Abstract
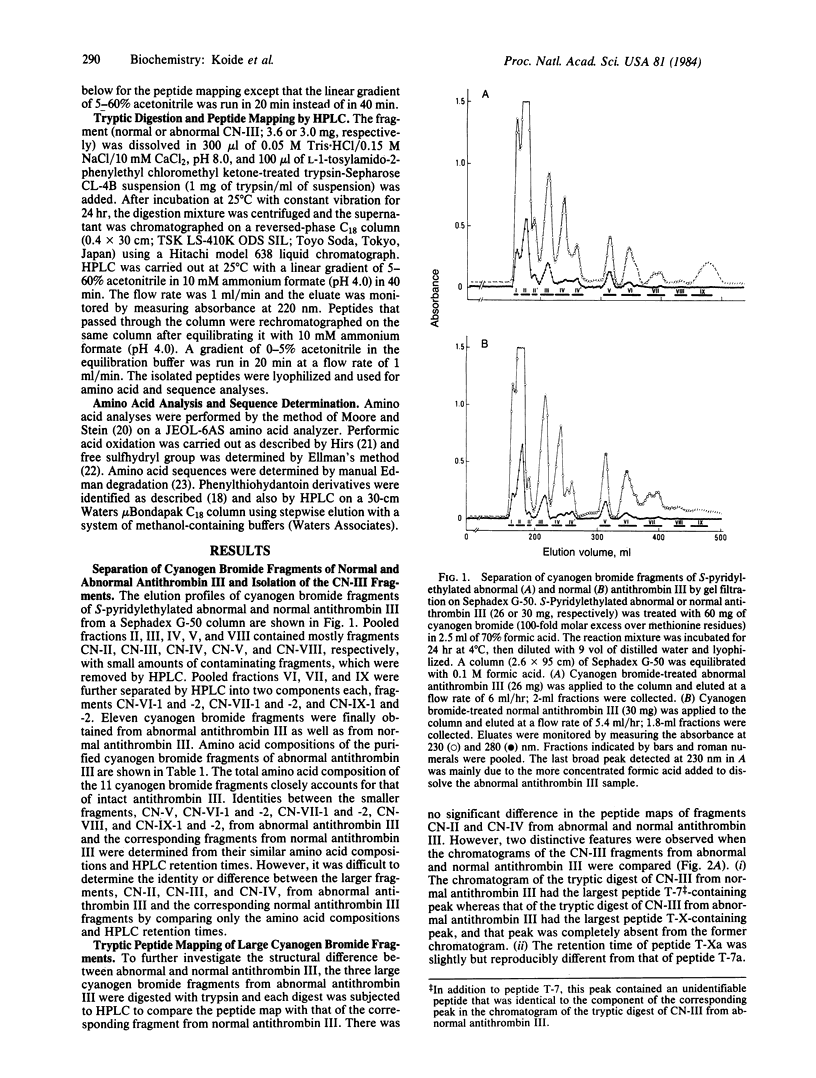
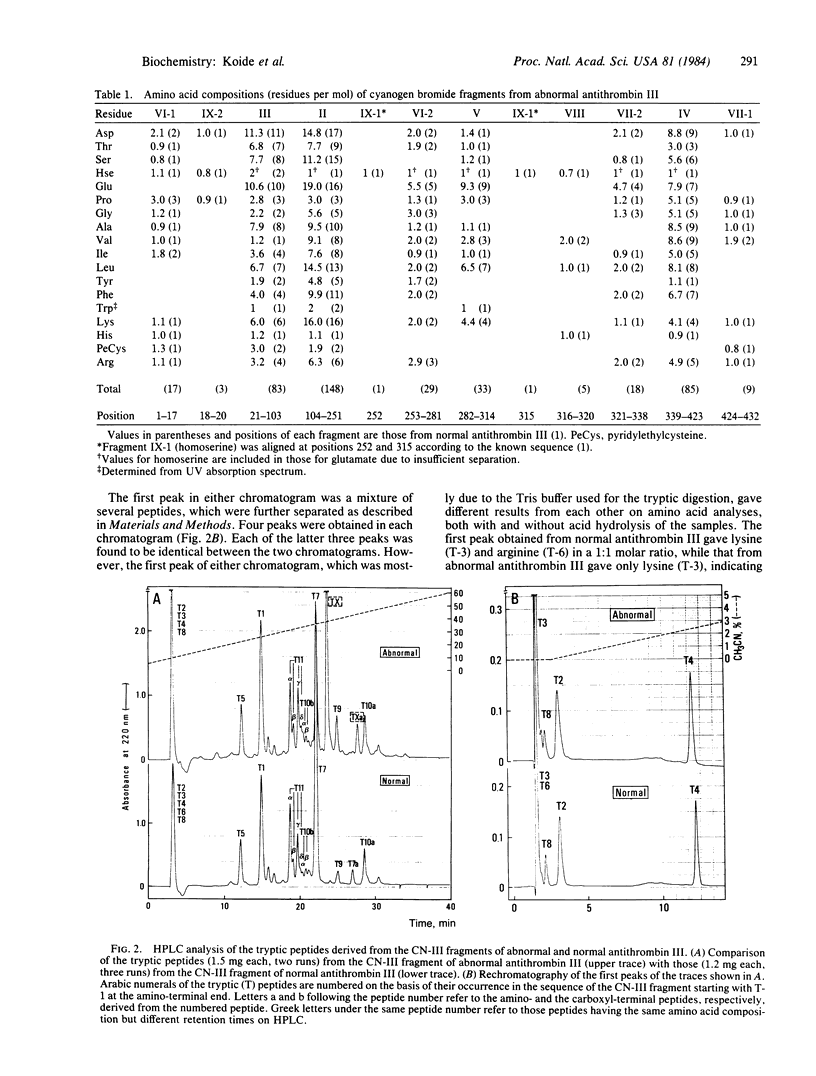
Structural analyses of a hereditary abnormal antithrombin III, antithrombin III Toyama, which has normal progressive antithrombin activity but no heparin cofactor activity, have been carried out to elucidate the molecular abnormality causing recurrent thrombophlebitis of a patient and to identify an amino acid residue essential for the binding with heparin. Abnormal antithrombin III was reduced, S-pyridylethylated, and treated with cyanogen bromide. Eleven fragments were isolated by the combination of Sephadex G-50 gel filtration and reversed-phase HPLC and compared with those from normal antithrombin III. One large fragment (CN-III) that appeared to have a different amino acid composition from that of the corresponding fragment from normal antithrombin III was digested with trypsin, and the digests were separated by HPLC. The abnormal peptide was identified by comparing the peptide map with that from normal antithrombin III. Amino acid sequence analysis of the abnormal peptide indicated that the arginine-47 of normal antithrombin III had been replaced by cysteine in antithrombin III Toyama. One base mutation, C leads to T, in the 5' terminal position of the arginine-47 genetic codon (CGT) is probably responsible for this substitution. These results also suggest that arginine-47 is an essential amino acid residue for the binding with heparin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björk I., Jackson C. M., Jörnvall H., Lavine K. K., Nordling K., Salsgiver W. J. The active site of antithrombin. Release of the same proteolytically cleaved form of the inhibitor from complexes with factor IXa, factor Xa, and thrombin. J Biol Chem. 1982 Mar 10;257(5):2406–2411. [PubMed] [Google Scholar]
- Brozovic M., Stirling Y., Hamlyn A. N. Thrombotic tendency and probable antithrombin III deficiency. Thromb Haemost. 1978 Jun 30;39(3):778–779. [PubMed] [Google Scholar]
- Chandra T., Stackhouse R., Kidd V. J., Woo S. L. Isolation and sequence characterization of a cDNA clone of human antithrombin III. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1845–1848. doi: 10.1073/pnas.80.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGEBERG O. INHERITED ANTITHROMBIN DEFICIENCY CAUSING THROMBOPHILIA. Thromb Diath Haemorrh. 1965 Jun 15;13:516–530. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Iwanaga S., Wallén P., Gröndahl N. J., Henschen A., Blombäck B. On the primary structure of human fibrinogen. Isolation and characterization of N-terminal fragments from plasmic digests. Eur J Biochem. 1969 Mar;8(2):189–199. doi: 10.1111/j.1432-1033.1969.tb00514.x. [DOI] [PubMed] [Google Scholar]
- Koide T. Isolation and characterization of antithrombin III from human, porcine and rabbit plasma, and rat serum. J Biochem. 1979 Dec;86(6):1841–1850. doi: 10.1093/oxfordjournals.jbchem.a132706. [DOI] [PubMed] [Google Scholar]
- Koide T., Odani S., Ono T. The N-terminal sequence of human plasma histidine-rich glycoprotein homologous to antithrombin with high affinity for heparin. FEBS Lett. 1982 May 17;141(2):222–224. doi: 10.1016/0014-5793(82)80052-5. [DOI] [PubMed] [Google Scholar]
- Koide T., Takahashi K., Odani S., Ono T., Sakuragawa N. Isolation and characterization of a hereditary abnormal antithrombin III 'Antithrombin III Toyama'. Thromb Res. 1983 Jul 15;31(2):319–328. doi: 10.1016/0049-3848(83)90334-1. [DOI] [PubMed] [Google Scholar]
- Longas M. O., Finlay T. H. The covalent nature of the human antithrombin III--thrombin bond. Biochem J. 1980 Sep 1;189(3):481–489. doi: 10.1042/bj1890481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuochi T., Fujii J., Kurachi K., Kobata A. Structural studies of the carbohydrate moiety of human antithrombin III. Arch Biochem Biophys. 1980 Aug;203(1):458–465. doi: 10.1016/0003-9861(80)90199-x. [DOI] [PubMed] [Google Scholar]
- Morii M., Odani S., Koide T., Ikenaka T. Human alpha1-antitrypsin. Characterization and N- and C-terminal sequences. J Biochem. 1978 Jan;83(1):269–277. doi: 10.1093/oxfordjournals.jbchem.a131900. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Sakuragawa N., Takahashi K., Kondo S., Koide T. Antithrombin III Toyama: a hereditary abnormal antithrombin III of a patient with recurrent thrombophlebitis. Thromb Res. 1983 Jul 15;31(2):305–317. doi: 10.1016/0049-3848(83)90333-x. [DOI] [PubMed] [Google Scholar]
- Sørensen P. J., Dyerberg J., Stoffersen E., Jensen M. K. Familial functional antithrombin III deficiency. Scand J Haematol. 1980 Feb;24(2):105–109. doi: 10.1111/j.1600-0609.1980.tb02352.x. [DOI] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus D. W., Blank M. K. Heparin cofactor II. Purification and properties of a heparin-dependent inhibitor of thrombin in human plasma. J Biol Chem. 1982 Mar 10;257(5):2162–2169. [PubMed] [Google Scholar]
- Tran T. H., Bounameaux H., Bondeli C., Honkanen H., Marbet G. A., Duckert F. Purification and partial characterization of a hereditary abnormal antithrombin III fraction of a patient with recurrent thrombophlebitis. Thromb Haemost. 1980 Oct 31;44(2):87–91. [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]


