Abstract
A condition of excess activity in the hippocampal formation is observed in the aging brain and in conditions that confer additional risk during aging for Alzheimer’s disease. Compounds that act as positive allosteric modulators at GABAA α5 receptors might be useful in targeting this condition because GABAA α5 receptors mediate tonic inhibition of principal neurons in the affected network. While agents to improve cognitive function in the past focused on inverse agonists, which are negative allosteric modulators at GABAA α5 receptors, research supporting that approach used only young animals and predated current evidence for excessive hippocampal activity in age-related conditions of cognitive impairment. Here, we used two compounds, Compound 44 [6,6-dimethyl-3-(3-hydroxypropyl)thio-1-(thiazol-2-yl)-6,7-dihydro-2-benzothiophen-4(5H)-one] and Compound 6 [methyl 3,5-diphenylpyridazine-4-carboxylate], with functional activity as potentiators of γ-aminobutyric acid at GABAA α5 receptors, to test their ability to improve hippocampal-dependent memory in aged rats with identified cognitive impairment. Improvement was obtained in aged rats across protocols differing in motivational and performance demands and across varying retention intervals. Significant memory improvement occurred after either intracereboventricular infusion with Compound 44 (100 μg) in a water maze task or systemic administration with Compound 6 (3 mg/kg) in a radial arm maze task. Furthermore, systemic administration improved behavioral performance at dosing shown to provide drug exposure in the brain and in vivo receptor occupancy in the hippocampus. These data suggest a novel approach to improve neural network function in clinical conditions of excess hippocampal activity.
Keywords: GABA agonist, inverse agonist, neural hyperactivity, water maze, radial arm maze, mild cognitive impairment
1. Introduction
GABAA receptors are pentameric assemblies of different subunits (α1-6, β1-3, γ1-3, δ, ε, θ, π) forming a chloride-permeable channel that is gated by the neurotransmitter γ-aminobutyric acid (GABA). GABAA receptors containing the α5 subunit have a restricted localization in rodents and humans (Sur et al., 1998) and mediate tonic inhibition of hippocampal pyramidal neurons (Glykys and Mody, 2006). Efforts to develop drugs with cognitive enhancing properties focused in the past on selective α5 inverse agonists which function as negative allosteric modulators (NAMs) (e.g., Chambers et al., 2003; Atack et al., 2006; Collinson et al., 2006; Dawson et al., 2006; Ballard et al., 2009), but such compounds have yet to prove efficacious in clinical trials designed to treat age-related cognitive impairment (Atack, 2010).
Observations in animal models now indicate that excessive neural activity in the hippocampal formation contributes to cognitive impairment in aging and in conditions that confer increased risk for Alzheimer’s disease (AD). In Long-Evans aged rats with cognitive impairment, CA3 pyramidal neurons have elevated firing rates along with a loss in the ability to encode new information (Wilson et al., 2005) and CA3/dentate gyrus network excitability is also elevated in aged Fisher 344 rats (Patrylo et al., 2007). Moreover, aberrant excitability is found in the hippocampal system in transgenic mice overexpressing amyloid precursor protein (Palop et al., 2007) and in mouse models of ApoE4 (Andrews-Zwilling et al., 2010), a genotype that confers greatly elevated risk for sporadic AD. In the latter models, ApoE4 relative to E3 augmented a detrimental effect of aging on hilar interneurons that provide an inhibitory network associated with the dentate gyrus-CA3. Consistent with evidence in such animal models, data from functional magnetic resonance imaging (fMRI) has revealed increased activation in the hippocampal system in elderly humans with memory impairment, including those meeting criteria for amnestic mild cognitive impairment (aMCI) and in carriers of an ApoE4 allele (Bookheimer et al., 2000; Dickerson et al., 2005; Putcha et al., 2011; for recent review see Ewers et al., 2011). Moreover, studies using high-resolution fMRI have isolated such excess activation to the dentate-CA3 subregion of the hippocampal formation (Yassa et al., 2010a, 2010b; Bakker et al., 2012).
Supporting the view that excess hippocampal activity is a dysfunctional condition, beneficial effects in the animal models are being reported using treatments to control it, including transfection of an inhibitory neuropeptide into CA3 and systemic use of certain antiepileptic drugs (AEDs) in both aged rats (Koh et al., 2010; Rose and Corbin, 2010) and in mouse models (Sanchez et al., 2011). Pentobarbital was also reported to rescue impairment in ApoE4 knock-in mice with deficits in the function of hippocampal inhibitory interneurons (Andrews-Zwilling et al., 2010). A translational clinical study using low doses of the AED levetiracetam in aMCI patients recently demonstrated significant reduction of dentate-CA3 overactivity as measured by fMRI and improved memory performance in the scanning task (Bakker et al., 2012).
In this context, GABAA α5 receptors could be an important therapeutic target for age-related cognitive impairment. Principal neurons in the hippocampus, including CA3 pyramidal neurons, express high levels of α5 mRNA and protein (Wisden et al., 1992; Fritschy and Mohler, 1995; Haberman et al., 2011), and receptor binding follows that same topography in rodents and humans (Sur et al., 1998, 1999). In conditions of excessive neural activity, therefore, the use of positive allosteric modulators (PAMs), rather than NAMs, might be indicated. Here, we tested that concept in a well-characterized model of age-related memory impairment using Long-Evans rats. Individual variability in the degree of hippocampal-dependent memory impairment in this model has shown strong test-retest reliability (Colombo et al., 1997) and is closely related to changes in hippocampal integrity and function (Gallagher and Rapp, 1997; Wilson et al., 2006).
Behavioral improvement was obtained with two different GABAA α5 PAMs in aged rats across protocols differing in motivational and performance demands and across varying retention intervals. Significant improvement occurred after either intracereboventricular (ICV) infusion or systemic administration. Finally, systemic administration with one of the test compounds improved behavioral performance at dosing shown to provide drug exposure in the brain and in vivo receptor occupancy in the hippocampus.
2. Materials and Methods
2.1. Subjects
Pathogen-free aged, male Long-Evans rats were obtained at 8-9 mo of age from Charles River Laboratories (Raleigh, NC) and housed in a vivarium at Johns Hopkins University until 24-26 mo of age. Young rats obtained from the same source were tested at 6 mo of age. Receptor occupancy studies were conducted at Covance Inc. (Indianapolis, IA) using adult, male Long-Evans rats (Charles River Laboratories, Portage, MI). All procedures were approved by Institutional Animal Care and Use Committees in accordance with the National Institutes of Health directive.
Young and aged rats were screened in a standardized assessment of hippocampal-dependent spatial cognition prior to behavioral studies with experimental treatments. Background characterization used a Morris water maze protocol as described in detail elsewhere (Gallagher et al., 1993). Briefly, rats were trained for eight days (3 trials per day) to locate a camouflaged escape platform that remained at the same location throughout training. Every sixth trial consisted of a probe (no escape platform for 30 s) that served to assess the development of a spatially localized search. A learning index was generated from the proximity of the rat to the escape platform during probe trials and was used to define impairment in the aged rats. The learning index is the sum of weighted proximity scores obtained during probe trials, with low scores reflecting a more accurate search. Cue training (visible escape platform; 6 trials) occurred on the last day of training to test for sensorimotor and motivational factors independent of spatial learning. Aged rats with impaired performance (i.e., those with learning index scores outside the young “normative” range) but successful cued training were used for the studies below.
2.2. Surgery and drug infusion
Aged behaviorally characterized rats selected for the drug study involving ICV infusion were implanted unilaterally with a guide cannula (26 gauge, Plastics One, Roanoke, VA) into the lateral ventricle under isoflurane anesthesia. Stereotaxic coordinates were 1.0 mm posterior to bregma, 1.5 mm lateral to midline, and 3.5 mm ventral to skull surface. The cannula was anchored to the skull with stainless steel screws and dental cement. A 33-gauge dummy cannula was inserted into each guide cannula to prevent clogging. Rats were given a week to recover before the start of behavioral training and drug treatment. Drug infusion was done through a 33-gauge injector cannula connected to a Hamilton microsyringe (Reno, NV). The injector extended 1.0 mm beyond the guide cannula. During the infusion, rats were held in the experimenter’s lap while the drug was slowly infused. The injector cannula was left in place for an additional minute to allow diffusion of the drug away from the cannula tip.
2.3. Behavioral assessment of drug treatments
Rats with ICV cannula were trained and tested in a novel water maze environment to assess the effect of drug treatment. The water maze used was housed in a different building and was surrounded by curtains with a novel set of patterns relative to the maze used for initial assessment of cognitive status. In order to eliminate any response tendencies associated with cue training at the end of background water maze characterization, the training protocol here consisted of two days of pre-training (6 trials per day) in which the hidden escape platform location was varied from day to day. No drug treatment was given during pre-training. Rats were then given two days of training (8 trials per day in 2 training blocks) in which the hidden escape platform remained in the same location. Each trial was 60 s, with a 60 s inter-trial interval. On the third day, the rats were given a probe test (120 s) in which the platform was removed. To assess memory for the target location, the time spent and the number of passes in an annulus (1.5 times the size of the platform) where the platform was located during training were compared to those registered in a control annulus in the opposite quadrant.
A hippocampal-dependent radial arm maze task was also used to assess the effect of drug treatment as described in detail elsewhere (Chappell et al., 1998; Koh et al., 2010). The protocol allowed repeated within-subject assessment using systemic administration of drugs at different doses and in combinations. Prior research using this model has shown that reliable individual differences in hippocampal-dependent memory among aged rats translate across the water maze used for initial characterization and the radial maze task (Koh et al., 2010).
Pre-training consisted of habituation, standard win-shift training, and win-shift training with delays interposed between information and memory test phases on the eight-arm maze. Drug treatments began a day after the completion of pre-training. Three arms were blocked at the beginning of each trial (information phase). The identity and configuration of the blocked arms were varied across trials. Food-deprived rats were allowed to retrieve food reward (Kellogg’s Froot Loops cereal) from the five unblocked arms. The rat was then removed from the maze for 2 hr (retention interval for aged rats) or 5 hr (retention interval for young rats to approximate the number of errors committed by memory-impaired aged rats under drug-free conditions), during which time the barriers on the blocked arms were removed allowing access to all eight arms. Rats were then placed back onto the center platform and allowed to retrieve the remaining food rewards (memory test phase). An error consisted of returning to an arm (all four paws on the arm) from which food had already been obtained. Note that young rats rarely commit errors in the post-delay retention test at the delay used for the aged rats in the current study (Chappell et al., 1998). The number of errors made in the retention phase was used to assess memory performance. Rats were tested with a series of drug doses in ascending/descending order; each dose, including vehicle alone, was thus tested twice.
2.4. Compounds
For the proof of concept study involving the water maze task, 6,6-dimethyl-3-(3-hydroxypropyl)thio-1-(thiazol-2-yl)-6,7-dihydro-2-benzothiophen-4(5H)-one, hereafter referred to as Compound 44 (a GABAA α5 receptor PAM, synthesized by DavosPharma, Upper Saddle River, NJ; Chambers et al., 2003), was dissolved in dimethyl sulfoxide. This compound has activity as a PAM at α5 receptors and high selective affinity to those binding sites (+25 ± 10% potentiation of GABA EC20 concentration, with a Ki of 4.7 nM at α5 relative to other GABAA binding sites, Ki > 48 nM, as reported in Chambers et al., 2003). Compound 44 (or vehicle of equal volume) was infused into the ICV at 100 μg in 5 μl approximately 40 min prior to water maze training and testing.
For behavioral studies on the radial arm maze, methyl 3,5-diphenylpyridazine-4-carboxylate, hereafter referred to as Compound 6 (a GABAA α5 receptor PAM, synthesized by Nox Pharmaceuticals, India; van Niel et al., 2005) and TB21007 (GABAA α5 receptor NAM, purchased from Tocris Bioscience, Ellisville, MO; Chambers et al., 2003) were dissolved in dimethyl sulfoxide (5% of final volume) and then with polyethylene glycol 300 (20%) and distilled water (75%); both drugs were injected intraperitoneally (IP) at 1 ml/kg. Drugs and vehicle were administered 30-40 min prior to training sessions. The newly synthesized Compound 6 was tested for functional activity in oocytes expressing GABAA receptors (α5β3γ2), which confirmed its PAM activity (+25.7 ± 4.2% potentiation of GABA EC50 concentration, consistent with +27% as reported in van Niel et al., 2005, with a Ki of 12 nM at α5 relative to other GABAA binding sites, Ki > 64 nM). TB21007 is a GABAA α5 receptor NAM, with −38 ± 2% negative modulation at GABA EC20 concentration and 10-13 folds selectivity at α5 versus other GABAA receptor subunits (Chambers et al., 2003).
In receptor occupancy studies, Ro 15-4513 (Tocris Bioscience, Ellisville, MO) was used as the tracer for GABAA α5 receptor sites based on its near 20-fold selectivity for GABAA α5 receptors relative to other α subunit containing GABAA receptors (Pym et al., 2005) and has been successfully used for GABAA α5 receptor occupancy studies both in animals and humans (Lingford-Hughes et al., 2002; Pym et al., 2005; Momosaki et al., 2010). Ro 15-4513 (1 μg/kg) was dissolved in 25% hydroxyl-propyl β-cyclodextrin and administered intravenously (IV) 20 min prior to the receptor occupancy evaluations. For receptor occupancy studies, Compound 6 was dissolved in 25% hydroxyl-propyl β-cyclodextrin and administered IV 15 min prior to tracer injection. Compounds were administered in a volume of 0.5 ml/kg except for the highest dose of Compound 6 (10 mg/kg), which was administered in a volume of 1 ml/kg.
2.5. Tissue preparation and receptor occupancy analysis
Rats were sacrificed by cervical dislocation 20 min after tracer injection. Trunk blood was collected in EDTA-coated Eppendorf tubes and stored on wet ice until study completion. Hippocampus and cerebellum were dissected and stored in Eppendorf tubes, and placed on wet ice until tissue extraction.
Acetonitrile containing 0.1% formic acid was added to each sample at a volume of four times the weight of the tissue sample. For the standard curve (0.1-30 ng/g) samples, a calculated volume of standard reduced the volume of acetonitrile. The sample was homogenized (FastPrep-24, Lysing Matrix D; 5.5 m/s, for 60 s or 7-8 watts power using sonic probe dismembrator) and centrifuged for 16 min at 14,000 rpm. The (100 μl) supernatant solution was diluted by 300 μl of sterile water (pH 6.5). This solution was then mixed thoroughly and analyzed via liquid chromatography-tandem mass spectrometry (LC/MS/MS) for Ro 15-4513 (tracer) and Compound 6.
Receptor occupancy was determined by the ratio method which compared tracer amount of Ro 15-4513 in the hippocampus with tracer amount of Ro 15-4513 in the cerebellum, a region with no detectable GABAA α5 binding (Sur et al., 1999), mRNA (Wisden et al., 1992) or protein expression (Fritschy and Mohler, 1995). Based on previous studies demonstrating that the highly selective GABAA α5 NAM L-655,708 demonstrates GABAA α5 receptor occupancy using Ro 15-4513 as ligand (Lingford-Hughes et al., 2002; Rosenzweig-Lipson et al., 2011), we further defined full receptor occupancy using L-655,708 (10 mg/kg, IV).
3. Results
3.1. Background assessment of cognitive status
Rats were tested for cognitive status prior to the experimental treatments using a protocol developed in this study population to assess hippocampal-dependent function. The proximity of the rat’s position to the escape platform location on probe tests interpolated over the course of training was used to calculate a learning index score for each rat, providing a composite graded measure of spatial learning capacity (Gallagher et al., 1993). Young rats routinely learn to swim directly to the escape platform, hence attaining low learning index scores. The performances of aged rats, on the other hand, reveal substantial individual differences, with approximately 40-50% of the aged rats having index scores that are distributed within the range of young performance (i.e., memory-unimpaired aged rats), and the remaining aged rats from the same cohort having index scores that fall outside the entire range of young performance (i.e., memory-impaired aged rats; see Gallagher et al., 1993; 2003; 2006). The higher learning index scores of the memory-impaired aged rats signify worse performance by reflecting search at a greater distance from the escape location during the memory probe tests.
The young adult rats used in the subsequent drug experiments had index scores ranging from 133-235, which are in the normative range for this study population, while index scores for memory-impaired aged rats selected for the drug experiments were 239-402, as routinely observed in this model. As aged rats were segregated based on their memory performance relative to that of young rats, it is not surprising then that the performance of memory-impaired aged rats was significantly impaired relative to the young rats, t(45) = 8.63, p = 0.001. All rats were tested in a hippocampal-independent cued task at the end of water maze training in which the escape platform was visible. Performance in these cued trials did not differ as a function of age or cognitive status (performance of young and impaired aged rats, or the impaired aged rats with unimpaired aged rats tested concurrently were p > 0.741 and p > 0.510, respectively; data not shown). Those data are consistent with the profile of animals in this model and indicate that the memory impairment observed in the memory-impaired aged rats were not due sensorimotor or motivational deficits.
3.2. Proof of concept study in a novel spatial environment
As a proof of concept, we tested the efficacy of a GABAA α5 receptor PAM, Compound 44 (Chambers et al., 2003), to modulate the memory of a subset of aged rats (n = 11) with identified hippocampal-dependent memory impairment. To ensure that the test compound reached the brain, ICV infusion was used to administer Compound 44 (or vehicle) prior to training and testing in a water maze task conducted in a novel spatial environment. Rats assigned to drug or vehicle groups were closely matched on learning index scores from background training, mean learning index scores were 275 (drug group) and 276 (vehicle group), p = 0.958. Figure 1A shows the escape latency of the rats during acquisition training in the drug treatment study. While rats in both groups performed similarly at the beginning of training, rats treated with Compound 44 appeared to escape more proficiently during the final block of training trials, which occurred on the second day of training. An analysis of variance with repeated measures revealed no main effect of group, F(1, 9) = 1.23, p = 0.297, a main effect of trial, F(3, 27) = 5.74, p = 0.004, but the interaction between group and trial block did not reach significance, F(3, 27) = 1.74, p = 0.183.
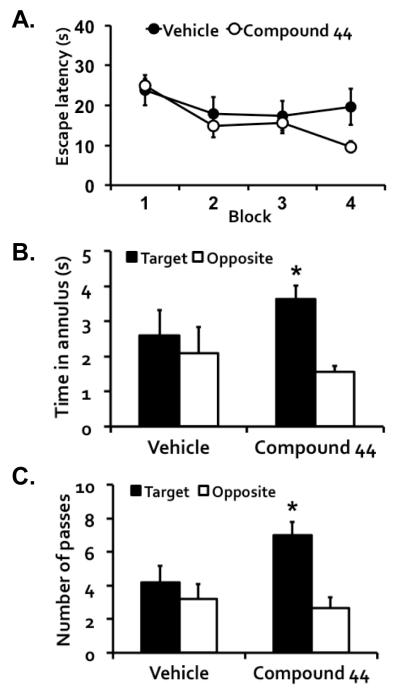
Figure 1.
Compound 44, a GABAA α5 receptor PAM, improved long-term memory of aged rats with cognitive impairment in a water maze task. (A) Rats treated with Compound 44 (n = 6) appeared to show more proficiency than those treated with vehicle (n = 5) in locating the hidden escape platform by the end of water maze training. Each training block has 4 trials, with trials in Blocks 1 and 2 conducted on the first training day, and those in Blocks 3 and 4 conducted on the second day. A probe test was given 24 hr after training to assess memory. Rats that received Compound 44, but not those that received vehicle, spent more time (B) and had more passes (C) in the target annulus than the opposite control annulus during the retention test.
The rats were tested for memory in a probe test 24 hr after training. Figures 1B and 1C show time spent and number of passes, respectively, in the target and opposite control annuli during the test. Rats treated with Compound 44 exhibited a significant spatial bias indicative of retention for the escape platform location, with more time spent and more passes in the target than the control annuli, t(5) = 4.62, p = 0.006 and t(5) = 3.88, p = 0.012, respectively. No such spatial bias, however, was observed in the vehicle group, consistent with their memory-impaired status (all ps > 0.600).
3.3. GABAA α5 receptor NAM for spatial memory on a radial maze
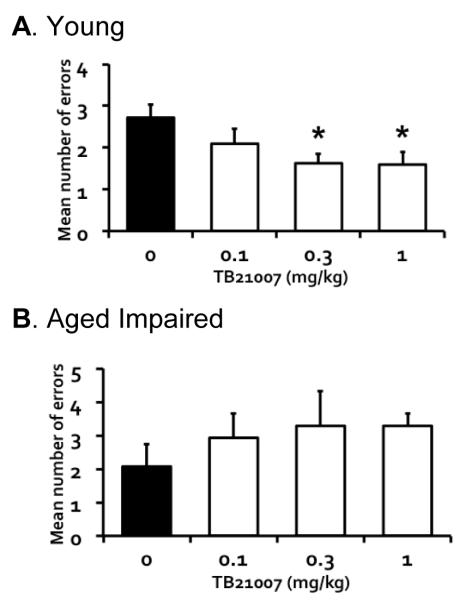
Here, we used a radial arm maze task that allows for repeated within-subject assessment of spatial memory to explore a range of drug doses. Figure 2 shows the performance of young and memory-impaired aged rats treated with varying doses of TB21007, a selective GABAA α5 receptor NAM (Chambers et al., 2003), as indexed by the number of errors made during post-delay memory tests. Note that a shorter delay was used for testing memory-impaired aged rats. Differences in memory performance were evident under drug treatment as a function of dose in young rats, F(3, 45) = 3.42, p = 0.025 (Figure 2A). The doses at 0.3 and 1 mg/kg significantly reduced memory errors relative to vehicle, t(15) = 2.84, p = 0.012 and t(15) = 2.29, p = 0.037, respectively. The same treatment in aged rats, however, failed to improve memory performance, F(3, 18) = 0.66, p = 0.588 (Figure 2B). These results confirm the efficacy of a GABAA α5 receptor NAM in young animals (Chambers et al., 2003; Atack et al., 2006; Dawson et al., 2006; Ballard et al., 2009) while showing that the same treatment fails to benefit aged rats with memory impairment.
Figure 2.
TB21007, a GABAA α5 receptor NAM, improved memory performance of young (A; n = 16) but not aged rats with memory impairment (B; n = 7) in a radial arm maze task. Rats were tested with a series of drug doses or vehicle (dimethyl sulfoxide and polyethylene glycol 300 in distilled water) in ascending/descending order; each dose was thus tested twice. Memory errors were recorded during test phases and consisted of a rat returning to an arm of the maze from which food reward had already been obtained. Treatment with TB21007 at 0.3 and 1 mg/kg in young rats (A) significantly reduced memory errors relative to vehicle (0 mg/kg). The same treatment, however, failed to affect memory performance in aged rats (B).
3.4. GABAA α5 receptor PAM for spatial memory on a radial maze
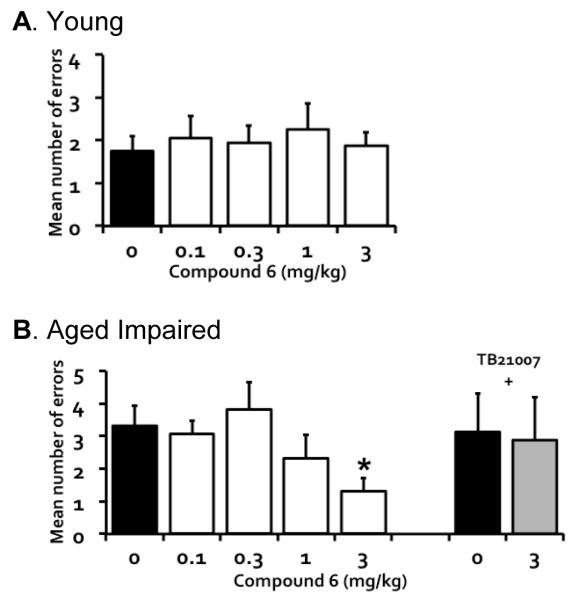
Next, we assessed a GABAA α5 receptor PAM over a range of doses using a within-subject protocol in the radial arm maze task. Figure 3 shows the performance of young and aged rats treated with varying doses of Compound 6, a highly selective PAM at GABAA α5 receptors (van Niel, et al., 2005). Treatment with Compound 6 in young rats failed to affect memory performance, F(4, 28) = 0.18, p = 0.949 (Figure 3A). Memory performance however was significantly improved by the drug treatment as a function of dose in aged rats with cognitive impairment, F(4, 28) = 3.57, p = 0.018 (Figure 3B), with a dose of 3 mg/kg significantly reducing errors relative to vehicle, t(7) = 4.23, p = 0.004. Additionally, memory improvement in those aged rats by Compound 6 (3 mg/kg) was blocked by concurrent administration of the GABAA α5 receptor NAM TB21007 (0.3 mg/kg), t(7) = 0.20, p = 0.845 compared to vehicle (Figure 3B).
Figure 3.
Compound 6, a GABAA α5 receptor PAM, failed to affect memory performance of young rats (A; n = 8) but significantly improved memory performance of aged rats with memory impairment (B; n = 8) in a radial arm maze task; and benefit was blocked by combination treatment with the GABAA α5 receptor NAM TB21007 (0.3 mg/kg). Rats were tested twice with each drug dose (or vehicle, dimethyl sulfoxide and polyethylene glycol 300 in distilled water) in ascending/descending order.
3.5. Receptor occupancy and exposure profiles
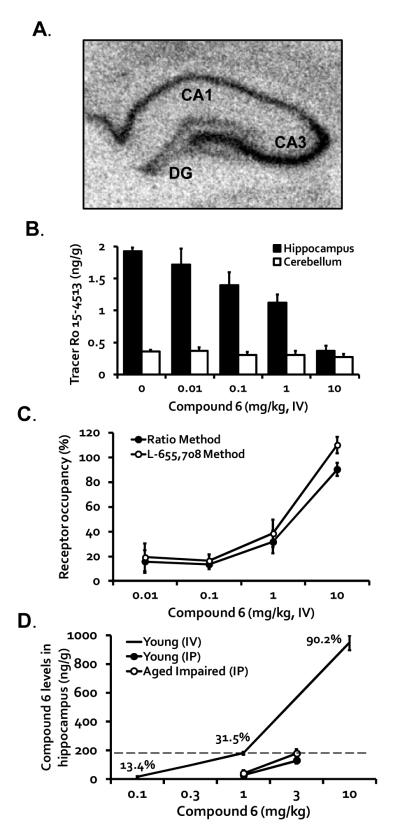
The principal neurons of the hippocampus, particularly those of CA3, show prominent expression of GABAA α5 receptor mRNA (Figure 4A; also see Haberman et al., 2011). To demonstrate that systemic administration of Compound 6 has good brain penetration and localizes to the hippocampus, we conducted receptor occupancy studies. As shown in Figures 4B-D, Compound 6 (0.01 – 10 mg/kg, IV, 15 min prior to tracer injection) dose-dependently reduced Ro 15-4513 (a tracer with high selectivity for the α5 receptor subunit) binding in hippocampus, without affecting cerebellum levels of Ro 15-4513 (Figure 4B), and a dose of 10 mg/kg, IV (20 min prior to receptor occupancy evaluations), demonstrated more than 90% occupancy (Figures 4C and 4D) with an ED50 value of 1.8 mg/kg, IV. We confirmed the dose-dependent α5 receptor occupancy of Compound 6 with a second method utilizing another selective GABAA α5 NAM, L-655,708, which has been previously shown to produce full receptor occupancy of the α5 receptor using Ro 15-4513 as a ligand (Lingford-Hughes et al., 2002; Rosenzweig-Lipson et al., 2011; Figure 4C). Utilizing L-655,708 to define receptor occupancy, Compound 6 produced dose-dependent occupancy with an ED50 value of 1.1 mg/kg, IV. Hippocampal exposure was linear as the dose of Compound 6 was increased from 0.1 to 1 mg/kg, IV, with a 10-fold increase in exposure corresponding to the 10-fold dose escalation, and was less than dose-proportional with a 5-fold increase in exposure upon escalation of dosing from 1 to 10 mg/kg, IV (Figure 4D).
Figure 4.
(A) An autoradiograph of GABAA α5 receptor mRNA in the hippocampus. The principal neurons of CA3 show prominent expression of α5 mRNA. See Haberman et al. (2011) for quantification and analysis of the mRNA expression levels. DG = dentate gyrus. (B) Vehicle administration followed by tracer Ro 15-4513 administration (1 g/kg, IV) resulted in more than 5-fold higher levels of Ro 15-4513 in hippocampus (1.93 ± 0.05 ng/g) compared with cerebellum (0.36 ± 0.02 ng/g). Compound 6 dose-dependently inhibited Ro 15-4513 tracer binding in the hippocampus, but not cerebellum (n = 4/group). (C) Compound 6 produced dose-dependent occupancy of GABAA α5 receptors as defined by using either a ratio method (comparison of hippocampal Ro 15-4513 exposure to cerebellar Ro 15-4513 exposure) or the selective GABAA α5-selective NAM, L-655,708 to define full occupancy. (D) Following administration of Compound 6, dose-dependent increases in Compound 6 levels were demonstrated in the hippocampus following IV administration in young, or IP administration in young or memory-impaired aged rats (n = 2-4/group). Numbers in percentages represent receptor occupancy at those doses as defined using the ratio method. Exposure at the behaviorally active dose of Compound 6 in aged rats (3 mg/kg, IP) corresponded to approximately 30% receptor occupancy observed at 1 mg/kg, IV.
Additional studies were conducted in aged Long-Evans rats in order to determine drug exposure at behaviorally relevant dosing for improved memory performance. Drug exposure in young rats after the same dosing with IP administration was determined to bridge with the receptor occupancy studies, which were conducted in young rats. Exposures in young and aged rats after IP administration of Compound 6 were relatively similar (Figure 4D). Increasing the dose of Compound 6 3-fold from 1 to 3 mg/kg, IP, resulted in a greater than dose-proportional increase in exposure in young and aged rats in both hippocampus and plasma with increases ranging from 4.3 to 6.6 folds. In the receptor occupancy studies, an exposure of 180 ng/g in hippocampus (1 mg/kg of Compound 6, IV) represented 31.5% occupancy. This exposure is similar to that observed in aged rats at 3 mg/kg of Compound 6, IP (177 ng/g), indicating that approximately 30% receptor occupancy is required for cognitive efficacy in this model.
4. Discussion
Treatment with a GABAA α5 receptor NAM enhanced behavioral performance in young rats, but no benefit of that drug treatment occurred in a model of age-related memory impairment in which principal neurons in CA3 have elevated firing rates (Wilson et al., 2005). Thus, those findings are consistent with prior preclinical studies on NAMs as cognitive enhancers in young adult rodents but indicate a potential limitation for benefit in the condition of cognitive aging. Instead, a GABAA α5 receptor PAM, Compound 6, improved memory in the cognitively impaired aged rats, but not in young rats. That finding is consistent with the role of GABAA α5 receptors in mediating tonic inhibition of principal hippocampal neurons (Glykys and Mody, 2006) and with other studies showing excess CA3 firing rates in memory-impaired aged rats (Wilson et al., 2005) and improved memory performance with treatments targeting that excess hippocampal activity (Koh et al., 2010). In addition to the benefit observed in the radial arm maze task using Compound 6, memory-impaired aged rats showed improved spatial memory using another GABAA α5 receptor PAM, Compound 44, in our exploratory proof-of-concept study. Thus, the benefit in aged rats with memory impairment generalizes across two different drugs that are GABAA α5 PAMs, and extends across tasks with different motivational and performance demands but share in their hippocampal-dependence for memory. Given the restricted localization of GABAA α5 receptors in the mammalian forebrain, with highest expression associated with the principal neurons of the hippocampus (Wisden et al., 1992; Sur et al., 1999; Haberman et al., 2011; also see Figure 4A), the current data support the hypothesis that the use of GABAA α5 receptor PAMs for memory loss in the context of brain aging may be beneficial.
The origin of excess CA3 activity and dysfunction in the hippocampal network is likely to come from several sources in the aging brain. Reduced cholinergic innervation and deficient post-synaptic M1 signaling may play a role. Interestingly, selective immunotoxic removal of the cholinergic input to hippocampus in young rats to mimic effects of aging produces increased burst firing of CA3 neurons without altering the firing properties of CA1 pyramidal neurons (Ikonen et al., 2002). A circuit specific loss of layer II entorhinal synaptic connections onto the dentate gyrus and CA3 distal dendrites also occurs in aged rats with memory loss (Smith et al., 2000), weakening signals for pattern separation that are needed to encode distinctive representations at the level of the CA3 region (for recent review, see Gallagher and Koh, 2011). The reduced input from entorhinal cortex may also have consequences by lowering control from the dentate gyrus over the extensive inhibitory interneuron network in the CA3 that is innervated by mossy fibers. Because the unimpaired aged phenotype retains hippocampal integrity on these measures, such as synaptic connections and encoding properties of hippocampal neurons (Smith et al., 2000; Wilson et al., 2003), GABAA α5 receptor PAMs would not be expected to boost memory performance in unimpaired aged rats. Finally, we have specifically confirmed a reduction in the expression GABAA α5 subunit mRNA in the CA3 region in our model of memory loss in aged Long-Evans rats (Haberman et al., 2011) and others have reported decreased receptor binding for those sites in the hippocampus of aged rodents (Hoekzema et al., 2011). Consequently, it may be especially important to potentiate the inhibitory activity of GABA at remaining GABAA α5 receptors in a condition of excess CA3 activity.
Ro 15-4513 was used as a tracer to determine receptor occupancy of the GABAA α5 receptor. The use of tracers and their measurement by LC/MS/MS, in lieu of radioligands, has been validated for multiple receptors including dopamine D2, serotonin 2A, opioid (mu, kappa, and delta) and neurokinin 1 receptors (Chernet et al., 2005, Need et al., 2007). Consistent with previous reports demonstrating that radiolabelled Ro 15-4513 can be used as a ligand for evaluating GABAA α5 receptor occupancy (Lingford-Hughes et al., 2002; Pym et al., 2005; Momosaki et al., 2010), the present studies demonstrating high hippocampal levels of Ro 15-4513 and low cerebellar levels of Ro 15-4513 coupled with findings that two GABAA α5 receptor NAMs dose-dependently occupy these sites (Rosenzweig-Lipson et al., 2011) suggest that Ro 15-4513 can be used as a tracer for evaluating GABAA α5 receptor occupancy of other compounds.
Compound 6 clearly crossed the blood brain barrier and demonstrated significant hippocampal exposure and occupancy of hippocampal GABAA α5 receptors. Exposures of Compound 6 in young and aged animals that correspond to the pro-cognitive doses in aged animals produced approximately 30% occupancy of the GABAA α5 receptors. These data, in combination with in vitro and in vivo data on Compound 6 GABAA α5 PAM activity, provide compelling evidence that potentiating the effects of GABA at GABAA α5 receptors has benefit for cognitive deficits associated with aging.
Control of excessive activity in the hippocampus could have benefit beyond improving memory performance. As recently reviewed in Ewers et al. (2011), increased hippocampal activation in fMRI has become recognized as a signature in the aging human brain. Such hippocampal activation is modestly increased in older subjects and in patients with aMCI greatly exceeds that of healthy age-matched controls. Such excess activation has also been reported to predict subsequent cognitive decline and conversion to AD (Dickerson et al., 2004; Miller et al., 2008) and has been linked to pathological AD degeneration in the brain (Putcha et al., 2011). As predicted by the animal research, studies using high-resolution fMRI have now demonstrated a restricted localization of excess hippocampal activation in the CA3/dentate gyrus subregions (Yassa et al., 2010a, 2010b; Bakker et al., 2012). Given evidence for neural activity playing a role in driving AD pathophysiology (Bero et al., 2011; Busche et al., 2012), reduction of excess neural activity could potentially improve both symptomatic function and progression in prodromal AD.
Although drugs with selectivity for subtypes of GABAA receptors have been under development for a number of psychiatric and neurological indications, attention surrounding α5 selective agents has largely focused on drugs that are negative modulators of GABA (for review, see Rudolph and Möhler, 2006; Atack 2011). But recently, using the methylazoxymethanol acetate developmental model of schizophrenia, Gill et al. (2011) showed that treatment with a GABAA α5 PAM reduced excess hippocampal output that occurs in that model and improved the behavioral abnormalities associated with psychiatric illness. Hence, a growing body of evidence, both in laboratory animal research and clinical studies indicates that augmenting rather than reducing GABAergic control is indicated in conditions of overactivity affecting hippocampal circuits.
Highlights.
Hippocampal hyperactivity occurs in cognitive impairment associated with aging.
GABAA α5 receptors mediate tonic inhibition of neurons in the affected network.
GABAA α5 positive allosteric modulators improved memory in an aged rat model.
At efficacious dosing, hippocampal GABAA α5 receptor occupancy was demonstrated.
Acknowledgments
This research was supported by National Institute of Aging Grant P01-AG-09973 (M.G.), U01-AG041140 and a grant from the Alzheimer’s Drug Discovery Foundation (ADDF) to AgeneBio, Inc. We appreciate the contribution of Dr. Daryl Davies for functional screening of newly synthesized Compound 6, the assistance of Dr. Patrick Love at Covance, Inc. in the development of the receptor occupancy assays, and the generosity of Dr. Rebecca Haberman for sharing the autoradiograph as shown in Figure 4A.
Footnotes
Disclosure/Conflict of Interest
M.G. is the founder of AgeneBio Incorporated, a biotechnology company that is dedicated to discovery and development of therapies to treat cognitive impairment in aging. She serves as Chair of the scientific advisory board at AgeneBio and has a financial interest in the company. Her conflict of interest is managed by Johns Hopkins University. The authors (M.T.K. and M.G.) are inventors on Johns Hopkins University intellectual property licensed to AgeneBio. M.G. serves as a member of the Board of Scientific Counselors to the National Institute on Aging and is also a member of the Scientific Advisory Board of the Stanley Center at the Broad Institute. Otherwise, she has had no consulting relationships with other public or private entities in the past three years and has no other financial holdings that could be perceived as constituting a potential conflict of interest. M.T.K has received no financial support or compensation from any individual or corporate entity for research or professional services, and has no financial holdings that could be perceived as constituting a potential conflict of interest. S.R.L is a consultant to AgeneBio and serves as the Vice President of Research. She does not have additional consulting relationships on aging or Alzheimer’s disease at the present time. S.R.L is a former employee of Pfizer and retains Pfizer stock and has no additional financial holdings that could be perceived as constituting a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J. Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Atack JR. Preclinical and clinical pharmacology of the GABAA receptor alpha5 subtype-selective inverse agonist alpha5IA. Pharmacol. Ther. 2010;125:11–26. doi: 10.1016/j.pharmthera.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Atack JR. GABAA receptor subtype-selective modulators. II. α5-selective inverse agonists for cognition enhancement. Curr. Top. Med. Chem. 2011;11:1203–1214. doi: 10.2174/156802611795371314. [DOI] [PubMed] [Google Scholar]
- Bakker A, Krauss G, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard TM, Knoflach F, Prinssen E, Borroni E, Vivian JA, Basile J, Gasser R, Moreau JL, Wettstein JG, Buettelmann B, Knust H, Thomas AW, Trube G, Hernandez MC. RO4938581, a novel cognitive enhancer acting at GABAA alpha5 subunit-containing receptors. Psychopharmacology. 2009;202:207–223. doi: 10.1007/s00213-008-1357-7. [DOI] [PubMed] [Google Scholar]
- Bero AW, Yan P, Roh JH, Cirrito JR, Stewart FR, Raichle ME, Lee JM, Holtzman DM. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14:750–756. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busche MA, Chen X, Henning HA, Reichwald J, Staufenbiel M, Sakmann B, Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2012;109:8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, Hobbs SC, Marshall G, Maubach KA, Pillai GV, Reeve AJ, Macleod AM. Identification of a novel, selective GABAA α5 receptor inverse agonist which enhances cognition. J. Med. Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Chappell J, McMahan R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacology. 1998;37:481–487. doi: 10.1016/s0028-3908(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Chernet E, Martin LJ, Li D, Need AB, Barth VN, Rash KS, Phebus LA. Use of LC/MS to assess brain tracer distribution in preclinical, in vivo receptor occupancy studies: Dopamine D2, serotonin 2A and NK-1 receptors as examples. Life Sci. 2005;78:340–346. doi: 10.1016/j.lfs.2005.04.075. [DOI] [PubMed] [Google Scholar]
- Collinson N, Atack JR, Laughton P, Dawson GR, Stephens DN. An inverse agonist selective for alpha5 subunit-containing GABAA receptors improves encoding and recall but not consolidation in the Morris water maze. Psychopharmacology. 2006;188:619–628. doi: 10.1007/s00213-006-0361-z. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc. Natl. Acad. Sci. USA. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J. Pharmacol. Exp. Ther. 2006;316:1335–1345. doi: 10.1124/jpet.105.092320. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer’s disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Bizon JL, Hoyt EC, Helm KA, Lund PK. Effects of aging on the hippocampal formation in a naturally occurring animal model of mild cognitive impairment. Exp. Gerontol. 2003;38:71–77. doi: 10.1016/s0531-5565(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman RP, Rapp PR, Tanila H, Wilson IA. Individual differences in neurocognitive aging of the medial temporal lobe. Age. 2006;28:221–233. doi: 10.1007/s11357-006-9017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer’s disease. Curr. Opin. Neurobiol. 2011;21:929–934. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABAAR-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. doi: 10.1038/npp.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Hippocampal network hyperactivity after selective reduction of tonic inhibition in GABAA receptor α5 subunit-deficient mice. J. Neurophysiol. 2006;95:2796–2807. doi: 10.1152/jn.01122.2005. [DOI] [PubMed] [Google Scholar]
- Haberman RP, Colantuoni C, Stocker AM, Schmidt AC, Pedersen JT, Gallagher M. Prominent hippocampal CA3 gene expression profile in neurocognitive aging. Neurobiol. Aging. 2011;32:1678–1692. doi: 10.1016/j.neurobiolaging.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Rojas S, Herance R, Pareto D, Abad S, Jiménez X, Figueiras FP, Popota F, Ruiz A, Flotats N, Fernández FJ, Rocha M, Rovira M, Víctor VM, Gisper JD. In vivo molecular imaging of the GABA/benzodiazepine receptor complex in the aged rat brain. Neurobiol. Aging. 2011 doi: 10.1016/j.neurobiolaging.2010.12.006. e-pub ahead of print 25 January 2011. doi:10.1016/j.neurobiolaging.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ikonen S, McMahan R, Gallagher M, Eichenbaum H, Tanila H. Cholinergic system regulation of spatial representation by the hippocampus. Hippocampus. 2002;12:386–397. doi: 10.1002/hipo.1109. [DOI] [PubMed] [Google Scholar]
- Koh MT, Haberman RP, Foti S, McCown TJ, Gallagher M. Treatment strategies targeting excess hippocampal activity benefit aged rats with cognitive impairment. Neuropsychopharmacology. 2010;35:1016–1025. doi: 10.1038/npp.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Hume SP, Feeney A, Hirani E, Osman S, Cunningham VJ, Pike VW, Brooks DJ, Nutt DJ. Imaging the GABA-benzodiazepine receptor subtype containing the alpha5-subunit in vivo with [11C]Ro 15 4513 positron emission tomography. J. Cereb. Blood Flow Metab. 2002;22:878–889. doi: 10.1097/00004647-200207000-00013. [DOI] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momosaki S, Hosoi R, Abe K, Inoue O. Remarkable selectivity of the in vivo binding of [3H]Ro15-4513 to α5 subtype of benzodiazepine receptor in the living mouse brain. Synapse. 2010;64:928–936. doi: 10.1002/syn.20812. [DOI] [PubMed] [Google Scholar]
- Need AB, McKinzie JH, Mitch CH, Statnick MA, Phebus LA. In vivo brain opioid receptor binding of LY255582 assessed with a novel method using LC/MS/MS and the administration of three tracers simultaneously. Life Sci. 2007;81:1389–1396. doi: 10.1016/j.lfs.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu GQ, Kreitzer A, Finkbeiner S, Noebels JL, Mucke L. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrylo PR, Tyagi I, Willingham AL, Lee S, Williamson A. Dentate filter function is altered in a proepileptic fashion during aging. Epilepsia. 2007;48:1964–1978. doi: 10.1111/j.1528-1167.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Putcha D, Brickhouse M, O’Keefe K, Sullivan C, Rentz D, Marshall G, Dickerson B, Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J. Neurosci. 2011;31:17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym LJ, Cook SM, Rosahl T, McKernan RM, Atack JR. Selective labelling of diazepam-insensitive GABAA receptors in vivo using [3H]Ro 15-4513. Br. J. Pharmacol. 2005;146:817–825. doi: 10.1038/sj.bjp.0706392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose GM, Corbin AE. Levetiracetam improves spatial memory in aged-impaired Fischer 344 rats. 2010 Neuroscience Meeting Planner; San Diego, CA. Society for Neuroscience; 2010. Program No. 205.6. [Google Scholar]
- Rosenzweig-Lipson S, Love P, Koh MT, Watson M, Leander JD, Gallagher M. GABAA α5 receptor occupancy of a GABAA α5 agonist: Receptor occupancy studies using Ro 15-4513 as a tracer. 2011 Neuroscience Meeting Planner; Washington, DC. Society for Neuroscience; 2011. Program No. 243.01. [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr. Opin. Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sanchez P, Vossel K, Ho K, Kim DH, Yu G, Verret L, Palop JJ, Mucke L. Reducing epileptiform activity by levetiracetam rescues synaptic and cognitive functions in a mouse model of Alzheimer’s disease. International Conference on Alzheimer’s Disease; Paris, France. 2011. Program No. P3-463. [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J. Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. Autoradiographic localization of α5 subunit-containing GABAA receptors in rat brain. Brain Res. 1999;822:265–270. doi: 10.1016/s0006-8993(99)01152-x. [DOI] [PubMed] [Google Scholar]
- Sur C, Quirk K, Dewar D, Atack J, McKernan R. Rat and human hippocampal α5 subunit-containing γ-aminobutyric acidA receptors have α5β3γ2 pharmacological characteristics. Mol. Pharmacol. 1998;54:928–933. doi: 10.1124/mol.54.5.928. [DOI] [PubMed] [Google Scholar]
- van Niel MB, Wilson K, Adkins CH, Atack JR, Castro JL, Clarke DE, Fletcher S, Gerhard U, Mackey MM, Malpas S, Maubach K, Newman R, O’Connor D, Pillai GV, Simpson PB, Thomas SR, MacLeod AM. A new pyridazine series of GABAA α5 ligands. J. Med. Chem. 2005;48:6004–6011. doi: 10.1021/jm050249x. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J. Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol. Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, Diencepahlon, Mesencephalon. J. Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2010a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic Mild Cognitive Impairment. Neuroimage. 2010b;51:1242–52. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]