Abstract
It is well established that the central nervous system (CNS), especially the hypothalamus, plays an important role in regulating energy homeostasis and lipid metabolism. We have previously shown that hypothalamic corticotropin-releasing hormone (CRH) is critical for stimulating fat loss in response to dietary leucine deprivation. The molecular mechanisms underlying the CNS regulation of leucine deprivation–stimulated fat loss are, however, still largely unknown. Here, we used intracerebroventricular injection of adenoviral vectors to identify a novel role for hypothalamic p70 S6 kinase 1 (S6K1), a major downstream effector of the kinase mammalian target of rapamycin, in leucine deprivation stimulation of energy expenditure. Furthermore, we show that the effect of hypothalamic S6K1 is mediated by modulation of Crh expression in a melanocortin-4 receptor–dependent manner. Taken together, our studies provide a new perspective for understanding the regulation of energy expenditure by the CNS and the importance of cross-talk between nutritional control and regulation of endocrine signals.
Energy homeostasis is maintained by a balance between energy intake and energy expenditure. The central nervous system (CNS), particularly the hypothalamus, has been shown to play a critical role in the regulation of these events by sensing changes in the concentrations of nutrients and hormones and coordinating subsequent physiological responses (1–5). P70 S6 kinase 1 (S6K1), a major downstream target of the kinase mammalian target of rapamycin (mTOR), is widely expressed in the CNS, including the hypothalamus. Recent studies have demonstrated a key role for S6K1 signaling in the regulation of energy homeostasis in Drosophila (6), in mice deficient in S6K1 (7,8), and in rats injected with S6K1 expression vector in the mediobasal hypothalamus (9). A role for hypothalamic S6K1 in the regulation of energy homeostasis and underlying mechanisms, however, requires further investigation.
Corticotropin-releasing hormone (CRH) is a 41–amino acid peptide, produced primarily in the paraventricular nucleus of the hypothalamus (PVN), but also in additional sites in the brain and peripheral tissues (10). Studies have shown that intracerebroventricular administration of CRH decreases food intake (11) and increases energy expenditure, possibly by increasing thermogenesis in brown adipose tissue (BAT) and lipolysis in white adipose tissue (WAT) (12). Melanocortin-4 receptor (MC4R), which is also expressed in the hypothalamus (13,14), is a component of the CNS melanocortin signaling system (15). Accumulating evidence has implicated MC4R in the regulation of energy intake and energy expenditure (15–17). MC4R also mediates the regulation of energy homeostasis by hormones secreted from peripheral tissues, including leptin, insulin, and ghrelin (2). Despite the importance of MC4R in the control of energy balance, its downstream signaling pathways are not well understood. Several studies have provided evidence that MC4R and CRH are expressed in the same regions of brain (18) and induce similar anorectic effects (11,16). It is also known that CRH neurons in the PVN are innervated by neuronal α-melanocyte–stimulating hormone terminals (19). These observations suggest that MC4R may regulate energy homeostasis by affecting Crh expression, although this has yet to be experimentally confirmed.
We have previously shown that leucine deprivation decreases abdominal fat mass largely by increasing energy expenditure (20,21), and that this effect is mediated by increased Crh expression in the hypothalamus and activation of the sympathetic nervous system (22). The molecular mechanisms underlying the CNS control of leucine deprivation–induced fat loss, however, are largely unknown. Given the fact that hypothalamic S6K1 plays a critical role in the regulation of energy balance and sensing of amino acids (7–9,23–25), we hypothesized that hypothalamic S6K1 may play a role in the regulation of leucine deprivation–induced fat loss. The current study aims to investigate these possibilities and elucidate the underlying molecular and cellular mechanisms.
In the current study, we demonstrate a key role for hypothalamic S6K1 in the regulation of energy expenditure during leucine deprivation, based on the attenuation of leucine deprivation–induced fat loss after intracerebroventricular injection of adenoviral vectors expressing constitutively active S6K1 (Ad-CAS6K1). Furthermore, we show that the effect of S6K1 is mediated by modulation of Crh expression in the PVN. Finally, we use the MC4R antagonist SHU9119 to demonstrate that S6K1 regulates Crh expression in an MC4R-dependent manner. Taken together, our results suggest a novel role for hypothalamic S6K1 in the regulation of energy expenditure under leucine deprivation. This is the first report, to our knowledge, demonstrating a role for S6K1 in regulating Crh expression in the hypothalamus.
RESEARCH DESIGN AND METHODS
Animals and diets.
Wild-type C57BL/6J mice were obtained from the Shanghai Laboratory Animals Co. Ltd. (Shanghai, China). Eight- to ten-week-old mice were maintained on a 12-h light/dark cycle at 24°C and provided free access to commercial rodent chow and tap water prior to the experiments. Control (nutritionally complete amino acid) and −leu (leucine-deficient) diets were obtained from Research Diets, Inc. (New Brunswick, NJ). All diets were isocaloric and compositionally the same in terms of carbohydrate and lipid component. As previously described (20,21), at the start of the feeding experiment, mice were acclimated to a control diet for 7–10 days and then randomly assigned to either control or −leu diet groups with free access to either control or −leu diet, respectively, for 7 days. Food intake and body weight were recorded daily. At the end of experiments, animals were killed by CO2 inhalation. WAT weight was recorded at the time they were killed. Hypothalamus, WAT, and BAT were isolated, snap frozen, and stored at −80°C for future analysis. These experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Institute for Nutritional Sciences.
Intracerebroventricular administration experiments.
Intracerebroventricular administration experiments were conducted as previously described (26). Three days after recovery, mice infused with 1 μL of 0.1 mmol/L SHU9119 (Tocris Bioscience, Bristol, U.K.) or 1 μL PBS once daily at 5:00 p.m. were maintained on a control or −leu diet for 7 days. For CRH administration experiments, mice were simultaneously injected with adenovirus expressing green fluorescent protein (Ad-GFP) or hemagglutinin (HA)-tagged CAS6K1 (Ad-CAS6K1), implanted with cannula, and allowed to recover for 3 days. After recovery, all the mice were provided with −leu diet for 7 days. During this period, mice were injected with 1 μL of 1 μg/1 μL CRH (Bachem, Bubendorf, Switzerland) or 1 μL artificial cerebrospinal fluid as control once daily at 5:00 p.m. through the implanted cannula. Ad-CAS6K1 was constructed as previously described (27). Ad-CAS6K1 or Ad-GFP control (1 μL 5 × 108 plaque forming units [pfu]/mouse) was administered to mice by intracerebroventricular injection using a microsyringe. Mice were killed on day 7.
Indirect calorimetry.
Mice were maintained in a comprehensive laboratory animal monitoring system (CLAMS; Columbus Instruments, Columbus, OH) for 20 h to allow them to adapt to this environment, and the volume of O2 consumption and CO2 production were continuously recorded during the next 24 h according to the instructions of the manufacturer. Regarding mice receiving intracerebroventricular injection or implanted cannula, mice were put in CLAMS on day 5 after initiation of control or −leu diets.
Rectal temperature measurement.
Rectal temperatures of mice were measured at 3:00 p.m. (basal metabolic state) using a rectal probe attached to a digital thermometer (Physitemp, Clifton, NJ).
Primary hypothalamic neuron isolation and treatments.
Primary cultures of hypothalamic neurons were prepared as previously described (22). On day 10, primary cultured hypothalamic neurons were infected with adenovirus expressing S6K1-specific short hairpin RNA (shRNA) (Ad-shS6K1; 108 pfu/60 cm2 cells) or scramble control shRNA, followed by treatment with 0.1 μmol/L SHU9119 (Tocris) or control PBS for 24 h. Ad-shS6K1 or control vector was generated with the BLOCK-iT Adenoviral RNAi Expression System (Invitrogen) according to the manufacturer’s instructions. The sequence designed for the knockdown of S6K1 was 5′- CACCGGGAGTTGGACCATATGAACTCGAA-AGTTCATATGGTCCAACTCCC-3′. Regarding treatment with MTII (melanocortin agonist), primary cultured hypothalamic neurons were infected with Ad-CAS6K1 (5 × 107 pfu/60 cm2 cells) or Ad-GFP and then incubated in −leu medium for 8 h in the presence or absence of treatment with 0.3 μmol/L MTII (Tocris) or PBS. The −leu medium was prepared from amino acid–free Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen) by adding back all the amino acids contained in regular DMEM but without leucine only.
RNA isolation and relative quantitative RT-PCR.
RNA isolation and RT-PCR were performed as previously described (22). The sequences of primers used in this study are available upon request.
Western blot analysis.
Western blot analysis was performed as previously described (21). Primary antibodies (anti–phosphorylated S6 [p-S6] [Ser235/236], anti-S6, anti–p-p70S6K1 [Thr389], anti–p70S6K1, anti–p-cAMP-responsive element-binding protein [p-CREB] [Ser133], and anti-CREB [Cell Signaling Technology, Beverly, MA], anti–uncoupling protein 1 [UCP1], anti-HA, and anti-MC4R [Santa Cruz Biotechnology], and anti–β-actin [Sigma-Aldrich]) were incubated overnight at 4°C and specific proteins were visualized by enhanced chemiluminescence (Amersham Biosciences).
Immunohistochemistry staining.
Immunohistochemistry staining was performed as described previously (23). In brief, brain coronal sections of 25 μm were cut using a frozen microtome (Leica Microsystems, Bensheim, Germany) and incubated with primary antibody (anti–p-S6 [Ser235/236]; Cell Signaling Technology), and pictures were taken by using an Olympus BX61 microscope (Olympus, Tokyo, Japan).
Immunofluorescence staining.
Immunofluorescence staining was performed as described previously (22). Brain sections were incubated with anti-CRH (Santa Cruz Biotechnology) and anti–p-S6 (Ser235/236) antibody (Cell Signaling Technology) overnight, followed by incubation with Alexa Fluor 555 and 488–conjugated anti-goat antibody (Invitrogen) at room temperature for 1 h. Pictures were taken using a fluorescence microscope (BX61; Olympus) and a Zeiss LSM 510 confocal microscope (Carl Zeiss Imaging, Oberkochen, Germany).
Statistical analysis.
All values are presented as mean ± SEM. Differences between groups were analyzed either by the Student t test or one-way ANOVA followed by the Student-Newman-Keuls (SNK) test, in which P < 0.05 was considered statistically significant.
RESULTS
Leucine deprivation decreases S6K1 activity in the hypothalamus.
To investigate the possible involvement of S6K1 in the regulation of fat loss during leucine deprivation, we examined levels of phosphorylation of S6K1 and its direct downstream target ribosomal protein S6, which reflects the activation status of S6K1, in the hypothalamus of mice maintained on a control or leucine-deficient diet for 7 days. Consistent with the changes in the liver (27), levels of p-S6K1 and p-S6 were significantly decreased in the hypothalamus of leucine-deprived mice compared with control diet–fed mice (Fig. 1A).
FIG. 1.
Leucine deprivation decreases S6K activity in the hypothalamus. Mice were fed a control (+leu) or leucine-deficient (−leu) diet for 7 days. Data are mean ± SEM for at least two independent experiments with mice maintained on each diet for each experiment (n = 5 for each group). Statistical significance was determined by the Student t test. *P < 0.01, for the effect of −leu vs. control diet. A: Hypothalamic S6K1 and S6 proteins (top, Western blot; bottom, quantitative measurements of p-S6K1 and p-S6 protein relative to total S6K1 and total S6, respectively). B: Immunohistochemistry staining for p-S6 in hypothalamus. Images shown are representative of several animals for each group. 3V, third ventricle. (A high-quality digital representation of this figure is available in the online issue.)
mTOR signaling in the PVN and arcuate nucleus (Arc) of the hypothalamus has been shown to regulate energy homeostasis (23,28). Here, we found that the levels of p-S6 were decreased in both the PVN and Arc of the hypothalamus in leucine-deprived mice compared with mice fed a control diet, as shown by immunohistochemistry staining (Fig. 1B).
Intracerebroventricular injection of Ad-CAS6K1 significantly reduces leucine deprivation–stimulated fat loss.
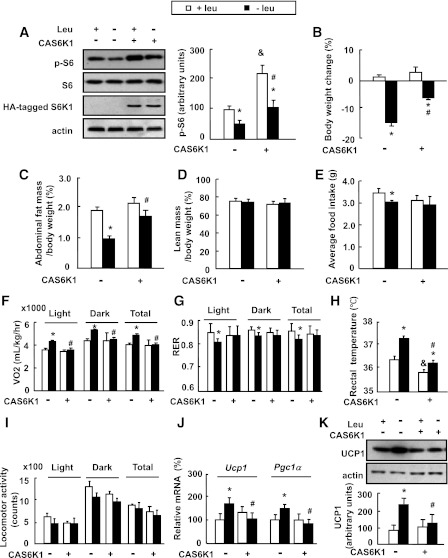
To investigate a role for S6K1 in the regulation of fat mass under leucine deprivation, we intracerebroventricularly injected mice with Ad-CAS6K1 or adenoviral vectors expressing GFP (Ad-GFP), and then maintained the mice on a control or leucine-deficient diet for 7 days. As predicted, overexpression of Ad-CAS6K1 in the hypothalamus (confirmed by Western blotting using an anti–HA-tagged antibody) increased S6 phosphorylation (Fig. 2A). Consistent with our previous results (21), leucine deprivation significantly decreased body weight (from day 3) (data not shown) and fat mass compared with the control diet–fed group in Ad-GFP–injected mice, both of which were largely prevented by Ad-CAS6K1 (Fig. 2B and C). Ad-CAS6K1 did not have an effect on body weight and fat mass in mice maintained on a control diet (Fig. 2B and C). Lean mass, however, was not affected by leucine deprivation or intracerebroventricular injection of CAS6K1 (Fig. 2D).
FIG. 2.
Intracerebroventricular injection of adenovirus expressing constitutively active S6K1 (Ad-CAS6K1) in the hypothalamus attenuates leucine deprivation–induced fat loss. Mice received intracerebroventricular injection of adenovirus expressing CAS6K1 (+CAS6K1) or GFP (−CAS6K1) and then were fed a control (+leu) or leucine-deficient (−leu) diet for 7 days. Energy expenditure was measured by indirect calorimetry. Data are means ± SEM of at least two independent experiments (n = 6 for each group). Statistical significance was determined by one-way ANOVA followed by the SNK test. *P < 0.05, for the effect of −leu vs. control diet within the same intracerebroventricular group; #P < 0.01, for the effect of with vs. without Ad-CAS6K1 under −leu diet; &P < 0.01, for the effect of with vs. without Ad-CAS6K1 under control diet. A: Hypothalamic HA-tagged S6K1 and S6 proteins (left, Western blot; right, quantitative measurements of p-S6 protein relative to total S6). B: Body weight change. C: Adipose tissue mass in proportion to body weight. D: Lean mass in proportion to body weight. E: Average food intake. F: Oxygen consumption (VO2; 24 h). G: RER. H: Rectal temperature. I: Physical activity. J: Ucp1 and Pgc-1α mRNA in BAT. K: UCP1 protein in BAT (top, Western blot; bottom, quantitative measurements of UCP1 protein relative to actin).
Intracerebroventricular injection of Ad-CAS6K1 significantly decreases leucine deprivation–stimulated energy expenditure.
The blocking effect of upregulation of S6K1 activity on leucine deprivation–induced fat loss may result from an increase in food intake, or a decrease in energy expenditure. Consistent with previous results (21), we found that leucine deprivation decreased food intake in Ad-GFP–injected mice. A similar reduction in food intake was observed in leucine-deprived mice injected with Ad-CAS6K1, although these mice did not show a statistically significant difference with mice fed a control diet and injected with Ad-CA6K1 (Fig. 2E). Furthermore, Ad-CAS6K1 did not have an effect on food intake in mice maintained on a control diet (Fig. 2E).
We therefore measured energy expenditure by indirect calorimetry, rectal temperature, and physical activity. Consistent with previous results, the total energy expenditure (24-h O2 consumption, normalized to lean body mass) was markedly increased and the respiratory exchange ratio (RER; VCO2/VO2) was low during both dark and light phases in the Ad-GFP group maintained on a leucine-deficient diet compared with mice maintained on a control diet (Fig. 2F and G). By contrast, the effects of leucine deprivation on energy expenditure and RER were abolished in Ad-CAS6K1 mice (Fig. 2F and G). Consistent with changes in energy expenditure, the increased body temperature by leucine deprivation was significantly attenuated in Ad-CAS6K1 mice (Fig. 2H). Overexpression of Ad-CAS6K1, however, also decreased body temperature, but did not change oxygen consumption in mice maintained on a control diet (Fig. 2H). We did not see significant differences in physical activity in any group (Fig. 2I).
We have previously shown that leucine deprivation increases UCP1 expression in BAT (21). Here, we found its expression, as well as its upstream regulator, peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) (29), were blocked by Ad-CAS6K1, whereas Ad-CAS6K1 had no effect on these changes in mice maintained on a control diet (Fig. 2J and K).
Potential regulation of Crh expression by S6K1 in the hypothalamus.
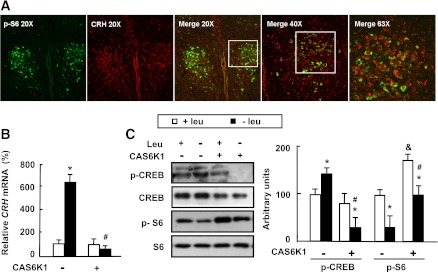
To test the possibility that CRH may mediate the effects of S6K1 in the hypothalamus, we conducted an immunofluorescence double-staining experiment and observed the colocalization of p-S6 and CRH at the cellular level in the PVN of mice maintained on a control diet (Fig. 3A).
FIG. 3.
Potential regulation of Crh expression by S6K1 in the hypothalamus. Mice received intracerebroventricular injection of adenovirus expressing constitutively active S6K1 (+CAS6K1) or GFP (−CAS6K1) and then were fed a control (+leu) or leucine-deficient (−leu) diet for 7 days. Data are means ± SEM of at least two independent experiments (n = 6 for each group). Statistical significance was determined by one-way ANOVA followed by the SNK test. *P < 0.05, for the effect of −leu vs. control diet within the same intracerebroventricular group; #P < 0.01, for the effect of with vs. without Ad-CAS6K1 under −leu diet; &P < 0.01, for the effect of with vs. without Ad-CAS6K1 under control diet. A: Immunofluorescence double staining of CRH (red) and p-S6 (green) in the PVN of mice maintained on a control diet. B: Hypothalamic Crh mRNA. C: Hypothalamic CREB and S6 proteins (left, Western blot; right, quantitative measurements of p-CREB and p-S6 protein relative to total CREB and total S6, respectively). (A high-quality digital representation of this figure is available in the online issue.)
The potential regulation of Crh expression by S6K1 was further investigated in Ad-GFP– or Ad-CAS6K1–injected mice maintained on control or leucine-deficient diets. Furthermore, we have shown previously that increased Crh expression in the hypothalamus of leucine-deprived mice was stimulated by increased phosphorylation of CREB (22). Here, we found that levels of Crh mRNA and phosphorylation of CREB in the hypothalamus were significantly increased by leucine deprivation in the Ad-GFP group compared with those maintained on a control diet, whereas this induction of Crh expression and CREB phosphorylation was largely blocked by Ad-CAS6K1 (Fig. 3B and C).
Hypothalamic S6K1 regulates energy expenditure via modulation of Crh expression under leucine deprivation.
To test whether hypothalamic S6K1 regulates leucine deprivation–induced fat loss by modulating Crh expression, we first intracerebroventricularly injected Ad-CAS6K1 (which, as described above, decreases Crh expression) and examined whether subsequent injection of CRH could reverse the suppressing effect of Ad-CAS6K1 on leucine deprivation–induced fat loss in mice maintained on a leucine-deficient diet.
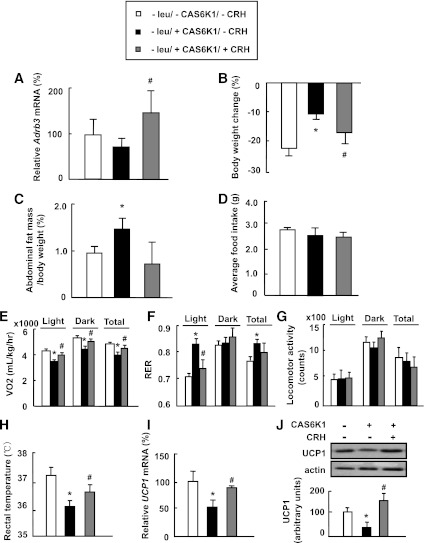
To validate the effect of intracerebroventricular injection of CRH, we examined mRNA levels of β-adrenergic receptors (Adrb3) in BAT, expression of which has previously been shown to be regulated by CRH (22). As predicted, CRH injection reversed the suppressing effect of Ad-CAS6K1 on the mRNA levels of Adrb3 in BAT (Fig. 4A). The possibility that CRH mediates the effect of S6K1 was strengthened by our observation that intracerebroventricular injection of CRH also reverses the suppressing effects of Ad-CAS6K1 on leucine deprivation–induced body weight and fat mass reduction (Fig. 4B and C). Because levels of CRH are already increased in leucine-deprived mice, we expected that this increased CRH may occlude the effect of injected CRH on food intake. Consistent with this expectation, the reduction in food intake after CRH-only injection was very small in mice maintained on a control diet (data not shown). Food intake, however, was also not significantly affected by intracerebroventricular injection of CAS6K1 under leucine deprivation (Fig. 4D). We therefore measured energy expenditure–related parameters and found that intracerebroventricular injection of CRH increased energy expenditure and decreased RER in Ad-CAS6K1 mice compared with control mice (Fig. 4E and F). Furthermore, intracerebroventricular injection of CRH did not affect physical activity significantly, but increased rectal temperature and UCP1 expression in BAT in Ad-CAS6K1 mice compared with control mice (Fig. 4G–J).
FIG. 4.
Hypothalamic S6K1 regulates leucine deprivation–stimulated energy expenditure via CRH. Mice received intracerebroventricular injection of adenovirus expressing constitutively active S6K1 (+CAS6K1) or GFP (−CAS6K1), followed by injection of 1 μL artificial cerebral spinal fluid (−CRH) or 1 μL of 1 μg/1 μL CRH (+CRH) once daily via an implanted cannula, synchronized with maintenance on a leucine-deficient (−leu) diet for 7 days. Data are means ± SEM of at least two independent experiments (n = 6 for each group). Statistical significance was determined by the Student t test. *P < 0.01, for the effect of with vs. without CAS6K1 in the absence of CRH; #P < 0.01, for the effect of with vs. without CRH in mice receiving CAS6K1. A: Adrb3 mRNA in BAT. B: Body weight change. C: Adipose tissue mass in proportion to body weight. D: Average food intake. E: Oxygen consumption (VO2; 24 h). F: RER. G: Physical activity. H: Rectal temperature. I: Ucp1 mRNA in BAT. J: UCP1 protein in BAT (top, Western blot; bottom, quantitative measurements of UCP1 protein relative to actin).
Hypothalamic S6K1 regulates energy expenditure by modulation of Crh expression in an MC4R-dependent manner.
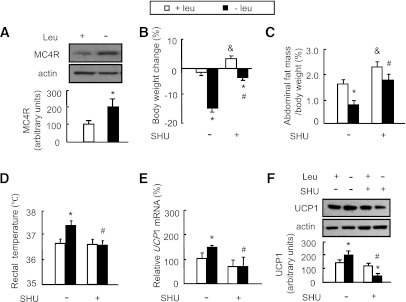
The possible involvement of MC4R, a G protein–coupled, seven-transmembrane receptor, in mediating the hypothalamic S6K1 regulation of Crh expression during leucine deprivation was investigated by examining MC4R protein levels in the hypothalamus, which were found to be significantly elevated by leucine deprivation (Fig. 5A). A role for MC4R in regulating Crh expression was then evaluated by intracerebroventricular injection of the MC4R antagonist SHU9119 or PBS as control daily in mice maintained on a control or leucine-deficient diet. We found that intracerebroventricular injection of SHU9119 not only significantly attenuated leucine deprivation–induced body weight and fat mass reduction (Fig. 5B and C) but also blocked leucine deprivation–increased rectal temperature and UCP1 expression in BAT (Fig. 5D–F).
FIG. 5.
MC4R regulates energy expenditure during leucine deprivation. Mice receiving daily intracerebroventricular injection of 1 μL of 0.1 mmol/L SHU9119 (+SHU) or 1 μL PBS (−SHU) via cannula implantation were maintained on a control (+leu) or leucine-deficient (−leu) diet for 7 days. Data are means ± SEM of at least two independent experiments (n = 6 for each group). Statistical significance was determined by one-way ANOVA followed by the SNK test. *P < 0.01, for the effect of −leu vs. control diet within the same intracerebroventricular group; #P < 0.01, for the effect of with vs. without SHU under −leu diet; &P < 0.01, for the effect of with vs. without SHU under control diet. A: Hypothalamic MC4R protein (top, Western blot; bottom, quantitative measurements of MC4R relative to actin). B: Body weight change. C: Abdominal fat mass in proportion to body weight. D: Rectal temperature. E: Ucp1 mRNA in BAT. F: UCP1 protein in BAT (top, Western blot; bottom, quantitative measurements of UCP1 protein relative to actin).
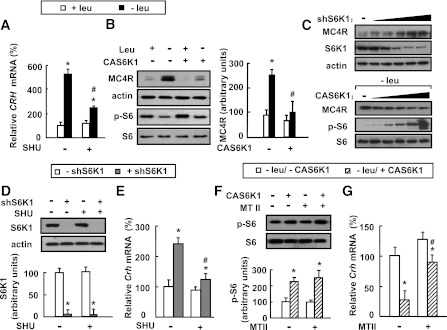
Consistent with a possible role for MC4R in regulating Crh expression and functions downstream from S6K1, we found that leucine deprivation–stimulated Crh expression and MC4R protein levels in the hypothalamus were blocked by SHU9119 and intracerebroventricular injection with Ad-CAS6K1, respectively (Fig. 6A and B). The effect of S6K1 on MC4R expression was further investigated by infecting primary cultured hypothalamic neurons with different concentrations of adenovirus expressing S6K1-specific shRNA (Ad-shS6K1, as confirmed by Western blot using anti-S6K1 antibody), or Ad-CAS6K1 in leucine-deficient medium, and examining MC4R expression. We found that MC4R protein levels were regulated by S6K1 in a dose-dependent manner (Fig. 6C).
FIG. 6.
Hypothalamic S6K1 modulates Crh expression via MC4R. A and B: Mice receiving intracerebroventricular injection of adenovirus expressing constitutively active S6K1 (+CAS6K1) or GFP (−CAS6K1), or daily intracerebroventricular injection of 1 μL of 0.1 mmol/L SHU9119 (+SHU) or 1 μL PBS (−SHU) via cannula implantation, were maintained on a control (+leu) or leucine-deficient (−leu) diet for 7 days. Data are means ± SEM of at least two independent experiments (n = 6 for each group). Statistical significance was determined by one-way ANOVA followed by the SNK test. *P < 0.01, for the effect of −leu vs. control diet within the same intracerebroventricular group; #P < 0.01, for the effect of with vs. without SHU or Ad-CAS6K1 under −leu diet. C–G: Primary cultured hypothalamic neurons infected with adenovirus expressing S6K1-specific shRNA (+shS6K1) or control scramble shRNA (−shS6K1) in the absence or presence of 0.1 μmol/L SHU9119 (+SHU) or PBS (−SHU), or infected with Ad-S6K1 (+CAS6K1) or Ad-GFP (−CAS6K1), were then incubated in −leu medium in the absence or presence of 0.3 μmol/L MTII (+MTII) or PBS (−MTII). The titer for ShS6K1 and CAS6K1 in C are from 103 to 107 pfu/60 cm2 cells. Data are representative of at least two independent experiments or are mean ± SEM for at least two independent experiments. Statistical significance was determined by ANOVA followed by the SNK test. *P < 0.05, for the effect of with vs. without Ad-ShS6K1 or Ad-CAS6K1 under the same treatment; #P < 0.05, for the effect of with vs. without Ad-ShS6K1 after SHU treatment in D and E, or for the effect of with vs. without Ad-CAS6K1 after MTII treatment in F and G. A: Hypothalamic Crh mRNA. B: Hypothalamic MC4R and p-S6 proteins (left, Western blot; right, quantitative measurements of MC4R protein relative to actin). C: MC4R protein. D: S6K1 protein (top, Western blot; bottom, quantitative measurements of S6K1 protein relative to actin). E: Crh mRNA. F: p-S6 protein (top, Western blot; bottom, quantitative measurements of p-S6 protein relative to their total protein). G: Crh mRNA.
To demonstrate a direct link between S6K1/MC4R and Crh expression, we examined whether activation of Crh expression mediated by decreased S6K1 activity after exposure to Ad-shS6K1 could be abrogated by SHU9119 in primary cultured hypothalamic neurons and found that was the case (Fig. 6D and E). On the other hand, Ad-CAS6K1–mediated repression of Crh expression was relieved by MTII treatment in primary cultured neurons incubated in leucine-deficient medium (Fig. 6F and G).
DISCUSSION
The CNS, particularly the hypothalamus, has been shown to play an important role in maintaining energy homeostasis by integrating signals from nutrients and hormones (1,2,4). In our current study, we demonstrate a novel role for hypothalamic S6K1 in the regulation of energy homeostasis during leucine deprivation and provide important information that elucidates the underlying mechanisms.
S6K1, an evolutionarily conserved nutrient-sensing enzyme, phosphorylates the 40S ribosomal protein S6 to regulate the translation of 5′-terminal oligopyrimidine tract (5′TOP) -containing mRNA transcripts and is a downstream target of mTOR (30). In addition to a large number of studies investigating a role for mTOR in the regulation of energy homeostasis (23,28,31,32), which provide indirect evidence for a role of S6K1 in the regulation of energy homeostasis, recent studies have also provided evidence for a role of S6K1 in the regulation of energy balance in mammals (7–9,24,25). S6K1−/− mice, for example, are protected against age- and diet-induced obesity due to their reduced ability to store lipids (7), whereas overexpression of S6K1 in mediobasal hypothalamus has been shown to reduce body weight due to reduced food intake (9).
Studies have shown that mTOR/S6K1 signaling is regulated by leucine availability (23,30,33,34). For example, mTOR activity is increased by elevated extracellular levels of leucine (33), and intracerebroventricular administration of leucine into the Arc stimulates S6K1 phosphorylation in the hypothalamus of rats (23). By contrast, removal of leucine from tissue culture media decreases phosphorylation of S6K1 (35), and S6K1 activity is decreased in the liver of mice under leucine deprivation (27). Consistent with these results, we found that leucine deprivation decreases S6K1 activity in the hypothalamus. A role for S6K1 in the regulation of energy homeostasis during leucine deprivation was demonstrated by the ability of intracerebroventricular injection of Ad-CAS6K1 to attenuate energy expenditure and fat loss induced by leucine deprivation. Our study thus provides a new perspective for understanding the role of S6K1 in the hypothalamus in the regulation of energy homeostasis.
BAT oxidizes fat to produce heat, a process that is stimulated by increased expression of UCPs. The role of UCP1 in thermogenesis regulation of body weight is demonstrated by the resistance of UCP1-ablated mice to develop obesity (36,37) and the observation that upregulation of UCP1 increases thermogenesis and energy expenditure (38). It has been reported that Ucp1 mRNA expression in response to overfeeding or cold exposure is upregulated due to increased activities of the sympathetic nervous system mediated by PGC-1α (29). We have shown previously that leucine deprivation stimulates fat loss by increasing expression of UCP1 and PGC-1α in BAT (21). Here, we show that these changes were largely blocked by intracerebroventricular injection of Ad-CAS6K1. Consistent with our observations, decreased fat accumulation in S6K1−/− mice was found to be caused by an enhanced metabolic rate, as demonstrated by increased oxygen consumption and UCP1 expression in BAT (7). Our study, however, provides the first direct evidence, to our knowledge, for a role of S6K1 in the hypothalamus in regulating UCP1 expression in BAT.
CRH has been shown to be important in the regulation of energy homeostasis (11,12,39). It is known that Crh expression is positively regulated by Gs (guanine nucleotide–binding protein)-mediated increases in adenylate cyclase cAMP-dependent activation of PKA (cAMP-dependent protein kinase) and phosphorylation of CREB (40) and negatively regulated by glucocorticoid receptors (41). The possibility that hypothalamic S6K1 regulates energy expenditure via CRH during leucine deprivation was confirmed by our observations of 1) colocalization of CRH and p-S6 in the PVN, 2) the blocking effect of overexpression of S6K1 by Ad-CAS6K1 on leucine deprivation–induced Crh expression, and 3) the ability of exogenous CRH to suppress the effects of Ad-CAS6K1 on energy expenditure and fat mass during leucine deprivation. This is the first study to report that hypothalamic S6K1 acts as an upstream regulator for Crh expression and thereby regulates peripheral energy expenditure under leucine deprivation. Our results also extend the role of S6K1 as a nutrient sensor to affect whole-body energy availability through cooperating with endocrine signals in the hypothalamus.
Based on our previous observation that Crh expression under leucine deprivation is stimulated by a Gs-dependent pathway (22), we explored the possible involvement of MC4R in the regulation of energy expenditure during leucine deprivation. MC4R, a component of the CNS melanocortin signaling system, has previously been shown to be important in regulating energy balance (42,43). For example, both knockdown of MC4R expression (15–17) and increased MC4R activity by intracerebroventricular infusion of the MC4R agonist α-melanocyte–stimulating hormone or MTII (a synthetic melanocortin agonist) influence energy balance in animal models (15,16). Several studies have also provided important hints of regulation of Crh expression by MC4R (44), but this possibility has not been investigated specifically.
In this study, we showed that MC4R protein levels in the hypothalamus are increased under leucine deprivation and that this increase could be blocked by overexpression of S6K1 via intracerebroventricular injection of Ad-CAS6K1. Furthermore, we used the MC4R antagonist SHU9119 to demonstrate that MC4R regulates Crh expression in the hypothalamus, as well as energy expenditure and UCP1 expression in BAT during leucine deprivation. In addition, we showed that Crh expression is regulated by S6K1 in an MC4R-dependent manner in primary cultured hypothalamic neurons under leucine deprivation. Taken together, these results suggest that hypothalamic S6K1 regulates energy expenditure by modulation of Crh expression in an MC4R-dependent manner in response to leucine deprivation. The blocking effects of SHU9119 on Crh expression, body weight, and adiposity in mice under leucine deprivation, however, were not complete, suggesting the existence of additional pathways involved in this regulation. Consistent with other reports (11,16,18), we found that SHU9119 treatment also attenuates the reduction in food intake under leucine deprivation (data not shown), which may also contribute to decreased fat loss in these mice. Finally, the molecular mechanisms underlying S6K1 regulation of MC4R expression, however, are still unclear. We speculate that S6K1 regulates MC4R expression at the level of translation, rather than transcription, as we did not observe increased Mc4r mRNA expression under leucine deprivation (data not shown). These possibilities will be investigated in future studies.
In contrast to previous reports showing that activation of S6K1 signaling in the hypothalamus decreases food intake in rats (9,45,46), we did not observe an effect of CAS6K1 on food intake in mice maintained on a control or leucine-deficient diet. The source of this inconsistency is unclear but may reflect a difference in the species, methods of S6K1 activation, time points examined, and/or methods of measuring food intake. In support of this possibility and consistent with our results, food intake is not altered in S6K1-deficient mice compared with control mice (7). Similar to differential effects of food intake, the effects of S6K1 on energy expenditure and body weight are also not always the same (7,9,45,46). Therefore, a role of hypothalamic S6K1 in regulating energy intake and energy expenditure could be very complicated, and special attention needs to be paid when evaluating possible roles for S6K1 in energy homeostasis regulation under different situations.
At present, the identification of the upstream regulator of S6K1 in the hypothalamus during leucine deprivation is poorly understood. GCN2 is an amino acid sensor that is activated in response to essential amino acid deprivation (47), which we have recently shown to play a role in regulating lipid metabolism and insulin sensitivity (20,27). Studies have shown that leucine deprivation decreases mTOR activity in liver, HepG2 cells, and primary hepatocytes and that the decreased mTOR activity is mediated by GCN2 (27,48). Although not yet demonstrated in brain, we think this is a plausible pathway for the response to leucine deprivation. This possibility will be investigated in our future studies.
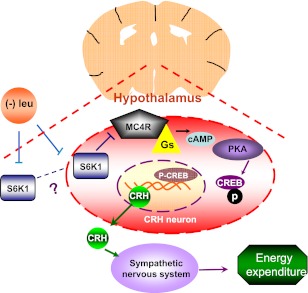
In summary, our data suggest a novel role for hypothalamic S6K1 in regulating fat loss by modulating energy expenditure during leucine deprivation. By regulating expression of CRH in an MC4R-dependent manner, hypothalamic S6K1 has a profound effect on the energy expenditure and overall energy homeostasis (Fig. 7). Because deficiencies of essential amino acids almost always occur in conjunction with deficiencies of other nutrients, we speculate that S6K1 in the hypothalamus may act as a master regulator of metabolic adaptation to nutrient deprivation, including deprivation of any other essential amino acid. It remains unclear which part of the hypothalamus is responsible for the fat loss during leucine deprivation. Our results suggest that S6K1 expressed in PVN might be important for regulating energy expenditure during leucine deprivation. Since additional regions along ventricles of the brain might be affected by intracerebroventricular injection of adenovirus (data not shown), we cannot exclude a role for other regions of the brain in this study. Such possibilities will be investigated in the future.
FIG. 7.
Hypothalamic S6K1 regulates energy expenditure via MC4R-dependent modulation of CRH under leucine deprivation. Dietary leucine deprivation inhibits hypothalamic S6K1 activity, which in turn increases the G protein–coupled, seven-transmembrane receptor MC4R protein levels and stimulates Gs activity. These events increase intracellular cAMP levels, which stimulates cAMP-dependent protein kinase (PKA)–mediated phosphorylation and activation of CREB. In the nucleus, Crh transcription is stimulated by the binding of phosphorylated CREB at the cAMP-responsive element site in its promoter. After translation and modification, CRH is secreted as a polypeptide and activates the sympathetic nervous system to stimulate energy expenditure and thermogenesis in the whole organism.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Science and Technology of China (973 Program 2009CB919001 and 2010CB912502), the State Key Program of National Natural Science Foundation (81130076), the National Natural Science Foundation (30871208 and 30890043), the Chief Scientist Program of Shanghai Institutes for Biological Sciences, the Chinese Academy of Sciences (SIBS2008006), the Science and Technology Commission of Shanghai Municipality (08DJ1400601), the 2010 Key Program of Clinical Research Center, the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, the Chinese Academy of Sciences (CRC2010005), the Key Program of Shanghai Scientific and Technological Innovation Action Plan (10JC1416900), and the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-R-09). F.G. was also supported by the One Hundred Talents Program of the Chinese Academy of Sciences.
No potential conflicts of interest relevant to this article were reported.
T.X. researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. Y.C. researched data. Q.Z. researched data, contributed to discussion, and reviewed and edited the manuscript. F.X., B.L., and S.C. provided research material and contributed to discussion. F.G. contributed to discussion and wrote, reviewed, and edited the manuscript. F.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank Dr. Chunxia Wang (Institute for Nutritional Sciences) for writing advice, Qingshu Meng (Institute for Nutritional Sciences) for calorimetry assistance, and Chun Feng (Institute for Nutritional Sciences) for confocal image analysis assistance.
REFERENCES
- 1.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res 2010;209:1–12 [DOI] [PubMed] [Google Scholar]
- 2.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 2002;36:199–211 [DOI] [PubMed] [Google Scholar]
- 3.Cota D, Proulx K, Seeley RJ. The role of CNS fuel sensing in energy and glucose regulation. Gastroenterology 2007;132:2158–2168 [DOI] [PubMed] [Google Scholar]
- 4.Elmquist JK, Coppari R, Balthasar N, Ichinose M, Lowell BB. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 2005;493:63–71 [DOI] [PubMed] [Google Scholar]
- 5.Chakravarthy MV, Zhu Y, López M, et al. Brain fatty acid synthase activates PPARalpha to maintain energy homeostasis. J Clin Invest 2007;117:2539–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q, Zhang Y, Xu J, Shen P. Regulation of hunger-driven behaviors by neural ribosomal S6 kinase in Drosophila. Proc Natl Acad Sci USA 2005;102:13289–13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004;431:200–205 [DOI] [PubMed] [Google Scholar]
- 8.Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J 1998;17:6649–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab 2008;8:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richard D, Rivest R, Naïmi N, Timofeeva E, Rivest S. Expression of corticotropin-releasing factor and its receptors in the brain of lean and obese Zucker rats. Endocrinology 1996;137:4786–4795 [DOI] [PubMed] [Google Scholar]
- 11.Momose K, Inui A, Asakawa A, et al. Intracerebroventricularly administered corticotropin-releasing factor inhibits food intake and produces anxiety-like behaviour at very low doses in mice. Diabetes Obes Metab 1999;1:281–284 [DOI] [PubMed] [Google Scholar]
- 12.LeFeuvre RA, Rothwell NJ, Stock MJ. Activation of brown fat thermogenesis in response to central injection of corticotropin releasing hormone in the rat. Neuropharmacology 1987;26:1217–1221 [DOI] [PubMed] [Google Scholar]
- 13.Kishi T, Aschkenasi CJ, Choi BJ, et al. Neuropeptide Y Y1 receptor mRNA in rodent brain: distribution and colocalization with melanocortin-4 receptor. J Comp Neurol 2005;482:217–243 [DOI] [PubMed] [Google Scholar]
- 14.Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endocrinol 1994;8:1298–1308 [DOI] [PubMed] [Google Scholar]
- 15.Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005;123:493–505 [DOI] [PubMed] [Google Scholar]
- 16.Huszar D, Lynch CA, Fairchild-Huntress V, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997;88:131–141 [DOI] [PubMed] [Google Scholar]
- 17.Chen AS, Metzger JM, Trumbauer ME, et al. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res 2000;9:145–154 [DOI] [PubMed] [Google Scholar]
- 18.Lu XY, Barsh GS, Akil H, Watson SJ. Interaction between alpha-melanocyte-stimulating hormone and corticotropin-releasing hormone in the regulation of feeding and hypothalamo-pituitary-adrenal responses. J Neurosci 2003;23:7863–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsuda K, Kojima K, Shimakura S, et al. Corticotropin-releasing hormone mediates alpha-melanocyte-stimulating hormone-induced anorexigenic action in goldfish. Peptides 2008;29:1930–1936 [DOI] [PubMed] [Google Scholar]
- 20.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 2007;5:103–114 [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Meng Q, Wang C, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010;59:17–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng Y, Zhang Q, Meng Q, et al. Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol Endocrinol 2011;25:1624–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cota D, Proulx K, Smith KAB, et al. Hypothalamic mTOR signaling regulates food intake. Science 2006;312:927–930 [DOI] [PubMed] [Google Scholar]
- 24.Aguilar V, Alliouachene S, Sotiropoulos A, et al. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab 2007;5:476–487 [DOI] [PubMed] [Google Scholar]
- 25.Pende M, Kozma SC, Jaquet M, et al. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature 2000;408:994–997 [DOI] [PubMed] [Google Scholar]
- 26.Kleinridders A, Schenten D, Könner AC, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab 2009;10:249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao F, Huang ZY, Li HK, et al. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 2011;60:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori H, Inoki K, Münzberg H, et al. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 2009;9:362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature 2000;404:652–660 [DOI] [PubMed] [Google Scholar]
- 30.Blouet C, Jo YH, Li XS, Schwartz GJ. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci 2009;29:8302–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roa JA, Tena-Sempere M. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metab 2010;21:519–528 [DOI] [PubMed] [Google Scholar]
- 32.Villanueva EC, Münzberg H, Cota D, et al. Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology 2009;150:4541–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 1984;4:409–454 [DOI] [PubMed] [Google Scholar]
- 34.Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab 2001;281:E466–E471 [DOI] [PubMed] [Google Scholar]
- 35.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 1998;273:14484–14494 [DOI] [PubMed] [Google Scholar]
- 36.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 2009;9:203–209 [DOI] [PubMed] [Google Scholar]
- 37.Enerbäck S, Jacobsson A, Simpson EM, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 1997;387:90–94 [DOI] [PubMed] [Google Scholar]
- 38.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab 2010;11:263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillhouse EW, Grammatopoulos DK. The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 2006;27:260–286 [DOI] [PubMed] [Google Scholar]
- 40.Nikodemova M, Kasckow J, Liu H, Manganiello V, Aguilera G. Cyclic adenosine 3′,5′-monophosphate regulation of corticotropin-releasing hormone promoter activity in AtT-20 cells and in a transformed hypothalamic cell line. Endocrinology 2003;144:1292–1300 [DOI] [PubMed] [Google Scholar]
- 41.Heitzer MD, Wolf IM, Sanchez ER, Witchel SF, DeFranco DB. Glucocorticoid receptor physiology. Rev Endocr Metab Disord 2007;8:321–330 [DOI] [PubMed] [Google Scholar]
- 42.Butler AA. The melanocortin system and energy balance. Peptides 2006;27:281–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi J, Balthasar N, Olson D, et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 2011;13:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thiele TE, van Dijk G, Yagaloff KA, et al. Central infusion of melanocortin agonist MTII in rats: assessment of c-Fos expression and taste aversion. Am J Physiol 1998;274:R248–R254 [DOI] [PubMed] [Google Scholar]
- 45.Cota D, Matter EK, Woods SC, Seeley RJ. The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 2008;28:7202–7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ropelle ER, Fernandes MFA, Flores MBS, et al. Central exercise action increases the AMPK and mTOR response to leptin. PLoS ONE 2008;3:e3856. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Hao SZ, Sharp JW, Ross-Inta CM, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science 2005;307:1776–1778 [DOI] [PubMed] [Google Scholar]
- 48.Anthony TG, McDaniel BJ, Byerley RL, et al. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 2004;279:36553–36561 [DOI] [PubMed] [Google Scholar]