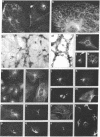
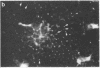
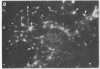
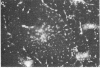
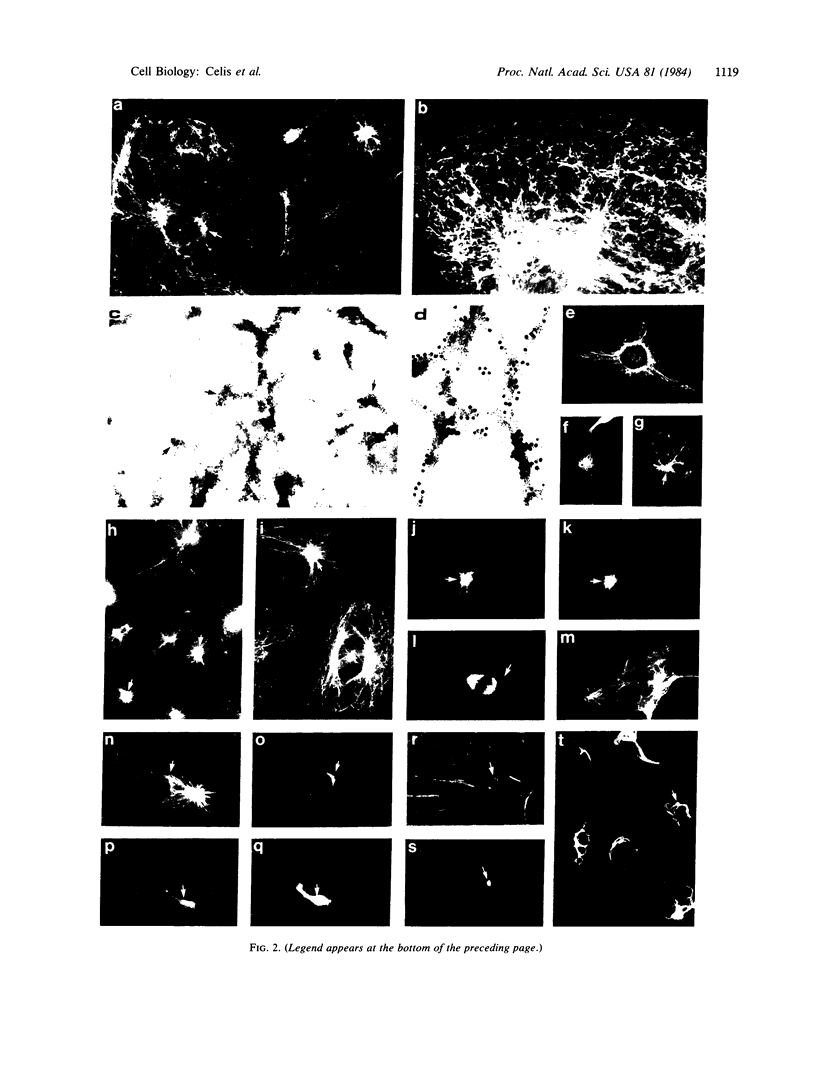
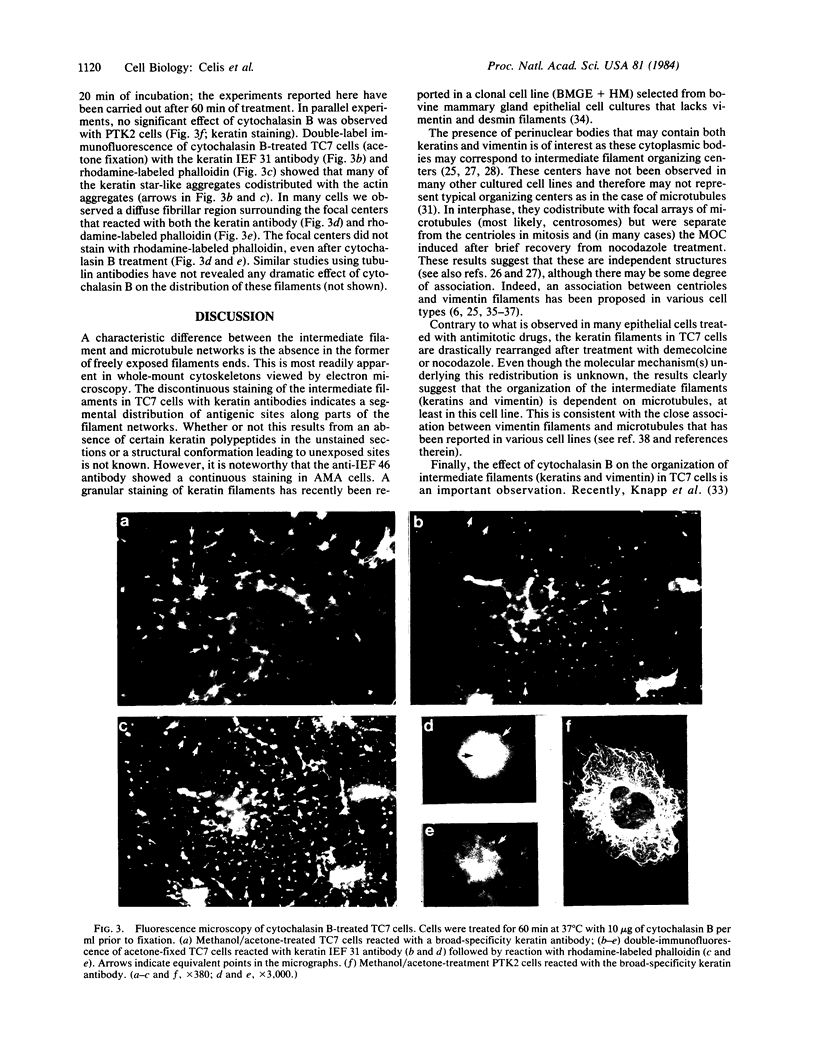
Abstract
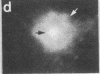
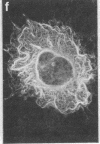
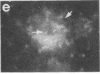
Two-dimensional gel electrophoresis of intermediate-sized filament-enriched cytoskeletons of epithelial monkey kidney TC7 cells has shown that they are composed of at least two keratins (isoelectric focusing 36, Mr = 48,500; IEF 46, Mr = 43,500; HeLa protein catalogue number) and vimentin. Indirect immunofluorescence as well as immunoelectron microscopy using antibodies directed against specific polypeptides sometimes revealed a discontinuous staining of keratin-containing filaments. Indirect immunofluorescence analysis of cells stained with keratin or vimentin antibodies also revealed a bright perinuclear staining in 58% of the cells in interphase. Of particular interest were focal centers from which filaments radiated. Double-label immunofluorescence using tubulin and keratin antibodies showed that these centers codistributed with focal arrays of microtubules (most likely centrosomes) in interphase cells but were not colocalized with centrioles in mitosis or, in many cases, with the microtubule organizing centers seen after release from nocodazole treatment. Treatment of TC7 cells with demecolcine (10 micrograms/ml, 20 hr) resulted in a drastic rearrangement of the keratin and vimentin filaments. Likewise, treatment with cytochalasin B (10 micrograms/ml, 1 hr) produced a star-like arrangement of the keratin and vimentin filaments and, in most cases, these codistributed with patches of actin. The results provide evidence for the interaction of intermediate filaments (keratins and vimentin) with both microtubules and microfilaments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubin J. E., Osborn M., Franke W. W., Weber K. Intermediate filaments of the vimentin-type and the cytokeratin-type are distributed differently during mitosis. Exp Cell Res. 1980 Sep;129(1):149–165. doi: 10.1016/0014-4827(80)90340-7. [DOI] [PubMed] [Google Scholar]
- Borenfreund E., Schmid E., Bendich A., Franke W. W. Constitutive aggregates of intermediate-sized filaments of the vimentin and cytokeratin type in cultured hepatoma cells and their dispersal by butyrate. Exp Cell Res. 1980 May;127(1):215–235. doi: 10.1016/0014-4827(80)90428-0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Bellatin J., Celis J. E. [35S]-methionine labelled polypeptides from HELA cells. Coordinates and percentage of some major polypeptides. Cell Biol Int Rep. 1981 Jan;5(1):93–96. doi: 10.1016/0309-1651(81)90162-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Up-dated catalogue of HeLa cell proteins: percentages and characteristics of the major cell polypeptides labeled with a mixture of 16 14C-labeled amino acids. Clin Chem. 1982 Apr;28(4 Pt 2):766–781. [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Larsen P. M., Coppard N., Celis J. E. Proteins IEF (isoelectric focusing) 31 and IEF 46 are keratin-type components of the intermediate-sized filaments: keratins of various human cultured epithelial cells. J Cell Biol. 1983 Feb;96(2):416–423. doi: 10.1083/jcb.96.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Small J. V., Larsen P. M., Celis J. E. Coexistence of three major isoactins in a single sarcoma 180 cell. Cell. 1981 Jul;25(1):195–202. doi: 10.1016/0092-8674(81)90244-0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Summerhayes I. C., Johnson L. V., Walsh M. L., Bernal S. D., Lampidis T. J. Probing mitochondria in living cells with rhodamine 123. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):141–155. doi: 10.1101/sqb.1982.046.01.018. [DOI] [PubMed] [Google Scholar]
- David-Ferreira K. L., David-Ferreira J. F. Association between intermediate-sized filaments and mitochondria in rat Leydig cells. Cell Biol Int Rep. 1980 Jul;4(7):655–662. doi: 10.1016/0309-1651(80)90204-0. [DOI] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- De Mey J., Moeremans M., Geuens G., Nuydens R., De Brabander M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol Int Rep. 1981 Sep;5(9):889–899. doi: 10.1016/0309-1651(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Eckert B. S., Daley R. A., Parysek L. M. Assembly of keratin onto PtK1 cytoskeletons: evidence for an intermediate filament organizing center. J Cell Biol. 1982 Feb;92(2):575–578. doi: 10.1083/jcb.92.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey S. J., Larsen P. M., Bravo R., Celis A., Celis J. E. Differential immunological crossreactivity of HeLa keratin antibodies with human epidermal keratins. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1905–1909. doi: 10.1073/pnas.80.7.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey S. J., Larsen P. M., Celis J. E. Evidence for coordinated phosphorylation of keratins and vimentin during mitosis in transformed human amnion cells. Phosphate turnover of modified proteins. FEBS Lett. 1983 Jun 27;157(1):165–169. doi: 10.1016/0014-5793(83)81138-7. [DOI] [PubMed] [Google Scholar]
- Geuens G., de Brabander M., Nuydens R., De Mey J. The interaction between microtubules and intermediate filaments in cultured cells treated with taxol and nocodazole. Cell Biol Int Rep. 1983 Jan;7(1):35–47. doi: 10.1016/0309-1651(83)90103-0. [DOI] [PubMed] [Google Scholar]
- Hoebeke J., Van Nijen G., De Brabander M. Interaction of oncodazole (R 17934), a new antitumoral drug, with rat brain tubulin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):319–324. doi: 10.1016/0006-291x(76)90524-6. [DOI] [PubMed] [Google Scholar]
- Hormia M., Linder E., Lehto V. P., Vartio T., Badley R. A., Virtanen I. Vimentin filaments in cultured endothelial cells form butyrate-sensitive juxtanuclear masses after repeated subculture. Exp Cell Res. 1982 Mar;138(1):159–166. doi: 10.1016/0014-4827(82)90101-x. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W. Vimentin and keratin intermediate filament systems in cultured PtK2 epithelial cells are interrelated. EMBO J. 1982;1(2):161–165. doi: 10.1002/j.1460-2075.1982.tb01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp L. W., O'Guin W. M., Sawyer R. H. Drug-induced alterations of cytokeratin organization in cultured epithelial cells. Science. 1983 Feb 4;219(4584):501–503. doi: 10.1126/science.6186022. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Morgan G., Wooding F. B. Mitochondria and mitochondria-tonofilament-desmosomal associations in the mammary gland secretory epithelium of lactating cows. J Cell Sci. 1979 Aug;38:125–135. doi: 10.1242/jcs.38.1.125. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Kurki P. Intermediate filaments anchor the nuclei in nuclear monolayers of cultured human fibroblasts. Nature. 1978 Mar 9;272(5649):175–177. doi: 10.1038/272175a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Mose-Larsen P., Bravo R., Fey S. J., Small J. V., Celis J. E. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell. 1982 Dec;31(3 Pt 2):681–692. doi: 10.1016/0092-8674(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Schmid E., Franke W. W., Grund C., Schiller D. L., Kolb H., Paweletz N. An epithelial cell line with elongated myoid morphology derived from bovine mammary gland. Expression of cytokeratins and desmosomal plaque proteins in unusual arrays. Exp Cell Res. 1983 Jul;146(2):309–328. doi: 10.1016/0014-4827(83)90133-7. [DOI] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Direct visualization of the 10-nm (100-A)-filament network in whole and enucleated cultured cells. J Cell Sci. 1978 Jun;31:393–409. doi: 10.1242/jcs.31.1.393. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T., Dutton A. H., Singer S. J. Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken skeletal muscle. J Cell Biol. 1983 Jun;96(6):1727–1735. doi: 10.1083/jcb.96.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nelson W. J., Kühn S., Vorgias C. E. The interaction in vitro of the intermediate filament protein vimentin with naturally occurring RNAs and DNAs. J Biol Chem. 1983 Feb 10;258(3):1456–1466. [PubMed] [Google Scholar]
- Wang E., Connolly J. A., Kalnins V. I., Choppin P. W. Relationship between movement and aggregation of centrioles in syncytia and formation of microtubule bundles. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5719–5723. doi: 10.1073/pnas.76.11.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]