Abstract
Background & Aims
Ethanol-inducible cytochrome P450 2E1 (CYP2E1) activity contributes to oxidative stress. However, CYP2E1 may have an important role in the pathogenesis of high-fat mediated nonalcoholic steatohepatitis (NASH). Thus, the role of CYP2E1 in high-fat mediated NASH development was evaluated.
Methods
Male wild-type (WT) and Cyp2e1-null mice were fed a low-fat diet (LFD, 10% energy-derived) or high-fat diet (HFD, 60% energy-derived) for 10 weeks. Liver histology and tissue homogenates were examined for various parameters of oxidative stress and inflammation.
Results
Liver histology showed that only WT mice fed a HFD developed NASH despite increased steatosis in both WT and Cyp2e1-null mice fed HFD. Markers of oxidative stress such as elevated CYP2E1 activity and protein amounts, lipid peroxidation, protein carbonylation, nitration, and glycation with increased phospho-JNK were all markedly elevated only in the livers of HFD-fed WT mice. Furthermore, while the levels of inflammation markers osteopontin and F4/80 were higher in HFD-fed WT mice, TNFα and MCP-1 contents were lower compared to the corresponding LFD-fed WT. Finally, only HFD-fed WT mice exhibited increased insulin resistance and impaired glucose tolerance.
Conclusions
These data suggest that CYP2E1 is critically important in NASH development by promoting oxidative/nitrosative stress, protein modifications, inflammation and insulin resistance.
Keywords: Liver, CYP2E1, null mice, high-fat diet, NAFLD, NASH, oxidative stress, protein modifications, inflammation, insulin resistance
Introduction
Nonalcoholic fatty liver disease (NAFLD), a major health burden [1 and references within], is a chronic liver disease characterized by fat accumulation (steatosis). However, a small portion of NAFLD can progress to advanced forms of liver injury such as necro-inflammation, fibrosis, cirrhosis, hepatocellular carcinoma and liver failure. NAFLD with inflammation is called nonalcoholic steatohepatitis (NASH) [2]. The “two-hit” hypothesis [3] described the pathophysiology of NASH. Steatosis (a primary insult) can sensitize the liver to secondary serious insults including reactive oxygen/nitrogen species (ROS/RNS), gut-derived endotoxins, pro-inflammatory cytokines like tumor necrosis factor-alpha (TNFα), resulting in NASH development [1–3].
Obesity and chronic over-nutrition are the major risk factors for the development of NAFLD [4]. NAFLD is also considered the hepatic manifestation of the metabolic syndrome [5]. Although several mechanisms of NAFLD/NASH development have been proposed, its pathophysiology is still elusive. The incidence of insulin resistance (IR) or type-2 diabetes mellitus with NAFLD suggests that there is a positive association between IR and the pathogenesis of NASH [6]. However, the role of IR in the development and progression of NAFLD is unclear.
Oxidative stress has been suggested to play a role in the advancement of NAFLD to NASH. Sources of oxidative stress include: cytochrome P450 2E1 (CYP2E1), lipid peroxidation, mitochondrial dysfunction, cytokine induction, NADPH oxidase, etc [7]. CYP2E1, a member of the oxido-reductase cytochrome family, can oxidize a variety of small molecule substrates including xenobiotics, ethanol and fatty acids [7–9]. Superoxide anion, a byproduct of the CYP2E1-mediated metabolism [7–9], is a very potent ROS, which can serve as part of the second hit to advance the severity of NAFLD. CYP2E1 expression and activity in the liver are increased in humans and in animal models of NAFLD [7,10,11] Indeed, CYP2E1 was increased in obesity, fatty liver, NASH in both human and rodents, and this increase appears to correlate well with the severity of NAFLD [7,10–12]. Thus, it would be of great interest to characterize the potential role of CYP2E1 in the advancement of NAFLD and development of NASH by using Cyp2e1-null mice as a negative control in high-fat diet (HFD)-mediated NASH models. The aim of this study was to examine this hypothesis by feeding wild-type (WT) and Cyp2e1-null mice with a HFD for 10 weeks. Steatosis, inflammatory response, oxidative stress, systemic IR and the indicators of NAFLD and NASH manifestations were monitored in both WT and Cyp2e1-null mice fed a HFD or a low-fat diet (LFD).
Materials and methods
Materials
All chemicals used in this study were from Sigma Chemical (St. Louis, MO, USA), unless indicated otherwise. Secondary antibodies and specific primary antibody to RAGE, JNK, and p-JNK were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to CYP2E1, p38 MAPK (p38), and 3-NT were from Abcam Inc. (Cambridge, MA, USA). Anti-AGE antibody was purchased from Fitzgerald Industries (Acton, MA, USA). Antibody for osteopontin (OPN) was obtained from University of IOWA, (Iowa City, IA, USA).
Animal treatment and histopathology analysis
Mice used in this study were age-matched Cyp2e1-null mice on 129/Svj background [13] and WT mice [stock number 002448 from Jackson Laboratory (Bar harbor, ME, USA)]. Male mice were randomly assigned to four groups fed on solid LFD (10% energy-derived calories) or HFD (60% energy-derived calories) (Research Diets, New Brunswick NJ, USA) for 10 weeks: (1) wild-type fed LFD (WT-LFD) (n=4); (2) wild-type fed HFD (WT-HFD) (n=5); (3) Cyp2e1-null fed LFD (null-LFD) (n=4); and (4) Cyp2e1-null fed HFD (null-HFD) (n=4). Weight of each mouse was recorded before the start of LFD/HFD regimen, once every week, and at the time of euthanasia. Tissue preparation for histological examination and tissue maintenance were performed as previously described [11]. The NAFLD histological scoring system (NAS) [14] was also used to evaluate the samples blindly. NASH was defined in the cases of NAS of ≥5. All animal experiments were performed in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee.
Tissue extraction
Total homogenate from each mouse was prepared in extraction buffer (50 mM Tris-Cl, pH 7.5, 1 mM EDTA, and 1% CHAPS), which was pre-equilibrated with nitrogen gas to remove the dissolved oxygen and liver homogenates were prepared as previously described [15,16].
Measurements of transaminase activities, CYP2E1 activity, MDA+HAE concentration and cytokine levels
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were monitored in each mouse using the clinical IDEXX Vet Test chemistry analyzer system (IDEXX Laboratories, West Brook, ME, USA). CYP2E1 activity levels were measured by the rate of p-nitrophenol (PNP) oxidation to p-nitrocatechol, as described [15]. The concentration of MDA+HAE (μM) was measured using commercially available kits (Oxford Biomedical Research, Oxford, MI, USA) following the manufacturer’s protocol. Intrahepatic TNFα and MCP-1 levels (in 0.5 mg liver extracts) were determined by ELISA using the manufacturer’s protocols (Thermoscientific, Rockford, IL, USA).
Protein oxidation and glycoprotein determination
The levels of carbonylated proteins were determined with the OxyBlot protein oxidation detection kit following the manufacturer’s protocol (Millipore, Billerica, MA, USA). Glycoproteins were evaluated using Pro-Q Emerald 488 Dye following the manufacturer’s protocol (Molecular Probes, Eugene, OR, USA).
Immunohistochemistry
Formalin-fixed liver samples were processed and 5 μm thick paraffin sections were used for F4/80 immunohistochemistry. Briefly, de-paraffinized liver sections were treated with 3% hydrogen peroxide followed by antigen retrieval. The sections were blocked with 10% horse serum, and incubated with primary antibody, which was then linked to the biotinylated secondary antibody followed by avidin-biotin complex (ABC Vectastain kit; Vector Laboratories, Burlingame, CA, USA), and diaminobenzidine, which gave a brown reaction product. The sections were then counterstained with hematoxylin. F4/80 expression levels were identified by the increased brown colored staining versus normal controls by using the NIH Image analysis software.
IR and Glucose Tolerance (GT) tests
After 10-weeks of HFD-feeding, IR and GT tests were evaluated in mice fasted for 6 hours and subsequently injected with insulin (0.75 U/kg; Eli Lilly) or glucose (2 g/kg). GT tests were performed 3 days after the IR test. Tail blood was collected at indicated times immediately after intraperitoneal injection of insulin (IR) or glucose (GT). Blood glucose levels were determined using the Elite glucometer (Bayer).
Data evaluation
Data represent results from at least two separate measurements, unless otherwise stated. Each point represents the mean ± SEM (n=4 for both control groups and n=5 for both HFD groups), unless otherwise indicated. Statistical significance was evaluated using the Student’s t-test at the 95% confidence level. Other materials and methods including immunoblot analysis not specified here were the same as described [11,16].
Results
Evaluation of NAFLD development
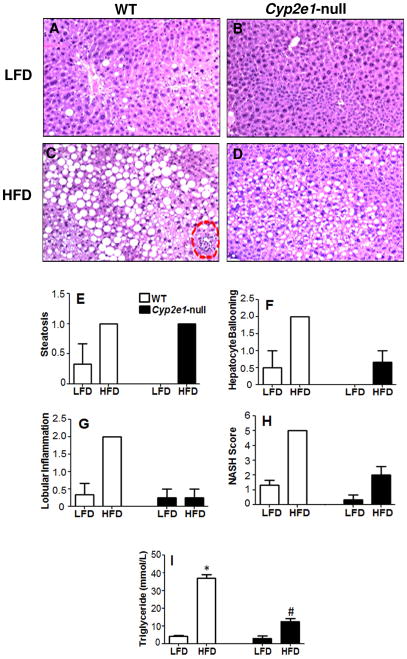
Livers of WT and Cyp2e1-null mice fed LFD look grossly (not shown) and histologically normal, although some occasional fat deposits were observed in the WT-LFD (Figs. 1A and B). In contrast, livers from both WT and Cyp2e1-null mice fed HFD exhibited much paler color compared with those of their corresponding LFD controls. Histological analysis (Figs. 1C and D) revealed accumulation of micro- and macro-vesicular intracellular lipid droplets in WT-HFD and to a lesser extent in Cyp2e1-null-HFD mice and the degrees of steatosis were markedly higher than their corresponding LFD groups (Figs. 1A–D,I). However, several inflammatory foci were monitored only in WT-HFD group (Fig. 1C). According to the histological scoring system for NAFLD which reflects the sum of steatosis, hepatocytes ballooning, and lobular inflammation [14], only the WT-HFD group achieved a NAS of 5 (Fig. 1E–H). These results were supported by the measurement of hepatic triglyceride (TG) contents where TG content in HFD-WT was the highest and significantly different from those of all other groups (Fig. 1I).
Fig. 1. Effects of LFD or HFD on hepatic steatosis and inflammation.
Photomicrographs (200×) following H&E staining from the indicated mouse livers are presented: (A) WT fed LFD, (B) Cyp2e1-null fed LFD, (C) WT fed HFD (necro-inflammatory foci shown with circular broken lines) (D) Cyp2e1-null fed HFD. Individual scores of hepatic steatosis (E), hepatocyte ballooning (F), lobular inflammation (G), NAFLD histological scoring system (H), and hepatic triglyceride levels (I) were tabulated for all four groups where WT-fed HFD was the only group to achieve a NAS of 5.
Effects of HFD on body weight, ratios of liver to body weights, and liver transaminases
Weight gains in both HFD-WT and HFD-Cyp2e1-null were significantly greater than their corresponding LFD-fed groups (P < 0.05) (Supplementary Fig. 1A). However, the ratios of liver to total body weight (liver index) were not significantly increased (Supplementary Fig. 1B) in any of the four groups despite the fact that histological analysis revealed that livers from both HFD-fed WT and Cyp2e1-null developed massive steatosis (Fig. 1). Plasma ALT and AST activities did not differ between WT and Cyp2e1-null groups regardless of different diets (Supplementary Fig. 1C), as previously described [10,11].
Evaluation of different oxidative stress and inflammation parameters
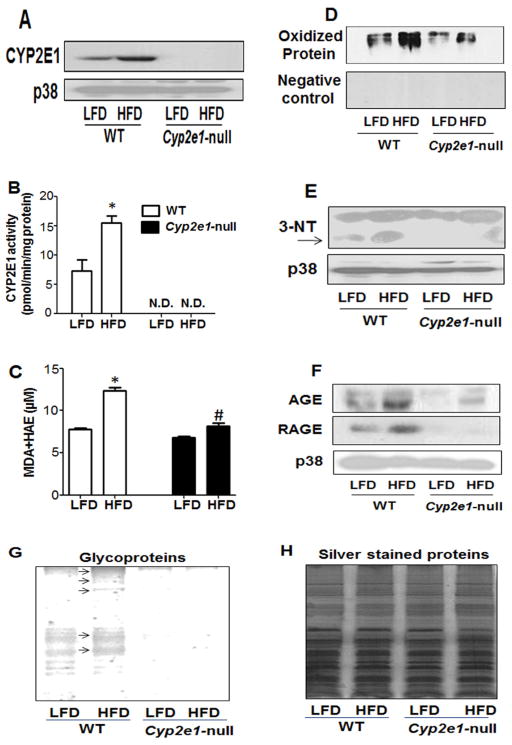
Elevated CYP2E1 is a marker for increased oxidative stress and is involved in the biotransformation of many small molecular weight xenobiotics and endogenous substrates such as fatty acids [7–9]. Furthermore, its activity and protein increased in response to HFD [7,10,11,17]. Thus, we evaluated whether HFD increased the activity of CYP2E1 in the four mouse groups. Indeed, both CYP2E1 activity and protein levels were significantly increased in the WT-HFD group compared to its corresponding LFD-fed group, while CYP2E1 was not detectable in Cyp2e1-null mice (Figs. 2A and B). p38 was used as a loading control since we observed that the levels of β-actin, glyceraldehyde 3-phosphate dehydrogenase, and tubulin are all fluctuated in response to the HFD (not shown). Staining with Coomassie blue also verified similar protein loading for all samples (not shown).
Fig. 2. Changes in the levels of CYP2E1 protein levels, CYP2E1 activity, MDA+HAE concentration, hepatic protein oxidation, nitration and glycosylation parameters.
(A–C) Equal amounts of whole liver lysates from different groups were used to determine: (A) CYP2E1 protein levels (upper panel) and p38 as a loading control (lower panel) by immunoblot analysis; (B) CYP2E1 activity evaluated by measuring the rate of PNP oxidation to p-nitrocatechol, and (C) hepatic MDA+HAE as a marker for lipid peroxidation. *Significantly different from all other groups. N.D.: not detected. (D) Protein oxidation was determined using Oxyblot analysis of total cells lysates from all four groups (upper panel) or negative control (lower panel). (E) Equal amounts of whole liver lysates (40 μg/well) from different groups were used to determine protein nitration using anti-3-NT antibody (upper panel) or p38 as a loading control (lower panel). (F) Total cell lysates were used to evaluate the levels of AGE (top), RAGE (middle), or p38 as a loading control (bottom) from all four groups using immunoblot analysis. (G) Whole liver homogenates from different groups were used to determine the levels of glycoproteins (arrows indicate increased levels of glycoproteins). (H) Equal protein loading for the samples analyzed for glycoproteins was verified by staining with silver.
In addition, as illustrated in Fig. 2C, MDA+HAE levels increased in both the WT and Cyp2e1-null groups fed HFD compared to their LFD corresponding groups; however the increased levels of lipid peroxidation were also significantly higher in the WT-HFD compared with the null-HFD (Fig. 2C). Furthermore, Oxyblot analysis demonstrated that the levels of oxidized proteins (Fig. 2D upper panel) were markedly increased in the WT-HFD groups compared to those of the other three groups. The specificity of the oxidized protein bands was confirmed by using 1 derivatization Control Solution (Fig. 2D, lower panel) where no bands were detected. Immunoblot analysis revealed that the levels of 3-NT proteins, as a marker for nitrosative stress, were prominently higher in the WT-HFD group compared to those of the other three groups (Fig. 2E, upper panel), while p38 bands used as the loading control (Fig. 2B, lower panel) were similar in all groups.
Advanced glycation end products (AGEs) and their receptor (RAGE) have also been suggested to contribute to the pathogenesis of NASH in HFD-fed rats [18,19]. Also, oxidative stress has been reported to increase glycated protein levels [20]. As shown in Fig. 2F, the levels of AGE (top) and RAGE (middle) were evidently higher in the WT-HFD group compared to those of the other three groups, while p38 (bottom) and Coomassie staining (not shown) verified similar protein loading for all samples. In addition, staining for glycoproteins (Fig. 2G) and silver staining (Fig. 2H) confirmed that HFD increased the levels of glycoproteins only in WT-HFD. In fact, very few glycoproteins were detected in the Cyp2e1-null mouse groups, suggesting an important role of CYP2E1 in protein glycosylation/glycation.
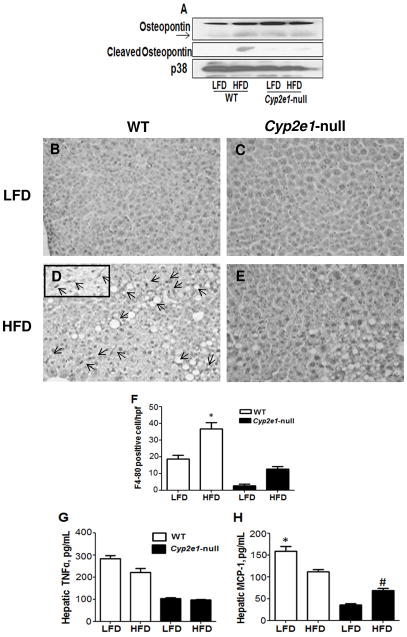
Since the inflammatory marker OPN, the pro-inflammatory cytokine TNFα, and the marker Kupffer cells F4/80, play important roles in NASH [11,21–23], the levels of these proteins were evaluated in mice fed HFD. As shown in Fig. 3A, immunoblot analysis revealed that the cleaved (active form) OPN levels were only increased in the WT-HFD. Furthermore, quantitative immunohistochemistry analysis revealed that F4/80 positive cells were markedly increased in the WT-HFD compared with the other three groups (Fig. 3B–F). However, intrahepatic levels of TNFα and MCP-1 were lower in the WT-HFD than their corresponding WT-LFD groups, while intrahepatic MCP-1 increased in HFD-null group compared to their corresponding control (Fig. 3G and H). We did not detect significant changes in apoptosis among the groups evaluated by TUNEL assay, and the levels of pro-apoptotic proteins such as Bax, Bcl2, and cleaved caspase 3 (data not shown).
Fig. 3. Effects of HFD on the levels of OPN, F4/80, TNFα, and MCP-1.
(A) Whole liver lysates were used to evaluate the levels of OPN (upper panel), cleaved OPN (middle panel), or the loading control p38 (lower panel) by immunoblot analyses. (B–E) F4/80 positive cells were also determined in all four groups using immunohistochemistry. Arrows indicate F4/80 positive cells. (F) The number of F4/80 positive cells was evaluated in at least five high power fields (HPF) (inset in D). Intrahepatic levels of TNFα (G) and MCP-1 (H) determined by ELISA are shown. *Significantly different from all other groups for Figures (F,H). #Significantly different from corresponding null group for Figure (H).
IR Development
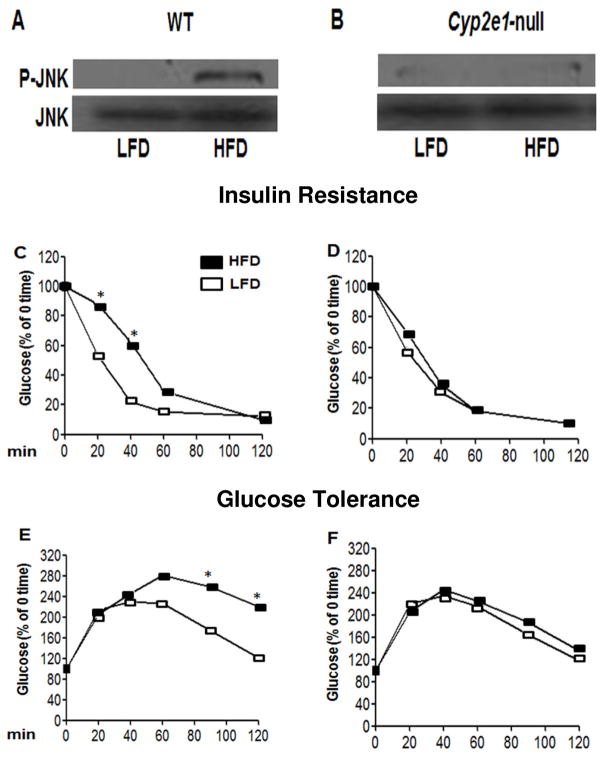
Activated (phosphorylated) JNK is partly responsible for promoting IR in CYP2E1 over-expressed cells [12]. Our results revealed that WT-HFD mice with higher levels of hepatic phospho-JNK developed systemic IR and impaired GT compared to the WT-LFD group (Fig. 4A,C,E). In contrast, no significant differences were observed in JNK phosphorylation, insulin sensitivity, or GT between HFD-fed Cyp2e1-null mice compared to their corresponding LFD-exposed group (Fig. 4B,D,F).
Fig. 4. Activation of JNK, increased IR, and impaired GT in WT-FHD.
A,B) Whole liver lysates were used to evaluate the levels of P-JNK (top) and JNK (bottom) by immunoblot analyses. C,D) IR was evaluated by injecting insulin (ip, 0.75 U/kg; Eli Lilly), while GT was determined by injecting glucose (ip, 2 g/kg) into each mouse fasted for 6 hours. Tail blood from each mouse was collected at indicated times following insulin or glucose injection. Each point represents the average value (C–F). *Significantly different from the corresponding time-course groups.
Discussion
Obesity and exogenous HFD are major risk factors for NAFLD development [1,10,11]. Thus, we used solid HFD (60% energy-derived from fat) to simulate the human conditions [10].
CYP2E1 produces high levels of ROS [7–9] and plays a role in the formation of peroxynitrite [16,24]. The pathological role of CYP2E1 in the development of both alcoholic steatohepatitis [25] and NASH [26,27] has been suggested and thus our main aim was to evaluate the hypothesis that HFD-exposed Cyp2e1-null mice are less susceptible to NASH compared with WT-HFD. Only the WT-HFD group achieved a NAS of 5, which is qualified as NASH [14], because of higher levels of TG and lobular inflammation in WT-HFD than the null-HFD. No significant differences in the ratios of liver to body weights and plasma transaminase levels were observed among all four groups. It was previously reported that an underlying hepatic disorder can exist without the concomitant elevation of liver transaminases [10,11,28]. Thus, the presence of CYP2E1 is important in NASH development in WT-HFD. This result is consistent with a previous study that Cyp2e1-null mice are less vulnerable to alcoholic fatty liver [29].
Hepatic steatosis increases the liver susceptibility to more serious insults like inflammation and increased oxidative/nitrosative stress, especially in the presence of oxidizable unsaturated fatty acids in steatotic livers [1,11]. The oxidation of unsaturated fatty acids can increase lipid peroxidation, which can activate hepatic stellate cells thus expediting the development of fibrosis [1,25]. Fatty acids upregulate both CYP2E1 mRNA and protein levels in cell culture models and that HFD increased CYP2E1 levels in the rat livers [7,17]. Furthermore, using ob/ob mice, obesity-mediated development of oxidative stress and liver injury were shown to be enhanced by CYP2E1 induction [30]. We also witnessed that both CYP2E1 activity and protein amounts were increased remarkably only in HFD-WT mice. This result suggests that the presence higher levels of oxidative/nitrosative stress in the WT-HFD group compared to the other groups since CYP2E1 is known to increase the generation of both ROS and RNS levels [8–10,16], leading to increased production of peroxynitrite, which can modify the function of various proteins [31]. Consistent with this view, we found that the lipid peroxidation levels and oxidized and nitrated proteins were highest in WT-HFD mice. Oxidative/nitrosative events are well-documented to cause DNA damage and oxidative post-translational modifications of Cys-residues of cellular proteins [15,31,32], which negatively affect essential functions essential proteins prior to the observation of full-blown liver diseases [15,32]. Earlier studies revealed that the amounts of nitrated proteins, marker of NO-dependent nitrosative stress, were increased in the livers of obese ob/ob mice [33], explaining a potential role of 3-NT in causing the low mitochondrial respiratory chain activity in patients suffering from NASH. Our data also showed that nitrated protein levels are remarkably increased in the WT-HFD group compared to the other three groups (Fig. 2), suggesting that elevated levels of oxidative/nitrosative stress caused by, but may not be limited to CYP2E1, have increased protein oxidation and nitration, both of which might have also contributed to the progression to NASH development following steatosis.
Protein glycation and its markers namely AGEs and their receptor RAGE, were reported to increase in rat fed HFD, contributing NASH pathogenesis [18,19]. Based on this information and the fact that liver is the main organ to clear AGEs, it was logical to evaluate protein glycation/glycosylation in the present model. Indeed, the WT-HFD group showed markedly higher levels of AGEs, RAGE, and glycoproteins compared to the other three groups (Fig. 2). These results suggest that protein glycation/glycosylation may also play a role in the faster development of NASH in the WT-HFD mice and that the absence of CYP2E1 ameliorates/attenuates the AGEs formation. Thus, the second hit in the NASH [3] represents increased lipid peroxidation, protein oxidation, nitration, and glycation/glycosylation in the WT-HFD (Supplementary Fig. 2).
ROS/RNS, lipid peroxidation, and protein glycation likely increase the expression of pro-inflammatory cytokines and hence increase inflammation [1,34]. Obesity and HFD are also known to induce inflammatory cytokines and increase hepatic inflammation in steatotic livers [1 and references within]. Furthermore, CYP2E1 sensitizes hepatocytes to LPS, TNFα, and oxidative injury in a p38- and JNK-dependent fashion [35]. However, the roles of various cytokines and chemokines in NAFLD are complex and sometimes inconsistent depending on the stimulating agents, exposure times (i.e., sample collection times), and evaluation of mRNA or protein levels since they might give paradoxical results [36,37]. For instance, hepatic TNFα increased first after 1 and 2 weeks of exposure to methionine-choline deficient diet and then actually decreased at 4 and 8 weeks despite the high levels of TNFα mRNA and OPN throughout the feeding periods [37]. In addition, F4/80 and high hepatic inflammatory foci have been found to be remarkably high in Mcp-1-null mice [38]. In the current model, we observed decreased intrahepatic levels of TNFα and MCP-1 in HFD-WT than the corresponding LFD-WT mice despite increased amounts of OPN and F4/80. These data suggest that there is a temporal change in the levels of hepatic cytokines in response to HFD where the decreased levels of TNFα and MCP-1 might actually follow earlier increases, as previously reported [37]. Furthermore, this temporal response in the liver may also differ from other organ response (e.g. fat tissues). Thus, the temporally different responses to HFD in liver, fat tissues, and muscles for oxidative stress and inflammation development should be further investigated to understand the underlying pathophysiological process.
Obesity, NAFLD, NASH, and HFD were reported to contribute to high incidence of IR which may worsen the NASH condition, mainly due to increased levels of oxidative/nitrative stress and inflammation [1,7,39]. Furthermore, it was shown that overexpression of hepatic CYP2E1 both in vitro and in vivo promotes the development of hepatic IR partly through activation (phosphorylation) of JNK [12,40]. Thus we also evaluated whether CYP2E1 deletion would lead to the development of IR and impaired GT. Our data revealed that only WT-HFD, but not the other groups developed IR and impaired GT (Fig. 4), which supports the notion that increased CYP2E1 in HFD for an extended period likely promotes IR and that the absence or blocking of CYP2E1 may exert beneficial effects. During the final preparation of this manuscript, a detailed report showed the role CYP2E1 deletion in the development of IR in the same mice strain, same diet and very similar duration of feeding [41]. Pessin and colleagues demonstrated that, using euglycemic-hyperinsulinemic clamps, the Cyp2e1-null mice were protected substantially against HFD-induced hepatic IR and exhibited better sensitivity of adipose tissue glucose uptake with decrease of hepatic glucose output. Although we did not directly determine hepatic IR while we evaluated the development of systemic IR and GT, it is likely that the WT-HFD group in our study would also have developed earlier hepatic IR than the Cyp2e1-null mice, based on the massive fat accumulation, remarkably higher levels of oxidative stress, OPN, F4/80, and phospho-JNK, which eventually impairs insulin signaling. Nonetheless, with the development of systemic IR, fat accumulation and inflammation will even be greater in the already-primed liver of WT-HFD mice and would accelerate the NASH development, which is the main focus of this study (Supplementary Figure 2).
In summary, CYP2E1, which appears to be involved in fat accumulation in NAFLD, plays an important role in the advancement of steatotic livers to inflammatory NASH and IR development. This can be possibly explained by the fact that up-regulation of CYP2E1 leads to increased production of ROS/RNS, which likely increase lipid peroxidation, protein nitration, oxidation, glycation, with inflammation and the IR development, all of which can negatively affect the vital functions of the hepatocytes and ultimately lead to the development of NASH.
Supplementary Material
(A) The changes of body weight, measured before and after the exposure to LFD or HFD, are presented as a percentage of weight gain. (B) Immediately following euthanasia, the liver from each mouse was excised and immediately weighed. Liver weight ratios are presented as a percentage of the corresponding body weight for each group. (C) ALT and AST activities were monitored in plasma samples from each mice and presented. Data are expressed as mean ± SEM of 4–5 mice per group.
The first and second hits for the development of NASH in HFD-exposed WT are presented. CYP2E1 appears to have a critical role in fat accumulation and the development of NASH in HFD-fed WT mice through increased oxidative/nitrosative stress, lipid peroxidation, protein modifications, inflammation and insulin resistance.
Acknowledgments
Financial support
This research was supported by the Intramural Research Program of National Institute on Alcohol Abuse and Alcoholism.
We are thankful to Dr. Klaus Gawrisch for supporting this study.
Abbreviations
- AGE
advance glycation end products
- ALT
alanine aminotransferase
- AST
asparagine aminotransferase
- CYP2E1
cytochrome P450 2E1
- GT
glucose tolerance
- HAE
hydroxyalkenal
- HFD
high-fat diet
- HNE
4-hydroxynonenal
- IR
insulin resistance
- MDA
malondialdehyde
- NAFLD
nonalcoholic fatty liver diseases
- NAS
NAFLD histological scoring system
- NASH
nonalcoholic steatohepatitis
- 3-NT
3-nitrotyrosine
- null-HFD
Cyp2e1-null fed HFD
- null-LFD
Cyp2e1-null fed LFD
- OPN
osteopontin
- p38
p38 kinase
- PBS
phosphate buffered saline
- PNP
p-nitrophenol
- RAGE
receptor for advanced glycation end products
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TG
triglyceride
- TNFα
tumor necrosis factor-alpha
- WT
wild-type
- WT-HFD
wild-type fed HFD
- WT-LFD
wild-type fed LFD
Footnotes
Conflict of interest
The authors declared no conflict of interest.
Author’s contributions
Study Design and concept: Mohamed A. Abdelmegeed and BJ Song Analysis and interpretation of data: Mohamed A. Abdelmegeed, Atrayee Banerjee, Seong-Ho Yoo, Se-Hwan Jang, and BJ Song. Drafting and finalizing the manuscript: Mohamed A. Abdelmegeed, Frank J. Gonzalez, and BJ Song
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mohamed A. Abdelmegeed, Email: abdelmegeedm@mail.nih.gov.
Atrayee Banerjee, Email: atrayee.banerjee@nih.gov.
Seong-Ho Yoo, Email: yoosh@snu.ac.kr.
Sehwan Jang, Email: sehwan.jang@nih.gov.
Frank J. Gonzalez, Email: gonzalef@mail.nih.gov.
References
- 1.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 3.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 4.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377–3389. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 6.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 7.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 8.Song BJ, Koop DR, Ingelman-Sundberg M, Nanji A, Cederbaum AI. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20 (Suppl):138A–146A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 9.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 10.Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, et al. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 11.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 13.Lee SS, Buters JT, Pineau T, Fernandez-Salguero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 14.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 15.Abdelmegeed MA, Moon KH, Hardwick JP, Gonzalez FJ, Song BJ. Role of peroxisome proliferator-activated receptor-alpha in fasting-mediated oxidative stress. Free Radic Biol Med. 2009;47:767–778. doi: 10.1016/j.freeradbiomed.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun YP, Casazza JP, Sohn DH, Veech RL, Song BJ. Pretranslational activation of cytochrome P450IIE during ketosis induced by a high fat diet. Mol Pharmacol. 1992;41:474–479. [PubMed] [Google Scholar]
- 18.Wu J, Zhao MY, Zheng H, Zhang H, Jiang Y. Pentoxifylline alleviates high-fat diet-induced non-alcoholic steatohepatitis and early atherosclerosis in rats by inhibiting AGE and RAGE expression. Acta Pharmacol Sin. 2010;31:1367–1375. doi: 10.1038/aps.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura Y, Hyogo H, Yamagishi S, Takeuchi M, Ishitobi T, Nabeshima Y, et al. Atorvastatin decreases serum levels of advanced glycation endproducts (AGEs) in nonalcoholic steatohepatitis (NASH) patients with dyslipidemia: clinical usefulness of AGEs as a biomarker for the attenuation of NASH. J Gastroenterol. 2010;45:750–757. doi: 10.1007/s00535-010-0203-y. [DOI] [PubMed] [Google Scholar]
- 20.Cai Z, Zhao B, Ratka A. Oxidative stress and beta-amyloid protein in Alzheimer’s disease. Neuromolecular Med. 2011;13:223–250. doi: 10.1007/s12017-011-8155-9. [DOI] [PubMed] [Google Scholar]
- 21.Chapman J, Miles PD, Ofrecio JM, Neels JG, Yu JG, Resnik JL, et al. Osteopontin is required for the early onset of high fat diet-induced IR in mice. PLoS One. 2010;5:e13959. doi: 10.1371/journal.pone.0013959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stienstra R, Mandard S, Patsouris D, Maass C, Kersten S, Muller M. Peroxisome proliferator-activated receptor alpha protects against obesity-induced hepatic inflammation. Endocrinology. 2007;148:2753–2763. doi: 10.1210/en.2007-0014. [DOI] [PubMed] [Google Scholar]
- 23.Matono T, Koda M, Tokunaga S, Kato J, Sugihara T, Ueki M, et al. Therapeutic effects of ezetimibe for non-alcoholic steatohepatitis in fatty liver shionogi-ob/ob mice. Hepatol Res. 2011;41:1240–1248. doi: 10.1111/j.1872-034X.2011.00888.x. [DOI] [PubMed] [Google Scholar]
- 24.Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- 25.Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517–544. doi: 10.1152/physrev.1997.77.2.517. [DOI] [PubMed] [Google Scholar]
- 26.Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, et al. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raucy JL, Lasker JM, Kraner JC, Salazar DE, Lieber CS, Corcoran GB. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–280. [PubMed] [Google Scholar]
- 28.Calvaruso V, Craxi A. Implication of normal liver enzymes in liver disease. J Viral Hepat. 2009;16:529–536. doi: 10.1111/j.1365-2893.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 30.Dey A, Cederbaum AI. Induction of cytochrome P450 2E1 promotes iver injury in ob/ob mice. Hepatology. 2007;45:1355–1365. doi: 10.1002/hep.21603. [DOI] [PubMed] [Google Scholar]
- 31.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, et al. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Ruiz I, Rodriguez-Juan C, Diaz-Sanjuan T, del Hoyo P, Colina F, Munoz-Yague T, et al. Uric acid and anti-TNF antibody improve mitochondrial dysfunction in ob/ob mice. Hepatology. 2006;44:581–591. doi: 10.1002/hep.21313. [DOI] [PubMed] [Google Scholar]
- 34.Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293. doi: 10.1038/ncpendmet0786. [DOI] [PubMed] [Google Scholar]
- 35.Wu D, Cederbaum A. Cytochrome P4502E1 sensitizes to tumor necrosis factor alpha-induced liver injury through activation of mitogen-activated protein kinases in mice. Hepatology. 2008;47:1005–1017. doi: 10.1002/hep.22087. [DOI] [PubMed] [Google Scholar]
- 36.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18:727–735. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahai A, Malladi P, Melin-Aldana H, Green RM, Whitington PF. Upregulation of osteopontin expression is involved in the development of nonalcoholic steatohepatitis in a dietary murine model. Am J Physiol Gastrointest Liver Physiol. 2004;287:G264–273. doi: 10.1152/ajpgi.00002.2004. [DOI] [PubMed] [Google Scholar]
- 38.Kassel KM, Guo GL, Tawfik O, Luyendyk JP. Monocyte chemoattractant protein-1 deficiency does not affect steatosis or inflammation in livers of mice fed a methionine-choline-deficient diet. Lab Invest. 2010;90:1794–1804. doi: 10.1038/labinvest.2010.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Millonig G, Nair J, Patsenker E, Stickel F, Mueller S, et al. Ethanol-induced cytochrome P4502E1 causes carcinogenic etheno-DNA lesions in alcoholic liver disease. Hepatology. 2009;50:453–461. doi: 10.1002/hep.22978. [DOI] [PubMed] [Google Scholar]
- 40.Kathirvel E, Morgan K, French SW, Morgan TR. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:973–983. doi: 10.1097/MEG.0b013e328328f461. [DOI] [PubMed] [Google Scholar]
- 41.Zong H, Armoni M, Harel C, Karnieli E, Pessin JE. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and IR. Am J Physiol Endocrinol Metab. 2012;302:E532–539. doi: 10.1152/ajpendo.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The changes of body weight, measured before and after the exposure to LFD or HFD, are presented as a percentage of weight gain. (B) Immediately following euthanasia, the liver from each mouse was excised and immediately weighed. Liver weight ratios are presented as a percentage of the corresponding body weight for each group. (C) ALT and AST activities were monitored in plasma samples from each mice and presented. Data are expressed as mean ± SEM of 4–5 mice per group.
The first and second hits for the development of NASH in HFD-exposed WT are presented. CYP2E1 appears to have a critical role in fat accumulation and the development of NASH in HFD-fed WT mice through increased oxidative/nitrosative stress, lipid peroxidation, protein modifications, inflammation and insulin resistance.