Abstract
The present review examines the role of perirhinal cortex (PRC) in Pavlovian fear conditioning. The focus is on rats, partly because so much is known, behaviorally and neurobiologically, about fear conditioning in these animals. In addition, the neuroanatomy and neurophysiology of rat PRC have been described in considerable detail at the cellular and systems levels. The evidence suggests that PRC can serve at least two types of mnemonic functions in Pavlovian fear conditioning. The first function, termed "stimulus unitization," refers to the ability to treat two or more separate items or stimulus elements as a single entity. Supporting evidence for this perceptual function comes from studies of context conditioning as well as delay conditioning to discontinuous auditory cues. In a delay paradigm, the conditional stimulus (CS) and unconditional stimulus (US) overlap temporally and co-terminate. The second PRC function entails a type of "transient memory." Supporting evidence comes from studies of trace cue conditioning, where there is a temporal gap or trace interval between the CS offset and the US onset. For learning to occur, there must be a transient CS representation during the trace interval. We advance a novel neurophysiological mechanism for this transient representation. These two hypothesized functions of PRC are consistent with inferences based on non-aversive forms of learning.
Keywords: unitization, trace conditioning, transient memory, persistent firing, muscarinic receptors
The present review brings together contemporary data and hypotheses pertinent to the role of perihinal cortex (PRC) in Pavlovian fear conditioning to cues and contexts. The focus is on rodents, partly because so much is known, behaviorally and neurobiologically, about fear conditioning in these animals (LeDoux, 2000; Maren, 2001; Kim & Jung, 2006). In addition, the neuroanatomy and neurophysiology of rat PRC have been described in considerable detail at the cellular and systems levels (Burwell, Witter, & Amaral, 1995; Liu & Bilkey, 1996; Brown & Xiang, 1998; Cousens & Otto, 1998; Faulkner & Brown, 1999; Beggs et al., 2000; Burwell, 2001; Moyer et al., 2002; McGann, Moyer, & Brown, 2001; Moyer & Brown, 2007; Furtak, Allen, & Brown, 2007a; Furtak, Moyer Jr, & Brown, 2007b; Furtak, Wei, Agster, et al., 2007c; Allen et al., 2007; Massey et al., 2008; Seoane et al., 2009; Kealy & Commins, 2011; Navaroli et al., 2012).
The experiments reviewed below suggest that PRC serves two types of mnemonic functions in Pavlovian fear conditioning. The first function, termed "stimulus unitization," refers to the ability to treat two or more separate items or stimulus elements as a single entity. Stimulus unitization is proposed to be necessary for fear conditioning to complex stimuli (Kholodar-Smith, Allen, Brown, 2008a; Bang & Brown, 2009a). The second suggested PRC function entails "transient memory" (Kholodar-Smith et al., 2008b; Bang and Brown, 2009b; Navaroli et al., 2012). We use this term rather than "working memory" because the latter has additional connotations (Baddeley, 1992). This transient-memory function is discussed at the molecular, cellular, and systems levels. Before proceeding to the evidence for these dual functions, we first introduce some pertinent terms, paradigms, and stimuli that will be used in describing the conditioning paradigms.
Fear conditioning terms, paradigms, and stimuli
The data reviewed here are based on both cue and context conditioning. In cue conditioning, the onset of a previously-neutral stimulus (the conditional stimulus or CS) precedes and predicts the onset of an aversive event (the unconditional stimulus or US; Fig. 1). Neurobiological studies of cued fear conditioning in rats usually employ auditory rather than visual CSs, for several reasons. Rats are nocturnal and, not surprisingly, their visual perception is limited (Prusky et al., 2002). For this reason, visual tasks might not illuminate high-level PRC functions in rats. Furthermore, some of the most extensively-studied strains of rats are albinos, which have extremely poor vision (Prusky et al., 2002).
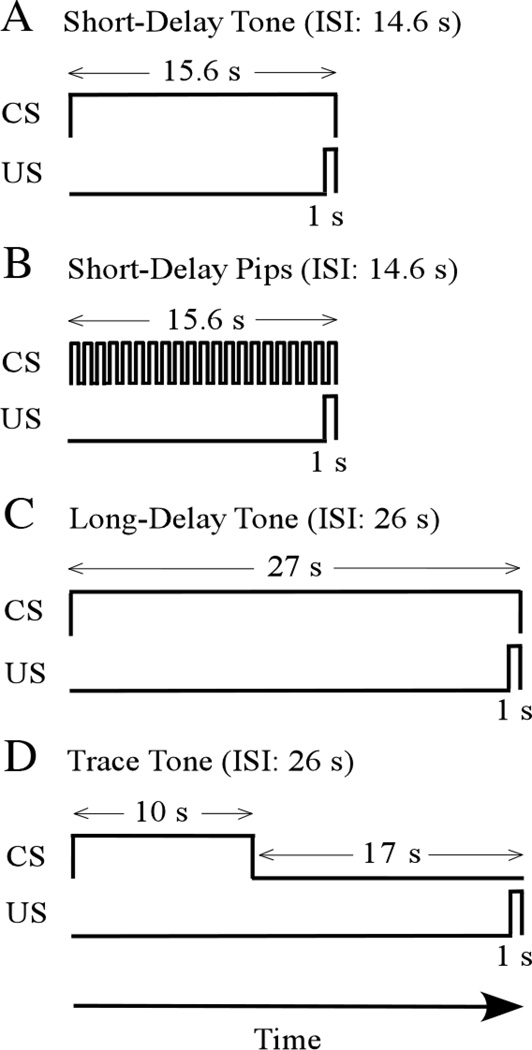
Figure 1.
Representative examples of temporal relationships between the conditional stimulus (CS) and the unconditional stimulus (US). A. Short-delay conditioning to a continuous tone CS. The CS duration is 15.6 s, the interstimulus interval (ISI) is 14.6 s, and the US duration is 1 s. Note that the CS and US overlap temporally. B. Short-delay conditioning to a discontinuous tone (termed "Pips"). The only difference from A is that the CS is discontinuous. C. Long-delay conditioning to a continuous tone. The CS duration is 27 s, the ISI is 26 s, and the US duration is 1 s. Again, the CS and US overlap temporally. D. Trace conditioning to a continuous tone. The CS duration is 10 s, the ISI is 26 s, and the US duration is 1 s. The CS and US do not overlap temporally. Instead, a 16 s "trace interval" separates the CS offset from the US onset. Note that the ISI is the same in parts C and D. Modified from Bang and Brown (2009b).
In contrast, hearing in albinos seems normal and, like pigmented rats, includes an impressive frequency range (Heffner et al., 1994). Importantly, rat PRC receives a major input from auditory cortex and it has reciprocal connections with the amygdala (Deacon et al., 1983; Burwell & Amaral, 1998; Shi & Cassell, 1999; Pitkänen et al., 2000; Burwell, 2000; Furtak et al., 2007c). Furthermore, auditory communication plays an absolutely-essential role in the social organization of rodents (Brudzynski, 2007; Wohr & Schwarting, 2007).
Cue-conditioning paradigms are defined in terms of temporal relationships between the CS and US. In a typical "delay" cue-conditioning paradigm, the CS onset precedes the US onset, the CS and US briefly overlap (co-occur), and they co-terminate (Fig. 1A–C). By contrast, in a "trace" cue-conditioning paradigm, a stimulus-free interval always separates the offset of the CS from the onset of the US (Fig 1 D). In trace conditioning, unlike delay conditioning, there is no time period during which the CS and US co-occur. Trace conditioning therefore requires some type of transient memory to bridge the temporal gap between the CS offset and the US onset. Studies of humans lead to the conclusion that trace, but not delay, conditioning engages cognitive processes and requires conscious awareness of the conditioning paradigm (Clark & Squire, 1998; Manns, Clark, & Squire, 2000; Smith et al., 2005). Subsequent evidence, however, shows that awareness can play a decisive role in both delay and trace conditioning (Lovibond et al., 2011).
Using either a delay or trace paradigm, cue conditioning typically involves several pairings of the CS and US. Representative time scales for these CS-US pairings are illustrated in Figure 1. Following these pairings, successful cue conditioning is revealed by showing that the presentation of the previously-neutral CS elicits a fearful conditional response (CR), typically measured in terms of freezing behavior. Several fear-related CRs are highly correlated with freezing behavior, both within and across experimental conditions (Lee et al, 2001; Choi & Brown, 2003). The CS-US inter-stimulus interval (ISI) is the time between the onset of the CS and the onset of the US. Figure 1 illustrates two representative ISIs (14.6 s and 26 s).
Cue conditioning is normally evaluated in a shifted context because the cue-conditioning procedure also causes context conditioning, which entails fear of the context in which the cue conditioning occurred. The shifted context may include changes in visual, tactile, auditory, and olfactory stimuli. Context conditioning is evaluated by returning the animals to the original cue-conditioning context without presenting the cue. The sequence of testing cue and context conditioning is normally counterbalanced. Context conditioning can also be induced in the absence of cue conditioning simply by presenting the US in a particular context. In the studies reviewed below, the US is always a shock, usually to the feet.
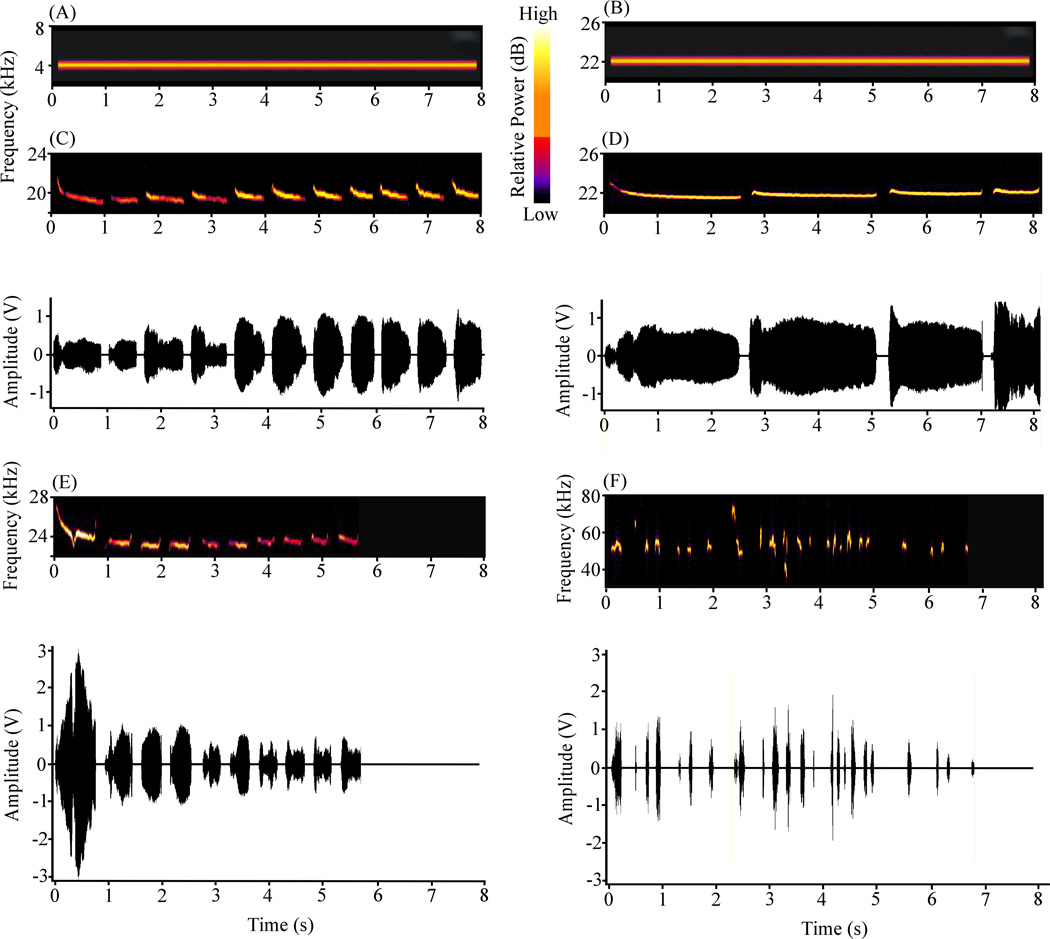
Figure 2 shows six examples of auditory CSs that have been used to study fear conditioning. Synthetic cues are usually continuous tones, traditionally within the frequency-range of human hearing (Fig. 2A), but sometimes at ultrasonic frequencies (Fig. 2B). Recent experiments have also examined fear conditioning to rat ultrasonic vocalizations (USVs; Allen, Furtak, & Brown, 2007; Endres et al., 2007; Furtak, Allen, & Brown, 2007a; Bang & Brown, 2009a,b; Kholodar-Smith et al., 2008a,b; Kim et al., 2010; Parsana et al., 2012a,b), which are ethologically-essential social signals (Brudzynski, 2007; Wohr & Schwarting, 2007, 2010). Parts C–F of Figure 2 show frequency spectrograms and amplitude plots of four different USVs. Three of these are "22 kHz USVs" (Fig. 2 C–E) and one is a "50 kHz USV" (Fig. 2F). The illustrated USVs are typical in that they contain temporal discontinuities and frequency and amplitude modulations. Discontinuous tones (termed "pips") have also been used as CSs. A schematic example of a 15.6 s discontinuous tone is shown in Figure 1B.
Figure 2.
Spectrograms and amplitude plots of six stimuli that have been used as CSs in fear conditioning. (A) Continuous 4 kHz tone (7.91 s). (B) Continuous 22 kHz tone (7.91 s). (C) 19 kHz USV (7.91 s, 11 calls). (D) 22 kHz USV (8.13 s, 4 calls). (E) 23 kHz USV (5.71 s, 10 calls). All of these are conventionally termed "22 kHz USVs" even though the principle frequency may not be exactly 22 kHz. (F) 53 kHz USV (6.74 s, 26 calls). This is termed a "50 kHz USV" even though the principle frequency is slighter higher. The four USVs were recorded from different rats. From Bang et al. (2008).
PRC role in unitizing complex stimuli
In what follows, we trace the evolution of our current perspective that PRC plays an important role in stimulus unitization. We begin with the "complexity hypothesis" and end with the "unitization hypothesis." Along the way, we consider a series of experiments that were designed to probe the complexity hypothesis. These two hypotheses differ in focus, but the perspectives are not mutually-exclusive. The complexity hypothesis attempts to identify the class of stimuli that cause conditioning to be PRC-dependent, whereas the unitization hypothesis focuses on the nature of the operation that PRC performs on such stimuli.
At the start of the 21st century, rat PRC was seen to be essential for normal fear conditioning to "complex" stimuli but not "simple" stimuli (Yaniv et al., 2004; Lindquist et al., 2004). Supporting evidence comes from the finding that PRC damage severely impairs fear conditioning to contexts (see Corodimas & LeDoux, 1995; Sacchetti et al., 1999; Bucci, Phillips, Burwell, 2000; Lindquist et al., 2004; Kholodar-Smith, Boguszewski, & Brown, 2008b; Kholodar-Smith, Allen, & Brown, 2008a; Bang & Brown, 2009a; Albrechet-Souza et al., 2011), while sparing delay fear-conditioning to continuous tones. This last claim is based on studies of delay fear conditioning to continuous tones at 0.8kHz (Romanski & LeDoux, 1992), 2 kHz (Campeau & Davis, 1995), 4 kHz (Lindquist et al., 2004; see Fig. 2A), 10 kHz (Bucci, Phillips, & Burwell, 2000), ~22 kHz (Kholodar-Smith, Allen, & Brown, 2008a; Lindquist et al., 2004), and 53 kHz (Bang & Brown, 2009a). In almost all of the fear-conditioning studies discussed here, the CR was freezing behavior.
The complexity hypothesis is appealing because it follows naturally from the fact that there are two neuroanatomical pathways through which information about the CS can reach the amygdala (AM), which is well-known to be essential for Pavlovian fear conditioning (Maren & Quirk, 2004; Poulos et al., 2009; Pape & Pare 2010; Johansen et al., 2011). One pathway entails a direct projection to AM from the thalamus (LeDoux, Farb, & Ruggiero, 1990; Bordi & LeDoux, 1994; Sacchetti et al., 1999; LeDoux, 2000). Obviously, complex percepts cannot be communicated through this subcortical route. For example, the thalamus cannot create unitized representations of contexts. There is general agreement that context conditioning requires cortical processing. However, the subcortical pathway is sufficient for fear conditioning to continuous tones at frequencies ranging from 800 Hz to 53 kHz.
A different interpretation of these same facts is that PRC supports context conditioning but not cue conditioning. This possibility was tested by examining the effects of PRC damage on delay fear conditioning to a 22 kHz ultrasonic social signal (Lindquist et al., 2004; Kholodar-Smith et al., 2008a). Ultrasonic vocalizations (USVs) can be regarded as "complex," relative to continuous tones, in that they consist of frequency modulations, amplitude modulations, and frank discontinuities (Fig. 2C–F). Twenty-two kHz USVs have been termed "alarm signals" (Blanchard et al., 1991) and are emitted in association with negative affective states (Brudzynski, 2007; Wohr & Schwarting, 2010; Parsana et al., 2012a,b).
USVs were selected as cues both because they are relatively complex and because they are natural social stimuli. It is easy to imagine that rat brains have evolved specialized neurophysiological circuitry to process these essential social signals. One potential complication is that these natural stimuli might not be "neutral" prior to fear conditioning. However, it is now clear that 22 kHz USVs do not elicit freezing in naive laboratory rats (Endres et al., 2007; Bang et al., 2008; Wohr & Schwarting, 2010; Kim et al., 2010; Parsana et al., 2012a,b). Furthermore, 22 kHz USVs are no more effective than continuous tones in supporting delay fear conditioning (Endres et al., 2007; Bang et al. 2008; Parsana et al., 2012a). Fear of 22 kHz USVs was recently hypothesized to be acquired through a novel learning mechanism termed "autoconditioning" (Kim et al., 2010; Parsana et al., 2012b), discussion of which is beyond the scope of this review. For reasons outlined elsewhere (Parsana et al., 2012b), autoconditioning is predicted to depend on PRC function.
Returning to the argument regarding stimulus complexity, if PRC is only important for context conditioning, then PRC damage should spare conditioning to USVs. On the other hand, if PRC is essential for conditioning to "complex" stimuli, then conditioning to a 22 kHz USV should also be impaired by PRC damage. Using freezing as a CR, two studies found that PRC damage severely impairs delay fear conditioning to 22 kHz USVs without affecting conditioning to a matched continuous tone (Lindquist et al., 2004; Kholodar-Smith et al., 2008a). Altogether, three different 22 kHz USVs were tested. In the first of these studies (Lindquist et al., 2004), aspirative PRC lesions were made through an opening in the lateral skull. Tissue was removed using a curved and blunted hypodermic needle that was attached to a vacuum pump. PRC removal impaired both context conditioning and delay conditioning to USVs, but had no significant effect on delay conditioning to continuous tones. Unfortunately, the lesions were larger than desired.
To improve on the precision of the lesions, the second study (Kholodar-Smith et al., 2008a) used neurotoxic lesions, which were produced by NMDA injections (8/side) into PRC, again using a lateral surgical approach. This method causes the most complete and selective PRC damage. During the procedure, PRC was directly visualized, which made it possible to position the injection needle accurately into PRC and to avoid damage to some of the larger blood vessels. Because of the short travel distances, there is no need for a guide cannula. A similar lateral approach has also been used for single-unit recordings from PRC (Allen et al., 2007; Furtak et al., 2007a). Three-dimensional reconstructions revealed extensive damage along the full anterior-posterior extent of PRC, including areas 35 and 36, with relatively little damage to nearby structures (Kholodar-Smith et al., 2008a). Control animals received comparable injections of the saline vehicle.
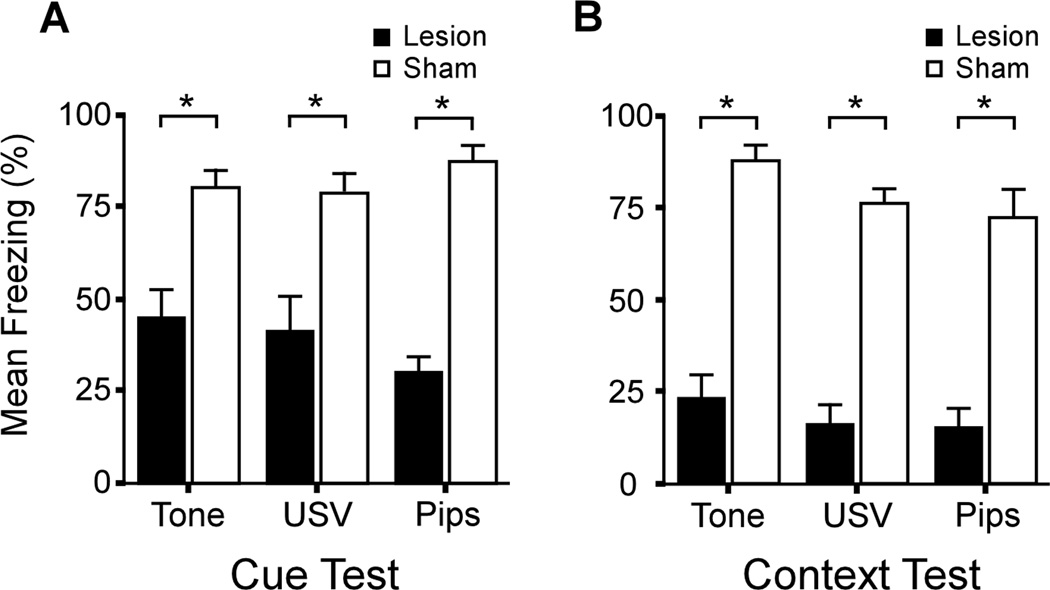
In addition to the goal of improving the size and shape of the PRC lesion, this study also evaluated which spectrotemporal sub-features of USVs determine whether fear conditioning is PRC-dependent. In principle, this could be any combination of principle frequency, amplitude modulations, frequency modulations, temporal discontinuities, or unidentified features. In agreement with the previous study, PRC damage significantly impaired conditioning to the USV (Kholodar-Smith et al., 2008a; Fig. 3A, labeled "USV"). As expected, the same lesions had no significant effect on conditioning to a continuous tone that matched the USV in terms of duration, loudness, and principle frequency (Fig. 3A, labeled "Tone"). The lesion effect size (Cohen's d, Cohen, 1988) for conditioning to the USV was 1.4. Predictably, PRC damage also severely impaired context conditioning (Fig 3B; mean d = 1.8). Thus, the results of both studies supported the complexity hypothesis.
Figure 3.
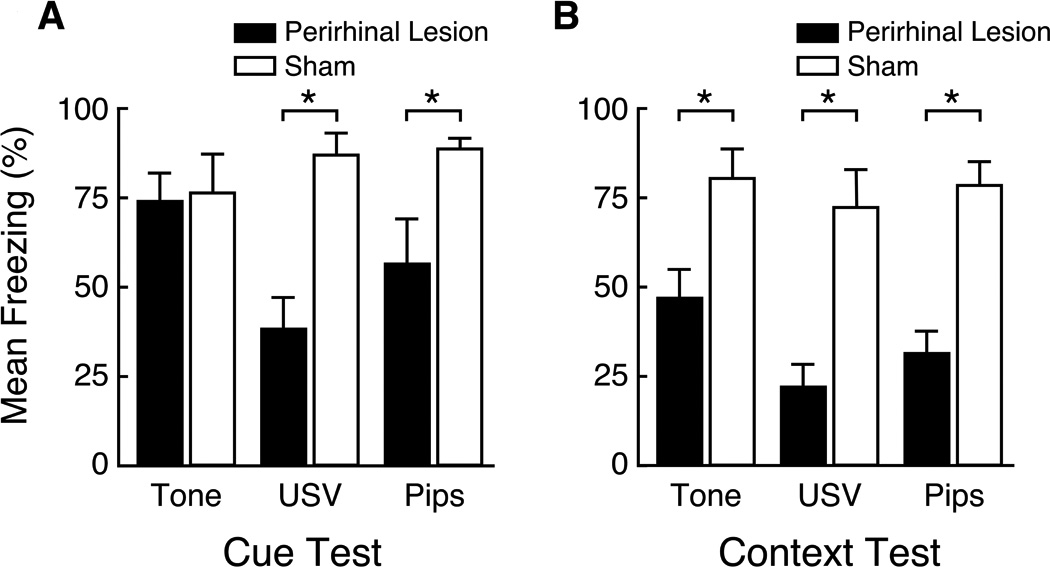
Neurotoxic PRC lesion effects on delay cue conditioning and context conditioning. Mean levels of freezing are shown in PRC-lesioned rats (solid bars) and sham operated controls (open bars). Asterisks denote significant differences between the sham-operated and PRC-lesioned rats (p < 0.05). Upward lines on the bars represent the standard error of the mean. A. Animals were conditioned to one of three CSs: a 19 kHz continuous tone (labeled Tone), a 19 kHz USV, or a 19 kHz discontinuous tone (labeled Pips). The USV is the one shown in Fig. 2C. After multiple CS-US pairings, all three cues elicited robust freezing in control animals. As expected, PRC damage had no significant effect on delay conditioning to the continuous tone. However, PRC damage significantly impaired conditioning to both the USV (Cohen's d = 1.4) and the discontinuous tone (d = 1.7). There were no significant differences between the USV and Pips groups. B. PRC damage profoundly impaired context conditioning in all three cue groups (mean d = 1.8). There were no significant differences among cue groups. Modified from Kholodar-Smith et al. (2008a).
Perhaps surprisingly, conditioning to the discontinuous tone was also severely impaired by PRC damage (Fig. 3A, Pips; d = 1.8). In fact, the effect of PRC damage on conditioning to the discontinuous tone was statistically indistinguishable from the effect on conditioning to the parent USV. This outcome left two possible interpretations. The first was that temporal discontinuity is the key stimulus attribute that causes conditioning to USVs to be PRC-dependent. The second possibility was that PRC is specifically required for fear conditioning to 22 kHz USVs and similar stimuli. PRC might house a "neural template" for 22 kHz USV-like stimuli (Wohr & Schwarting, 2010).
This second interpretation was tested by comparing conditioning to a 50 kHz USV, a frequency-matched continuous tone, and a frequency-matched discontinuous tone with the same on/off pattern as the USV (Bang & Brown, 2009a). In contrast to 22 kHz USVs, 50 kHz USVs are emitted in association with positive emotional states (Brudzynsky, 2007; Panksepp, 2007). They have been referred to as "rat laughter" (Panksepp, 2007). Figure 2F shows the spectrogram and amplitude plot of the 50 kHz USV that was used in this study. This 50 kHz USV is typical in that, compared to a 22 kHz USV (see Figs. 2C, D and E), the duty cycle is much smaller, the bandwidth is much larger, and, of course, the principle frequency is more than two-fold higher. In spite of these considerable physical differences, the results were the same. Neurotoxic PRC damage severely and comparably impaired fear conditioning to both the 50 kHz USV (d = 2.1) and the 50 kHz discontinuous tone (d = 1.9), but had no significant effect on conditioning to the 50 kHz continuous tone.
Thus, temporal discontinuity of the CS causes fear conditioning to require intact PRC function. Sub-features of the CS that seem unimportant in terms of this requirement include the principle frequency, bandwidth, duty cycle, amplitude modulations, and frequency modulations. These results immediately raise the question of why, in conceptual or computational terms, temporal discontinuity should determine the requirement for PRC function. What PRC-dependent operation could be pertinent to fear conditioning to temporally discontinuous sounds?
The proposed answer (Kholodar-Smith et al., 2008a; Bang & Brown, 2009a) was that PRC supports stimulus "unitization" or stimulus "binding," an experience-dependent form of perceptual learning. Specifically, PRC was suggested to be necessary for fusing discontinuous auditory segments into a unitary representation. The idea was that these discontinuous cues need to be unitized in order for effective fear conditioning to occur. Unitized representations of chunks of natural sounds have been termed "auditory objects" (see Griffiths & Warren, 2004). PRC-damaged rats were hypothesized to treat the discontinuous CSs as a set of statistically-unrelated, sub-second, sound segments; a circumstance that was argued to be unfavorable for fear conditioning (Bang & Brown, 2009a).
Because all natural sounds contain discontinuities and modulations, mechanisms have evolved to recognize and classify chunks of spectrotemporal patterns. Our interpretation is that rat PRC functions as an advanced stage in this auditory pattern analysis, at a point when certain spectrotemporal patterns are represented as auditory objects (Kholodar-Smith et al., 2008a; Bang and Brown, 2009a). In terms of auditory fear conditioning, PRC may serve as the most advanced stage in the ventral auditory stream (see Murray & Bussey; 1999; Bussey & Saksida, 2007; Murray and Wise, this volume). Of course, it is possible that auditory unitization occurs, partly or completely, in auditory cortex. In either case, PRC is proposed to play a pivotal role in maintaining and distributing this information to the amygdala and other structures that are essential for certain aspects or types of fear conditioning. Since PRC is a high-level polymodal cortex, one expects some integration of auditory and non-auditory stimuli, along with contextual representations. Indeed, some PRC neurons may encode an object in a particular place (Burke et al., this volume).
Recall that PRC damage reliably and severely impairs context conditioning (see Figs. 3b and 4b), a fact that was previously discussed in relationship to the stimulus-complexity hypothesis. However, in terms of understanding PRC function, the deficits in context conditioning are better understood in terms of stimulus unitization. Fear conditioning to a context is presumed to require a unitized representation that distinguishes that particular context (Fanselow, 1986; Rudy & O’Reilly, 1999; Anagnostaras, Gale, & Fanselow, 2001; Rudy, Huff, & Matus-Amat, 2004; Kholodar-Smith et al., 2008a).
Figure 4.
Neurotoxic PRC lesion effects on trace cue conditioning and context conditioning. Mean levels of freezing are shown in PRC-lesioned rats (solid bars) and sham operated controls (open bars). Asterisks denote significant differences between the sham-operated and PRC-lesioned rats (p < 0.05). Upward lines on the bars represent the standard error of the mean. A. Animals were conditioned to one of three CSs: a 23 kHz continuous tone (labeled Tone), a 23 kHz USV, or a 23 kHz discontinuous tone (labeled Pips). Following multiple CS-US pairings, all three cues elicited robust freezing in control animals. However, PRC lesions significantly impaired conditioning to all three cues (mean d = 2.7). The type of cue had no significant effect on freezing. (B) PRC damage profoundly impaired context conditioning in all three cue groups (mean d = 1.8). There were no significant differences among cue groups. From Kholodar-Smith et al. (2008b).
The well-known "immediate-shock deficit" (Fanselow, 1986; Landeira-Fernandez et al. 2006) is generally interpreted in terms of stimulus unitization or a related concept. The basic finding is that rats do not show contextual fear conditioning if they are shocked immediately after being placed in a context. By contrast, if the shock is delayed sufficiently, context conditioning reliably occurs. The theory is that context conditioning requires a unitized representation of the context, the creation of which takes time. Our conclusion is that the effect of PRC damage on fear conditioning to both cues and contexts can be easily accommodated by the stimulus unitization hypothesis.
This conclusion accords with a leading hypothesis that has emerged from studies that have used non-aversive paradigms. This hypothesis proposes that PRC is responsible for encoding conjunctions among sensory features (Bussey & Saksida, 2007; Murray et al., 2007; Murray & Wise, this volume). Indeed, numerous studies have shown that PRC is essential for tasks, such as object recognition, that require a unitized stimulus representation (Eichenbaum et al., 1996; Murray & Bussey, 1999; Brown & Aggleton, 2001; Petrulis & Eichenbaum, 2003; Norman & Eacott, 2004; Barense et al., 2005; Buffalo, Bellgowan, & Martin, 2006; Taylor et al., 2006; Winters, Saksida, & Bussey, 2006; Bartko et al., 2007; Murray, Bussey, & Saksida, 2007; Haskins et al., 2008; Brown & Eldridge, 2009; Parsana & Brown, 2010; Murray & Wise, this volume). Interestingly, individual PRC neurons in macaques have been reported to abstract a unitized representation of paired stimuli (Fujimichi et al., 2010). Murray and Wise (this volume) have recently written an excellent review of the evolution of current thinking regarding the perceptual functions of PRC.
PRC role in transient memory
The second suggested PRC function in fear conditioning involves the ability to maintain a neural representation of a stimulus after that stimulus is no longer physically present. This transient-memory function was identified using a "trace" fear-conditioning paradigm, an example of which is shown in Figure 1 (part D). In this Pavlovian paradigm, a temporal gap or "trace interval" separates the offset of the CS from the onset of the US (Fig. 1D). In the example illustrated in Figure 1D, the trace interval is 16 s. Successful trace fear conditioning requires that a neural representation of the CS persist until the onset of the US. Otherwise, there is no known mechanism by which an associative modification can occur.
The effects of neurotoxic PRC lesions, performed using the lateral surgical approach, were examined using a 16 s trace interval (Kholodar-Smith et al., 2008b), shown in Figure 1D. Three CSs were examined: a 10-call 22 kHz USV (USV group), a matched discontinuous tone (Pips group), and a matched continuous tone (Tone group). The neurotoxic lesions were relatively complete and selective. PRC removal caused a severe impairment in trace fear conditioning, measured by CS-elicited freezing behavior, in all three cue groups (Fig. 4A). The lesion effect sizes for the three cue groups were as follows: USV (d = 1.7); Tone (d = 1.9); and Pips (d = 4.6). There were no significant differences among the cues in terms of elicited freezing. As anticipated from the previous results, the lesions also impaired context conditioning (Fig. 4B; mean d = 3.9).
The concept of stimulus unitization is not useful in explaining the PRC lesion-produced deficit in trace conditioning. Trace conditioning is not conceived as causing the CS and US to become unitized. An intense flash of lightning may reliably predict subsequent thunder, but the two events remain distinct in perception and memory. The explanation of the deficit presumably involves the disruption of a PRC-dependent transient-memory function. We suggest that this function is not localized within PRC, but is supported by a spatially-distributed system that includes several, reciprocally-connected, brain regions.
Diverse ideas exist regarding the neurophysiological basis of transient memory (Durstewitz et al., 2000; Major & Tank, 2004; Teramae & Fukai, 2005; Fransen et al., 2006; Mongillo et al., 2008). Most of these can be broadly divided into two types, which are not mutually exclusive (Navaroli et al, 2012). The most common theory imagines that transient memory is supported by re-circulating activity within recurrent networks of neurons. Other theories have emphasized non-synaptic mechanisms that are intrinsic to individual neurons.
In the latter category, one interesting candidate mechanism is the phenomenon known as "endogenous persistent firing" (EPF), which was first described in brain slices of the entorhinal cortex (EC; Egorov et al., 2002; Fransen et al., 2006; Tahvildari et al., 2007) and the lateral nucleus of the amygdala (LA; Egorov et al., 2006). EPF neurons continue to fire action potentials long after the termination of the original, spike-eliciting, current. In some neurons, the capacity to exhibit EPF depends on the activation of muscarinic cholinergic receptors (mAChRs). The sustained, depolarizing, current responsible for mAChR-dependent EPF is produced by non-selective cation channels whose opening depends on agonist binding to extracellular mAChRs and elevated intracellular Ca2+ (Egorov et al, 2002; Fransen et al., 2006). The ion pores responsible for this persistent current are thought to be comprised of subunits of TRPC channels (Robereda et al., 2011; Zhang et al., 2011).
mAChR-dependent EPF was recently also discovered in pyramidal neurons from layer II/III of rat PRC (Navaroli et al., 2012). When these PRC brain slices were bathed in a cholinergic agonist, EPF occurred in 85% of the sampled cells. Note that the agonist did not cause spontaneous firing. Instead, repetitive firing only occurred after the cells were stimulated with a suprathreshold current. By contrast, in the absence of a cholinergic agonist, EPF has never been observed in PRC neurons of any type and from any layer.
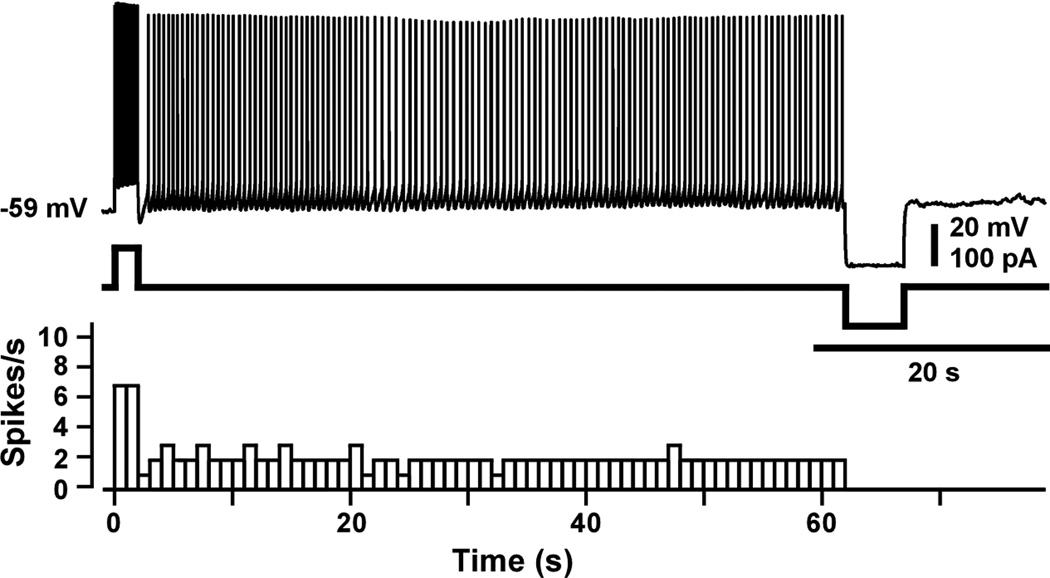
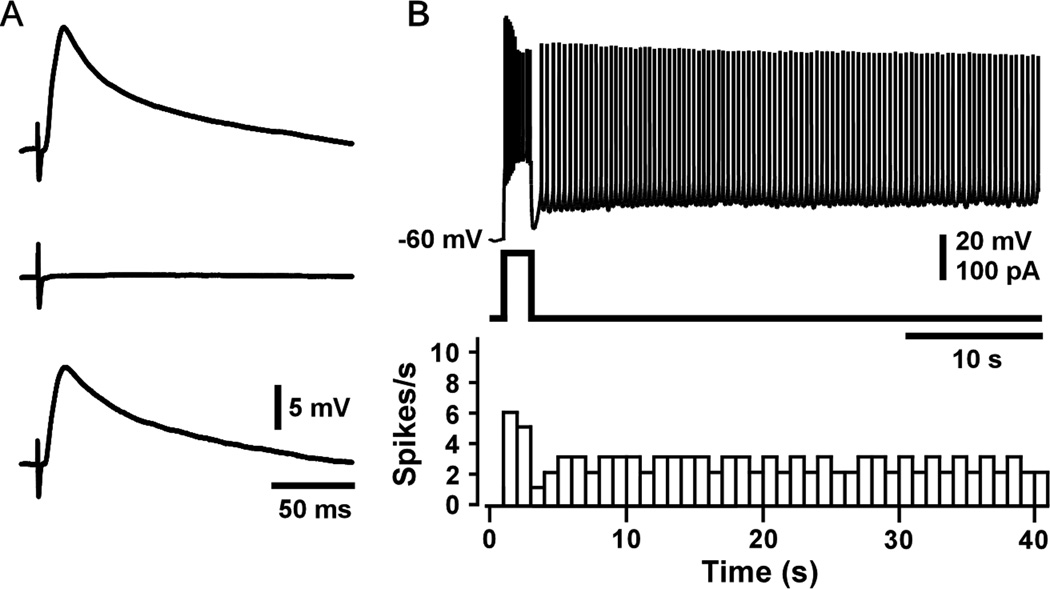
An example of persistent firing in a layer II/III pyramidal neuron is shown in Figure 5. As illustrated, a brief, supra-threshold, current injection caused repetitive firing that lasted for a minute, at which time firing was experimentally terminated by a hyperpolarizing current step. Under natural conditions, firing might be stopped by a decrease in the local concentration of acetylcholine (ACh), which can change on a second-by-second basis, depending on the task (Sarter, et al., 2009; Hasselmo and Sarter, 2011).
Figure 5.
Elicitation and termination of persistent firing. (Top) Voltage responses showing repetitive spiking and current steps used to elicit or terminate firing. Persistent firing was ended after one minute by a hyperpolarizing current step. (Bottom) The histogram shows the number of spikes in successive 1-s time bins. From Navaroli et al. (2012).
As in LA and EC, the occurrence of persistent firing in PRC neurons does not depend upon recirculating synaptic activity. Instead, persistent firing can be elicited even after fast synaptic transmission has been pharmacologically blocked by antagonists for ionotropic glutamate and GABA receptors. Figure 5A (top trace) shows a large excitatory postsynaptic potential (EPSP) that was evoked in a layer II/III pyramidal neuron, by extracellular stimulation, before adding the receptor antagonists. The middle trace in Figure 5A shows that the antagonists completely blocked the postsynaptic response. During this block of synaptic transmission, EPF was readily elicited by a depolarizing current injection (Fig. 5B), which is why this phenomenon is termed "endogenous" persistent firing. The EPSP returned following a washout of the antagonists (Fig. 5A, bottom trace), demonstrating that the synaptic input remained intact. Of course, the fact that EPF can be elicited after blocking fast synaptic transmission does not imply that recurrent circuitry normally plays no role in supporting persistent firing. In fact, we assume just the opposite.
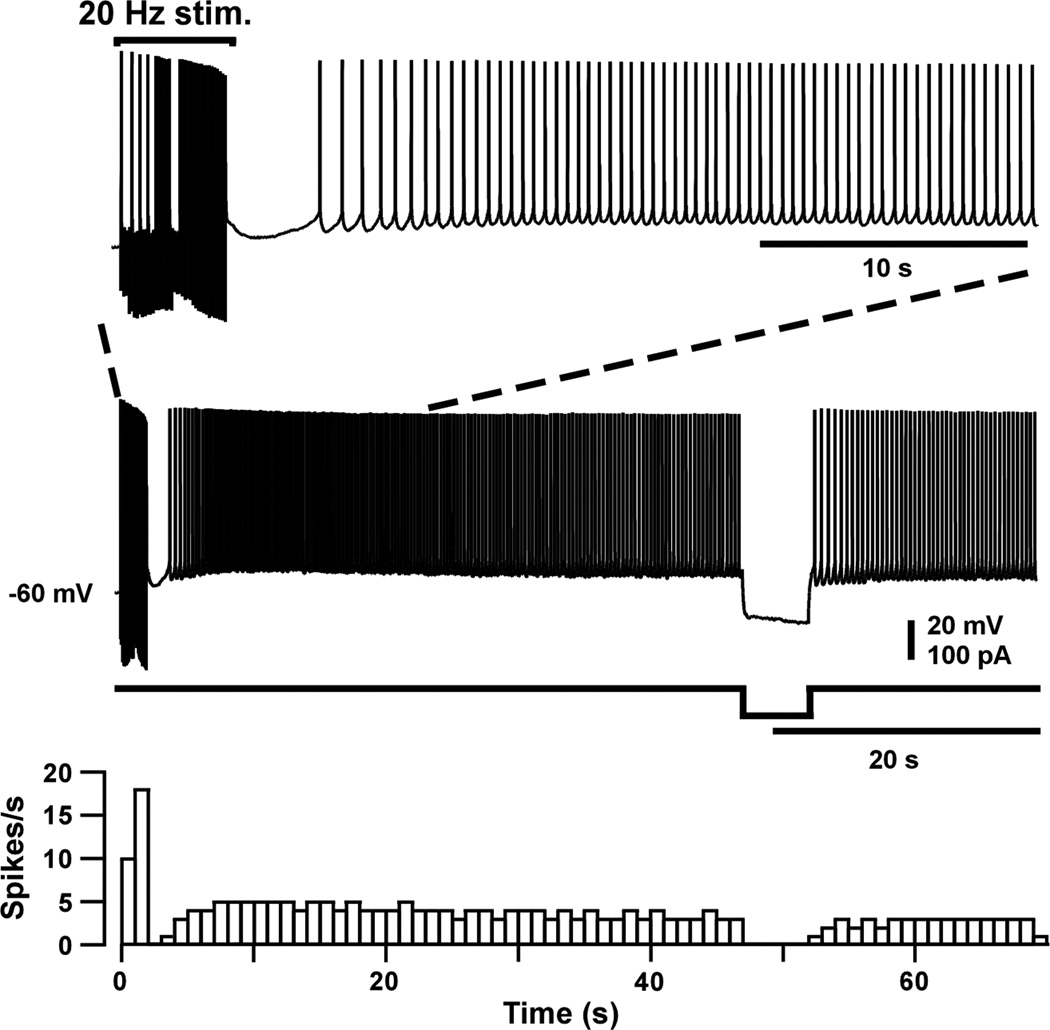
The results in Figures 5 and 6 show that EPF can be elicited by injecting a suprathreshold depolarizing current. An example of synaptically-triggered persistent firing in PRC is shown in Figure 7. Using extracellular stimulation, the synaptic input was stimulated at 20 Hz for 2 s, resulting in repetitive firing in the postsynaptic cell. After the synaptic stimulation ended, there was a brief pause in repetitive firing. However, repetitive firing quickly resumed and continued for the next 44 s, at which time a hyperpolarizing current was injected into the neuron.
Figure 6.
Persistent firing in the absence of fast synaptic transmission. (A) Top trace shows a large excitatory postsynaptic potential (EPSP). Middle trace shows that the EPSP was completely blocked by the addition of APV, DNQX, and picrotoxin. Bottom trace shows that the synaptic input was still intact after washing out the three drugs. Each of the traces is the average of five synaptic stimulations. (B) Persistent firing elicited by a depolarizing current step during the time when the EPSP was blocked. From Navaroli et al. (2012).
Figure 7.
Persistent firing elicited by synaptic stimulation. (Top) Synaptic stimulation (at 20 Hz for 2 s) caused repetitive firing during and after the stimulation. Firing is shown on two different time scales. A hyperpolarizing current step temporarily blocked repetitive firing, which resumed as soon as the hyperpolarization ended. (Bottom) Histogram showing the number of spikes in successive 1-s time bins. From Navaroli et al. (2012).
The hyperpolarization completely blocked repetitive firing, which then resumed after the hyperpolarization ended. Whether a hyperpolarization permanently terminates repetitive firing depends on its size and duration. In every PRC neuron that was studied, a sufficiently large and prolonged hyperpolarization always terminated EPF. Once persistent firing has been initiated, subsequent depolarizations and hyperpolarizations can, respectively, increase or decrease the rate of persistent firing, as in EC (Egorov et al., 2002; Fransen et al., 2006; Tahvildari et al., 2007) and LA (Egorov et al., 2006). This effect is termed "graded persistent firing," indicating that persistent firing is not an all-or-nothing event. Thus far, everything that is known about EPF in PRC matches what is known in EC and LA (Navaroli et al., 2012).
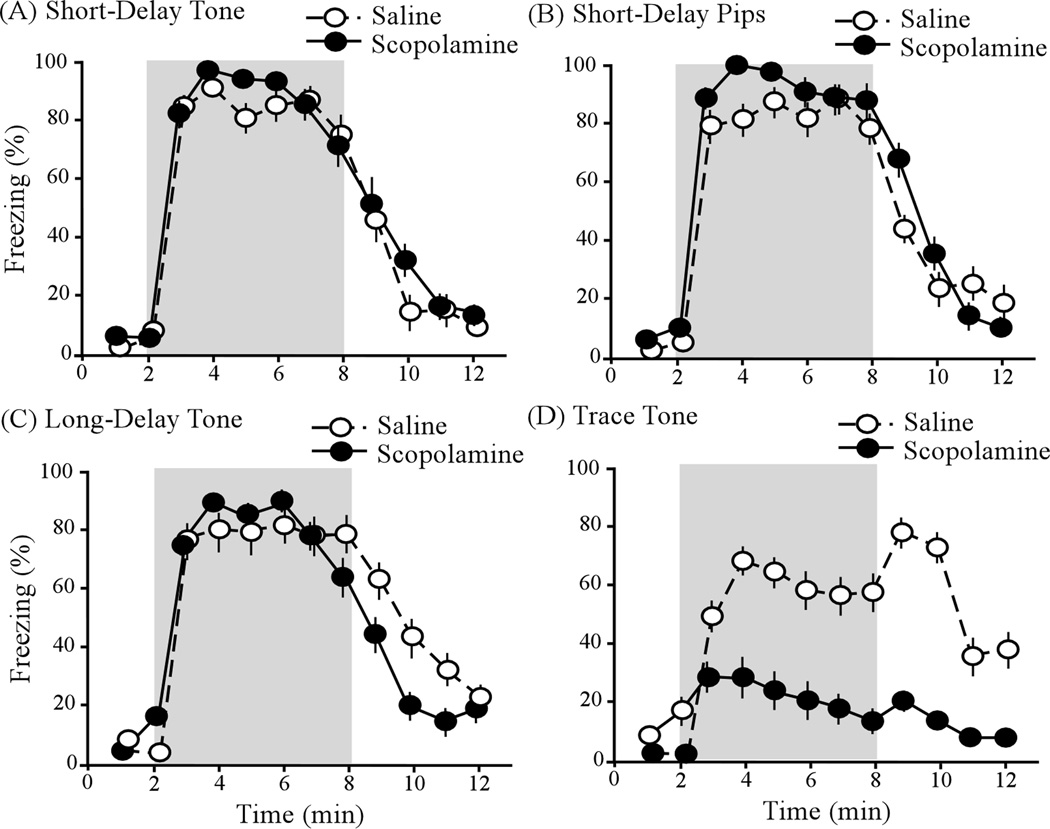
If trace conditioning is supported by EPF in PRC, then infusion of this structure with a broad-spectrum mAChR antagonist, such as scopolamine, would be expected to impair trace conditioning, possibly without affecting either delay or context conditioning. This is exactly what was found (Fig. 8). In the trace-conditioning paradigm (shown in Fig. 1D), the CS was a 10 s 22 kHz tone and the US was a 1 s grid shock. The ISI was 26 s and the trace interval was 16 s. As indicated, scopolamine infusion into PRC greatly impaired trace conditioning (Fig. 8D; d = 1.2). By contrast, the infusion had no significant effect on short-delay conditioning to a 22 kHz tone (Fig. 8A), short-delay conditioning to 22 kHz pips (Fig. 8B), or long-delay conditioning to a 22 kHz tone (Fig. 8C). The temporal relationships between the CS and US in these last three groups are shown in Figure 1 (parts A–C). The infusion had no significant effect on context conditioning.
Figure 8.
Effects of PRC infusions with scopolomine on delay and trace cue conditioning. The infusion had no effect on short-delay conditioning to a continuous tone (A, Tone), short-delay conditioning to a discontinuous tone (B, Pips), or long-delay conditioning to a continuous tone (C, Tone). By contrast, the infusion significantly impaired trace conditioning to a tone (D, Tone). Black and white circles correspond to groups receiving PRC infusions with scopolamine or a saline vehicle, respectively. Shading represents the 6 min CS presentation period. From Bang & Brown (2009b).
There was some concern that the drug might have spread into LA. In fact, a subsequent study found that scopolamine infusion directly into LA greatly impairs trace fear conditioning but has no significant effect on delay or context conditioning (Kent, Baysinger, & Brown, 2011). The possible extent of drug spread in either direction, from LA to PRC or the reverse, is somewhat uncertain.
However, the amount of drug spread between PRC and LA might not be a critical issue. According to one theory (Navaroli et al., 2012), trace fear conditioning is supported by EPF in a spatially-distributed memory system that minimally includes LA, PRC, EC, and the hippocampus (HC). Surprisingly, persistent firing was only recently observed in HC (Jochems & Yoshida, 2012; Knauer et al., 2012; Yoshida et al., 2012). Lesions of ventral HC (Yoon & Otto, 2007; Czerniawski et al., 2009, 2011; Esclassan et al., 2009b) also impair trace but not delay conditioning. Similarly, EC lesions severely impair trace fear conditioning without affecting delay conditioning (Esclassan et al., 2009a). Injecting EC with an M1 muscarinic receptor antagonist (pirenzepine), impairs trace conditioning without affecting delay conditioning. In brain slices, pirenzepine blocks EPF in both EC (Egorov et al., 2002) and PRC (Navaroli et al. 2012).
Persistent-firing neurons have also been observed in anterior cingulate cortex (ACC; Zhang & Sequela, 2010) and, importantly, ACC lesions impair trace but not delay fear conditioning (Han et al., 2003). Still unknown is whether mAChR blockade in ACC selectively impairs trace fear conditioning. Persistent-firing neurons have also been discovered in the postsubiculum, where they have been hypothesized to support sustained activity of head-direction neurons in the absence of exteroceptive spatial cues (Yoshida & Hasselmo, 2009).
The effects of lesions and drug infusions show that the transient memory system that enables trace fear conditioning can be disrupted at multiple sites. Thus it seems that there is relatively little redundancy in this memory buffer. This conclusion naturally leads to the question, What kind of spatially-distributed memory buffer might behave in this manner? An obvious possibility is that the buffer depends on re-circulating activity among several structures. Several decades ago, Donald Hebb (1949) theorized that reverberating signals in ensembles of neurons furnish temporary memory storage. A new twist on this old idea is that EPF helps drive and maintain this re-circulating activity. One important goal will be to understand the computational implications of the prospect that EPF supports reverberating circuits.
The EPF hypothesis makes numerous predictions that have not yet been tested. The most obvious one is that there should exist, in multiple parts of this memory system, CS-elicited neural activity that persists during the trace interval of trace fear-conditioning trials. Firing during the trace interval might only exist during the first few conditioning trials, making it difficult to detect. Ultimately, it will be important to demonstrate directly that EPF occurs in vivo. Obviously, it will be technically challenging to duplicate in vivo the essential neurophysiological and neuropharmacological manipulations that have thus far defined EPF in brain slices. Additional tools for testing the EPF hypothesis should become available as more is learned about the underlying mechanisms. One would certainly welcome the development of soluble agents that can selectively modulate subtypes of TRPC receptors. Another potentially valuable approach might entail transfecting PRC so as to inhibit the production of sub-types of TRPC receptors in adult animals. The lateral surgical approach, described earlier, is perfectly suited for selective transfection.
In closing this section, we note that studies using non-aversive behavioral paradigms have also suggested a role for PRC in transient memory. For example, PRC lesions are known to impair object recognition in a delay-dependent fashion (Mumby & Pinel, 1994; Wiig & Bilkey, 1995; Ennaceur et al., 1996; Norman & Eacott, 2005; Winter & Bussey, 2005; Cowell, Bussey, & Saksida, 2006). At the cellular level, PRC neurons in both rats and monkeys have been observed to show stimulus-selective firing during the delay period of recognition tasks (Nakamura & Kubota, 1995; Young et al., 1997). Firing during the delay period has been suggested to reflect EPF (Hasselmo & Stern, 2006).
Conclusions regarding PRC role in fear conditioning
The simplest conclusion is that rat PRC participates in two types of mnemonic operations in conjunction with Pavlovian fear conditioning. The first of these functions, which we termed unitization, may be localized to some extent within PRC. This can be conceived as a form of perceptual plasticity that can become enduring. The second function, transient memory, clearly is not localized within PRC. To the contrary, this memory system is supported by cooperative activity in multiple structures, minimally including HC, EC, LA, and PRC. The phenomenon of EPF, exhibited by neurons in all four structures, is a fascinating candidate mechanism for supporting transient memory. We speculate that EPF may furnish the needed drive to maintain Hebbian "reverberating circuits."
Whereas these dual functions of PRC were inferred from studies of auditory fear conditioning and context conditioning in rats, they dovetail nicely with an earlier theory (Murray & Bussey, 1999) that emerged from studies of visually-guided, instrumental, behaviors in primates, based on non-aversive incentives. This well-established theory (reviewed in Murray & Wise, this volume) explicitly proposes that PRC has a perceptual-mnemonic function. We suggest that the perceptual-mnemonic theory be expanded to include perception of auditory and other stimuli.
Acknowledgments
Grant sponsor: This work was supported by MH58405 to THB, NSERC Fellowship to BAK, and Yale University.
References
- Albrechet-Souza L, Borelli KG, Almada RC, Brandao ML. Midazolam reduces the selective activation of the rhinal cortex by contextual fear stimuli. Behavioural Brain Research. 2011;216(2):631–638. doi: 10.1016/j.bbr.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Allen TA, Furtak SC, Brown TH. Single-unit responses to 22 kHz ultrasonic vocalizations in rat perirhinal cortex. Behavioural Brain Research. 2007;182(2):327–336. doi: 10.1016/j.bbr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: Recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Bang SJ, Allen TA, Jones LK, Boguszewski P, Brown TH. Asymmetrical stimulus generalization following differential fear conditioning. Neurobiology of Learning and Memory. 2008;90(1):200–216. doi: 10.1016/j.nlm.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Brown TH. Perirhinal cortex supports acquired fear of auditory objects. Neurobiology of Learning and Memory. 2009a;92(1):53–62. doi: 10.1016/j.nlm.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang SJ, Brown TH. Muscarinic receptors in perirhinal cortex control trace conditioning. The Journal of Neuroscience. 2009b;29(14):4346–4350. doi: 10.1523/JNEUROSCI.0069-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee ACH, Rogers TT, Davies RR, Saksida LM, Murray EA. Graham KS. Functional specialization in the human medial temporal lobe. Journal of Neuroscience. 2005;25(44):10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perceptual functions of perirhinal cortex in rats: zero-delay object recognition and simultaneous oddity discriminations. Journal of Neuroscience. 2007;27:2548–2559. doi: 10.1523/JNEUROSCI.5171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggs JM, Moyer JR, Jr, McGann JP, Brown TH. Prolonged synaptic integration in perirhinal cortical neurons. Journal of Neurophysiology. 2000;83(6):3294–3298. doi: 10.1152/jn.2000.83.6.3294. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiology & Behavior. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Bordi F, LeDoux JE. Response properties of single units in areas of rat auditory thalamus that project to the amygdala. I. Acoustic discharge patterns and frequency receptive fields. Experimental Brain Research. 1994;98(2):261–274. doi: 10.1007/BF00228414. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nature reviews. Neuroscience. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Brown MW, Eldridge M. Perirhinal Cortex: Neural Representations. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol.7. Oxford: Academic Press; 2009. pp. 565–577. [Google Scholar]
- Brown MW, Xiang JZ. Recognition memory:neuronal substrates of the judgement of prior occurrence. Progress in Neurobiology. 1998;55(2):149–189. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Ultrasonic calls of rats as indicator variables of negative or positive states: Acetylcholine-dopamine interaction and acoustic coding. Behavioural Brain Research. 2007;182(2):261–273. doi: 10.1016/j.bbr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience. 2000;114(5):882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & Memory. 2006;13(5):638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Maurer AP, Hartzell S, Nematollahi S, Uprety A, Wallace JL, Barnes CA. 3-dimensional object-associated perirhinal cortical neuron activity is not modulated by novelty. Hippocampus. this volume. [Google Scholar]
- Burwell RD, Amaral DG. Cortical afferents of the perirhinal, postrhinal, and entorhinal cortices of the rat. The Journal of Comparative Neurology. 1998;398(2):179–205. doi: 10.1002/(sici)1096-9861(19980824)398:2<179::aid-cne3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Annals of the New York Academy of Sciences. 2000;911(1):25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. Journal of Comparative Neurology. 2001;437(1):17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. Perirhinal and postrhinal cortices of the rat: A review of the neuroanatomical literature and comparison with findings from the monkey. Hippocampus. 1995;5(5):390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Memory, perception, and the ventral visual-perirhinal-hippocampal stream: thinking outside of the boxes. Hippocampus. 2007;17:898–908. doi: 10.1002/hipo.20320. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience. 1995;15(3):2312–2327. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J-S, Brown TH. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. Journal of Neuroscience. 2003;23(25):8713–8721. doi: 10.1523/JNEUROSCI.23-25-08713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: the role of awareness. Science. 1998;280(5360):77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical analysis for the behavioral sciences. New Jersey: USA: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- Corodimas KP, LeDoux JE. Disruptive effects of posttraining perirhinal cortex lesions on conditioned fear: contributions of contextual cues. Behavioral Neuroscience. 1995;109(4):613–619. doi: 10.1037//0735-7044.109.4.613. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto TA. Induction and transient suppression of long-term potentiation in the peri- and postrhinal cortices following theta-related stimulation of hippocampal field CA1. Brain Research. 1998;780(1):95–101. doi: 10.1016/s0006-8993(97)01151-7. [DOI] [PubMed] [Google Scholar]
- Cowell RA, Bussey TJ, Saksida LM. Why does brain damage impair memory? A connectionist model of object recognition memory in perirhinal cortex. The Journal of Neuroscience. 2006;26(47):12186–12197. doi: 10.1523/JNEUROSCI.2818-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Otto T. Dorsal versus ventral hippocampal contributions to trace and contextual conditioning: Differential effects of regionally selective nmda receptor antagonism on acquisition and expression. Hippocampus. 2011 doi: 10.1002/hipo.20992. In Press. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Eichenbaum H, Rosenberg P, Eckmann KW. Afferent connections of the perirhinal cortex in the rat. The Journal of Comparative Neurology. 1983;220(2):168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature Neuroscience. 2000;3(Suppl):1181–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420(6912):173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. European Journal of Neuroscience. 2006;24(11):3183–3194. doi: 10.1111/j.1460-9568.2006.05200.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Schoenbaum G, Young B, Bunsey M. Functional organization of the hippocampal memory system. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(24):13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behavioural Brain Research. 1996;80(1–2):9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Endres T, Widmann K, Fendt M. Are rats predisposed to learn 22kHz calls as danger-predicting signals? Behavioural Brain Research. 2007;185:69–75. doi: 10.1016/j.bbr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. A cholinergic-dependent role for the entorhinal cortex in trace fear conditioning. The Journal of Neuroscience. 2009a;29(25):8087–8093. doi: 10.1523/JNEUROSCI.0543-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2009b;19(1):33–44. doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Faulkner B, Brown TH. Morphology and physiology of neurons in the rat perirhinal-lateral amygdala area. The Journal of Comparative Neurology. 1999;411:613–642. [PubMed] [Google Scholar]
- Fanselow MS. Associative vs topographical accounts of the immediate shock-freezing deficit in rats: implications for the response selection rules governing species-specific defensive reactions. Learning and Motivation. 1986;17:16–39. [Google Scholar]
- Fransen E, Tahvildari B, Egorov AV, Hasselmo ME, Alonso AA. Mechanism of graded persistent cellular activity of entorhinal cortex layer v neurons. Neuron. 2006;49:735–746. doi: 10.1016/j.neuron.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Fujimichi R, Naya Y, Koyano KW, Takeda M, Takeuchi D, Miyashita Y. Unitized representation of paired objects in area 35 of the macaque perirhinal cortex. The European Journal of Neuroscience. 2010;32(4):659–667. doi: 10.1111/j.1460-9568.2010.07320.x. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Allen TA, Brown TH. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. The Journal of Neuroscience. 2007a;27(45):12277–12291. doi: 10.1523/JNEUROSCI.1653-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Moyer JR, Brown TH. Morphology and ontogeny of rat perirhinal cortical neurons. The Journal of Comparative Neurology. 2007b;505(5):493–510. doi: 10.1002/cne.21516. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Wei S, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007c;17:709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Griffiths TD, Warren JD. What is an auditory object? Nature Reviews Neuroscience. 2004;5(11):887–892. doi: 10.1038/nrn1538. [DOI] [PubMed] [Google Scholar]
- Han CJ, O’Tuathaigh CMO, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, Koch C, Anderson DJ. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. PNAS. 2003;100(22):13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59(4):554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends in Cognitive Sciences. 2006;10(11):487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. Organization of Behavior: A Neuropsychological Theory. New York: John Wiley and Sons; 1949. [Google Scholar]
- Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hearing Research. 1994;73:244–247. doi: 10.1016/0378-5955(94)90240-2. [DOI] [PubMed] [Google Scholar]
- Jochems A, Yoshida M. Intrisic perisitent firing in hippocampal CA3 pyramidal neurons in vitro. FENS. 2012 2012 Abstract, 6, A-471-0031-00827. [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, Ledoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kealy J, Commins S. The rat perirhinal cortex: A review of anatomy, physiology, plasticity, and function. Progress in Neurobiology. 2011;93(4):522–548. doi: 10.1016/j.pneurobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Kent BA, Baysinger AN, Brown TH. Selective control of trace fear conditioning by muscarinic receptors in the amygdala. Society for Neuroscience. 2011 2011 Abstract. [Google Scholar]
- Kholodar-Smith DB, Allen TA, Brown TH. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behavioral Neuroscience. 2008a;122(5):1178–1185. doi: 10.1037/a0012902. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Boguszewski P, Brown TH. Auditory trace fear conditioning requires perirhinal cortex. Neurobiology of Learning and Memory. 2008b;90(3):537–543. doi: 10.1016/j.nlm.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience & Biobehavioral Reviews. 2006;30(2):188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5:1–8. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer B, Jochems A, Valero MJ, Yoshida M. Cholinergic-dependent intrinsic perisistent firing of in vitro rat CA1 pyramidal neurons- A possible mechanism for short-term information retention. FENS. 2012 2012 Abstract 6, A 471-0031-01890. [Google Scholar]
- Landeira-Fernandez J, Decola JP, Kim JJ, Fanselow M. Immediate shock deficit in fear conditioning: Effects of shock manipulations. Behavioral Neuroscience. 2006;120(4):873–879. doi: 10.1037/0735-7044.120.4.873. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb C, Ruggiero DA. Topographic organization of neurons in the acoustic thalamus that project to the amygdala. Journal of Neuroscience. 1990;10(4):1043–1054. doi: 10.1523/JNEUROSCI.10-04-01043.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Choi J-S, Brown TH, Kim JJ. Amygdalar N-Methyl-D-Aspartate (NMDA) receptors are critical for the expression of multiple conditioned fear responses. J. Neuroscience. 2001;21(11):4116–4124. doi: 10.1523/JNEUROSCI.21-11-04116.2001. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist DH, Jarrard LE, Brown TH. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. The Journal of Neuroscience. 2004;24(14):3610–3617. doi: 10.1523/JNEUROSCI.4839-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Bilkey DK. Long-term potentiation in the perirhinal-hippocampal pathway is NMDA dependent. Neuroreport. 1996;7:1241–1244. doi: 10.1097/00001756-199605170-00003. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Liu JCJ, Weidermann G, Mitchell CJ. Awareness is necessary for differential trace and delay eyeblink conditioning in humans. Biological Psychology. 2011;87(3):393–400. doi: 10.1016/j.biopsycho.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Current Opinion Neurobiology. 2004;15:334–342. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Awareness predicts the magnitude of single-cue trace eyeblink conditioning. Hippocampus. 2000;10:181–186. doi: 10.1002/(SICI)1098-1063(2000)10:2<181::AID-HIPO7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nature Reviews in Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Massey PV, Phythian D, Narduzzo K, Warburton EC, Brown MW, Bashir ZI. Learning-specific changes in long-term depression in adult perirhinal cortex. The Journal of Neuroscience. 2008;28(30):7548–7554. doi: 10.1523/JNEUROSCI.1935-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann JP, Moyer JR, Jr, Brown TH. Predominance of late-spiking neurons in layer VI of rat perirhinal cortex. The Journal of Neuroscience. 2001;21(14):4969–4976. doi: 10.1523/JNEUROSCI.21-14-04969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science. 2008;319:1543–1546. doi: 10.1126/science.1150769. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Brown TH. Visually-guided patch-clamp recordings in brain slices. In: Walz W, editor. Patch-Clamp Analysis: Advanced Techniques. 2nd Edition. Totowa, NJ: Humana Press; 2007. pp. 169–227. [Google Scholar]
- Moyer JR, McNay E, Brown TH. Three classes of pyramidal neurons in layer V of rat perirhinal cortex. Hippocampus. 2002;12:218–234. doi: 10.1002/hipo.1110. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Pinel JPJ. Rhinal cortex lesions and object recognition in rats. Behavioral Neuroscience. 1994;108(1):11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP. Why is there a special issue on perirhinal cortex in a journal called Hippocampus?: The perirhinal cortex in historical perspective. Hippocampus. doi: 10.1002/hipo.22055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Kubota K. Mnemonic firing of neurons in the monkey temporal pole during a visual recognition memory task. Journal of Neurophysiology. 1995;74(1):162–178. doi: 10.1152/jn.1995.74.1.162. [DOI] [PubMed] [Google Scholar]
- Navaroli VL, Zhao Y, Boguszewski P, Brown TH. Muscarinic receptor activation enables persistent firing in pyramidal neurons from superficial layers of dorsal perirhinal cortex. Hippocampus. 2012;22:1392–1404. doi: 10.1002/hipo.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural Brain Research. 2004;148(1–2):79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacot MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behavioral Neuroscience. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behavioural Brain Research. 2007;182(2):231–244. doi: 10.1016/j.bbr.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Parsana AJ, Brown TH. Temporal lobe and object recognition. Encyclopedia of Behavioral Neuroscience. 2010;3:375–382. [Google Scholar]
- Parsana AJ, Li N, Brown TH. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behavioural Brain Research. 2012a;226(1):77–86. doi: 10.1016/j.bbr.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsana AJ, Moran EE, Brown TH. Rats learn to freeze to 22-kHz ultrasonic vocalizations through autoconditioning. Behavioural Brain Research. 2012b;232(2):395–399. doi: 10.1016/j.bbr.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiology Review. 2010;90(2):419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Eichenbaum H. The perirhinal–entorhinal cortex, but not the hippocampus, is critical for expression of individual recognition in the context of the Coolidge effect. Neuroscience. 2003;122(3):599–607. doi: 10.1016/j.neuroscience.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Poulos AM, Li V, Sterlace SS, Tokushige F, Ponnusamy R, Fanselow MS. Persistence of fear memory across time requires the basolateral amygdala complex. PNAS. 2009;106(28):11737–11741. doi: 10.1073/pnas.0905257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Harker KT, Douglas RM, Wishaw IQ. Variation in visual acuity within pigmented, and between pigmented and albino rat strains. Behavioural Brain Research. 2002;136(2002):339–348. doi: 10.1016/s0166-4328(02)00126-2. [DOI] [PubMed] [Google Scholar]
- Reboreda A, Jiménez-Díaz L, Navarro-López JD. TRP channels and neural persistent activity. Adv Exp Med Biol. 2011;704:595–613. doi: 10.1007/978-94-007-0265-3_32. [DOI] [PubMed] [Google Scholar]
- Romanski LM, LeDoux JE. Bilateral destruction of neocortical and perirhinal projection targets of the acoustic thalamus does not disrupt auditory fear conditioning. Neuroscience Letters. 1992;142(2):228–232. doi: 10.1016/0304-3940(92)90379-l. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O’Reilly RC. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behavioral Neuroscience. 1999;113(5):867–880. doi: 10.1037//0735-7044.113.5.867. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Huff NC, Matus-Amat P. Understanding contextual fear conditioning: insights from a two-process model. Neuroscience & Biobehavioral Reviews. 2004;28(7):675–685. doi: 10.1016/j.neubiorev.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sacchetti B, Lorenzini CA, Baldi E, Tassoni G, Bucherelli C. Auditory thalamus, dorsal hippocampus, basolateral amygdala, and perirhinal cortex role in the consolidation of conditioned freezing to context and to acoustic conditioned stimulus in the rat. The Journal of Neuroscience. 1999;19(21):9570–9578. doi: 10.1523/JNEUROSCI.19-21-09570.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe M. Opinion: Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nature Reviews Neuroscience. 2009;10:383–390. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane A, Massey PV, Keen H, Bashir ZI, Brown MW. L-type voltage-dependent calcium channel antagonists impair perirhinal long-term recognition memory and plasticity processes. The Journal of Neuroscience. 2009;29(30):9534–9544. doi: 10.1523/JNEUROSCI.5199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CJ, Cassell MD. Perirhinal cortex projections to the amygdaloid complex and hippocampal formation in the rat. The Journal of Comparative Neurology. 1999;406(3):299–328. doi: 10.1002/(sici)1096-9861(19990412)406:3<299::aid-cne2>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Smith CN, Clark RE, Manns JR, Squire LR. Acquisition of differential delay eyeblink classical conditioning is independent of awareness. Behavioural Neuroscience. 2005;119:78–86. doi: 10.1037/0735-7044.119.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvildari B, Fransen E, Alonso AA, Hasselmo ME. Switching between “On” and “Off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17:257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(21):8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramae JN, Fukai T. A cellular mechanism for graded persistent activity in a model neuron and its implications in working memory. Journal of Computational Neuroscience. 2005;18:105–121. doi: 10.1007/s10827-005-5474-6. [DOI] [PubMed] [Google Scholar]
- Wiig KA, Bilkey DK. Lesions of rat perirhinal cortex exacerbate the memory deficit observed following damage to the fimbria-fornix. Behavioral Neuroscience. 1995;109(4):620–630. doi: 10.1037//0735-7044.109.4.620. [DOI] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience. 2005;25(1):52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Paradoxial facilitation of object recognition memory after infusion of scopolamine into perirhinal cortex: implications for cholinergic system function. Journal of Neuroscience. 2006;26(37):9520–9529. doi: 10.1523/JNEUROSCI.2319-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Schwarting RKW. Ultrasonic communication in rats: can playback of 50-kHz calls induce approach behavior? PLoS ONE. 2007;2(12):e1365. doi: 10.1371/journal.pone.0001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohr M, Schwarting RKW. Activation of the limbic system structures by replay of ultrasonic vocalization in rats. In: Brudzynski SM, editor. Handbook of mammalian vocalization. Oxford: Academic Press; 2010. pp. 113–124. [Google Scholar]
- Yaniv D, Desmedt A, Jaffard R, Richter-Levin G. The amygdala and appraisal processes: stimulus and response complexity as an organizing factor. Brain Research Brain Research Reviews. 2004;44(2–3):179–186. doi: 10.1016/j.brainresrev.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of the dorsal and ventral hippocampus in rats to trace fear conditioning. Neurobiology of Learning and Memory. 2007;87:464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Hasselmo ME. Persistent firing supported by an intrinsic cellular mechanism in a component of the head direction system. Journal of Neuroscience. 2009;29:4945–4952. doi: 10.1523/JNEUROSCI.5154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Knauer B, Jochems A. Cholinergic modulation of the CAN current may adjust neural dynamics for active memory maintenance, spatial navigation and time-compressed replay. Frontiers in Neural Circuits. 2012;6(9):1–15. doi: 10.3389/fncir.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. Journal of Neuroscience. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sequela P. Metabotropic induction of persistent activity in layers II/III of anterior cingulate cortex. Cerebral Cortex. 2010;20(12):2948–2957. doi: 10.1093/cercor/bhq043. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Reboreda A, Alonso AA, Barker PA, Séguéla P. TRPC channels underlie cholinergic plateau potentials and persistent activity in entorhinal cortex. Hippocampus. 2011;21:386–397. doi: 10.1002/hipo.20755. [DOI] [PubMed] [Google Scholar]