Abstract
A commonly held view of the mechanism of flavin mixed-function oxidases is that enzyme-bound 4a-hydroperoxyflavin (4a-FIHOOH) undergoes ring opening to provide a carbonyl oxide (IV), which, after transferring an oxene equivalent to substrate, yields a 6-arylamino-5-oxo(3H,5H)-uracil (I). The latter is then thought to undergo ring closure to form a 4a-hydroxyflavin (4a-FIHOH), which by loss of water yields flavin (scheme I). A close structural analogue of I (i.e., III) has been synthesized. Comparison of the spectra of III (and II), taken in solvents of widely differing dielectric constants and in a strongly basic medium, with those of the intermediate(s) observed to be formed in time between 4a-FlHOOH and 4a-FlHOH has shown that the enzyme-bound intermediate(s) does not resemble spectrally I nor its iminol tautomers.
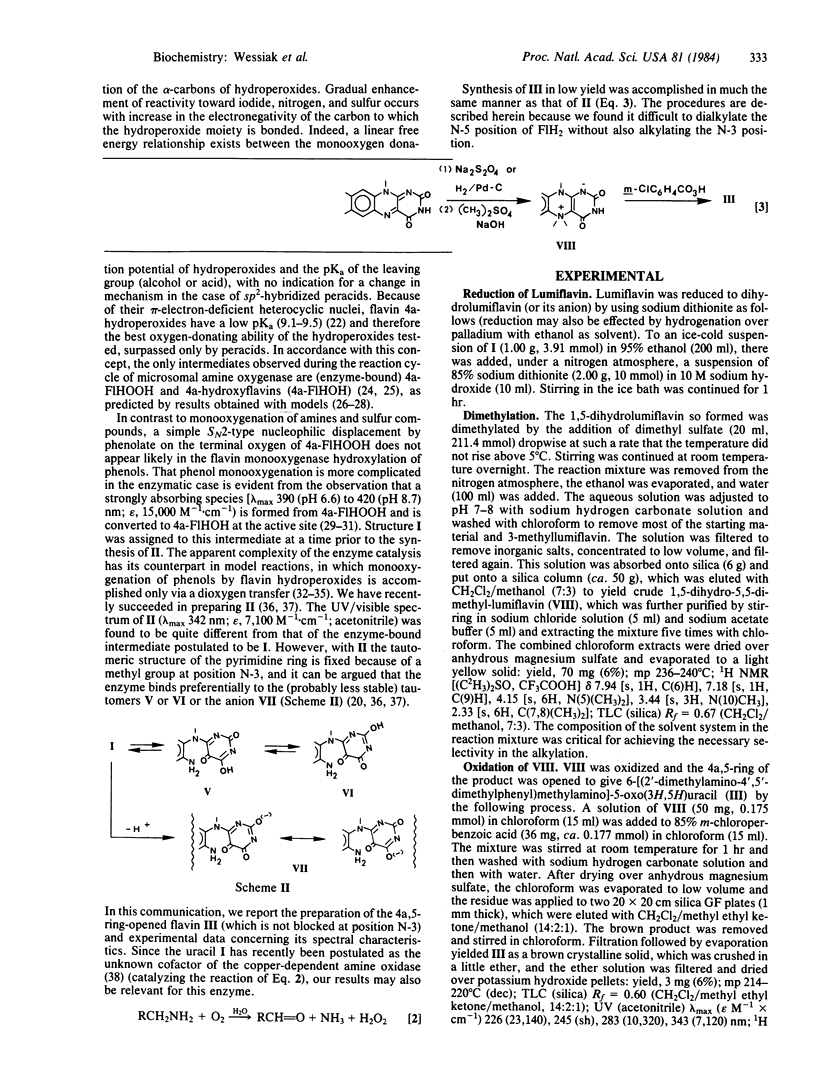
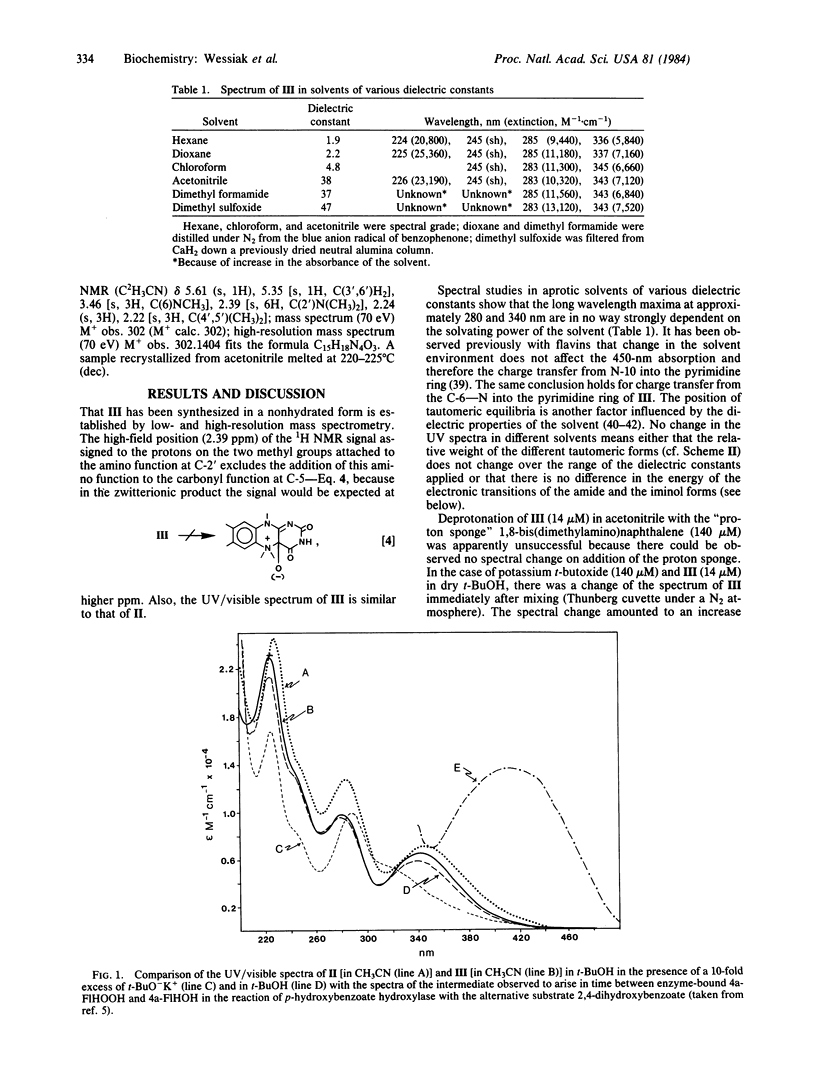
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaty N. B., Ballou D. P. The oxidative half-reaction of liver microsomal FAD-containing monooxygenase. J Biol Chem. 1981 May 10;256(9):4619–4625. [PubMed] [Google Scholar]
- Entsch B., Ballou D. P., Massey V. Flavin-oxygen derivatives involved in hydroxylation by p-hydroxybenzoate hydroxylase. J Biol Chem. 1976 May 10;251(9):2550–2563. [PubMed] [Google Scholar]
- Eweg J. K., Müller F., van Berkel W. J. On the enigma of old yellow enzyme's spectral properties. Eur J Biochem. 1982 Dec 15;129(2):303–316. doi: 10.1111/j.1432-1033.1982.tb07053.x. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., HASTINGS J. W. The oxidation of reduced flavin mononucleotide by molecular oxygen. Biochem J. 1962 May;83:368–377. doi: 10.1042/bj0830368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall D. W., Dietrich R. F., Benkovic S. J., Shiman R. Phenylalanine hydroxylase. Correlation of the iron content with activity and the preparation and reconstitution of the apoenzyme. J Biol Chem. 1982 Jan 25;257(2):845–849. [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- Hajjar N. P., Hodgson E. Flavin adenine dinucleotide--dependent monooxygenase: its role in the sulfoxidation of pesticides in mammals. Science. 1980 Sep 5;209(4461):1134–1136. doi: 10.1126/science.7403873. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Andree P. J., Fisher H. F. Cryoenzymological studies of the oxidative deamination of L-glutamate by glutamate dehydrogenase. Spectral resolution of transient and product complexes. J Biol Chem. 1981 Apr 25;256(8):3817–3821. [PubMed] [Google Scholar]
- Lazarus R. A., Wallick D. E., Dietrich R. F., Gottschall D. W., Benkovic S. J., Gaffney B. J., Shiman R. The mechanism of phenylalanine hydroxylase. Fed Proc. 1982 Jul;41(9):2605–2607. [PubMed] [Google Scholar]
- Massey V., Müller F., Feldberg R., Schuman M., Sullivan P. A., Howell L. G., Mayhew S. G., Matthews R. G., Foust G. P. The reactivity of flavoproteins with sulfite. Possible relevance to the problem of oxygen reactivity. J Biol Chem. 1969 Aug 10;244(15):3999–4006. [PubMed] [Google Scholar]
- Muto K., Yamamoto T., Atsumi K., Abe T., Shimizu K., Nohara Y. [Sequential changes of P loop in hypertension monitored by high-gain atrial vectorcardiography]. Nihon Rinsho. 1980 Nov;38(11):4470–4472. [PubMed] [Google Scholar]
- Poulsen L. L., Ziegler D. M. The liver microsomal FAD-containing monooxygenase. Spectral characterization and kinetic studies. J Biol Chem. 1979 Jul 25;254(14):6449–6455. [PubMed] [Google Scholar]
- SHUGAR D., FOX J. J. Spectrophotometric studies of nucleic acid derivatives and related compounds as a function of pH. I. Pyrimidines. Biochim Biophys Acta. 1952;9(2):199–218. doi: 10.1016/0006-3002(52)90147-9. [DOI] [PubMed] [Google Scholar]
- Schmidt W. On the environment and the rotational motion of amphiphilic flavins in artificial membrane vesicles as studied by fluorescence. J Membr Biol. 1979 May 7;47(1):1–25. doi: 10.1007/BF01869044. [DOI] [PubMed] [Google Scholar]
- Wierzchowski K. L., Litońska E., Shugar D. Infrared and ultraviolet studies on the tautomeric equilibria in aqueous medium between monoanionic species of uracil, thymine, 5-fluorouracil, and other 2,4-diketopyrimidines. J Am Chem Soc. 1965 Oct 20;87(20):4621–4629. doi: 10.1021/ja00948a039. [DOI] [PubMed] [Google Scholar]


