Abstract
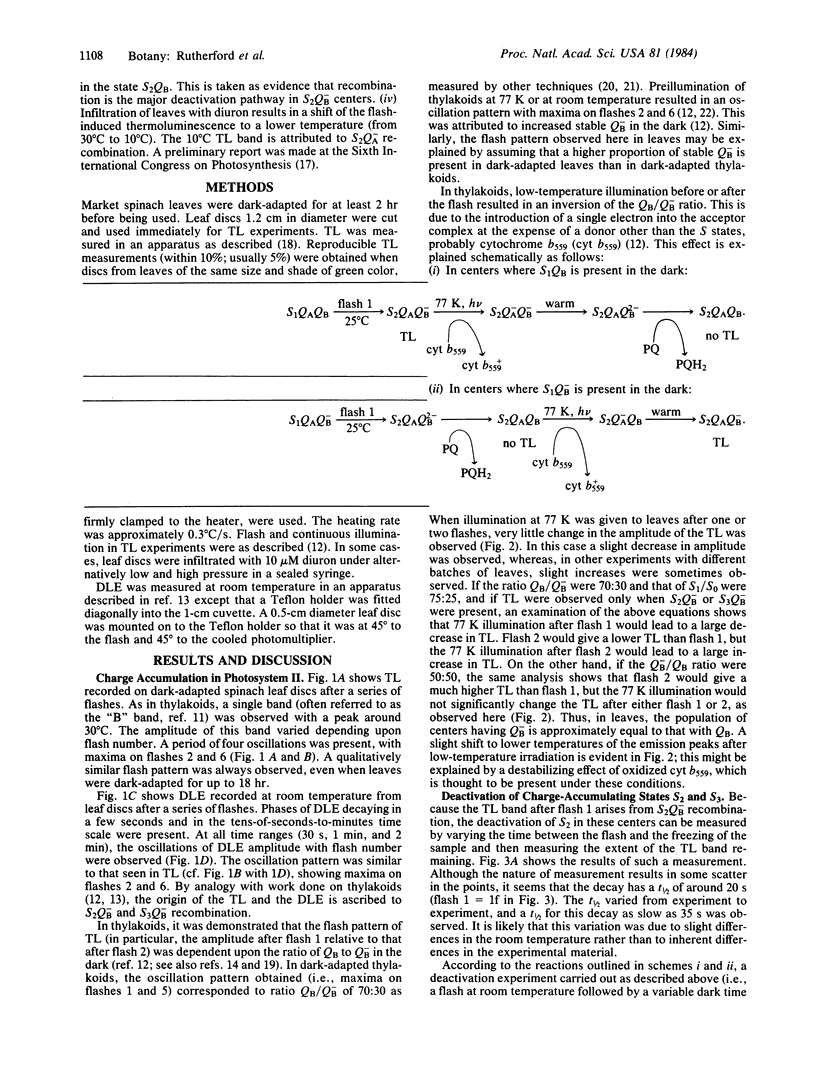
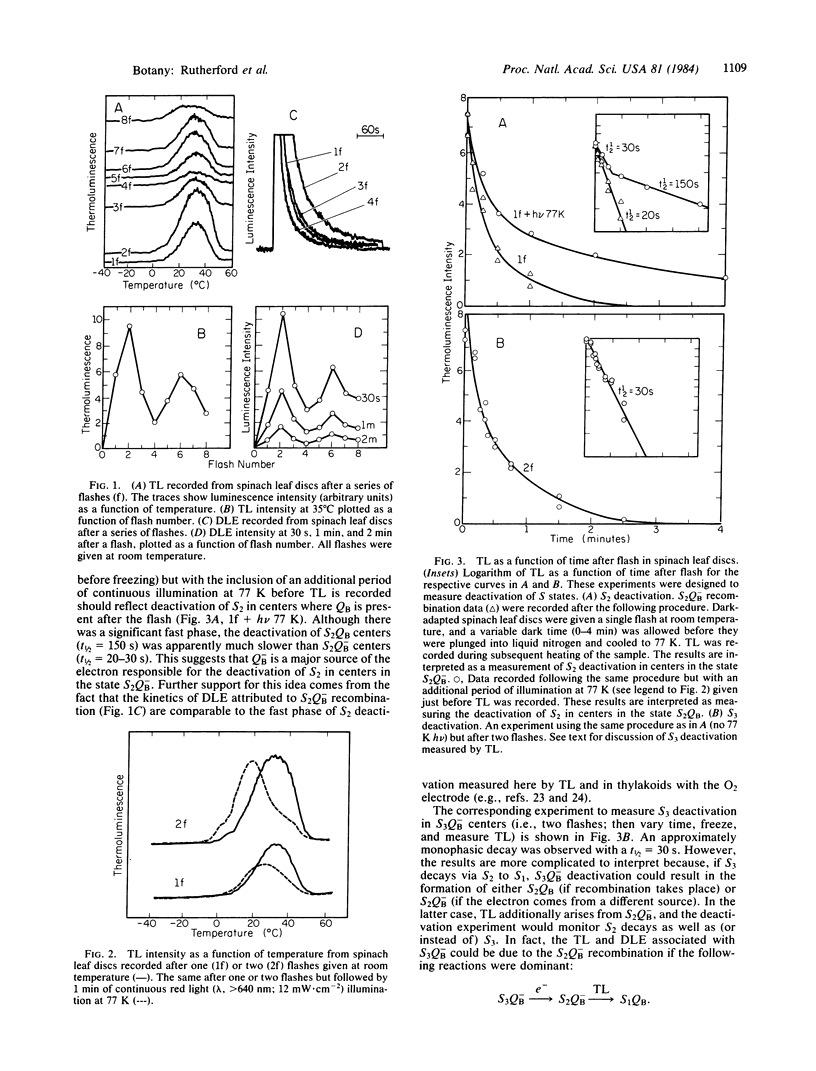
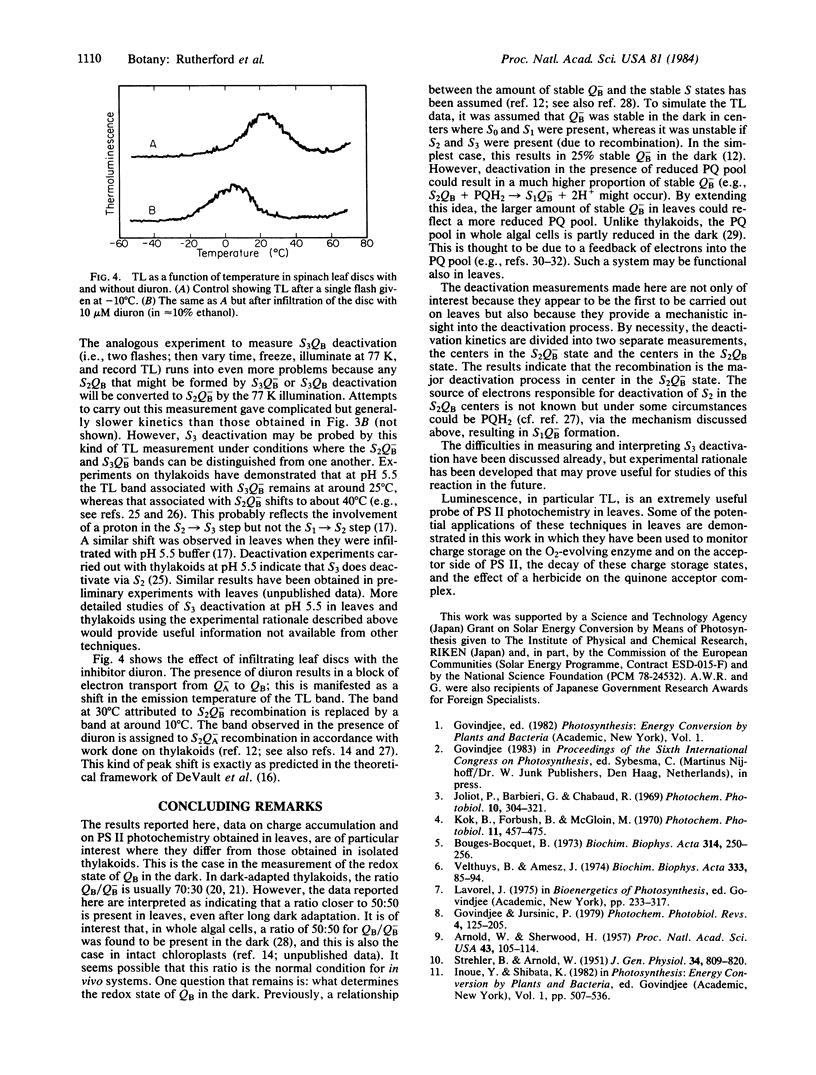
A major breakthrough in our understanding of how plants oxidize water to molecular O2 was the discovery by P. Joliot and co-workers that the O2 yield per flash, in a series of light flashes, oscillates with a periodicity of 4. This led to the concept by B. Kok and co-workers that these reactions involve accumulation of four positive charges in independent “O2-evolving centers,” which undergo a series of changes in their redox state (the so-called S states). In the present paper, we have applied optical techniques (such as thermoluminescence and delayed light emission, both discovered by W. Arnold and co-workers) to monitor charge storage on the O2-evolving system in leaves from higher plants. We observed a period of four oscillations in both thermoluminescence and delayed light emission, with maxima on flashes 2 and 6, establishing a relationship with the charge accumulation process in photosynthesis. These measurements provided additional new information: the deactivation of the “O2-evolving centers,” which cannot be measured by the O2 method in the leaves, is in the 20- to 30-s range; and in the dark-adapted leaves, the secondary bound plastoquinone molecule (the so-called secondary electron acceptor QB) is in equal concentration in its reduced and oxidized forms. The origin of thermoluminescence and delayed light emission, in terms of the recombination of charges on the O2-evolving and plastoquinone sides, is also discussed.
Keywords: photosynthesis, electron transfer, oxygen-evolving enzyme, deactivation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Sherwood H. K. ARE CHLOROPLASTS SEMICONDUCTORS? Proc Natl Acad Sci U S A. 1957 Jan 15;43(1):105–114. doi: 10.1073/pnas.43.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I., Chain R. K. Regulatory electron transport pathways in cyclic photophosphorylation: reduction in C-550 and cytochrome b6 by ferrodoxin in the dark. FEBS Lett. 1979 Jun 1;102(1):133–138. doi: 10.1016/0014-5793(79)80944-8. [DOI] [PubMed] [Google Scholar]
- Bouges-Bocquet B. Electron transfer between the two photosystems in spinach chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):250–256. doi: 10.1016/0005-2728(73)90140-0. [DOI] [PubMed] [Google Scholar]
- Devault D., Govindjee, Arnold W. Energetics of photosynthetic glow peaks. Proc Natl Acad Sci U S A. 1983 Feb;80(4):983–987. doi: 10.1073/pnas.80.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. F. Proton translocation in chloroplasts and its relationship to electron transport between the photosystems. Biochim Biophys Acta. 1977 Mar 11;459(3):351–363. doi: 10.1016/0005-2728(77)90037-8. [DOI] [PubMed] [Google Scholar]
- Ichikawa T., Inoue Y., Shibata K. Characteristics of thermoluminescence bands of intact leaves and isolated chloroplasts in relation to the water-splitting activity in photosynthesis. Biochim Biophys Acta. 1975 Dec 11;408(3):228–239. doi: 10.1016/0005-2728(75)90126-7. [DOI] [PubMed] [Google Scholar]
- Inoue Y. Charging of the A band of thermoluminescence, dependent on the S3 state in isolated chloroplasts. Biochim Biophys Acta. 1981 Feb 12;634(2):309–320. doi: 10.1016/0005-2728(81)90149-3. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Shibata K. Oscillation of thermoluminescence at medium-low temperature. FEBS Lett. 1978 Jan 15;85(2):193–197. doi: 10.1016/0014-5793(78)80453-0. [DOI] [PubMed] [Google Scholar]
- Kok B., Forbush B., McGloin M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem Photobiol. 1970 Jun;11(6):457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- Lavergne J., Etienne A. L. Prompt and delayed fluorescence of chloroplasts upon mixing with dichlorophenyldimethylurea. Biochim Biophys Acta. 1980 Nov 5;593(1):136–148. doi: 10.1016/0005-2728(80)90015-8. [DOI] [PubMed] [Google Scholar]
- Mills J. D., Crowther D., Slovacek R. E., Hind G., McCarty R. E. Electron transport pathways in spinach chloroplasts. Reduction of the primary acceptor of photosystem II by reduced nicotinamide adenine dinucleotide phosphate in the dark. Biochim Biophys Acta. 1979 Jul 10;547(1):127–137. doi: 10.1016/0005-2728(79)90101-4. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., ARNOLD W. Light production by green plants. J Gen Physiol. 1951 Jul;34(6):809–820. doi: 10.1085/jgp.34.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F. A. Determination and modification of the redox state of the secondary acceptor of photosystem II in the dark. Biochim Biophys Acta. 1978 Aug 8;503(2):263–273. doi: 10.1016/0005-2728(78)90187-1. [DOI] [PubMed] [Google Scholar]


