Abstract
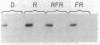
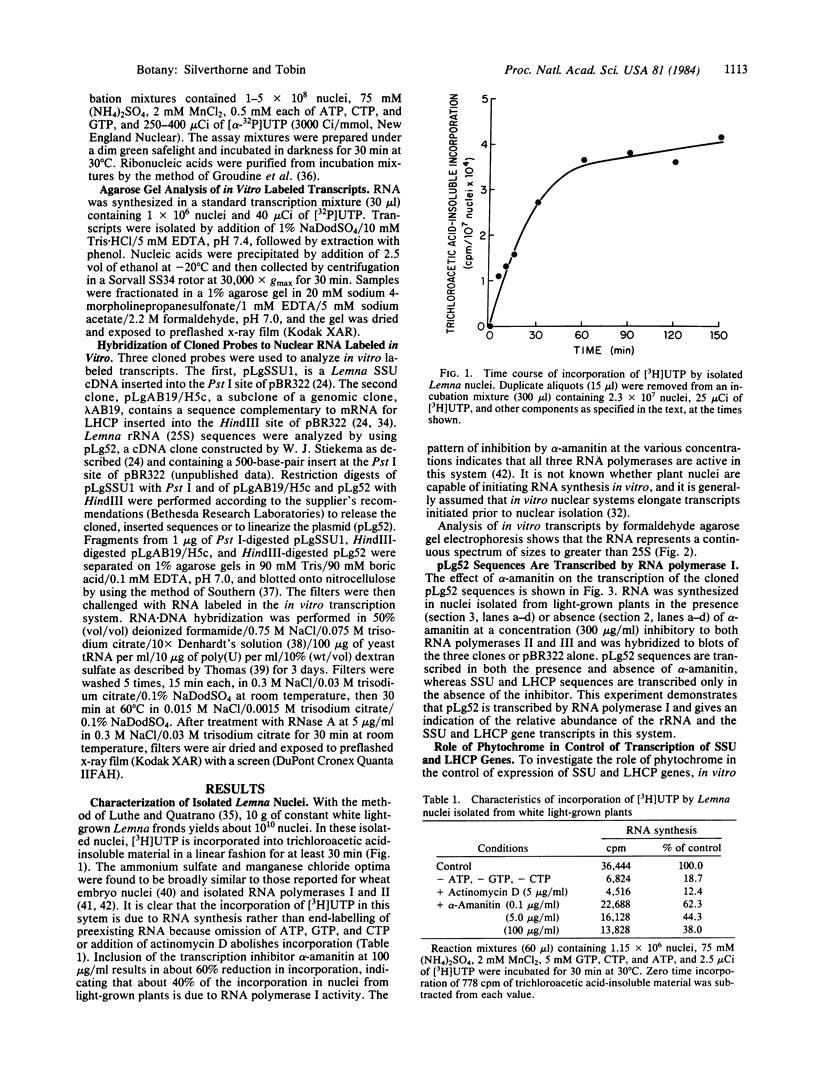
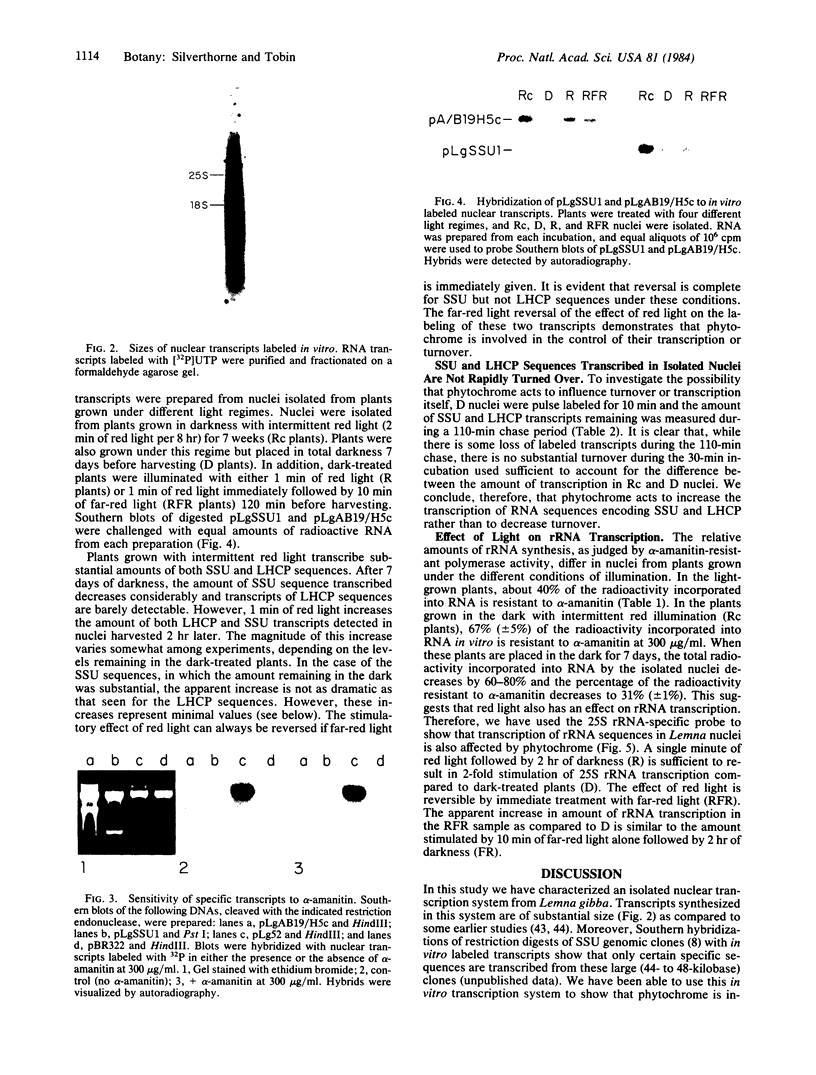
We have developed an in vitro transcription system that uses nuclei isolated from Lemna gibba G-3. The in vitro transcripts include sequences homologous to hybridization probes for the small subunit of ribulose-1,5-bisphosphate carboxylase [3-phospho-D-glycerate carboxy-lyase (dimerizing), EC 4.1.1.39], the light-harvesting chlorophyll a/b-protein, and rRNA. Light-harvesting chlorophyll a/b-protein sequences are transcribed to a greater extent in nuclei isolated from plants grown in darkness with 2 min of red light every 8 hr than in nuclei isolated from dark-treated plants. Furthermore, the amount of these transcripts measured in plants given a single minute of red light after dark treatment is increased over the amount measured in dark-treated plants. The effect of red light is at least partially reversible by 10 min of far-red light given immediately after the red light pulse. Transcription of both rRNA and small subunit sequences is also stimulated by a single minute of red light as compared to dark-treated tissue. However, the relative magnitudes of the increases compared to the dark levels are smaller than the increase seen for the chlorophyll a/b-protein, possibly because of the higher level of transcription of these sequences in the dark. The effect of red light on the transcription of small subunit and rRNA sequences is also reversible by immediate treatment with 10 min of far-red light. Pulse chase studies of dark-treated nuclei for up to 110 min do not show substantial turnover of in vitro labeled small subunit and chlorophyll a/b-protein transcripts. We therefore conclude that phytochrome action has induced specific changes in transcription of these genes.
Keywords: photoinduction; isolated nuclei; transcriptional control; light-harvesting chlorophyll a/b-protein; ribulose-1,5-bisphosphate carboxylase
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Apel K. Phytochrome-induced appearance of mRNA activity for the apoprotein of the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare). Eur J Biochem. 1979 Jun;97(1):183–188. doi: 10.1111/j.1432-1033.1979.tb13101.x. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Link G., Coen D. M., Bogorad L. Maize plastid gene expressed during photoregulated development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3060–3064. doi: 10.1073/pnas.75.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Lowe S. L., Mc Knight T. D., Shah D. M., Meagher R. B. The nucleotide sequence, expression, and evolution of one member of a multigene family encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in soybean. J Mol Appl Genet. 1982;1(6):483–498. [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert J. T., Hershey H. P., Quail P. H. Autoregulatory control of translatable phytochrome mRNA levels. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2248–2252. doi: 10.1073/pnas.80.8.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dunsmuir P., Smith S. M., Bedbrook J. The major chlorophyll a/b binding protein of petunia is composed of several polypeptides encoded by a number of distinct nuclear genes. J Mol Appl Genet. 1983;2(3):285–300. [PubMed] [Google Scholar]
- Dunsmuir P., Smith S., Bedbrook J. A number of different nuclear genes for the small subunit of RuBPCase are transcribed in petunia. Nucleic Acids Res. 1983 Jun 25;11(12):4177–4183. doi: 10.1093/nar/11.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. F., Ellis R. J. Light-stimulated transcription of genes for two chloroplast polypeptides in isolated pea leaf nuclei. EMBO J. 1982;1(12):1493–1498. doi: 10.1002/j.1460-2075.1982.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollmer I., Apel K. The phytochrome-controlled accumulation of mRNA sequences encoding the light-harvesting chlorophyll a/b protein of barley (Hordeum vulgare L.). Eur J Biochem. 1983 Jun 15;133(2):309–313. doi: 10.1111/j.1432-1033.1983.tb07463.x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrisak J., Becker W. M. Isolation, purification and characterization of RNA polymerases from wheat germ. Biochim Biophys Acta. 1973 Aug 10;319(1):48–54. doi: 10.1016/0005-2787(73)90039-7. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction I protein. IV. Mode of inheritance of primary structure in relation to whether chloroplast or nuclear DNA contains the code for a chloroplast protein. Biochim Biophys Acta. 1972 Feb 23;262(1):42–49. doi: 10.1016/0005-2787(72)90217-1. [DOI] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Asami S., Akazawa T. Development of Enzymes Involved in Photosynthetic Carbon Assimilation in Greening Seedlings of Maize (Zea mays). Plant Physiol. 1980 Feb;65(2):198–203. doi: 10.1104/pp.65.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung S. D., Thornber J. P., Wildman S. G. Nuclear DNA codes for the photosystem II chlorophyll-protein of chloroplast membranes. FEBS Lett. 1972 Aug 1;24(2):185–188. doi: 10.1016/0014-5793(72)80763-4. [DOI] [PubMed] [Google Scholar]
- Link D. P., Lantz B. M. Vasodilator response in the lower extremity induced by contrast medium. I Canine model. Acta Radiol Diagn (Stockh) 1982;23(2):81–86. doi: 10.1177/028418518202300201. [DOI] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: I. ISOLATION OF NUCLEI AND ELIMINATION OF ENDOGENOUS RIBONUCLEASE ACTIVITY. Plant Physiol. 1980 Feb;65(2):305–308. doi: 10.1104/pp.65.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthe D. S., Quatrano R. S. Transcription in Isolated Wheat Nuclei: II. CHARACTERIZATION OF RNA SYNTHESIZED IN VITRO. Plant Physiol. 1980 Feb;65(2):309–313. doi: 10.1104/pp.65.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli D. F., Briggs W. R. Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol. 1981 Apr;67(4):733–739. doi: 10.1104/pp.67.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel H. J., Apel K. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). The effect of light on the NADPH:protochlorophyllide oxidoreductase. Eur J Biochem. 1981 Nov;120(1):95–103. doi: 10.1111/j.1432-1033.1981.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Sakihama T., Kamikubo T., Shinozaki K. Phytochrome-mediated regulation of two mRNAs, encoded by nuclei and chloroplasts of ribulose-1,5-bisphosphate carboxylase/oxygenase. Eur J Biochem. 1983 Jul 1;133(3):617–620. doi: 10.1111/j.1432-1033.1983.tb07507.x. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Sasaki Y., Sakihama T., Kamikubo T. Coordinate light-induction of two mRNAs, encoded in nuclei and chloroplasts, of ribulose 1,5-bisphosphate carboxylase/oxygenase. FEBS Lett. 1982 Jul 19;144(1):73–76. doi: 10.1016/0014-5793(82)80571-1. [DOI] [PubMed] [Google Scholar]
- Sims T. L., Hague D. R. Light-stimulated increase of translatable mRNA for phosphoenolpyruvate carboxylase in leaves of maize. J Biol Chem. 1981 Aug 25;256(16):8252–8255. [PubMed] [Google Scholar]
- Smith S. M., Ellis R. J. Light-stimulated accumulation of transcripts of nuclear and chloroplast genes for ribulosebisphosphate carboxylase. J Mol Appl Genet. 1981;1(2):127–137. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stiekema W. J., Wimpee C. F., Silverthorne J., Tobin E. M. Phytochrome Control of the Expression of Two Nuclear Genes Encoding Chloroplast Proteins in Lemna gibba L. G-3. Plant Physiol. 1983 Jul;72(3):717–724. doi: 10.1104/pp.72.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M., Djiane J. Prolactin induces release of a factor from membranes capable of stimulating beta-casein gene transcription in isolated mammary cell nuclei. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6729–6733. doi: 10.1073/pnas.78.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Thien W., Schopfer P. Control by Phytochrome of Cytoplasmic Precursor rRNA Synthesis in the Cotyledons of Mustard Seedlings. Plant Physiol. 1982 May;69(5):1156–1160. doi: 10.1104/pp.69.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien W., Schopfer P. Control by Phytochrome of Cytoplasmic and Plastid rRNA Accumulation in Cotyledons of Mustard Seedlings in the Absence of Photosynthesis. Plant Physiol. 1975 Nov;56(5):660–664. doi: 10.1104/pp.56.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M. Light regulation of specific mRNA species in Lemna gibba L. G-3. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4749–4753. doi: 10.1073/pnas.75.10.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M., Suttie J. L. Light Effects on the Synthesis of Ribulose-1,5-Bisphosphate Carboxylase in Lemna gibba L. G-3. Plant Physiol. 1980 Apr;65(4):641–647. doi: 10.1104/pp.65.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin E. M. White Light Effects on the mRNA for the Light-Harvesting Chlorophyll a/b-Protein in Lemna gibba L. G-3. Plant Physiol. 1981 Jun;67(6):1078–1083. doi: 10.1104/pp.67.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P. S., Bennett J. Transcription in nuclei isolated from a higher plant. Biochem Soc Trans. 1976;4(4):812–813. doi: 10.1042/bst0040812. [DOI] [PubMed] [Google Scholar]