Abstract
The high-affinity copper transporter (Ctr1) (SCLC31A1) plays an important role in regulating Cu homeostasis because copper (Cu) is an essential micronutrient and Cu deficiency is detrimental to many important cellular functions but excess Cu is toxic. Recent research has revealed that human Cu homeostasis is tightly controlled by interregulatory circuitry involving Cu, Sp1 and hCtr1. This circuitry uses Sp1 transcription factor as a Cu sensor in modulating hCtr1 expression which in turn controls cellular Cu and Sp1 levels in a three-way mutual regulatory loop. Posttranslational regulation of hCtr1 expression by Cu stresses has also been described in the literature. Because hCtr1 can also transport platinum (Pt) drugs, this finding underscores the important role of hCtr1 in Pt drug sensitivity in cancer chemotherapy. Consistent with this notion is the findings that elevated hCtr1 expression was associated with favorable treatment outcomes in cisplatin-based cancer chemotherapy. Moreover, cultured cell studies demonstrated that elevated hCtr1 expression can be induced by depleting cellular Cu levels, resulting in enhanced cisplatin uptake and its cell killing activity. A phase 1 clinical trial using a combination of trientine (a Cu chelator) and carboplatin has been performed with encouraging results. This review discusses new insights into the role of hCtr1 in regulating Cu homeostasis and explains how modulating cellular Cu availability could influence treatment efficacy in platinum-based cancer chemotherapy through hCtr1 regulation.
Keywords: Cisplatin, High-affinity copper transporter, Cu-lowering agents, Drug-resistance, Drug transport
Introduction
Platinum (Pt)-based antitumor agents, including cisplatin (CDDP) carboplatin (CBDCA) and oxaliplatin (L-OHP), have been used for treating human solid tumors for more than three decades, especially testicular and ovarian cancers for which high response rates are attainable. Drug resistance is an important obstacle to achieving maximal therapeutic efficacy of these drugs. Overcoming drug resistance is very important for improving treatment outcome because once drug resistance has developed, other effective treatment options are limited. No effective strategy for combating cDDP resistance in cancer chemotherapy is currently available.
Mechanisms of cDDP resistance are complex. Here, we focus on a drug transport-associated resistance. This resistance mechanism has gained substantial attention after the discovery that Ctr1 (hCtr1 for humans) is the major transporter for cDDP (1). In this review, we discuss some inconsistencies published in the literature regarding mechanisms of hCtr1 regulation in Cu homeostasis maintenance that are relevant to Pt drug sensitivity.
It has been well established that the primary target of antitumor Pt drugs is DNA by the formation of intra- and inter-stranded DNA-drug adducts. Many previous reports have shown that cDDP cytotoxicity is correlated with the amount of DNA adducts, which is proportional to the total Pt content inside the cells. These findings underscore the importance of the transport system in Pt drug cancer chemotherapy. These studies implicated that cDDP enters cells by means of passive diffusion driven by concentration gradients or by endocytotic pathway (see reviews in ref. (2, 3)). The discovery of hCtr1 as a Pt-drug transporter is intriguing because the physiological and chemical properties in Cu and cDDP are different (controversy #1). However, a recent study using site-specific mutagenization approach demonstrated that amino acid residues involved in hCtr1-mediated cDDP transport are generally also required for Cu transport, although differences in transport kinetics (Km and Vmas values) exist (4). There are also reports that hCtr1 copy numbers in genetically engineered cell lines were not proportionally correlated with rates of Cu and cDDP uptake (5, 6), despite many studies demonstrating that elevated expression of hCtr1 confers cDDP sensitivity (7, 8). However, these genetically engineered cell lines were generated by transfection with recombinant hCtr1 cDNA. Whether all of the transfected hCtr1 is functional is not known in light of our new understanding of hCtr1 regulation mechanism which is constrained within the context of overall Cu homeostasis regulation (see below).
The importance of Ctr1 in cDDP transport overall can be found in Ctr1-knockout mouse embryonic fibroblast Ctr1(−/−) cells. These Ctr1 null-cells display only 30% to 35% of residual cDDP transport activity in reference to the Ctr1(+/+) counterpart (9). This study demonstrated that hCtr1 can also transport CBDCA with reduced efficiency, and that L-OHP is a poor substrate for hCtr1 (9).
The clinical relevance of hCtr1 expression and Pt drug cancer chemotherapy was reported by Ishida et al (10). These investigators analyzed an array-based hCtr1 expression dataset consisting of 91 ovarian cancer patients who had been treated with a Pt drug and a taxane and found a correlation between high hCtr1 expression levels and longer disease-free survival times. We analyzed an independent gene expression database published by Tothill et al. (11) derived from 285 serous and endometrioid tumors of ovary, peritoneum and fallopian tube, and found that patients with elevated hCtr1 expression in their tumors had more favorable outcomes after Pt-drug treatment than those expressing low hCtr1 levels (unpublished results). It is important to emphasize that this correlation was at the hCtr1 mRNA expression level. A similar correlation was found in Pt-based therapy for lung cancer (12).
Importantly, recent studies have demonstrated that levels of hCtr1 expression can be modulated by manipulating the cellular bioavailable Cu pool (13–15). These findings have resulted in a phase 1 clinical trial in advanced ovarian cancer chemotherapy at MD Anderson Cancer Center that uses a Cu chelator (trientine) as an enhancer for CBDCA treatment (16).
Key finding: Cu-chelation induces hCtr1 expression and chemosensitization to cDDP treatment
The conceptual development of using Cu chelators as chemosensitizers for Pt-drug treatment evolved from our previous study using cell lines overproducing glutathione (GSH) by transfection with recombinant DNA encoding the heavy subunit of γ-glutamylcysteine synthetase (γ-GCSh), a rate-limiting enzyme for de novo biosynthesis of GSH. These γ-GCSh/GSH-overproducing cells exhibited elevated hCtr1 expression and increased sensitivity to cDDP treatment. These transfected cell lines exhibited reduced cellular bioavailable Cu content, as evidenced by the reduced activities of several Cu-containing enzymes because GSH is an abundant physiological Cu chelator (13). These phenotypes were reversed when the elevated GSH levels were reduced. This finding was contradictory to the vast amount of previously published results showing that induction of γ-GCSh/GSH expression was responsible for cDDP resistance, because GSH is an important cytoprotector (inconsistency #2). However, it is important to note that all these cDDP-resistant cells were published before the discovery that hCtr1 is a cDDP transporter and that recent results have shown that most, if not all, cDDP-resistant variants contain various degrees of reduced hCtr1 levels (7). Elevated expression of γ-GCSh/GSH levels in these cDDP-resistant cells could be explained as due to the oxidative stress-induced mechanism during drug treatment, because GSH is an important ROS sensor/regulator. Consistent with this notion is the observations that elevated γGCSh/GSH expression can be found in cells treated with other pro-oxidants including cytokines, antitumor agents, and carcinogens, but no enhanced cDDP resistance was associated with these treatments (see review in (17) and references therein). Induction of hCtr1 expression by Cu-lowering agents was further confirmed using additional Cu-lowering agents (14, 15).
The role of hCtr1 in the regulation of Cu homeostasis in cultured cells
To understand how Cu chelation induces hCtr1 expression, it is important to elucidate how mammalian Cu homeostasis is regulated. Mechanisms of Cu homeostasis maintenance are evolutionarily conserved from yeasts, fruit flies, plants, to humans. Steady-state cellular Cu levels are maintained by a balance among Cu transporters (Ctr1), Cu chaperons (Atox1 for intracellular distribution), Cu storage (Ctr2), and Cu exporters (ATP7A and ATP7B). All of these transporters can also transport cDDP (7). However, Ctr1 is an important regulator in response to environmental Cu stresses.
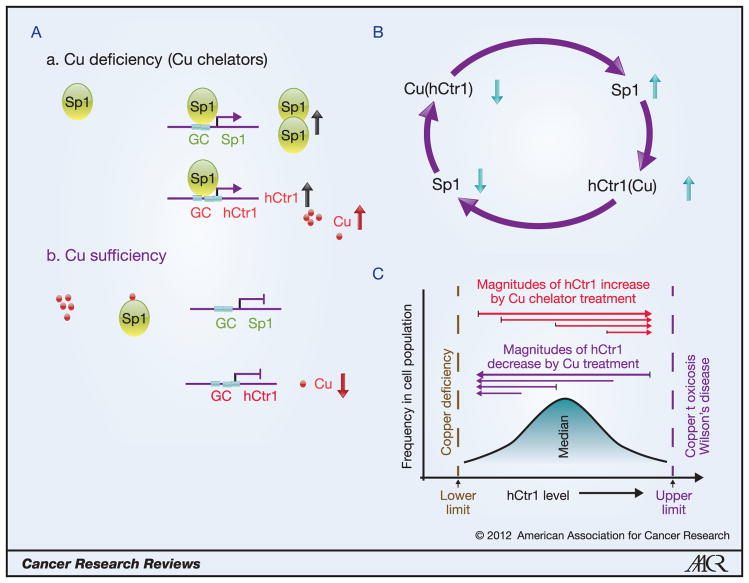
We recently demonstrated that Cu deficiency induced by treating human cancer cells with Cu chelators or by expressing a dominant-negative hCtr1 recombinant, upregulates hCtr1 expression; whereas Cu sufficiency achieved by treating cells with CuSO4 or by overexpressing the wild-type hCtr1 recombinant, downregulates endogenous hCtr1 expression (4,14,15). The upregulation or downregulation of hCtr1 under these two different Cu-stressed conditions is respectively controlled by Sp1 binding to or dissociating from the GC boxes located at the hCtr1 promoter. Not only hCtr1, but also Sp1 itself, is regulated by Cu bioavailability (Fig. 1A). Thus, human Cu homeostasis is regulated by the Cu-Sp1-hCtr1 interregulatory loop (Fig. 1B). This regulatory loop utilizes Sp1 oscillation in regulating hCtr1 expression to rebalance the cellular Cu budget (14). The sensing mechanism of Cu bioavailability by Sp1 lies on the zinc finger (ZF) domains located at the C-terminus of Sp1. Cu stresses-induced transcription regulation of Ctr1 in other organisms also uses ZF motifs in the transcription regulators as sensors in response to Cu inadequacies (18).
Figure 1.
Regulation of hCtr1 and Sp1 expression by Cu bioavailability. A, Upregulation of Sp1 and hCtr1 expression under Cu deficiency by enhanced promoter binding of Sp1 to these genes (a); Cu sufficiency prevents Sp1 binding to these promoters (b). B, The Cu-Sp1-hCtr1 interregulatory loop for Cu homeostasis regulation. Reducing the Cu levels (downward arrow), either by treating cells with Cu chelators or by expressing the hCtr1 dominant-negative recombinant, upregulates Sp1 and hCtr1 expression (upward arrows); likewise, increasing the Cu levels, either by treating cells with CuSO4 or by transfection with wild-type hCtr1 cDNA, downregulates Sp1 and hCtr1 expression. C, Magnitudes of hCtr1 regulation by Cu bioavailabilities depend upon the intrinsic hCtr1 levels. Low hCtr1-expressing cells exhibit greater magnitudes of hCtr1 induction than do high hCtr1-expressing cells by Cu chelator (red lines); likewise, greater reduction in hCtr1 induction was observed in high hCtr1-expressing cells by Cu treatment than in low hCtr1-expressing cells (purple lines).
Posttranslational regulation mechanisms have also been reported for regulating hCtr1 by Cu stresses. High Cu levels induce trafficking of hCtr1 from the plasma membrane to the endosomal/lysosomal compartments, where hCtr1 may be (19) or may be not (20) degraded. cDDP-induced internalization of hCtr1 was also reported (21), but another report, cDDP treatment did not internalize hCtr1 but rather induced hCtr1 stabilization through protein cross-linking (22). While the source for this inconsistency (#3) is not certain, but most likely due to different anti-hCtr1 antibodies used in these studies. Because Ctr1 is an evolutionarily conserved protein and good anti-hCtr1 antibody has been difficult to obtain. Anti-hCtr1 antibodies prepared from different laboratories often showed different antigen recognition patterns by the western blotting. Even the “monomeric hCtr1” detected by these antibodies often showed different molecular masses. In addition, there is report that Cu bioavailability also regulates hCtr1 protein stability (23). Regardless, the mechanistic insight into these posttranslational regulation mechanisms requires further elucidation, particularly from the Cu homeostasis point of view.
This Cu-Sp1-hCtr1 interregulatory mechanism suggests the following scenarios in the Cu homeostasis control: One, this regulatory mechanism is dynamic: changes in one component would feed-forward to affect levels of the other two, resulting in a new equilibrium. This dynamic regulation mechanism may explain why the magnitudes of hCtr1 and Sp1 regulated by Cu stresses are small (most often within 2-fold) in most cell settings, and may often be disregarded as being within normal experimental variations. This scenario underscores the importance of using qualitatively and quantitatively reliable measurement methods, such as isoform-specific hCtr probes in the RNase protection assay (14, 15).
Two, this regulation loop is tightly controlled, implicating that there are maximal and minimal ranges wherein each of these components can swing. This scenario suggests that the magnitudes of hCtr1 regulation depend on the intrinsic (basal) hCtr1 levels and that cells with high levels of hCtr1 expression would have greater magnitudes of hCtr1 downregulation than would those expressing reduced levels of hCtr1 when exposed to Cu. Likewise, cells expressing reduced levels of hCtr1 would have a greater amplitude of hCtr1 upregulation by Cu chelators than would those expressing higher levels (Fig. 1C). This second scenario is particularly relevant to Pt-based chemotherapy, given the observations that tumor cells expressing reduced levels of hCtr1 are often associated with cDDP resistance due to reduced cDDP transport capacity (7, 8). Greater induction of hCtr1 expression by Cu chelators would have greater Pt transport for cell killing. This has been demonstrated in three pairs of cDDP-resistant cell lines (Liang ZD et al., manuscript submitted). In cancer chemotherapy, initial treatment using Pt drugs would eradicate high hCtr1-expressing population and spare cells intrinsically resistant to cDDP. The ability of re-sensitizing these intrinsic Pt-resistant cells by Cu-chelators would improve the treatment efficacy of Pt drugs in cancer chemotherapy.
Issues relevant to the clinical use of Cu-lowering agents as cDDP enhancers
The phase 1 pilot study performed at MD Anderson Cancer Center to evaluate the concept of using Cu chelators for improving the effectiveness of Pt drugs involved five patients with Pt-resistant high-grade epithelial ovarian cancers. These patients were treated with trientiene and CBDCA. Two patients experienced severe adverse events that included myelosuppression, especially anemia requiring transfusion. Dose-limiting toxicity was not observed within the first 28 days. After two cycles of therapy, partial remission was achieved in one patient (10+ months), stable disease in three patients (2, 3.5+ and 5 months, respectively), and progressive disease in one patient. Better tumor responses were associated with greater decreases in Cu levels using the surrogate biomarkers serum ceruloplasmin (16). This study provides the first-in-human preliminary data showing that Pt-resistance in tumors could be at least partially overcome in some patients through the use of a Cu-lowering agent. Further study using larger patient population with improved strategies (see below) is warranted. In the meantime, several relevant issues are discussed below:
First, Cu-lowering agents such as trientine and D-penicillamine have been used for more than four decades for treating Cu toxicosis in Wilson’s disease, a genetic disorder caused by defects in ATP7B. Another Cu-lowering agent, tetrathiomolybdate (TM), has been in clinical trials as an antitumor agent. Cu is a cofactor required for several angiogenic mediators, including VEGF, bFGF, IL-1 and IL-8 (24). It has been shown that many patients with malignancies of the breast, colon, lung, prostate, and brain, display elevated Cu contents in their serum and tumors (24). Cu-lowering agents have been used in monotherapy by targeting the angiogenic growth mechanisms of the tumors (25). Combining Cu-lowering agents and Pt drugs in cancer chemotherapy may have additive antitumor effects (and may also have additive cytotoxicities, see below). This strategy is essentially a new use of old drugs, and therefore, if proven, would be a low cost treatment option for cancer patients.
Second, although the trientine/CBDCA protocol is targeting Pt-resistant tumors with reduced hCtr1 expression, however, mechanisms of cDDP resistance are multifactoral and reduced hCtr1 alone is not the only reason for drug resistance. Other factors including elevated ATP7A/ATP7B transporters (26) and hCtr2 (27), may also affect the treatment efficacy. To maximize the effect of Cu-chelators, determination of biomarkers associated with various cDDP resistance mechanisms should be very helpful to stratify patients who may benefit most from the treatment.
Third, the effectiveness of Cu-lowering agents in enhancing hCtr1 expression in vivo is a critical issue. A previous study of rats fed a Cu-deficient diet failed to show increased Ctr1 mRNA levels in livers and small intestines despite a substantial loss in Cu levels in these organs (69 ~ 89% reduction), as correspondingly compared with those in animals fed Cu-adequate diet (28). A cervical tumor model developed in transgenic HPV16/E2 mouse showed elevated mCtr1 protein levels as compared with those in the normal cervix. TM treatments did not show further induction of mCtr1 expression in the tumor lesions of transgenic mice, nor in the cervix of the wild-type animals (10). In contrast, increased mCtr1 expression was found in several organs (kidney, duodenum, brain) in Cu-deficient, postnatal day-16, mice fed Cu-deficient diets (29). Aside from the technical issues related to Ctr1 measurement as mentioned and different tissue sources were used in these studies, this inconsistency (#4) may reflect the complexity of in vivo Ctr1 regulation mechanisms. Further investigations are needed to address the following important issues: (i) The in vivo Ctr1 regulation mechanism by Cu deprivation may be more stringent than the in vitro system using cultured cells. (ii) Elevated Ctr1 expression may be an intrinsic mechanism associated with tumor development. This may explain why elevated Cu levels were observed in many human malignancies (24) and in the HPV16/E2 cervical tumors (10). Moreover, failure to further induce tumoric mCtr1 expression in the HPV16/E2 tumors by TM may be explained that these tumors already express elevated levels of mCtr1 (Fig. 1C). (iii) The effectiveness of Cu-lowering agents in enhancing cDDP sensitivity may be tissue-specific: tissues expressing high levels of hCtr1 may be less sensitive to Cu chelation-induced Ctr1 expression than those expressing reduced hCtr1 levels. A better understanding of hCtr1 expression in response to Cu chelation treatment in different settings may eventually lead to the use of hCtr1 inducibility as a predictor for the treatment outcome of Pt drug therapy.
Fourth, drug-induced toxicity is also an important issue. Both Cu chelators and cDDP are redox-active compounds and cause oxidative damage to cells. Combination therapy may exacerbate toxicity resulting in a myriad of pathophysiological consequences. In cancer chemotherapy, although cDDP is known to cause toxicities in many organs including kidney, nerve, ear, and bone marrow, CBDCA which is known as the second generation antitumor Pt drug, displays much reduced toxicities in these organs except bone marrow. Cu chelators are also known to cause bone marrow damage associated with anemia/leukopenia and worsen neurologic symptoms in Wilson’s disease (25). Accordingly, bone morrow seems to be the most likely target for the adverse events associated with Cu chelator/CBDCA combination therapy. This indeed occurred in our preliminary trientine/CBDCA trial which involved only five advanced ovarian cancer patients (16).
Clinical experience shows that the adverse effects inflicted by CBDCA and by Cu chelator therapy could be overcome when treatments were discontinued, implicating that the treatment-induced adverse events may be manageable. In this regard, some suggestions may be offered: (i) by modifying the treatment schedule such as sequential administration of a Cu chelator first to induce hCtr1 expression followed by CBDCA treatment to optimize the antitumor efficacy; (ii) by carefully designing a treatment holiday to minimize the cytotoxicities; and (iii) by critically evaluating the combinations between CBDCA and various Cu-lowering agents. These strategies may eventually improve the overall therapeutic index for the use of Cu chelator and CBDCA combinations.
And fifth, recent studies have demonstrated that cancer-initiating cells in human malignancies are resistant to cDDP (30). Although these cDDP-resistant cells may be in small population and present in special niches, they are highly tumorgenic in the immunocompromised mice. Currently we know very little about cDDP resistance mechanisms associated with this tumor population. This drug-resistant population may be the most difficult target for complete elimination by chemotherapy. The role of hCtr1 in cDDP resistance in these cancer-initiating cells remains to be investigated.
Concluding Remarks and Perspectives
The discovery that Ctr1 can also transport Pt drugs has paved the bridge between two seemingly unrelated research fields, i.e., Cu physiology and Pt-based cancer chemotherapy. Studies of the molecular basis of Cu homeostasis regulated by Cu stressed conditions have resulted in the ongoing clinical studies using Cu-lowering agent in enhancing Pt treatment efficacy for cancer chemotherapy. Although results await future large randomized studies, this treatment strategy is particularly attractive because it targets cDDP-resistant tumors which are known to be difficult. While both Cu-lowering agents and Pt drugs have been extensively studied for a long time, much remains to be learned. Importantly, more investigations are needed to critically address many relevant issues mentioned in this communication. Research aimed at a better understanding of how the Pt drug transport capacity can be enhanced through hCtr1 upregulation and how the unwanted cytotoxicity can be simultaneously minimized, may eventually facilitate the development of effective Cu chelation strategy to combat drug resistance problem in Pt-based cancer chemotherapy.
Acknowledgments
Supported in part by grants from the National Institutes of Health National Cancer Institute (R01 CA149620 to MTK), the National Science Council, Taiwan [Grant NSC97-2314-B-006-043], and the National Cheng Kung University Hospital, Tainan, Taiwan (NCKUH9904005 to HHWC).
The authors thank the Senior/Deputy Editor and reviewers for their insightful comments and Tamara Locke (Department of Scientific Publications, MD Anderson Cancer Center) for editing the manuscript. We apologize for not citing additional important publications due to space limitation.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol. 2008;48:495–535. doi: 10.1146/annurev.pharmtox.48.080907.180426. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 4.Liang ZD, Stockton D, Savaraj N, Tien Kuo M. Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol. 2009;76:843–53. doi: 10.1124/mol.109.056416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatti L, Chen D, Beretta GL, Rustici G, Carenini N, Corna E, et al. Global gene expression of fission yeast in response to cisplatin. Cell Mol Life Sci. 2004;61:2253–63. doi: 10.1007/s00018-004-4218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beretta GL, Gatti L, Tinelli S, Tinelli S, Corona E, Colangelo D, et al. Cellular pharmacology of cisplatin in relation to the expression of human copper transporter CTR1 in different pairs of cisplatin-sensitive and -resistant cells. Biochem Pharmacol. 2004;68:283–91. doi: 10.1016/j.bcp.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Howell SB, Safaei R, Larson CA, Sailor MJ. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol. 2010;77:887–94. doi: 10.1124/mol.109.063172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 9.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter CTR1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–4. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 10.Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–83. doi: 10.1016/j.ccr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–208. doi: 10.1158/1078-0432.CCR-08-0196. ( www.ncbi.nlm.nih.gov/geo, accession #GSE9899) [DOI] [PubMed] [Google Scholar]
- 12.Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, et al. Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer. 2012;75:228–34. doi: 10.1016/j.lungcan.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen HH, Song IS, Hossain A, Choi M-K, Yamane Y, Liang ZD, et al. Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol. 2008;74:697–704. doi: 10.1124/mol.108.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang ZD, Tsai WB, Lee MY, Savaraj N, Kuo MT. Specificity protein 1 (Sp1) oscillation is involved in copper homeostasis maintenance by regulating human high-affinity copper transporter 1 expression. Mol Pharmacol. 2012;81:455–64. doi: 10.1124/mol.111.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, et al. Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol. 2008;74:705–13. doi: 10.1124/mol.108.046771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu S, Naing A, Fu C, Kuo MT, Kurzrock R. Overcoming Platinum Resistance Through the Use of a Copper-Lowering Agent. Mol Cancer Ther. doi: 10.1158/1535-7163.MCT-11-0864. in press( http://clinicaltrials.gov/ct2/show/NCT01178112) [DOI] [PMC free article] [PubMed]
- 17.Chen HH, Kuo MT. Role of glutathione in the regulation of Cisplatin resistance in cancer chemotherapy. Met Based Drugs. 2010:1–7. doi: 10.1155/2010/430939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Festa RA, Thiele DJ. Copper: an essential metal in biology. Curr Biol. 21:R877–83. doi: 10.1016/j.cub.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem. 2004;279:17428–33. doi: 10.1074/jbc.M401493200. [DOI] [PubMed] [Google Scholar]
- 20.Molloy SA, Kaplan JH. Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem. 2009;284:29704–13. doi: 10.1074/jbc.M109.000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–52. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Smith K, Petris MJ. Cisplatin stabilizes a multimeric complex of the human Ctr1 copper transporter: requirement for the extracellular methionine-rich clusters. J Biol Chem. 2004;279:46393–9. doi: 10.1074/jbc.M407777200. [DOI] [PubMed] [Google Scholar]
- 23.Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 285:32385–92. doi: 10.1074/jbc.M110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat Rev. 2009;35:32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Brewer GJ. The use of copper-lowering therapy with tetrathiomolybdate in medicine. Expert Opin Investig Drugs. 2009;18:89–97. doi: 10.1517/13543780802621859. [DOI] [PubMed] [Google Scholar]
- 26.Dmitriev OY. Mechanism of tumor resistance to cisplatin mediated by the copper transporter ATP7B. Biochem Cell Biol. 89:138–47. doi: 10.1139/o10-150. [DOI] [PubMed] [Google Scholar]
- 27.Blair BG, Larson CA, Adams PL, Abada PB, Pesce CE, Safaei R, et al. Copper transporter 2 regulates endocytosis and controls tumor growth and sensitivity to cisplatin in vivo. Mol Pharmacol. 79:157–66. doi: 10.1124/mol.110.068411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J, Prohaska JR, Dagenais SL, Glover TW, Thiele DJ. Isolation of a murine copper transporter gene, tissue specific expression and functional complementation of a yeast copper transport mutant. Gene. 2000;254:87–96. doi: 10.1016/s0378-1119(00)00287-0. [DOI] [PubMed] [Google Scholar]
- 29.Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136:21–6. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Pratesi G, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–6. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]