatment, which is known to significantly enhance first-positive curvature in plants (Steintiz and Poff, 1986), should be largely ineffective in case of phytochrome-deficient mutants. The results obtained after exposure of wild-type and mutant seedlings to multiple blue light pulses interspersed by 10-min dark intervals are in conformity with this view. The manifestation of blue light response also needs a parallel action of phytochrome, which is evident by the fact that multiple pulses amplify the first-positive curvature in the au mutant, which possesses residual level of phytochrome, by nearly 5-fold. In contrast, the fri mutant is rather similar to wild type, as it shows only a 2-fold increase of first-positive curvature on exposure to multiple pulses. It is likely that in au mutant, the multiple pulses, in addition to activating blue light photoreceptors, may have also stimulated the residual pool of phytochrome to bring about this effect. Taken together, these results indicate that the simultaneous activation of phyA along with phototropins is also necessary for the full expression of blue light-mediated phototropism in tomato.
The phytochrome regulation of phototropism in tomato somewhat differs from Arabidopsis with respect to usage of different species of phytochromes. In tomato, only phyA seems to be used for the regulation of second-positive curvature. Even for the first-positive curvature, the severe impairment of response in the fri mutant indicates a major role of phyA. At the same time, additional phytochrome species may also participate in regulation of first-positive curvature as evident by slight stimulation of curvature by red light pretreatment of fri and fritri mutants. Contrastingly, in Arabidopsis, whereas only phyA is needed for amplification of the blue light-mediated first-positive curvature (Parks et al., 1996), phyA and phyB are required to elicit the second-positive curvature (Janoudi et al., 1997a, 1997b). A similar requirement for phytochrome activation for the expression of second-positive curvature has also been observed for etiolated maize (Zea mays) seedlings (Liu and Iino, 1996). Taken together, these results indicate that in a range of species the expression of phototropic curvature seems to have an obligatory requirement for coaction of phytochrome.
The possibility that the loss of phytochrome in mutants may have in turn affected the level or function of blue light photoreceptors is least likely. The fritri double mutant of tomato, which is essentially blind in the red and far-red region of the spectrum, shows blue light-mediated anthocyanin induction, indicating a normal operation of blue photoreceptors even in absence of phyA and phyB (Kerckhoffs et al., 1997). Moreover, the blue light-mediated phosphorylation of a 121-kD protein is seen in wild-type as well as in hp1 and fri seedlings, showing normal operation of phototropin action in the phyA mutant seedlings. Similarly, the phytochrome-deficient seedlings were normal in exhibiting differential growth responses to endogenous auxin as the fri mutant, and tri mutant seedlings showed geotropic curvatures similar to wild type.
Deficiency of phyA does not seem to affect other phototropin-mediated responses such as chloroplast movement (Wada et al., 2003) in tomato seedlings. The blue light-mediated accumulation of chloroplasts at low fluence light intensity and avoidance of chloroplast at high light intensity was similar in au, fri, and frihp1 mutants, and in wild type. First, it indicates that phot1 and phot2 operates normally in phyA-deficient tomato seedlings, at least for chloroplast accumulation. Second, it also indicates that the action of phyA in regulating phototropin action is restricted to only the signal transduction chain of phototropism, whereas the signal transduction of chloroplast movement takes place independently of participation of phytochrome. However, one has to take in account the fact that the chloroplast movement was observed in de-etiolated seedlings, and the de-etiolation per se removes the impairment of phototropism in mutant seedlings. The exposure of etiolated fri mutant seedlings to a prolonged blue light irradiation (12 h) leads to recovery of phototropism. Similarly, after deetiolation under white light, the phytochrome-deficient fri seedlings show normal phototropic curvature similar to that of wild type. The plausible mechanism of the recovery of phototropism is not known and can only be speculated. It is possible that the de-etiolation leads to a shift in usage of the phytochrome species where phytochromes other than phyA regulate phototropic curvature (Ballaré et al., 1992). Equally plausible is the possibility that deetiolated seedlings use phot2 as the photoreceptor for phototropism. The mutants defective in phot1 show phototropic curvature on exposure to high fluence blue light (Sakai et al., 2001), indicating that a second photoreceptor such as phot2 mediates phototropism, but functions only at high light intensities. In essence, our results indicate that phyA regulation of phototropism essentially appears to be a feature of etiolated seedlings under low fluence blue light.
HP1 Gene Product May Be a Negative Regulator of Phototropic Signal Transduction Pathway
The precise role of phytochrome in enhancement of blue light-mediated phototropism is still not known and may be quite complex. There are number of photoresponses where coaction of blue light photoreceptors and phytochrome is needed to elicit the response (Mohr, 1994); however, the manner in which these two photoreceptors interact is yet to be deciphered. It has been observed in Arabidopsis and tomato that blue light photoreceptor cryptochrome-mediated responses such as hypocotyl elongation and anthocyanin accumulation show functional dependence on levels of phyA and phyB, and consequently are reduced in mutants deficient in these phytochrome species (Ahmad and Cashmore, 1997; Weller et al., 2001). One of the likely explanations of these interactions is that whereas the photoresponse is triggered by one photoreceptor, another photoreceptor is needed to regulate the levels of downstream elements of the signal transduction pathway. The molecular-genetic analysis in Arabidopsis has revealed that in etiolated seedlings, photomorphogenesis is blocked by negative regulators such as DET and COP, which may perhaps act by regulating gene expression in nucleus (Serino and Deng, 2003). Light exposure to etiolated seedlings brings about its morphogenic action by down-regulating the level/distribution of these negative regulators. We assume that a negative regulator similar to COP regulates the phototropic signal transduction, and phytochrome activation down-regulates the level of the above negative regulator, allowing manifestation of phototropic curvature. The results obtained in this study strongly suggest for such a mechanism, with the gene product of HP1 as one of potential negative regulators, at least for second-positive curvature, whose level needs to be down-regulated by phyA to allow the blue light-mediated phototropic curvature. This view is strengthened by the finding that the HP2 gene, whose mutation elicits a phenotype similar to the hp1 mutation, encodes for a protein homologous to DET1 protein of Arabidopsis (Mustilli et al., 1999), which is a negative regulator of photomorphogenic development.
The physiological studies on tomato have shown that mutation at the HP1 locus amplifies the phytochrome-mediated responses in the seedlings (Peters et al., 1989, 1992; Goud and Sharma, 1994). Based on its recessive (loss-of-function) nature, it is proposed that the phytochrome action in etiolated seedlings is under the constraint of the HP1 gene product (Peters et al., 1992, 1998). The hp1 mutation does not affect the level of phytochrome protein or processes, but exaggerates the phytochrome-mediated responses in plants. This is consistent with the proposed hypothesis that HP1 is a component of the phytochrome-signaling pathway that modulates signal transduction. We propose that phytochrome regulates the time-dependent phototropic response by down-regulating HP1 level, which impedes the phototropic signal chain. The absence of phyA in the fri mutant makes the seedlings nonphototropic as the down-regulation of HP1 level is strictly under phyA control. This also explains the restoration of phototropic responsiveness in the frihp1 double mutants. In the frihp1 double mutant, the hp1 mutation would diminish HP1 level or makes a nonfunctional HP1 gene product, which would bypass the need for phyA to lower the HP1 level and would allow the phototropic signal to proceed normally. The study on time threshold of time-dependent phototropism in hp1 also favors this view. The hp1 mutant seedlings show reduced time threshold for second-positive curvature, whereas in wild-type seedlings, red preirradiation of seedlings is needed to reduce the time threshold. The role of the hp1 mutation in first-positive curvature appears to be complex. The dark-grown seedlings of the hp1 mutant showed higher curvature than the wild-type control. The frihp1 mutant seedlings showed slightly higher curvature than the fri seedlings. On the other hand, in the auhp1 mutant, the curvature was similar to the au mutant. This could be perhaps due to the pleiotropic effect of the au mutation that could have masked the effect of the hp1 mutation on first-positive curvature. Nevertheless, the effect of the hp1 mutation on first-positive curvature is not as conspicuous as observed for the second-positive curvature. The hp1 mutant does not show a mutant phenotype in dark-grown plants, but exhibits its phenotype only when plants are grown under light (Weller et al., 2000). It is plausible that the HP1 accumulates in the dark, and, on exposure to light, undergoes phytochrome-mediated degradation in light. Our results indicate that HP1 defines a point of crosstalk between phot1 and phyA signal transduction pathways and its removal is essential for the operation of phototropic signal transduction pathways.
The results of this study also provide a reasonable explanation for similar observation in Arabidopsis where it is observed that whereas red exposure to wild-type seedlings reduces the time threshold (Janoudi et al., 1992), in the phyAphyB double mutant, the time threshold is increased by almost 6-fold (Janoudi et al., 1997b). It is likely that similar to tomato, the operation of phototropic signal pathway in Arabidopsis may also be under constrain of a negative regulator, but requires coaction of two phytochromes to reduce its level. Similarly, in maize, where manifestation of first- and second-positive curvature are obligatorily dependent on phytochrome activation, phytochrome perhaps brings about blue light-mediated phototropism by down-regulating a negative regulator. Taken together, the observations in tomato and other plants indicate the likely existence of a negative regulator of phototropic signal transduction pathways. The information about the product encoded by the HP1 gene in tomato and its homologs in other systems is required to decipher its role in higher plant phototropism.
Our studies complement and extend similar observations made in Arabidopsis where null alleles NPH4 are nonphototropic under low fluence blue light, but retain phototropic response to high fluence of blue light or to light conditions that simultaneously activate phytochrome and phot1 (Liscum, 2002). Similarly, the phototropic responses are impaired in tomato diageotropica (dgt) mutant seedlings but could be suppressed through simultaneous activation of phytochrome and phototropin (Stowe-Evans et al., 1999). Both mutants are defective in the auxin signaling pathway, and whereas Arabidopsis NPH4 loci encodes for ARF7, the tomato DIAGEOTROPICA gene encodes for a cyclophilin-like protein (Oh et al., 2003). The likely role of auxin signaling in regulation of the phototropic curvature is also apparent from observations that overexpression of iaa1-GR transgene impaired phototropism in Arabidopsis seedlings, in gain-of-function of IAA1 (Park et al., 2002). The above observation indicates that the members of Aux/IAA family may act as negative regulators of phototropic response (Park et al., 2002). A connection between phytochrome and auxin signaling is beginning to emerge from the finding that the proteins belonging to the Aux/IAA family could be targets for phosphorylation by phytochrome, at least in vitro (Colón-Carmona et al., 2001). Given the importance of auxin in regulation of phototropic responses and the emerging role of phytochrome in regulation of auxin signaling, a crosstalk between the two pathways appears to be reasonable. It is plausible that the gene product of HP-1 may be a common element that is shared by both pathways, which could be perhaps related in some form to auxin action. The identification of gene product of HP1 and studies of regulation of its level would allow further insight into interaction of photoreceptors in regulating phototropism.
MATERIALS AND METHODS
Plant Material
The tomato (Lycopersicon esculentum) genotypes used were au, hp1, auhp1 and its isogenic wild type in the background AC, fri and its wild type in the background MM, and tri and its wild type in the breeding line GT; the frihp1 used was in the mixed background MM and AC, and the fritri used was in the mixed background MM and GT. Seed stock carrying the mutant lines were a generous gift from Prof. Maarten Koornneef and Prof. Richard E. Kendrick (Wageningen Universitat and Research Centrum, The Netherlands). The seeds used for experiments were of similar age and were a minimum of 2 years old. Seeds were surface sterilized by 1.5% (w/v) NaClO4 solution for 15 min, rinsed with distilled water, and were germinated on wet germination papers in the dark. The germinated seeds that showed emergences of radicle were individually transferred to screw caps (10-mm diameter × 5-mm height) filled with vermiculite/peat mixture. The seedlings were grown in darkness and used when hypocotyls were about 3 cm long, i.e. 4 d after germination. The seedlings for experiments on effect of varying fluence of blue lights were grown on 0.4% (w/v) agar in Eppendorf tubes. The plants for studies on chloroplast movements were grown in plastic cups filled with vermiculite and were irrigated with the tap water. The plants were then grown in a greenhouse and kept under weak white light conditions (5 μmol m–2) at least several hours before experiments. All of the experiments were carried out at 25°C ± 1°C and were repeated five to seven times. Unless otherwise indicated, all curvature data are mean values ± se.
Light Sources
Blue light for pulse treatments (0.1 μmol m–2 s–1) was obtained by passing light from a projector (150 W) through two CBS Blue filters (450 nm; Carolina Biological Supply, Burlington, NC). The continuous unidirectional blue light (0.1 μmol m–2 s–1) was provided by passing the output of a cool white fluorescent lamp (20 W) through a CBS blue filter (450 nm). The red light (25.0 μmol m–2 s–1) was obtained by passing light from a halogen lamp (150 W) through a CBS red filter (650 nm) and a 1-cm layer of water. Similarly, the far-red light (21 μmol m–2 s–1) was obtained by passing light from a halogen lamp (150 W) through a CBS far-red filter (750 nm) and a 1-cm layer of water. The photon fluence rate of light was measured by using a quantum photometer (SKY, Powys, UK).
The effect of different fluence of light was examined by exposing seedlings to varying fluences of light ranging from 0.1 to 40 μmol m–2 s–1 in a threshold box with seven chambers. The light source consisted of a 300-W Xenon lamp and the output of lamp was passed through a 10-cm layer of water and a broadband blue light filter. For 100 μmol m–2 s–1 light exposure, the light source consisted of a 1,000-W halogen lamp, and its light output was passed through a 10-cm layer of water and four layers of blue cellophane sheets (λmax = 450 nm).
Curvature Measurement
Seedlings of right length and orientation were picked up, and adhering seed coats were removed under dim green safe light; thereafter, the seedlings were exposed to continuous or pulse(s) of unidirectional blue light. For continuous blue light treatment, the seedlings were exposed with cotyledons in front of the light source. For blue light pulse treatment, seedlings were exposed with hook in front of light source, because pulse treatment induced only small angles of curvature with cotyledons in front of the light source. At defined time points, seedlings were removed, placed on a transparency sheet, and photocopied on a photocopier (Modi-Xerox, Rampur, India). The angle of curvature was measured from photocopied images. For gravitropism experiments, seedlings were kept horizontally, and at different time intervals, the curvatures were measured similar to phototropism.
In Vitro Phosphorylation
The in vitro phosphorylation was carried out essentially using the procedure outlined by Salomon et al. (1997). Ten 1-cm segments of hypocotyls were harvested under dim green safelight from 3-d-old etiolated seedlings and were frozen in liquid nitrogen in Eppendorf tubes. The frozen tissue was homogenized within Eppendorf tubes using a microhomogenizer with buffer containing 50 mm HEPES/KOH, pH 8.0, 5 mm MgSO4, 0.5% (w/v) Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 0.6 μm aprotinin, 16.5 μm chymostatin, and 3 μm pepstatin. The homogenates were centrifuged for 2 min at 10,000g. From the supernatant, a 30-μL (150 μg of protein) aliquot was drawn and used for each phosphorylation reaction. The reaction was started by addition of 10 μCi carrier-free (γ-32P) ATP (specific activity of 4,000 Ci mol–1). One set of samples was irradiated with blue light at a fluence rate 2,000 μmol m–2 s–1 for 1 min and another set of samples was kept in the dark for control. Reactions were allowed to proceed at 25°C for 2 min and were then terminated by adding 10 μL of 4× SDS-PAGE sample buffer consisting of 200 mm Tris-HCl, pH 6.8, 8% (w/v) SDS, 40% (v/v) glycerol, 20% (v/v) β-mercaptoethanol, and 0.4% (w/v) bromphenol blue followed by heating the sample for 4.5 min at 100°C. Aliquots from each sample containing equal amounts of protein were electrophoresed on 8% (w/v) SDS-PAGE followed by Coomassie staining and drying the gel. The radiolabeled protein bands were detected using a PhosphorImager (model no. STORM 840; Molecular Dynamics, Sunnyvale, CA). The molecular mass of the protein was determined by comparison with prestained molecular markers.
Chloroplast Movement
The chloroplast movement was examined in the cotyledons of 2-week-old tomato and its mutant seedlings. The cotyledons were evacuated with deionized water and the cells in the cotyledons were then observed under a microbeam irradiator (modified BX-50; Olympus, Tokyo) with a computer recording system. The monochromatic blue light was obtained through an interference filter (17-nm half-bandwidth centered at 449 nm). Other details were described in a previous paper (Kagawa and Wada, 2000).
Acknowledgments
We thank Prof. M. Koornneef and Prof. R.E. Kendrick (Wageningen, The Netherlands) for providing the mutant seeds of tomato.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.030650.
This work was supported by University Grants Commission, New Delhi, India (research fellowship support to A.S.), by Council of Scientific and Industrial Research, New Delhi, India (to R.K.B.), by Department of Science and Technology, New Delhi, India (research grant to R.S.), and by a Japanese Society for the Promotion of Science, Tokyo, Japan Senior, Visiting Fellowship (to R.S.).
References
- Ahmad M, Cashmore AR (1997) The blue light photoreceptor cryptochrome shows functional dependence on phytochrome A and phytochrome B in Arabidopsis thaliana. Plant J 11: 421–427 [DOI] [PubMed] [Google Scholar]
- Alba R, Kelmenson PM, Cordonnier-Pratt MM, Pratt LH (2000) The phytochrome gene family in tomato and the rapid differential evolution of this family in angiosperms. Mol Biol Evol 17: 362–373 [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Radosevich SR, Kendrick RE (1992) Phytochrome mediated phototropism in de-etiolated seedlings: occurrence and ecological significance. Plant Physiol 100: 170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A et al. (2001) The phototropin family of photoreceptors. Plant Cell 13: 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs WR, Christie JM (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7: 204–210 [DOI] [PubMed] [Google Scholar]
- Colón-Carmona A, Chen DL, Yeh K-C, Abel S (2001) Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol 124: 1728–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud KV, Sharma R (1994) Retention of photoinduction of cytosolic enzymes in aurea mutant of tomato (Lycopersicon esculentum). Plant Physiol 105: 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M (1990) Phototropism: mechanisms and implications. Plant Cell Environ 19: 633–650 [Google Scholar]
- Janoudi A-K, Gordon WR, Wagner D, Quail P, Poff KL (1997a) Multiple phytochromes are involved in red-light-induced enhancement of first-positive phototropism in Arabidopsis. Plant Physiol 113: 975–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Konjevic R, Poff KL (1992) Time threshold for second positive phototropism is decreased by a pre-irradiation with red light. Plant Physiol 99: 1422–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoudi A-K, Konjevic R, Whitlam GC, Gordon W, Poff KL (1997b) Both phytochrome A and phytochrome B are required for the normal expression of phototropism in Arabidopsis thaliana. Physiol Plant 101: 278–282 [Google Scholar]
- Janoudi A-K, Poff KL (1992) Action spectrum of enhancement of phototropism by Arabidopsis thaliana seedlings. Photochem Photobiol 56: 655–659 [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M (2001) Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M (2000) Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant Cell Physiol 41: 84–93 [DOI] [PubMed] [Google Scholar]
- Kerckhoffs LH, Kelmenson PM, Schreuder ME, Kendrick CI, Kendrick RE, Hanhart CJ, Koornneef M, Pratt LH, Cordonnier-Pratt MM (1999) Characterization of the gene encoding the apoprotein of phytochrome B2 in tomato, and identification of molecular lesions in two mutant alleles. Mol Gen Genet 261: 901–907 [DOI] [PubMed] [Google Scholar]
- Kerckhoffs LHJ, Schreuder MEL, van Tuinen A, Koornneef M, Kendrick RE (1997) Phytochrome control of anthocyanin biosynthesis in tomato seedlings: analysis using photomorphogenic mutants. Photochem Photobiol 65: 374–381 [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Lascève G, Leymarie J, Olney MA, Liscum E, Christie JM, Vavasseur A, Briggs WR (1999) Arabidopsis contains at least four independent blue-light-activated signal transduction pathways. Plant Physiol 120: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Shalitin D (2003) Cryptochrome structure and signal transduction Annu Rev Plant Biol 54: 469–496 [DOI] [PubMed] [Google Scholar]
- Liscum E (2002) Phototropism, Mechanisms and Outcomes. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD. http://www.aspb.org/downloads/arabidopsis/lis.pdf
- Liscum E, Briggs WR (1995) Mutations in the NPH1 locus of Arabidopsis disrupt the perception of phototropic stimuli. Plant Cell 7: 473–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Briggs WR (1996) Mutations of Arabidopsis in potential transduction and response components of the phototropic signaling pathway. Plant Physiol 112: 291–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Stowe-Evans EL (2000) Phototropism: a “simple” physiological response modulated by multiple interacting photosensory-response pathways. Photochem Photobiol 72: 273–282 [DOI] [PubMed] [Google Scholar]
- Liu YJ, Iino M (1996) Phytochrome is required for the occurrence of time-dependent phototropism in maize coleoptiles. Plant Cell Environ 19: 1379–1388 [DOI] [PubMed] [Google Scholar]
- Mohr H (1994) Coaction between pigment systems. In Kendrick RE, Kronenberg GHM, eds, Photomorphogenesis in Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 353–372
- Motchoulski A, Liscum E (1999) Arabidopsis NPH3: an NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Mustilli AC, Fenzi F, Ciliento R, Alfano F, Bowler C (1999) Phenotype of the tomato high pigment-2 mutant is caused by a mutation in the tomato homolog of DEETIOLATED1. Plant Cell 11: 145–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KC, Ivanchenko MG, White TJ, Lomax T (2003) The Diageotropica gene of tomato encodes a novel player, a cyclophilin, in auxin signaling. http://abstracts.aspb.org/pb2003/public/P46/0680.html [DOI] [PubMed]
- Park JY, Kim HJ, Kim J (2002) Mutation in domain II of IAA1 confers diverse auxin-related phenotypes and represses auxin-activated expression of Aux/IAA genes in steroid regulator-inducible system. Plant J 32: 669–683 [DOI] [PubMed] [Google Scholar]
- Parks BM, Quail PH, Hangarter RP (1996) Phytochrome A regulates red light induction of phototropic enhancement in Arabidopsis. Plant Physiol 110: 155–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, Schreuder MEL, Verduin SJW, Kendrick RE (1992) Physiological characterization of high pigment mutant of tomato. Photochem Photobiol 56: 75–82 [Google Scholar]
- Peters JL, Szell M, Kendrick RE (1998) The expression of light-regulated genes in the high-pigment-1 mutant of tomato. Plant Physiol 117: 797–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, van Tuinen A, Adamse P, Kendrick RE, Koornneef M (1989) High-pigment mutant of tomato exhibits high sensitivity for phytochrome action. J Plant Physiol 134: 661–666 [Google Scholar]
- Quail PH (2002) Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 3: 85–93 [DOI] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K (2001) Arabidopsis nph1 and npl1: blue light receptors that mediate both phototropism and chloroplast relocation. Proc Natl Acad Sci USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K (2000) RPT2: a signal transducer of the phototropic response in Arabidopsis. Plant Cell 12: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon M, Knieb E, Von Zeppelin T, Rudiger W (2003) Mapping of low- and high-fluence autophosphorylation sites in phototropin 1. Biochemistry 42: 4217–4225 [DOI] [PubMed] [Google Scholar]
- Salomon M, Zacherl M, Rüdiger W (1997) Phototropism and protein phosphorylation in higher plants: Unilateral blue light irradiation generates a directional gradient of protein phosphorylation across the oat coleoptile. Bot Acta 110: 214–216 [Google Scholar]
- Serino G, Deng X-W (2003) The COP9signalosome: regulating plant development through the control of proteolysis. Annu Rev Plant Biol 54: 165–182 [DOI] [PubMed] [Google Scholar]
- Sharma R (2001) Phytochrome: A serine kinase illuminates the nucleus. Curr Sci 80: 178–188 [Google Scholar]
- Sharma R, López-Juez E, Nagatani A, Furuya M (1993) Identification of photo-inactive phytochrome A in etiolated seedlings and photo-active phytochrome B in green leaves of the aurea mutant of tomato. Plant J 4: 1035–1042 [DOI] [PubMed] [Google Scholar]
- Short TW, Briggs WR (1994) The transduction of blue light signals in higher plants. Annu Rev Plant Physiol Plant Mol Biol 45: 143–171 [Google Scholar]
- Steintiz B, Poff KL (1986) A single positive response induced with pulsed light in hypocotyls of Arabidopsis thaliana. Planta 168: 305–315 [DOI] [PubMed] [Google Scholar]
- Stowe-Evans E, Lesser D, Loma T, Liscum E (1999) Phytochrome-dependent enhancement of phototropism in response to long-term irradiation. http://www.biotech.missouri.edu/mbp/exchange/mbw99/abstracts/stowe-evanse.html
- Stowe-Evans EL, Harper RM, Motchoulski AV, Liscum E (1998) NPH4, a conditional modulator of auxin-dependent differential growth responses in Arabidopsis. Plant Physiol 118: 1265–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe-Evans EL, Luesse DR, Liscum E (2001) The enhancement of phototropin-induced phototropic curvature in Arabidopsis occurs via a photoreversible phytochrome A-dependent modulation of auxin responsiveness. Plant Physiol 126: 826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry MJ, Kendrick RE (1996) The aurea and yellow-green-2 mutants of tomato are deficient in phytochrome chromophore synthesis. J Biol Chem 271: 21681–21686 [DOI] [PubMed] [Google Scholar]
- van Tuinen A, Kerckhoffs L, Nagatani A, Kendrick RE, Koornneef M (1995a) A temporarily red light-insensitive mutant of tomato lacks a light-stable, B-like phytochrome. Plant Physiol 108: 939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuinen A, Kerckhoffs LH, Nagatani A, Kendrick RE, Koornneef M (1995b) Far-red light-insensitive, phytochrome A-deficient mutants of tomato. Mol Gen Genet 246: 133–141 [DOI] [PubMed] [Google Scholar]
- Wada M, Kagawa T, Sato Y (2003) Chloroplast movement. Annu Rev Plant Biol 54: 455–468 [DOI] [PubMed] [Google Scholar]
- Weller JL, Perrotta G, Schreuder ME, van Tuinen A, Koornneef M, Giuliano G, Kendrick RE (2001) Genetic dissection of blue-light sensing in tomato using mutants deficient in cryptochrome 1 and phytochromes A, B1 and B2. Plant J 25: 427–440 [DOI] [PubMed] [Google Scholar]
- Weller JL, Schreuder ME, Smith H, Koornneef M, Kendrick RE (2000) Physiological interactions of phytochromes A, B1 and B2 in the control of development in tomato. Plant J 24: 345–356 [DOI] [PubMed] [Google Scholar]
- Whippo CW, Hangarter RP (2003) Second positive phototropism results from coordinated co-action of the phototropins and cryptochromes. Plant Physiol 132: 1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]