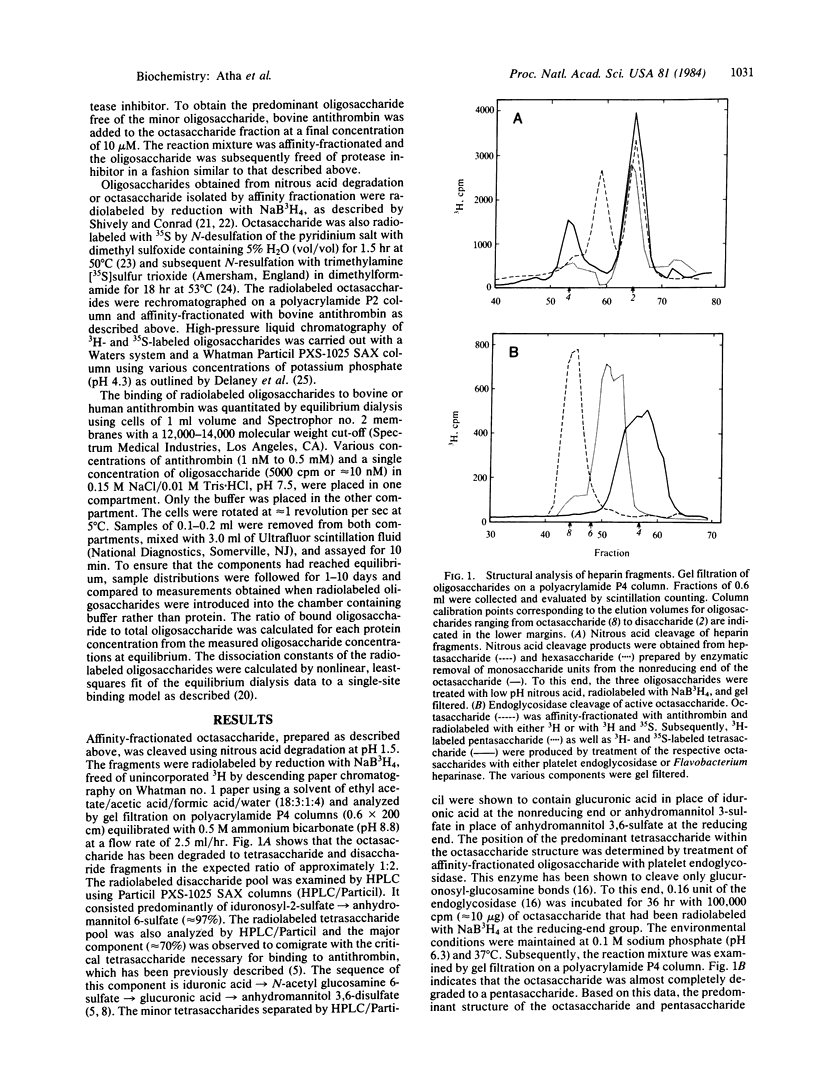
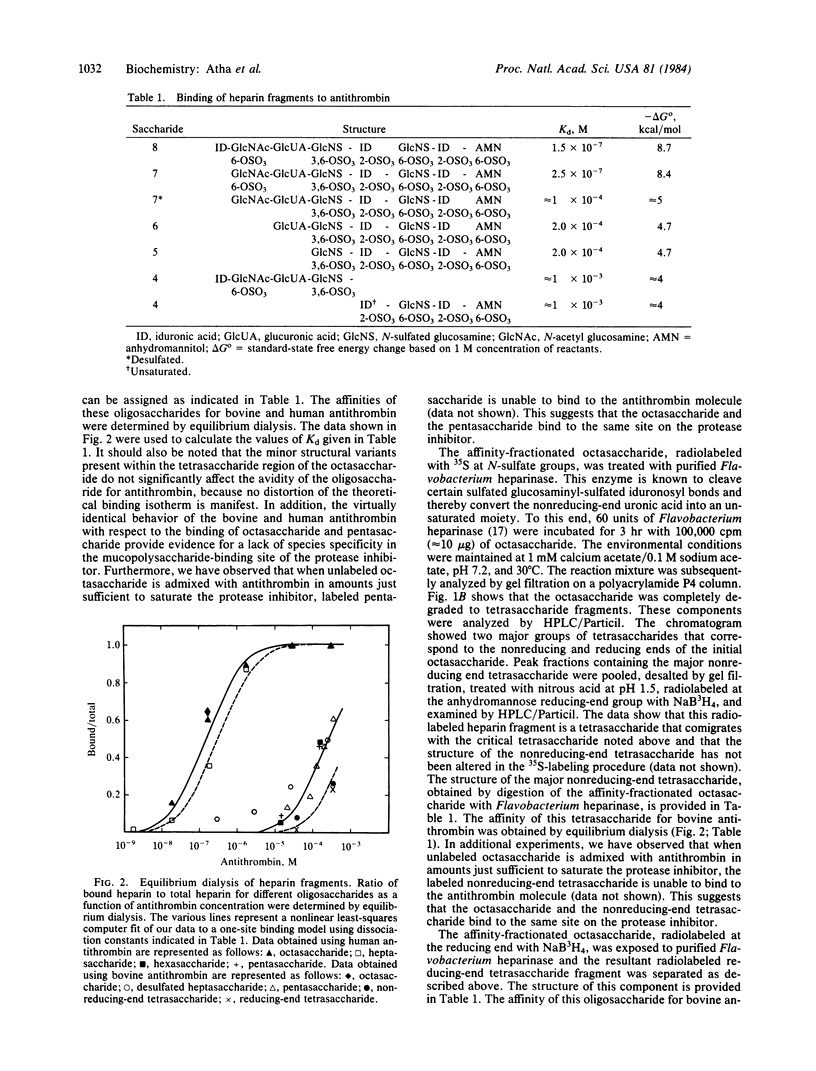
Abstract
We have examined the quantitative importance of various monosaccharide residues of an octasaccharide domain of heparin that are responsible for the binding of this oligosaccharide to antithrombin. Different fragments of the octasaccharide were prepared by enzymatic digestion and the avidities of these oligosaccharides for antithrombin were determined by equilibrium dialysis. The data show that the non-reducing-end and the reducing-end tetrasaccharides contribute equally to the binding energy of the octasaccharide. The O6-sulfate group of the N-acetyl glucosamine moiety within the nonreducing-end tetrasaccharide is responsible for approximately equal to 45% of the binding energy of the octasaccharide. Neither the two non-sulfated uronic acid groups that flank this residue nor the N-sulfated glucosamine residue on the reducing end of this tetrasaccharide sequence that bears the unique O3-sulfate substituent contribute significantly to the binding energy of the octasaccharide. We suggest that the lack of sulfation of the two uronic acid moieties within the nonreducing-end tetrasaccharide may be required to permit the N-acetyl glucosamine O6-sulfate group to interact with a specific region on the antithrombin molecule. However, we cannot exclude the possibility that the O3-sulfate group plays an important role in orienting this O6-sulfate group within the nonreducing-end tetrasaccharide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Beeler D., Rosenberg R., Jordan R. Fractionation of low molecular weight heparin species and their interaction with antithrombin. J Biol Chem. 1979 Apr 25;254(8):2902–2913. [PubMed] [Google Scholar]
- Castellot J. J., Jr, Addonizio M. L., Rosenberg R., Karnovsky M. J. Cultured endothelial cells produce a heparinlike inhibitor of smooth muscle cell growth. J Cell Biol. 1981 Aug;90(2):372–379. doi: 10.1083/jcb.90.2.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casu B., Oreste P., Torri G., Zoppetti G., Choay J., Lormeau J. C., Petitou M., Sinäy P. The structure of heparin oligosaccharide fragments with high anti-(factor Xa) activity containing the minimal antithrombin III-binding sequence. Chemical and 13C nuclear-magnetic-resonance studies. Biochem J. 1981 Sep 1;197(3):599–609. doi: 10.1042/bj1970599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choay J., Lormeau J. C., Petitou M., Sinay P., Casu B., Oreste P., Torri G., Gatti G. Anti-Xa active heparin oligosaccharides. Thromb Res. 1980 May 1;18(3-4):573–578. doi: 10.1016/0049-3848(80)90356-4. [DOI] [PubMed] [Google Scholar]
- Damus P. S., Rosenberg R. D. Antithrombin-heparin cofactor. Methods Enzymol. 1976;45:653–669. doi: 10.1016/s0076-6879(76)45056-5. [DOI] [PubMed] [Google Scholar]
- Delaney S. R., Leger M., Conrad H. E. Quantitation of the sulfated disaccharides of heparin by high performance liquid chromatography. Anal Biochem. 1980 Jul 15;106(1):253–261. doi: 10.1016/0003-2697(80)90145-1. [DOI] [PubMed] [Google Scholar]
- Hovingh P., Linker A. The enzymatic degradation of heparin and heparitin sulfate. 3. Purification of a heparitinase and a heparinase from flavobacteria. J Biol Chem. 1970 Nov 25;245(22):6170–6175. [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1976 Jan;46(1):87–95. doi: 10.1016/s0008-6215(00)83533-8. [DOI] [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Leder I. G. A novel 3-O sulfatase from human urine acting on methyl-2-deoxy-2-sulfamino-alphs-D-glucopyranoside 3-sulfate. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1183–1189. doi: 10.1016/0006-291x(80)90544-6. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Hök M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L., Leder I. G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L. The antithrombin-binding sequence in heparin. Identification of an essential 6-O-sulfate group. J Biol Chem. 1983 Aug 25;258(16):9826–9830. [PubMed] [Google Scholar]
- Lloyd A. G., Embery G., Fowler L. J. Studies on heparin degradation. I. Preparation of ( 35 S) sulphamate derivatives for studies on heparin degrading enzymes of mammalian origin. Biochem Pharmacol. 1971 Mar;20(3):637–648. doi: 10.1016/0006-2952(71)90150-x. [DOI] [PubMed] [Google Scholar]
- Nordenman B., Nyström C., Björk I. The size and shape of human and bovine antithrombin III. Eur J Biochem. 1977 Aug 15;78(1):195–203. doi: 10.1111/j.1432-1033.1977.tb11730.x. [DOI] [PubMed] [Google Scholar]
- Oosta G. M., Favreau L. V., Beeler D. L., Rosenberg R. D. Purification and properties of human platelet heparitinase. J Biol Chem. 1982 Oct 10;257(19):11249–11255. [PubMed] [Google Scholar]
- Oosta G. M., Gardner W. T., Beeler D. L., Rosenberg R. D. Multiple functional domains of the heparin molecule. Proc Natl Acad Sci U S A. 1981 Feb;78(2):829–833. doi: 10.1073/pnas.78.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenfeld J., Thunberg L., Hök M., Lindahl U. The antithrombin-binding sequence of heparin. Location of essential N-sulfate groups. J Biol Chem. 1981 Mar 10;256(5):2389–2394. [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Rosenberg R. D., Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1218–1222. doi: 10.1073/pnas.76.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Stoichiometry of the nitrous acid deaminative cleavage of model amino sugar glycosides and glycosaminoglycuronans. Biochemistry. 1970 Jan 6;9(1):33–43. doi: 10.1021/bi00803a005. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]


