Abstract
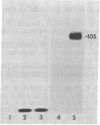
Mouse monoclonal antibodies to several cell surface antigens of human ovarian and endometrial carcinomas have been produced. The distribution of the antigens was determined by mixed hemagglutination assays on 153 normal and malignant cell cultures of various types and by immuno-peroxidase staining of frozen sections of 27 normal adult and 24 fetal tissues. Five distinct antigens were characterized. MD144 antigen was detected on only a single ovarian carcinoma cell line and has the biochemical properties of a lipid. MH55 antigen is weakly expressed on ovarian and uterine cancer cell lines but not on other cells and tissues tested. MF61 antigen was detected on an ovarian carcinoma and some renal carcinoma cell lines but not on other cell lines tested. It was also detected by immunoperoxidase staining in the noncellular follicles of the thyroid and in uterine glandular epithelial cells. This antigen also has the properties of a lipid. MF116 antigen was detected on a proportion of ovarian, uterine, renal, and bladder carcinoma and neuroblastoma cell lines and on normal kidney epithelial cell cultures but not on other cell lines tested. It was not detected in sections of any normal tissue tested using the immunoperoxidase method. MF116 was readily detected in the spent culture medium but not in detergent-solubilized extracts of metabolically radiolabeled cells. This shed antigen is a glycoprotein of Mr 105,000 and isoelectric point lower than pH 4.0. MH94 antigen was detected on a proportion of ovarian, uterine, colon, breast, lung, cervical, and pancreatic carcinoma cell lines. In tissue sections it was detected in many but not all epithelia, predominantly in secretory epithelial cells. Antibody MH94 did not immunoprecipitate a detectable antigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Audubert F., Semmel M. Lipophilic proteins extracted from chick embryo cell cultures by different methods. Biochem Biophys Res Commun. 1979 Nov 28;91(2):416–426. doi: 10.1016/0006-291x(79)91538-9. [DOI] [PubMed] [Google Scholar]
- BALFOUR B. M., DONLACH D., ROITT I. M., COUCHMAN K. G. Fluorescent antibody studies in human thyroiditis: auto-antibodies to an antigen of the thyroid colloid distinct from thyroglobulin. Br J Exp Pathol. 1961 Aug;42:307–316. [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. W. Immunological aspects of chemical carcinogenesis. Adv Cancer Res. 1973;18:1–75. doi: 10.1016/s0065-230x(08)60750-2. [DOI] [PubMed] [Google Scholar]
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya M., Chatterjee S. K., Barlow J. J., Fuji H. Monoclonal antibodies recognizing tumor-associated antigen of human ovarian mucinous cystadenocarcinomas. Cancer Res. 1982 May;42(5):1650–1654. [PubMed] [Google Scholar]
- Cairncross J. G., Mattes M. J., Beresford H. R., Albino A. P., Houghton A. N., Lloyd K. O., Old L. J. Cell surface antigens of human astrocytoma defined by mouse monoclonal antibodies: identification of astrocytoma subsets. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5641–5645. doi: 10.1073/pnas.79.18.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Lloyd K. O., Takahashi T., Travassos L. R., Old L. J. AU cell-surface antigen of human malignant melanoma: solubilization and partial characterization. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2898–2902. doi: 10.1073/pnas.76.6.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey T. E., Takahashi T., Resnick L. A., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: mixed hemadsorption assays for humoral immunity to cultured autologous melanoma cells. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3278–3282. doi: 10.1073/pnas.73.9.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dippold W. G., Lloyd K. O., Li L. T., Ikeda H., Oettgen H. F., Old L. J. Cell surface antigens of human malignant melanoma: definition of six antigenic systems with mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6114–6118. doi: 10.1073/pnas.77.10.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois G. C., Law L. W., Appella E. Purification and biochemical properties of tumor-associated transplantation antigens from methylcholanthrene-induced murine sarcomas. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7669–7673. doi: 10.1073/pnas.79.24.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Nakane P. K. Immunohistochemistry with enzyme labeled antibodies: a brief review. J Immunol Methods. 1981;47(2):129–144. doi: 10.1016/0022-1759(81)90114-9. [DOI] [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Welch W. R., Knapp R. C., Colvin R. B. Immunopathologic characterization of a monoclonal antibody that recognizes common surface antigens of human ovarian tumors of serous, endometrioid, and clear cell types. Am J Clin Pathol. 1983 Jan;79(1):98–104. doi: 10.1093/ajcp/79.1.98. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lloyd K. O., Ng J., Dippold W. G. Analysis of the biosynthesis of HLA-DR glycoproteins in human malignant melanoma cell lines. J Immunol. 1981 Jun;126(6):2408–2413. [PubMed] [Google Scholar]
- Magnani J. L., Brockhaus M., Smith D. F., Ginsburg V., Blaszczyk M., Mitchell K. F., Steplewski Z., Koprowski H. A monosialoganglioside is a monoclonal antibody-defined antigen of colon carcinoma. Science. 1981 Apr 3;212(4490):55–56. doi: 10.1126/science.7209516. [DOI] [PubMed] [Google Scholar]
- Mattes M. J., Cairncross J. G., Old L. J., Lloyd K. O. Monoclonal antibodies to three widely distributed human cell surface antigens. Hybridoma. 1983;2(3):253–264. doi: 10.1089/hyb.1983.2.253. [DOI] [PubMed] [Google Scholar]
- Mattes M. J., Sharrow S. O., Herberman R. B., Holden H. T. Identification and separation of Thy-1 positive mouse spleen cells active in natural cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Immunol. 1979 Dec;123(6):2851–2860. [PubMed] [Google Scholar]
- Mattes M. J., Tanimoto M., Pollack M. S., Maurer D. H. Preparing monolayers of non-adherent mammalian cells. J Immunol Methods. 1983 Jul 15;61(2):145–150. doi: 10.1016/0022-1759(83)90156-4. [DOI] [PubMed] [Google Scholar]
- Nudelman E., Hakomori S., Kannagi R., Levery S., Yeh M. Y., Hellström K. E., Hellström I. Characterization of a human melanoma-associated ganglioside antigen defined by a monoclonal antibody, 4.2. J Biol Chem. 1982 Nov 10;257(21):12752–12756. [PubMed] [Google Scholar]
- Ogata S., Ueda R., Lloyd K. O. Comparison of [3H]glucosamine-labeled glycoproteins from human renal cancer and normal kidney epithelial cell cultures by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1981 Feb;78(2):770–774. doi: 10.1073/pnas.78.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old L. J. Cancer immunology: the search for specificity--G. H. A. Clowes Memorial lecture. Cancer Res. 1981 Feb;41(2):361–375. [PubMed] [Google Scholar]
- Pukel C. S., Lloyd K. O., Travassos L. R., Dippold W. G., Oettgen H. F., Old L. J. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. J Exp Med. 1982 Apr 1;155(4):1133–1147. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda R., Shiku H., Pfreundschuh M., Takahashi T., Li L. T., Whitmore W. F., Oettgen H. F., Old L. J. Cell surface antigens of human renal cancer defined by autologous typing. J Exp Med. 1979 Sep 19;150(3):564–579. doi: 10.1084/jem.150.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]