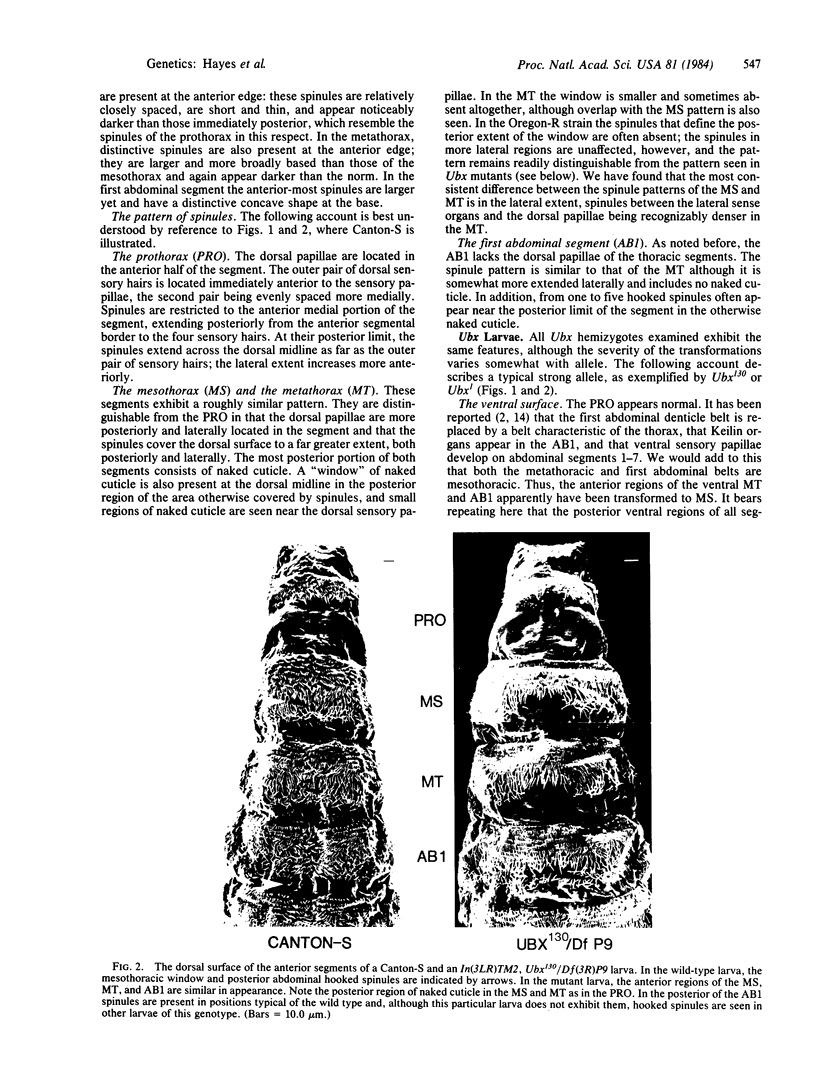
Abstract
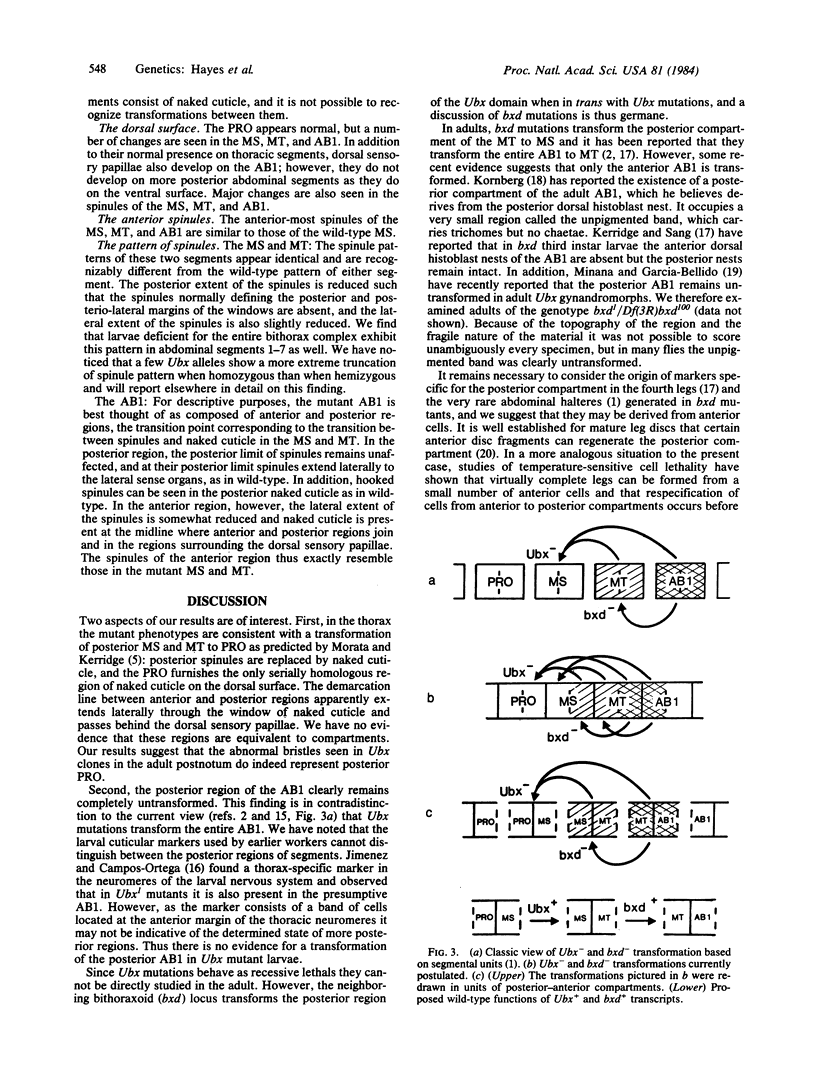
Recent results [Morata, G. & Kerridge, S. (1981) Nature (London) 290, 778-781] have shown that early Ultrabithorax- clones transform the posterior compartments of the adult meso- and metathoracic legs to prothorax. These transformations have not been seen in Ultrabithorax homozygous larvae, which are reported to show only transformations of the metathorax and the first abdominal segment to mesothorax [Lewis, E. B. (1978) Nature (London) 276, 565-570]. However, as the ventral surface of the larva does not exhibit sufficient markers to distinguish the posterior regions of these segments, cryptic larval transformations similar to those in the adult have been suggested (by Morata and Kerridge). We have further examined larvae of wild-type and various Ultrabithorax mutant genotypes, with particular attention to the dorsal surface. We find that Ultrabithorax homozygous larvae exhibit dorsal abnormalities consistent with transformations of the anterior metathorax and anterior first abdominal segment to mesothorax and of the posterior meso- and metathorax to prothorax as predicted by Morata and Kerridge; however, the posterior of the first abdominal segment remains untransformed. We suggest that in both larvae and adults the posterior first abdominal segment remains untransformed by Ultrabithorax mutations and that the unit of development with regard to the proximal bithorax complex consists of adjoining posterior and anterior compartments from neighboring segments rather than of segments themselves.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. Mutants of the bithorax complex and determinative states in the thorax of Drosophila melanogaster. Dev Biol. 1978 Aug;65(2):447–461. doi: 10.1016/0012-1606(78)90040-4. [DOI] [PubMed] [Google Scholar]
- Bender W., Akam M., Karch F., Beachy P. A., Peifer M., Spierer P., Lewis E. B., Hogness D. S. Molecular Genetics of the Bithorax Complex in Drosophila melanogaster. Science. 1983 Jul 1;221(4605):23–29. doi: 10.1126/science.221.4605.23. [DOI] [PubMed] [Google Scholar]
- Bjerke J. M., Freeman T. P., Anderson A. W. A new method of preparing insects for scanning electron microscopy. Stain Technol. 1979 Jan;54(1):29–31. doi: 10.3109/10520297909110672. [DOI] [PubMed] [Google Scholar]
- Girton J. R., Russell M. A. An analysis of compartmentalization in pattern duplications induced by a cell-lethal mutation in Drosophila. Dev Biol. 1981 Jul 15;85(1):55–64. doi: 10.1016/0012-1606(81)90235-9. [DOI] [PubMed] [Google Scholar]
- Kerridge S., Morata G. Developmental effects of some newly induced Ultrabithorax alleles of Drosophila. J Embryol Exp Morphol. 1982 Apr;68:211–234. [PubMed] [Google Scholar]
- Kerridge S., Sang J. H. Developmental analysis of the homoeotic mutation bithoraxoid of Drosophila melanogaster. J Embryol Exp Morphol. 1981 Feb;61:69–86. [PubMed] [Google Scholar]
- Kornberg T. Compartments in the abdomen of Drosophila and the role of the engrailed locus. Dev Biol. 1981 Sep;86(2):363–372. doi: 10.1016/0012-1606(81)90194-9. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G. Further studies of the engrailed phenotype in Drosophila. EMBO J. 1982;1(7):827–833. doi: 10.1002/j.1460-2075.1982.tb01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. B. A gene complex controlling segmentation in Drosophila. Nature. 1978 Dec 7;276(5688):565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lohs-Schardin M., Cremer C., Nüsslein-Volhard C. A fate map for the larval epidermis of Drosophila melanogaster: localized cuticle defects following irradiation of the blastoderm with an ultraviolet laser microbeam. Dev Biol. 1979 Dec;73(2):239–255. doi: 10.1016/0012-1606(79)90065-4. [DOI] [PubMed] [Google Scholar]
- Morata G., Kerridge S. Sequential functions of the bithorax complex of Drosophila. Nature. 1981 Apr 30;290(5809):778–781. doi: 10.1038/290778a0. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980 Oct 30;287(5785):795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Schubiger G. Regeneration, duplication and transdetermination in fragments of the leg disc of Drosophila melanogaster. Dev Biol. 1971 Oct;26(2):277–295. doi: 10.1016/0012-1606(71)90127-8. [DOI] [PubMed] [Google Scholar]
- Szabad J., Schüpbach T., Wieschaus E. Cell lineage and development in the larval epidermis of Drosophila melanogaster. Dev Biol. 1979 Dec;73(2):256–271. doi: 10.1016/0012-1606(79)90066-6. [DOI] [PubMed] [Google Scholar]
- Wieschaus E., Gehring W. Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Dev Biol. 1976 Jun;50(2):249–263. doi: 10.1016/0012-1606(76)90150-0. [DOI] [PubMed] [Google Scholar]
- Wright D. A., Lawrence P. A. Regeneration of the segment boundary in Oncopeltus. Dev Biol. 1981 Jul 30;85(2):317–327. doi: 10.1016/0012-1606(81)90263-3. [DOI] [PubMed] [Google Scholar]