Abstract
Analgesia is particularly susceptible to placebo responses. Recent studies in humans have provided important insights into the neurobiology underlying placebo-induced analgesia. However, human studies provide incomplete mechanistic explanations of placebo analgesia because of limited capacity to use cellular, molecular, and genetic manipulations. To address this shortcoming, we describe here the development of a rat model of conditioned analgesia in an operant pain assay. Specifically, rats were conditioned to associate a placebo manipulation with the analgesic effect of 1 mg/kg morphine (s.c.) on facial thermal pain. We found that conditioned (placebo) responding bore three of the hallmarks of placebo-induced analgesia: (1) strong inter-animal variability in the response, (2) suppression by the opiate antagonist naloxone (5 mg/kg, s.c.), and (3) a positive predictive relationship between the unconditioned analgesic effect and the conditioned (placebo) effect. Due to the operant nature of the assay and the use of only a mild noxious thermal stimulus, we suggest these results provide evidence of placebo-induced analgesia in a preclinical model that utilizes an affective behavioral endpoint. This finding may provide opportunities for invasive preclinical studies allowing greater understanding of placebo-induced analgesia, thus paving the way for avenues to harness its benefits.
Keywords: placebo, operant, analgesia, morphine, opioid, preclinical
Introduction
Placebo effects have been observed across diverse clinical studies, including immune responses [1; 26; 49] Parkinson's disease [17], drug abuse [61], and in particular pain and analgesia [2; 4; 9; 10; 18; 19; 35; 38-40; 50; 51; 54; 60; 62; 65]. In this context, the placebo effect is usually defined as a sham or simulated medical intervention that improves a given outcome, such as pain relief [52]. Although placebo effects are often viewed as confounding in clinical trials [13], understanding this phenomenon could provide valuable insight into psychosomatic effects, which in turn could be harnessed for therapeutic benefit.
The causes of placebo effects have been the subject of much discussion. Major mechanistic factors include learning, perception, and simulation of therapy, among others [2; 5; 9; 14; 22; 50; 52]. However, one factor that seems to apply to placebo responses across species is that of conditioning, including Pavlovian conditioning. Indeed, Gliedman and colleagues [27] forwarded this account as early as 1957. Subsequently, Herrnstein provided early pre-clinical evidence supporting this hypothesis, showing that a conditioned stimulus (i.e., placebo) paired with the amnesic agent scopolamine causes animals to perform poorly in a memory test. Certainly, when viewed in the context of classical conditioning, many examples of such “placebo responses” can be found throughout the fields of learning, memory and addiction [1; 9; 12; 24; 28; 31; 49; 61]. However, remarkably few preclinical studies have focused on placebo-induced analgesia. Thus, we present here evidence of placebo-induced analgesia in a preclinical model.
As far as we are aware, there have been only two major preclinical studies of placebo-induced analgesia using animal models. Both studies used reflex-based pain assays and relatively high doses of opiate drugs [11; 29]. In the first, Bryant, et al. demonstrated that placebo-induced analgesia in mice conditioned with the potent opiate fentanyl, measured using a hot plate [11]. In the second, Guo, et al. showed that a cue paired with morphine or aspirin elicited analgesic responses, also on a hot plate [29].
While these studies provide important foundations for further studies of placebo-induced analgesia, we reason that they are not equipped to assess aversive behaviors indicative of affective components of pain. The affective, or “emotional”, components of pain are particularly important to chronic pain sufferers and constitute one of the most common and debilitating aspects [42; 57]. In light of this, we report here use of an operant-based pain assay for studying placebo-induced analgesia. In what is essentially a conflict-reward assay, rodents express their willingness to withstand thermal pain to obtain sweet reward [46-48; 56]. As this paradigm tests mild non-injurious pain and utilizes operant responses, we reason that, unlike the more reflexive tests of other models, this model better incorporates cognitive and affective components of pain. As such, this assay could support detailed neurobiological investigations of both spinal and brain mechanisms of placebo analgesia, including cortical mechanisms known to be critically important in humans [6; 10; 17; 21; 22; 36; 50-52; 54].
Methods
Animals
Hairless male Sprague-Dawley rats (250 - 300 g, Charles River, Raleigh, NC) were used and housed with a cage mate in a standard 12:12 h light/dark cycle. Water and standard laboratory chow were available ad libitum when not being tested and weights were recorded weekly. Experiments were conducted in accordance with the guidelines of the Committee for Research and Ethical issues of the IASP on using laboratory animals [64]. The University of Florida Committee for the Care and Use of Animals approved all experimental procedures.
Drugs
Morphine sulfate (15 mg/ml, uncorrected for molecular salt and water content, Baxter Healthcare Corporation, Deerfield, IL) was diluted in phosphate buffered saline (PBS), pH 7.4 and injected subcutaneously (s.c.) at a dose of 1 mg/kg. Naloxone hydrochloride dihydrate (Sigma, St. Louis, MO) was dissolved in PBS at 25 mg/ml (uncorrected for molecular salt and water content) and injected subcutaneously at a dose of 5 mg/kg. All drugs were diluted to a final volume of 0.3 ml with PBS before injection.
Thermal facial operant testing
A detailed description of the reward-conflict operant testing paradigm used to assess facial analgesia can be found in our previous reports [47; 56]. Briefly, this employed an acrylic testing chamber with an adjustable slit (approx. 1 to 2 cm wide) opening lined with aluminum tubing. The tubing served as an adjustable thermode by circulating heated water via polyethylene tubing. A standard rodent water bottle containing diluted (1:2 with water) sweetened condensed milk solution at room temperature (Carnation, Nestlé USA, Glendale, CA) was mounted outside the cage as a palatable reward to incentivize rats to expose their faces to the thermode. Room temperature was maintained at 22 ± 1 °C for all behavioral tests.
Unrestrained animals were placed individually in testing chambers. The reward bottle was positioned such that access to the sweetened milk reward was possible contingent on facial contact with the thermode. The metal spout of the watering bottle was connected to a 13 V DC power supply and, in series, to a data acquisition module (WinDaq Lite Data Acq DI-194, DATAQ Instruments, Inc., Akron, OH). When licking from the bottle, the skin of the rat's muzzle contacted the thermode, and the tongue contacted the metal spout, thus completing two separate electrical circuits that registered as analog signals referred to as “contact” and “licking” events respectively [45-47; 56].
Acquisition of operant responding
Animals were fasted for 16 hours prior to testing sessions. All sessions were 20 mins long, separated by 48 hours, and were conducted between 08:00 h and 12:00 h. Animals were first trained with the thermodes set to the non-noxious temperature of 37±1°C (see fig. 1A for overview) for 6 sessions to reach criteria previously described [39]. Subsequently, animals underwent a single training session at 48 ± 1°C (termed “BASE48”). This session served to introduce animals to the noxious temperature at which analgesia testing would ultimately be performed, as well as to obtain baseline measurements for subsequent data normalization. This session was followed by one additional session at 37 ± 1°C.
Figure 1.
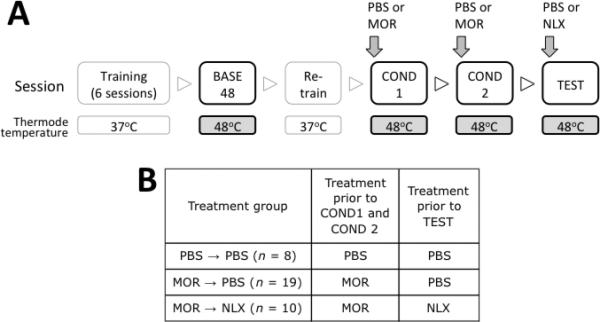
(A) Schematic representation of the behavioral paradigm used. Boxes represent exposure to the operant training apparatus wherein rats had access to palatable reward contingent upon contacting their muzzles on a heated thermode stimulus. Variations of PBS vehicle, morphine (MOR, 1mg /kg), or naloxone (NLX, 5 mg/kg) were administered 30 mins before the final three sessions. (B) Summary of major groups in the current study.
Induction of placebo-induced analgesia
The first two sessions following the training described above were performed with the thermode set at 48 °C and were designed to condition animals to associate the handling procedure with morphine-induced analgesia (fig. 1B). The handling procedure itself consisted of the s.c. injection procedure and light shaking of the animal for 3 s. These two sessions were referred to as “COND1” and “COND2” respectively. Following this, a final session referred to as “TEST” was performed (also at 48 °C) wherein rats were administered PBS (s.c.) or naloxone (s.c.). Throughout all three sessions (COND1, COND2 and TEST), rats were injected PBS or drugs 30 min before exposure to the training apparatus, during which time they remained in their home cages. The main experimental groups are described in fig. 2B. Additional groups of rats were included to compare any conditioned effects of morphine with unconditioned effects. In this case, rats were injected either morphine or PBS (controls) in their home cages 24 h before COND1 and COND2 training sessions.
Figure 2.
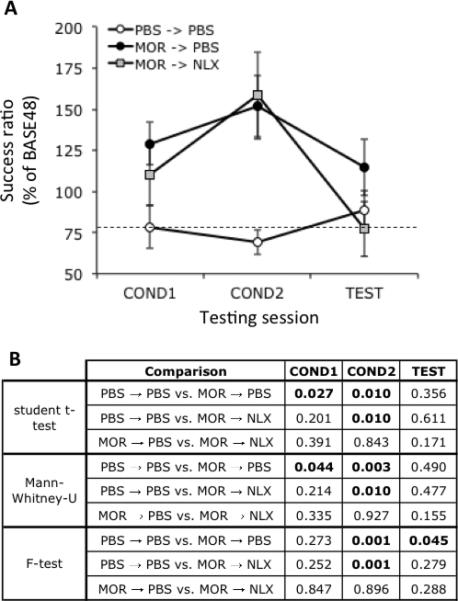
(A) Mean operant licking responses for palatable reward under various drug treatment conditions as described in fig. 1B. Dashed line indicates responding in the PBS→PBS-treated group during COND1 as a point of reference. Data is expressed as responses normalized to mean responding during BASE48 as depicted in fig. 1A within each treatment groups. Data is expressed as mean ± S.E.M. Group sizes (n): PBS→PBS, 8; MOR→PBS, 19; MOR→NLX, 10. (B) p values for t-test, Mann Whitney-U and F-test comparisons for responses at all time points shown in A. p values (t-test or F-test) less than 0.05 are bolded.
Data and statistical analyses
The primary outcome measure in this study was the ratio of successful licks to lick attempts, termed a “success ratio”. This ratio reflected the number of successful lick events occurring coincident with facial contact with the thermode. We have previously reported this measure is sensitive to manipulations altering pain perception. For instance, increasing the thermode temperature decreases the number of successful licks, whereas analgesics increase the number of successful licks [47]. Here, success ratios during COND1, COND2 and TEST are presented normalized as a percentage of those during BASE48 within animals to account for between-subject variability in unconditioned responding.
Various statistical analyses were applied to investigate the effects of drug treatment, relationship between responding at various time points, and the probability of specific responses occurring. These included repeated measures ANOVA, linear regression, chi-squared tests, student's t-tests and F-tests. The rational for applying specific methods of analyses is introduced in the relevant sections below. All statistical analyses were made using Statview (Abacus Concepts, Berkeley, CA), Microsoft Excel or online resources. Unless otherwise stated, data are presented as the mean ± standard error of the mean (S.E.M.) and probability (p) values less than 0.05 were considered statistically significant.
Results
Effects of drug treatment on responding during conditioning
Based on our previous studies, rats were considered trained upon reaching 1,000 successful licks during a 20 min testing session with the thermode heated to 37°C [45]. In the current study, rats attained a mean of 2443 ± 571 licks per 20 min session when considered across all groups prior to drug treatments. A single rat was removed from the MOR→NLX group due to extremely high (649%) responding during COND1, which proved to be an outlier according to Grubbs’ test.
Fig. 2A shows the normalized success ratios during COND1, COND2 and TEST in rats conditioned to 1 mg/kg s.c.morphine. Data are shown normalized to responding in the BASE48 session. Taking responding as the dependent measure over the three testing sessions, repeated measure ANOVA over the three sessions showed a statistically significant main effect of treatment (F2,34=4.501, p=0.0184), but no effect of session (F2,68=2.582. p=0.0831) or interaction between treatment and session (F4,68=1.639, p=0.1746). The results of post-hoc t-test and Mann-Whitney U comparisons between the effects of specific treatments within sessions are shown in fig. 2B. These showed statistically significant elevations in responding in both groups of morphine-treated rats (labeled MOR→ PBS and MOR→NLX in fig. 2A) during COND2 relative to PBS-treated animals. Notably, the mean success ratio increased approximately 30% between COND1 and COND2 in both groups of morphine-treated rats. However, as shown in table 1, no correlation was found in responding between these sessions.
Table 1.
Pearson product-moment correlation coefficient (r) and probability values (p) for comparisons within groups during specific sessions as depicted in figure 1A.
| Treatment group | COND1 vs COND2 | COND1 vs TEST | COND2 vs. TEST | COND2-COND1 vs. TEST | |
|---|---|---|---|---|---|
| r | PBS → PBS | 0.380 | 0.216 | 0.302 | -0.050 |
| MOR → PBS | -0.021 | -0.251 | 0.459 | 0.516 | |
| MOR → NLX | 0.158 | 0.616 | 0.108 | -0.284 | |
| p | PBS → PBS | 0.353 | 0.607 | 0.467 | 0.906 |
| MOR → PBS | 0.930 | 0.301 | 0.048 | 0.024 | |
| MOR → NLX | 0.662 | 0.078 | 0.781 | 0.459 |
p values (Pearson's test) less than 0.05 are bolded.
An additional control experiment was performed to test if morphine administration 48 hr before testing influences operant pain responding per se. For this purpose, morphine or PBS were injected 48 hr in the home cage before operant facial testing The mean normalized success ratios were 123.2 ± 31.0% and 100 ± 14.5% (n = 6 per group) for morphine-treated and vehicle-treated rats respectively. There was no statistically significant difference between these responses (t-test, p = 0.127).
Animals treated with morphine during COND1 and COND2, and then treated with PBS during TEST showed approximately 50% more responding during TEST than animals treated with PBS throughout. However, t-test comparison yielded a p value of 0.356 due to large variation in responding in the MOR→ PBS group. A statistically significant difference in the distribution of responding between the MOR→ PBS and animals treated with PBS throughout was confirmed using an F-test, which yielded a p value of 0.045 when comparing these two groups during TEST (fig. 2B). Animals treated with morphine during COND1 and COND2, and then treated with NLX during TEST showed slightly less responding during TEST than animals treated PBS throughout, suggesting that naloxone reversed the enhanced responding during TEST seen in MOR→ PBS-treated animals. A separate group of rats that did not undergo conditioning sessions COND1 and COND2 but treated with 5 mg/kg naloxone (n = 6) showed no difference in responding per se compared to PBS (n = 7, 101.5 ± 46.7 versus 100.0 ± 20.7, t-test, p = 0.941) suggesting that the suppressive effects of naloxone presented in fig. 2A depended on previous morphine treatment. Together, these data suggest that the enhanced responding seen during TEST in the MOR→ PBS-treated group depended on increased endogenous opioid activity.
Given the large variability in responses during TEST in MOR→ PBS-treated rats, a more refined analysis was performed to determine the characteristics of the distribution of the responses. Fig. 3 shows the responses of individual rats in the three groups of rats during TEST. Comparing the distribution of responses between MOR→PBS and PBS→PBS rats shows that several rats stand out within the MOR→ PBS group as displaying exceptionally high responses. Given this, we sought to determine the statistical likelihood of such high responses occurring based on the assumption that the responses of PBS→PBS-treated rats would follow a normal distribution.
Figure 3.
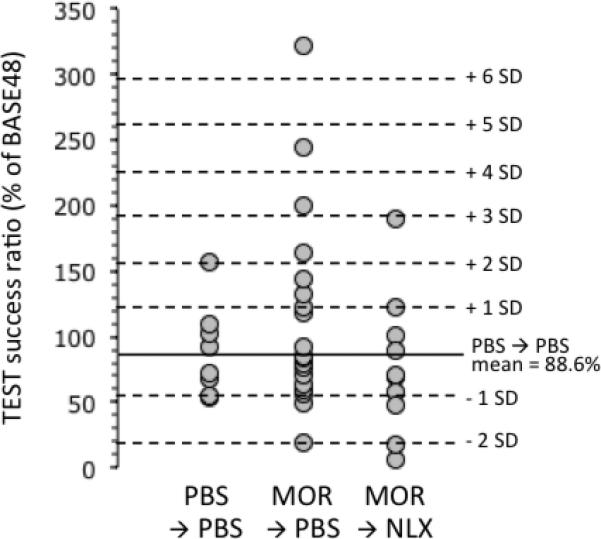
Operant licking responses for palatable reward in individual rats during the TEST session as described in fig. 1A. Solid horizontal line indicates mean responding in PBS→PBS-treated rats. Dashed horizontal lines indicate increasing standard deviations (SD) away from the mean responding for the PBS→PBS-treated group.
First, Anderson-Darling normality testing showed the distribution of responses during the TEST session of the PBS→PBS and MOR→ PBS groups to have p values of 0.4051 and 0.0146 respectively (table 2). This indicated that the PBS→PBS group displayed a normal distribution. In contrast, MOR→ PBS-treated animals did not. The apparent lack of normality in the distribution of responses in the MOR→ PBS-treated group was most likely due to skewing towards higher values. This was reflected in higher skewness and kurtosis values in the MOR→ PBS group (1.48 and 2.23 respectively) than the PBS→PBS group (1.07 and 1.12 respectively).
Table 2.
Results of Anderson-Darling normality testing for distributions of responses during COND1, COND2 and TEST.
| Treatment group | COND1 | COND2 | TEST |
|---|---|---|---|
| PBS → PBS | 0.525 | 0.090 | 0.015 |
| MOR → PBS | 0.610 | 0.345 | 0.405 |
| MOR → NLX | 0.346 | 0.430 | 0.678 |
Subsequently, the probability of the occurrence of rats responding at various levels during TEST in the MOR→ PBS relative to PBS→PBS-treated rats was estimated. To achieve this, the mean responding and standard deviation during TEST in PBS→PBS-treated rats was calculated (shown as horizontal dashed lines in fig. 3). This value was used to predict the expected number of responders in any given range of standard deviations from this mean based on a normal distribution, as shown in table 3. The expected number of responders was statistically compared to the actual number of responders using a chi-squared test (table 3). Based on this method of analysis, during TEST, MOR→PBS-treated animals included responders up to 6 standard deviations from the mean, yielding extremely low p values using a chi-squared test. No such effects were seen in the MOR→NLX group. It is important to note that this analysis was based on the hypothesis that TEST responding in the MOR→PBS group would be higher than in the PBS→PBS-treated group, as would be expected of a placebo response.
Table 3.
Estimation of probabilities (p) of the number of subjects responding at given levels relative to PBS→PBS-treated rats during TEST given groups size and a normal distribution by chi-squared test.
| PBS → PBS (n = 8) | MOR → PBS (n = 19) | MOR → NLX (n= 10) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Subjects during TEST in PBS → PBS group | Expected number of subjects | Number of subjects observed | p | Expected number of subjects | Number of subjects observed | p | Expected number of subjects | Number of subjects observed | p |
| > 0 SDs | 4 | 4 | 0.317 | 9.5 | 9 | 0.819 | 5 | 4 | 0.527 |
| > 1 SDs | 1.269 | 1 | 0.365 | 3.014 | 6 | 0.061 | 1.587 | 1 | 0.612 |
| > 2 SDs | 0.182 | 0 | 0.374 | 0.432 | 4 | 4×10-8 | 0.228 | 0 | 0.629 |
| > 3 SDs | 0.0108 | 0 | 0.472 | 0.026 | 3 | 4×10-77 | 0.014 | 0 | 0.907 |
| > 4 SDs | 3×10-4 | 0 | 0.479 | 0.001 | 2 | 10-99< | 3×10-4 | 0 | 0.986 |
| > 5 SDs | 2×10-6 | 0 | 0.479 | 5×10-6 | ` | 10-99< | 3×10-6 | 0 | 0.999 |
| > 6 SDs | 8×10-9 | 0 | 0.480 | 2×10-8 | 1 | 10-99< | 1×10-8 | 0 | 1.000 |
See Results for complete explanation of analysis method. p values (chi-squared test) less than 0.05 are bolded.
Relationships between operant responding between sessions
Linear regression (Pearson's correlation) showed weak non-significant relationships between responding during COND1 and TEST within all groups (table 1). Additionally, there were no statistically significant correlations between responding between COND1 and COND2. However, responding in COND2 was significantly positively correlated (r = 0.459. p = 0.048) with responding during the TEST session in MOR→PBS-treated animals (fig. 4), indicating that on a subject-by-subject basis, animals showing the strongest response during COND2 showed the strongest responding during TEST. Moreover, despite a lack of any significant correlation between COND1 and TEST, the strongest and most statistically significant correlation (r = 0.516, p = 0.024) with TEST was found when comparing the difference between COND2 and COND1 with TEST. This indicated that those animals showing the largest increase in morphine-induced analgesic responses between COND1 and COND2 showed the strongest conditioned (placebo) response during TEST.
Figure 4.
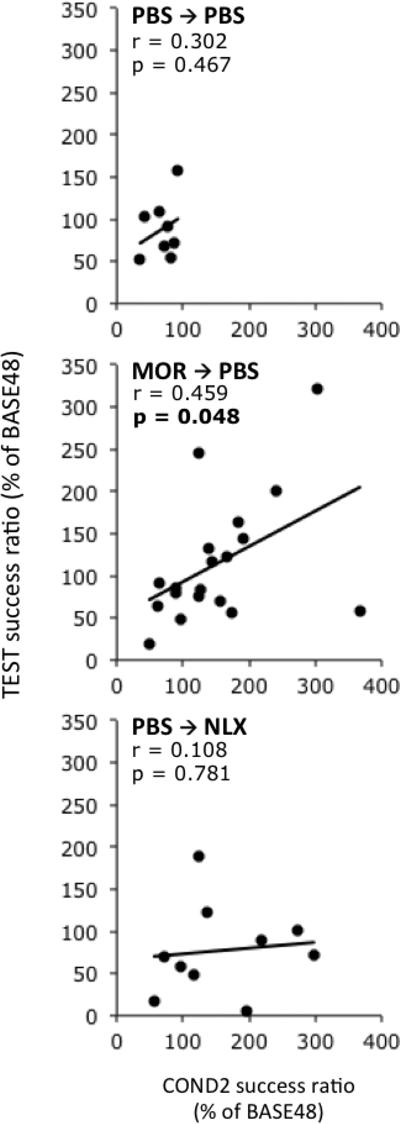
Correlative relationships between operant lick responding during COND2 and TEST for the three main treatment groups. Each point represents an individual rat. Solid lines indicate linear regressions. Additional analysis of correlational relationships is shown in table 2. p values (Pearson's test) less than 0.05 are bolded.
Discussion
As far as we are aware, the current report is the first presenting placebo effects in a preclinical operant pain assay. These results suggest that rats, like humans, show opioid-conditioned placebo analgesia. We believe this finding provides evidence that, in addition to nociceptive reflexes [42; 57], operant responses involving cognitive functions are also susceptible to placebos. As these responses better index the affective dimension of pain, they are especially relevant clinically [38; 40]. We suggest the approach used here may provide pre-clinical researchers a means for studying the affective dimension of placebo-induced analgesia using an animal model.
Characteristic of placebo responses observed in the current study
Placebo effects have several notable features. Among them is high variability in response size [9; 34; 53]. To a large extent, the apparent degree of this variability depends on the criteria used for defining the placebo response. Over the years, there have been numerous nuanced definitions of placebo responses (see [52]), but most agree that any sham or inert intervention with a beneficial effect beyond changes that occur due to natural history may be called a placebo effect [5; 22; 52]. Defining how large that effect need be is matter of discussion. Some studies specify a specific magnitude of symptom change and claim to identify placebo responses in individual subjects. However, rather than define a specific criterion, an alternative and objective approach is to study effect size, of which a d statistic is particularly common. In a recent discussion of this approach applied to meta-analytic findings on placebo effects in clinical settings, effects sizes for placebo effects of 0.24 and above were observed (see [32]). In the study presented here, we estimate the effect size (d) to be 0.45 (as defined in [60]), though it is important to note that relative to clinical studies, our preclinical study relied on considerably less subjects, thus decreasing the reliability of effect sizes.
Here, rather than defining a specific criterion, we determined the likelihood of responses of given magnitudes arising naturally. We based our analysis on the assumption that responses within control rats follow a normal (Gaussian) distribution. Indeed, normality testing suggested this was the case for PBS→PBS-treated rats. However, our group sizes were relatively small, which may have impacted the reliability of the statistical analysis. Nonetheless, as such, our analysis showed incidences of responding during TEST in the MOR→PBS group that statistically speaking were extremely unlikely to occur by chance. We suggest that these rats, which fell many standard deviations from the mean, could be considered placebo responders. It is also important to note that while our analysis was biased towards identifying animals that responded positively, one animal responded more than one standard deviation below the mean of the group treated PBS throughout.
A second major characteristic of placebo responses is that they often have strong relationships with genuine (unconditioned) responses [2; 8]. Indeed, we found a statistically significant correlation between responding in the COND2 and TEST sessions in MOR→PBS-treated animals. It was notable, and somewhat surprising, that no such correlation existed between COND1 and TEST in this group. On first look, that correlations between COND1 or COND2 and TEST sessions might exist may be unremarkable, as presumably animals should be consistent in their responses if experimental conditions remain constant. However, this was not the case in the current study, as no correlations were found between sessions in animals treated PBS throughout. This may reflect small but significant day-to-day changes in experimental conditions beyond the control of the experimenter. If so, that a correlation was found in morphine-treated animals reinforces the view that this relationship can overcome daily variation and may be physiologically important. This relationship may directly reflect individual differences in the analgesic efficacy of morphine or effects on learning capability.
A slightly stronger correlation was found between changes in responding between COND1 and COND2 sessions and responding during TEST, albeit it only marginally more statistically significant than the correlation discussed above. This is interesting when viewed in the context of recent theories in the psychobiology of substance abuse, one of which emphasizes that sensitization to cues predicting the availability of rewarding drugs is critical to the development of addiction [55; 59]. The increased responding between COND1 and COND2 sessions (rather than tolerance) seen in both morphine-treated groups is reminiscent of such sensitization. Furthermore, that such sensitization best predicted placebo responding suggests an involvement of incentive learning and expectancy in acquiring placebo responses. Indeed, our previous studies show that sensitization of locomotion and mesolimbic dopamine (a neural pathway considered fundamental to reward prediction) is predictive of the rewarding effects of drugs [63].
A third known feature of placebo responses is sensitivity to opioid antagonism [21; 29]. Here too, we confirmed this in our operant facial analgesia model, revealing the involvement of endogenous opioids by using the opiate antagonist naloxone. As discussed above, many have reasoned that placebo effects are fundamentally similar in their neurobiology to other expectancy effects [8; 39]. Indeed, recent human imaging studies show that the expectancy of reward is accompanied by increased endogenous opioid activity in limbic and cortical areas [25]. Additionally, opiate antagonists such as naloxone and naltrexone block various conditioned effects on pain [3; 21; 29]. Genetic deletion of some (but not all) endogenous opioids attenuates the expectancy of rewards [15; 23; 37; 41]. However, an involvement of endogenous opioids does not appear universal to all placebo effects. For instance, Amanzio and Benedetti found that naloxone blocks placebo analgesia induced by opioid conditioning but not by conditioning with the non-opioid ketarolac [2]. Amongst the few preclinical studies of placebo-induced analgesia, Guo and colleagues reported that placebo-induced analgesia was sensitive to naloxone, but that induced by aspirin was insensitive [29]. This raises the possibility, as suggested by human imaging studies discussed above, that placebo-induced analgesia stems from the brain simulating the original (unconditioned) effect of the drug; an idea also put forward by Guo and colleagues. In the case of morphine, this would be increased endogenous opioid release, thus being susceptible to naloxone.
Application to human placebo responses
Conditioning is one of several causes of human placebo analgesic responses, yet other causes also exist [5; 9; 14; 52]. Indeed, recent studies by Bryant and colleagues suggest rodents exhibit analgesic responses simply by being exposed to neighboring rodents exposed to opiates [11]. Thus, an operant model of placebo analgesia has the potential to analyze cognitive and brain mechanisms related to several causes/types of placebo analgesia such as social observational learning just mentioned for mice [11], as well as humans [14].
Alternative explanations and limitations
Though the current study suggests rats show placebo responses in an operant facial pain assay, other factors may be involved. First, the assay employed incorporates several psychological processes that may act synergistically or competitively before being emitted as a behavioral output. These not only include pain, but also reward, incentive motivation, learning, memory recall and appetite. For instance, previous studies show opiates can modulate appetitiveness and learning related to rewards [20; 33; 43; 44]. In this regard, our previous studies indicate that at the dose administered here and over the time scale applied, morphine does not influence licking per se under non-pain conditions, though more complex interactions may occur. Second, other studies show that morphine can stimulate locomotion [30; 58]. Although we did not perform an exhaustive analysis here, studies elsewhere show morphine can increase locomotion in Sprague-Dawley rats at doses as low as 1mg/kg (e.g. [16]). Such increases in locomotion could encourage rats to approach the reward, though these seems unlikely as again, under non-pain conditions, morphine as applied here does not influence licking behavior.
Summary and future implications
Our study introduces a novel preclinical model for studying placebo-induced analgesia that shows canonical features of placebo effects observed in humans. Due to its operant nature and use of mild noxious stimuli only, this paradigm may better address the emotional aspects of placebo-induced analgesia and executive cortical control placed over them. In doing so, these experiments provide the foundation for methodologies that would be extremely difficult in human studies, such as single neuron recordings, neurotransmitter depletion, focal lesions, and molecular/genetic manipulations. As an example, human studies have shown that, unlike conditioning with morphine, placebo analgesia (on a tourniquet tolerance test) generated by conditioning with ketarolac was not prevented by naloxone [2] but was prevented by a specific cannabinoid antagonist CB1 [7]. This distinction could be tested with the operant assay used here and could lead to its molecular and neuropharmacological characterization. Another possibility is that the genetic distinctions between placebo “responders” and “non-responders” might also be characterized using this same behavioral assay.
Summary.
Rats show canonical features of human placebo-induced analgesia in an assay designed to evaluate pain affect
Acknowledgements
TAN, NPM and JKN are funded by NIH grant 5R21DA027570-02
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
RC, NPM and JKN are employees of Velocity Laboratories, a company that provides fee-for-service behavioral testing using operant pain assays.
References
- 1.Ader R. Conditioned immunomodulation: research needs and directions. Brain, behavior, and immunity. 2003;17(Suppl 1):S51–57. doi: 10.1016/s0889-1591(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 2.Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(1):484–494. doi: 10.1523/JNEUROSCI.19-01-00484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedetti F. The opposite effects of the opiate antagonist naloxone and the cholecystokinin antagonist proglumide on placebo analgesia. Pain. 1996;64(3):535–543. doi: 10.1016/0304-3959(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 4.Benedetti F. Placebo analgesia. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2006;27(Suppl 2):S100–102. doi: 10.1007/s10072-006-0580-4. [DOI] [PubMed] [Google Scholar]
- 5.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annual review of pharmacology and toxicology. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 6.Benedetti F. No prefrontal control, no placebo response. Pain. 2010;148(3):357–358. doi: 10.1016/j.pain.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Benedetti F, Amanzio M, Rosato R, Blanchard C. Nonopioid placebo analgesia is mediated by CB1 cannabinoid receptors. Nature medicine. 2011;17(10):1228–1230. doi: 10.1038/nm.2435. [DOI] [PubMed] [Google Scholar]
- 8.Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(45):10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(10):4315–4323. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1-2):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Bryant CD, Roberts KW, Culbertson CS, Le A, Evans CJ, Fanselow MS. Pavlovian conditioning of multiple opioid-like responses in mice. Drug and alcohol dependence. 2009;103(1-2):74–83. doi: 10.1016/j.drugalcdep.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlino E, Pollo A, Benedetti F. Placebo analgesia and beyond: a melting pot of concepts and ideas for neuroscience. Current opinion in anaesthesiology. 2011;24(5):540–544. doi: 10.1097/ACO.0b013e328349d0c2. [DOI] [PubMed] [Google Scholar]
- 13.Colloca L, Benedetti F. Placebos and painkillers: is mind as real as matter? Nature reviews Neuroscience. 2005;6(7):545–552. doi: 10.1038/nrn1705. [DOI] [PubMed] [Google Scholar]
- 14.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144(1-2):28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH. Lack of reward and locomotor stimulation induced by heroin in mu-opioid receptor-deficient mice. European journal of pharmacology. 2002;446(1-3):103–109. doi: 10.1016/s0014-2999(02)01812-5. [DOI] [PubMed] [Google Scholar]
- 16.Craft RM, Clark JL, Hart SP, Pinckney MK. Sex differences in locomotor effects of morphine in the rat. Pharmacology, biochemistry, and behavior. 2006;85(4):850–858. doi: 10.1016/j.pbb.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science. 2001;293(5532):1164–1166. doi: 10.1126/science.1060937. [DOI] [PubMed] [Google Scholar]
- 18.Diederich NJ, Goetz CG. The placebo treatments in neurosciences: New insights from clinical and neuroimaging studies. Neurology. 2008;71(9):677–684. doi: 10.1212/01.wnl.0000324635.49971.3d. [DOI] [PubMed] [Google Scholar]
- 19.Dinnerstein AJ, Lowenthal M, Blitz B. The interaction of drugs with placebos in the control of pain and anxiety. Perspectives in biology and medicine. 1966;10(1):103–117. doi: 10.1353/pbm.1966.0031. [DOI] [PubMed] [Google Scholar]
- 20.Dum J, Herz A. Endorphinergic modulation of neural reward systems indicated by behavioral changes. Pharmacology, biochemistry, and behavior. 1984;21(2):259–266. doi: 10.1016/0091-3057(84)90224-7. [DOI] [PubMed] [Google Scholar]
- 21.Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63(4):533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59(2):195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Filliol D, Ghozland S, Chluba J, Martin M, Matthes HW, Simonin F, Befort K, Gaveriaux-Ruff C, Dierich A, LeMeur M, Valverde O, Maldonado R, Kieffer BL. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nature genetics. 2000;25(2):195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 24.Flor H, Birbaumer N, Schulz R, Grusser SM, Mucha RF. Pavlovian conditioning of opioid and nonopioid pain inhibitory mechanisms in humans. Eur J Pain. 2002;6(5):395–402. doi: 10.1016/s1090-3801(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 25.Gardner EL. Addiction and brain reward and antireward pathways. Advances in psychosomatic medicine. 2011;30:22–60. doi: 10.1159/000324065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giang DW, Goodman AD, Schiffer RB, Mattson DH, Petrie M, Cohen N, Ader R. Conditioning of cyclophosphamide-induced leukopenia in humans. The Journal of neuropsychiatry and clinical neurosciences. 1996;8(2):194–201. doi: 10.1176/jnp.8.2.194. [DOI] [PubMed] [Google Scholar]
- 27.Gliedman LH, Gantt WH, Teitelbaum HA. Some implications of conditional reflex studies for placebo research. The American journal of psychiatry. 1957;113(12):1103–1107. doi: 10.1176/ajp.113.12.1103. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein AP. Participant expectancies in psychotherapy. Psychiatry. 1962;25:72–79. doi: 10.1080/00332747.1962.11023298. [DOI] [PubMed] [Google Scholar]
- 29.Guo JY, Wang JY, Luo F. Dissection of placebo analgesia in mice: the conditions for activation of opioid and non-opioid systems. J Psychopharmacol. 2010;24(10):1561–1567. doi: 10.1177/0269881109104848. [DOI] [PubMed] [Google Scholar]
- 30.Hecht A, Schiorring E. Behavioral effects of low and high acute doses of morphine in solitary mice. Psychopharmacology. 1979;64(1):73–79. doi: 10.1007/BF00427348. [DOI] [PubMed] [Google Scholar]
- 31.Herrnstein RJ. Placebo effect in the rat. Science. 1962;138:677–678. doi: 10.1126/science.138.3541.677. [DOI] [PubMed] [Google Scholar]
- 32.Hunsley J, Westmacott R. Interpreting the magnitude of the placebo effect: mountain or Molehill? Journal of clinical psychology. 2007;63(4):391–399. doi: 10.1002/jclp.20352. [DOI] [PubMed] [Google Scholar]
- 33.Johnson PI, Stellar JR, Paul AD. Regional reward differences within the ventral pallidum are revealed by microinjections of a mu opiate receptor agonist. Neuropharmacology. 1993;32(12):1305–1314. doi: 10.1016/0028-3908(93)90025-x. [DOI] [PubMed] [Google Scholar]
- 34.Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128(1-2):31–39. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain practice : the official journal of World Institute of Pain. 2007;7(1):4–20. doi: 10.1111/j.1533-2500.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- 36.Krummenacher P, Candia V, Folkers G, Schedlowski M, Schonbachler G. Prefrontal cortex modulates placebo analgesia. Pain. 2010;148(3):368–374. doi: 10.1016/j.pain.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 37.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiological reviews. 2009;89(4):1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine JD, Gordon NC, Bornstein JC, Fields HL. Role of pain in placebo analgesia. Proceedings of the National Academy of Sciences of the United States of America. 1979;76(7):3528–3531. doi: 10.1073/pnas.76.7.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2(8091):654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 40.Levine JD, Gordon NC, Smith R, Fields HL. Analgesic responses to morphine and placebo in individuals with postoperative pain. Pain. 1981;10(3):379–389. doi: 10.1016/0304-3959(81)90099-3. [DOI] [PubMed] [Google Scholar]
- 41.Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383(6603):819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- 42.Merskey H. The taxonomy of pain. The Medical clinics of North America. 2007;91(1):13–20. vii. doi: 10.1016/j.mcna.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 43.Morley JE, Levine AS, Yim GK, Lowy MT. Opioid modulation of appetite. Neuroscience and biobehavioral reviews. 1983;7(2):281–305. doi: 10.1016/0149-7634(83)90020-9. [DOI] [PubMed] [Google Scholar]
- 44.Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain research. 1986;397(2):214–224. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- 45.Neubert JK, King C, Malphurs W, Wong F, Weaver JP, Jenkins AC, Rossi HL, Caudle RM. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Molecular pain. 2008;4:43. doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neubert JK, Rossi HL, Malphurs W, Vierck CJ, Jr., Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural brain research. 2006;170(2):308–315. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr., Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116(3):386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Nolan TA, Caudle RM, Neubert JK. Effect of caloric and non-caloric sweet reward solutions on thermal facial operant conditioning. Behavioural brain research. 2011;216(2):723–725. doi: 10.1016/j.bbr.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. Journal of developmental and behavioral pediatrics : JDBP. 1992;13(2):124–125. doi: 10.1097/00004703-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Pollo A, Benedetti F. The placebo response: neurobiological and clinical issues of neurological relevance. Progress in brain research. 2009;175:283–294. doi: 10.1016/S0079-6123(09)17520-9. [DOI] [PubMed] [Google Scholar]
- 51.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127(1-2):63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annual review of psychology. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 53.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83(2):147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 54.Qiu YH, Wu XY, Xu H, Sackett D. Neuroimaging study of placebo analgesia in humans. Neuroscience bulletin. 2009;25(5):277–282. doi: 10.1007/s12264-009-0907-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96(1):103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 56.Rossi HL, Vierck CJ, Jr., Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Molecular pain. 2006;2:37. doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Treede RD, Kenshalo DR, Gracely RH, Jones AK. The cortical representation of pain. Pain. 1999;79(2-3):105–111. doi: 10.1016/s0304-3959(98)00184-5. [DOI] [PubMed] [Google Scholar]
- 58.Tulunay FC, Ayhan IH, Sparber SB. The effects of morphine and delta-9-tetrahydrocannabinol on motor activity in rats. Psychopharmacology. 1982;78(4):358–360. doi: 10.1007/BF00433741. [DOI] [PubMed] [Google Scholar]
- 59.Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Current topics in behavioral neurosciences. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- 60.Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99(3):443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 61.Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(36):11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 63.Weitemier AZ, Murphy NP. Accumbal dopamine and serotonin activity throughout acquisition and expression of place conditioning: correlative relationships with preference and aversion. The European journal of neuroscience. 2009;29(5):1015–1026. doi: 10.1111/j.1460-9568.2009.06652.x. [DOI] [PubMed] [Google Scholar]
- 64.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 65.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Annals of the New York Academy of Sciences. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


