Abstract
In the billion years that bacteriophage (or phage) have existed together with bacteria the phage have evolved systems that may be exploited for our benefit. One of these is the lytic system used by the phage to release their progeny from an infected bacterium. Endolysins (or lysins) are highly evolved enzymes in the lytic system produced to cleave essential bonds in the bacterial cell wall peptidoglycan for progeny release. Small quantities of purified recombinant lysin added externally to gram-positive bacteria results in immediate lysis causing log-fold death of the target bacterium. Lysins have now been used successfully in a variety of animal models to control pathogenic antibiotic resistant bacteria found on mucosal surfaces and in infected tissues. The advantages over antibiotics are their specificity for the pathogen without disturbing the normal flora, the low chance of bacterial resistance, and their ability to kill colonizing pathogens on mucosal surfaces, a capacity previously unavailable. Lysins therefore, may be a much-needed anti-infective (or enzybiotic) in an age of mounting antibiotic resistance.
Keywords: bacteriophage, endolysin, gram-positive bacteria, lytic enzymes, mucosal colonization, phage, prophylaxis, therapeutic
Bacteriophages are viruses that specifically infect bacteria and have no direct effect on humans. In fact, with an estimated 1031 phage on earth it would be impossible to avoid ingesting phage regularly. After replicating inside its bacterial host the phage is faced with a problem, it needs to efficiently exit the bacterium to disseminate its progeny phage to begin a new cycle. To solve this, double-stranded DNA phages have evolved a lytic system to weaken the bacterial cell wall resulting in bacterial lysis. Phage lytic enzymes or lysins are highly efficient molecules that have been refined over millions of years of evolution for this very purpose. These enzymes target the integrity of the cell wall, and are designed to attack one of the five major bonds in the peptidoglycan. With few exceptions,1 lysins do not have signal sequences, so they are not translocated through the cytoplasmic membrane to attack their substrate in the peptidoglycan, this movement is tightly controlled by a second phage gene product in the lytic system, the holin.2 During phage development in the infected bacterium, lysin accumulates in the cytoplasm in anticipation of phage maturation. At a genetically specified time, holin molecules are inserted in the cytoplasmic membrane forming patches, ultimately resulting in generalized membrane disruption,3 allowing the cytoplasmic lysin to access the peptidoglycan, thereby causing cell lysis and the release of progeny phage.2 In contrast to large DNA phage, small RNA and DNA phages use a different release strategy. They call upon a phage-encoded protein to interfere with bacterial host enzymes responsible for peptidoglycan biosynthesis4,5 resulting in misassembled cell walls and ultimate lysis.
The scientific community has been aware of the lytic activity of phage for nearly a century, and while whole phage have been used to control infection,6 not until recently have lytic enzymes been exploited for bacterial control in vivo.7-9 One of the main reasons that such an approach is now even being considered is the sharp increase in antibiotic resistance among pathogenic bacteria. Current data indicate that lysins work only with gram-positive bacteria, since they are able to make direct contact with the cell wall carbohydrates and peptidoglycan when added externally, whereas the outer membrane of gram-negative bacteria, with rare exception,10 prevents this interaction. This review will outline the remarkable potency these enzymes have in killing bacteria both in vitro and in vivo.
Most human infections (viral or bacterial) begin at a mucous membrane site (upper and lower respiratory, intestinal, urogenital and ocular). In addition, the human mucous membranes are the reservoir (and sometimes the only reservoir) for many pathogenic bacteria found in the environment (i.e., pneumococci, staphylococci, streptococci, hemophilus) some of which are resistant to antibiotics. In most instances, it is this mucosal reservoir that is the focus of infection in the population.11-13 To date, except for polysporin and mupirocin ointments, which are the most widely used topically primarily to remove methicillin resistant Staphylococcus aureus (MRSA) and other colonizing staphylococci from the anterior nares, there are no anti-infectives that are designed to control colonizing pathogenic bacteria on mucous membranes14; we usually first wait for infection to occur before treating. Because of the fear of increasing the resistance problem, antibiotics are not indicated to control the carrier state of disease bacteria. It is acknowledged however, that by reducing or eliminating this human reservoir of pathogens in the community and controlled environments (i.e., hospitals and nursing homes), the incidence of disease will be markedly reduced.11,14 Toward this goal, lysins have been developed to prevent infection by safely and specifically destroying disease bacteria on mucous membranes. For example, based on extensive animal results, enzymes specific for S. pneumoniae, S. pyogenes and S. aureus may be used nasally and orally to control these organisms in the community as well as in nursing homes and hospitals to prevent or markedly reduce serious infections caused by these bacteria. This has been accomplished by capitalizing on the efficiency by which phage lysins kill bacteria.15 Like antibiotics, which are used by bacteria to control the organisms around them in the environment, phage lysins are the culmination of millions of years of development by the bacteriophage in their association with bacteria. Specific lysins have now been identified and purified that are able to kill specific gram-positive bacteria seconds after contact.7,16 For example, nanogram quantities of lysin could reduce 107 S. pyogenes by > 6 logs seconds to minutes after enzyme addition. No known biological compounds, except chemical agents, kill bacteria this quickly. Because of their highly effective activity against bacteria for the control of disease, the term “enzybiotics” was coined7 to describe these novel anti-infectives.
Lysin Structure
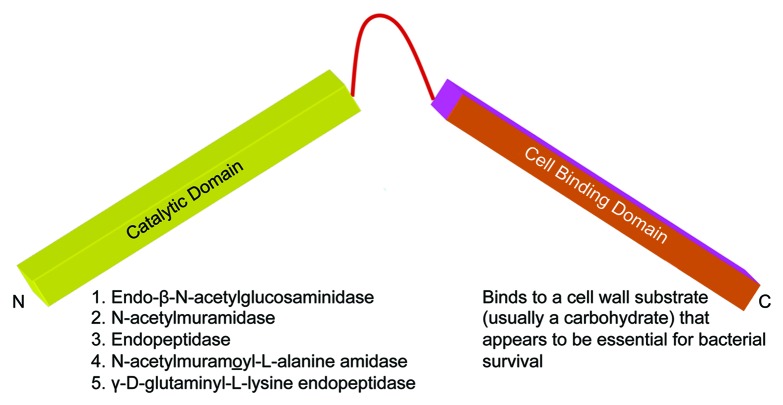
Lysins from DNA-phage that infect gram-positive bacteria are generally between 25–40 kDa in size except the PlyC lysin for streptococci, which is 114 kDa. PlyC is unique because it is composed of two separate gene products, PlyCA and PlyCB. Based on biochemical and biophysical studies, the catalytically active PlyC holoenzyme is composed of eight PlyCB subunits for each PlyCA.17 A feature of all other gram-positive phage lysins is their two-domain structure (Fig. 1). With rare exception,18,19 the N-terminal domain contains the catalytic activity of the enzyme. This activity may be either an endo-β-N-acetylglucosaminidase or N-acetylmuramidase (lysozymes), both of which act on the sugar moiety of the bacterial wall, an endopeptidase which acts on the peptide moiety, or an N-acetylmuramoyl-l-alanine amidase (or amidase), which hydrolyzes the amide bond connecting the glycan strand and peptide moieties.15,20 Recently an enzyme with γ-D-glutaminyl-l-lysine endopeptidase activity has also been reported.21 In some cases, particularly staphylococcal lysins and a lysin from group B streptococci, two and perhaps even three different catalytic domains may be linked to a single binding domain.22,23 The C-terminal cell binding domain on the other hand binds to a specific substrate (usually carbohydrate) found in the cell wall of the host bacterium.24-26 Efficient cleavage requires that the binding domain bind to its cell wall substrate, offering some degree of specificity to the enzyme since these substrates are only found in enzyme-sensitive bacteria.
Figure 1. Basic structure of phage lysins. In general, lysins range between 25 kDa to 40 kDa in size and have a domain structure. The N-terminal domain is invariably the catalytic domain, which cleaves one of the five major bonds in the peptidoglycan, and the C-terminal domain binds to a carbohydrate determinant in the cell wall.
The first complete crystal structure for the free and choline-bound states of the Cpl-1 lytic enzyme has been published.27 As suspected, the data suggest that choline recognition by the choline-binding domain of Cpl-1may allow the catalytic domain to be properly oriented for efficient cleavage. An interesting feature of this lysin is its hairpin conformation suggesting that the two domains interact with each other prior to binding its substrate in the bacterial cell wall (in this case choline). While this is not obvious with some reported lysins28,29 others show that these interactions do in fact occur30 (Dias et al., submitted). In the latter case it appears that the C-terminal binding domain is completely unstructured and interacts with the catalytic domain to prevent catalytic activity. When this autoinhibited lysin contacts its cell wall receptor after traversing the membrane through the hole produced by the holin, the binding domain folds and dimerizes with another lysin molecule (Dias et al., submitted). This mechanism, which is concentration dependant, may have evolved to prevent killing adjacent host cells and assure phage survival.
When the sequences between lytic enzymes of the same enzyme class are compared, high sequence homology is seen within the N-terminal catalytic region and very little homology within in the C-terminal cell-binding region. It seemed counterintuitive that the phage would design a lysin that was uniquely lethal for its host organism, however as we learned more about how these enzymes function, a possible reason for this specificity became apparent (see below, Resistance). Because of the specificity, enzymes that spilled out after cell lysis had a good chance of killing potential bacterial hosts in the vicinity of the released phage progeny. To prevent this, we believe that the gram-positive lysins have evolved to bind to their cell wall binding receptors at a high affinity31 to limit the release of free enzyme. This is not the case for lysins produced by gram-negative phage. Since spilled lysin after lysis is unable to penetrate through the outer membrane to cleave the peptidoglycan, nearly all lysins from gram-negative phage did not evolve binding domains.
Because of their domain structure, it seemed plausible that different enzyme domains could be swapped resulting in lysins with different bacterial and catalytic specificities. This was actually accomplished by early detailed studies of García and colleagues,19,32 in which the catalytic domains of lytic enzymes for S. pneumoniae phage could be swapped resulting in a new enzyme having the same binding domain for pneumococci, but able to cleave a different bond in the peptidoglycan. This capacity allows for enormous potential in creating designer enzymes with high specificity and equally high cleavage potential. In recent years, lysins have been engineered to achieve certain characteristics not present in the native lysin.33,34
Mechanism of Action
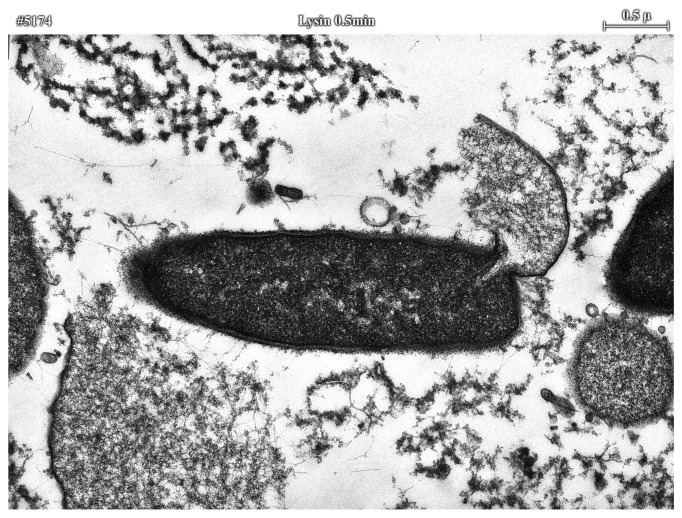
When examined by thin section electron microscopy, it seems obvious that lysins exert their lethal effects by forming holes in the cell wall through peptidoglycan digestion. The high internal pressure of gram-positive bacterial cells (roughly 15–25 atmospheres) is controlled by the highly cross-linked cell wall. Any disruption in the wall’s integrity will result in the extrusion of the cytoplasmic membrane and ultimate hypotonic lysis (Fig. 2). Catalytically, a single enzyme molecule should be sufficient to cleave an adequate number of bonds to kill an organism; however, it is uncertain at this time whether this theoretical limit is possible. The reason comes from the work of Loessner,31 showing that a listeria phage enzyme had a binding affinity approaching that of an IgG molecule for its substrate, suggesting that phage enzymes, like cellulases35 are one-use enzymes, likely requiring several molecules attacking a local region to sufficiently weaken the cell wall. The evolution of a high affinity binding for the binding domain for its cell wall substrate assured that lysins would not be released to kill potential hosts.
Figure 2. Electron microscopy of lysin treated bacilli. Thirty seconds after treatment of B. cereus with lysin, membrane extrusion is observed prior to lysis and ultimate death of the bacterium. Surrounding this lysing bacillus, is the debris of other lysed bacilli.
Lysin Efficacy
In general lysins only kill the species (or subspecies) of bacteria from which they were produced. For instance, enzymes produced from streptococcal phage kill certain streptococci, and enzymes produced by pneumococcal phage kill pneumococci.7,16 Specifically, a lysin from a group C streptococcal phage (PlyC) will kill group C streptococci, as well as groups A and E streptococci, the bovine pathogen S. uberis and the horse pathogen, S. equi, but essentially no effect on streptococci normally found in the oral cavity of humans and other gram-positive bacteria. Similar results are seen with a pneumococcal-specific lysin, however in this case, the enzyme was also tested against strains of penicillin-resistant pneumococci and the killing efficiency was the same. Unlike antibiotics, which are usually broad spectrum and kill many different bacteria found in the human body, some of which are beneficial, lysins may be identified which kill only the disease organism with little to no effect on the normal human bacterial flora. The most specific lysin reported is the lysins for B. anthracis (PlyG), this enzyme only kills B. anthracis with rare but unique B. cereus strains.8 Another highly specific lysin is a chimeric lysin for staphylococci called ClyS.34 Because this enzyme is an endopepdidase that cleaves the peptidoglycan cross bridge, and only staphylococci have penta glycine in their cross bridge, this enzyme was shown to have lytic activity on all staphylococci and no other species of bacteria tested.34 In some cases however, phage enzymes may be identified with broad lytic activity. For example, an enterococcal phage lysin has recently been reported to not only kill enterococci but a number of other gram-positive pathogens such as S. pyogenes, group B streptococci and MRSA, making it one of the broadest acting lysins identified.36 However, its activity for these other pathogens was somewhat lower than for enterococci.
A significant lysin with respect to infection control is one directed to MRSA.37-41 However in most cases these enzymes show low activity or are difficult to produce in large quantities. In one recent publication,37 a staphylococcal enzyme was described that could be easily produced recombinantly and had a significant lethal effect on methicillin resistant MRSA both in vitro and in a mouse model. In the animal experiments the authors show that the enzymes may be used to decolonize staphylococci from the nose of the mice as well as protect the animals from an intraperitoneal challenge with MRSA. However, in the latter experiments, the best protection was observed if the lysin was added up to 30 min after the MRSA. Very similar results were published recently using a lysin termed LysGH1542 In a more recent publication a chimera was produced linking the catalytic enzyme of the Twort lysin with the binding domain of a PhiNM3 lysin.34 This chimera had eliminated many of the bad features of native staphylococcal phage lysins in its activity and production.
Antibiotic and Lysin Synergy
Several lysins have been identified from pneumococcal bacteriophage which are classified into two groups: amidases and lysozymes. Exposure of pneumococci to either of these enzymes leads to efficient lysis. Both enzymes have very different N-terminal catalytic domains but share a similar C-terminal choline-binding domain. These enzymes were tested to determine whether their simultaneous use is competitive or synergistic and the results clearly show that they are synergistic.43 In vivo, the combination of two lysins with different peptidoglycan specificities was found to be more effective in protecting against disease than each of the single enzymes.43,44 Thus, in addition to more effective killing, the application of two different lysins may significantly retard the emergence of enzyme-resistant mutants.
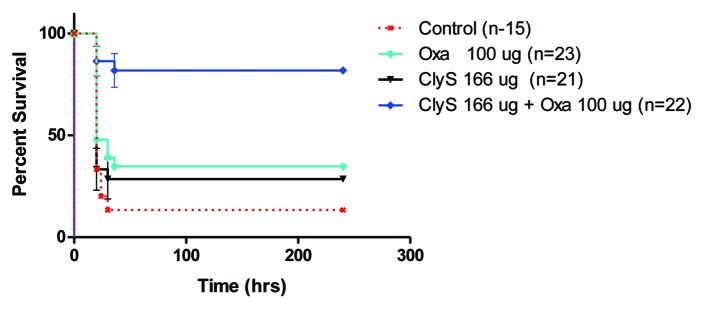
When the pneumococcal lysin Cpl-1 was used in combination with certain antibiotics a similar synergistic effect was seen. Cpl-1 and gentamicin were found to be increasingly synergistic in killing pneumococci with a decreasing penicillin MIC, while Cpl-1 and penicillin showed synergy against an extremely penicillin-resistant strain.45 Synergy was also observed with staphylococcal-specific lysins and antibiotics both in vivo (Fig. 3)34 as well as in vitro.34,37 Thus, the right combination of enzyme and antibiotic could help in the control of antibiotic resistant bacteria as well as reinstate the use of certain antibiotics for which resistance has been established.
Figure 3. Synergistic effects of ClyS and oxacillin protected mice from MRSA septicemia-induced death. Mice were intraperitoneally injected with ~5 × 105 CFU of MRSA strain MW2 in 5% mucin. Three hours post-infection, mice received an IP injection of a suboptimal concentration of ClyS (166 μg) or 20 mM phosphate buffer along with an IM injection of oxacillin (100 μg) or saline control. Mice were monitored for survival for 10 d and the results of 5 independent experiments were combined and plotted in a Kaplan Meier Survival curve.34
Lysins Tested in Animal Models
Animal models of mucosal colonization were used to test the capacity of lysins to kill organisms on these surfaces; perhaps the most important use for these enzymes. An oral colonization model was developed for S. pyogenes,7 a nasal model for pneumococci,16 and a vaginal model for group B streptococci.23 In all three cases, when the animals were colonized with their respective bacteria and treated with a single dose of lysin, specific for the colonizing organism, these organisms were reduced by several logs (and in some cases below the detection limit of the assay) when tested again two to four hours after lysin treatment. These results lend support to the idea that such enzymes may be used in specific high-risk populations to control the reservoir of pathogenic bacteria and thus control disease. A perfect example is the development of secondary infections after influenza. Recent studies reveal that 50–90% of deaths resulting from influenza are due to a secondary infection usually from S. pneumoniae, S. aureus, S. pyogenes or H. influenzae, in that order.46,47
Similar to other proteins delivered intravenously to animals and humans, lysins have a short half life (T½ = 15–20 min).9 However, the action of lysins for bacteria is so rapid, that this may be sufficient time to observe a therapeutic effect.9,44,48,49 Mice intravenously infected with type 14 S. pneumoniae and treated 1 h later with a single bolus of 2.0 mg of Cpl-1 survived through the 48h endpoint, whereas the median survival time of buffer-treated mice was only 25 h, and only 20% survival at 48h. Blood and organ cultures of the euthanized surviving mice showed that only one Cpl-1-treated animal was totally free of infection at 48h, suggesting that multiple enzyme doses or a constant infusion of enzyme would be required to eliminate the organisms completely in this application. Similar results were obtained when animals were infected and treated intraperitoneally with lysin.37,44 Because of lysin’s short half-life, it may be necessary to modify the lysins with polyethylene glycol or the Fc region of IgG, to extend the residence time in vivo to several hours.50 In recent studies, phage lysins have also been shown to be successful in the treatment of meningitis by adding the lysin directly to the brain intrathecally51 and endocarditis by delivering the lysin intravenously by constant IV infusion.52 Both these applications would also benefit from modified long-acting lysins.
The crucial challenge for lysins would be to determine whether they are able to cure an established infection. To approach this, a mouse pneumonia model was developed in which mice were transnasally infected with pneumococci and treated with Cpl-1 by repeated intraperitoneal injections after infection was established.49 From a variety of clinical measurements, as well as morphologic changes in the lungs, it was shown that at 24 h mice suffered from severe pneumonia. When treatment was initiated at 24 h and every 12 h thereafter, 100% of the mice survived otherwise fatal pneumonia and showed rapid recovery. Cpl-1 dramatically reduced pulmonary bacterial counts, and prevented bacteremia.
Bacterial Resistance to Lysins
Though attempts have been made to identify resistant bacteria, thus far bacteria that are resistant to the lytic action of lysins have not been reported. In experiments similar to those that would reveal antibiotic resistance, lysin resistance has not been found. For example, exposure of bacteria grown on agar plates to low concentrations of lysin did not lead to the recovery of resistant strains even after over 40 cycles. Organisms in colonies isolated at the periphery of a clear lytic zone created by a 10 ul drop of dilute lysin on a lawn of bacteria always resulted in enzyme sensitive bacteria. Enzyme resistant bacteria could also not be identified after > 10 cycles of bacterial exposure to low concentrations of lysin (from 5–20 units) in liquid culture.8,16 These results may be explained by the fact that the cell wall receptor for the pneumococcal lysin is choline,53 a molecule that is essential for pneumococcal viability. While not yet proven, it is possible that during a phage's association with bacteria over the millennia, to avoid becoming trapped inside the host, the binding domain of their lytic enzymes has evolved to target a unique and essential molecule in the cell wall, making resistance to these enzymes a rare event.
Identifying and Isolating New Lysins
There are a few ways in which lysins may be identified. The first and simplest is to identify a phage, shotgun clone its DNA and identify lytic activity by overlaying the plated clones with the phage-sensitive bacterium. In this case you usually have a lysin for the organism or species that the phage infects. A more general way of isolating lysins in order to understand the diversity in this class of enzymes in the environment is through functional metagenomic analysis.54 This technique uses random environmental phage populations processed for metagenomic analysis. The twist here is to add an amplification step and an expression step to express and produce the products of the isolated lysin genes. This approach has the potential of identifying novel lysins with powerful biotechnological value. Another approach, which combines the general and the specific approach mentioned above, is to exploit the lysogens in a host genome. This approach, termed multigenomics, identifies the lysin genes in the lysogens within many strains of the same species. In this case the DNA from tens to hundreds of strains of the same species is processed as in the metagenomic analysis, except here the enzymes are from the variety of lysogens in the single species.55
Concluding Remarks
Lysins are a new reagent to control bacterial pathogens, particularly those found on the human mucosal surface. For the first time we may be able to specifically kill pathogens on mucous membranes without affecting the surrounding normal flora thus reducing a significant pathogen reservoir in the population. Since this capability has not been previously available, its acceptance may not be immediate. Nevertheless, like vaccines, we should be striving to developing methods to prevent rather than treat infection. Whenever there is a need to kill bacteria, and contact can be made with the organism, lysins may be freely utilized. Such enzymes will be of direct benefit in environments where antibiotic resistant gram-positive pathogens are a serious problem, such as hospitals, day care centers and nursing homes. The lysins isolated thus far are remarkably heat stable (up to 60°C) and are relatively easy to produce in a purified state and in large quantities, making them amenable to these applications. The challenge for the future is to use this basic strategy and improve upon it, as was the case for second and third generation antibiotics. Protein engineering, domain swapping and gene shuffling all could lead to better lytic enzymes to control bacterial pathogens in a variety of environments. Since there are 1031 phage on earth, the potential to identify new lytic enzymes as well as those that kill gram-negative bacteria is enormous. Perhaps some day phage lytic enzymes will be an essential component in our armamentarium against pathogenic bacteria.
Acknowledgments
I acknowledge the members of my laboratory who are responsible for much of the phage lysin work, Qi Chang, Mattias Collin, Anu Daniel, Sherry Kan, Jutta Loeffler, Daniel Nelson, Chad Euler, Jonathan Schmitz, Raymond Schuch and Pauline Yoong, and with the excellent technical assistance of Peter Chahales, Adam Pelzek, Rachel Shively, Mary Windels and Shiwei Zhu. I am indebted to my collaborators Stephen Leib, Jon McCullers, Philippe Moreillon, Brian Volkman and Martin Witzenrath for their excellent work with the lysins in their model systems. Supported by DARPA and USPHS Grants AI057472 and AI11822.
Footnotes
Previously published online: www.landesbioscience.com/journals/bacteriophage/article/17747
References
- 1.Loessner MJ, Maier SK, Daubek-Puza H, Wendlinger G, Scherer S. Three Bacillus cereus bacteriophage endolysins are unrelated but reveal high homology to cell wall hydrolases from different bacilli. J Bacteriol. 1997;179:2845–51. doi: 10.1128/jb.179.9.2845-2851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang IN, Smith DL, Young R. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol. 2000;54:799–825. doi: 10.1146/annurev.micro.54.1.799. [DOI] [PubMed] [Google Scholar]
- 3.Wang IN, Deaton J, Young R. Sizing the Holin Lesion with an Endolysin-beta-Galactosidase fusion. J Bacteriol. 2003;185:779–87. doi: 10.1128/JB.185.3.779-787.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young I, Wang IN, Roof WD. Phages will out: strategies of host cell lysis. Trends Microbiol. 2000;8:120–8. doi: 10.1016/S0966-842X(00)01705-4. [DOI] [PubMed] [Google Scholar]
- 5.Bernhardt TG, Wang IN, Struck DK, Young R. A protein antibiotic in the phage Q-beta virion: diversity in lysis targets. Science. 2001;292:2326–9. doi: 10.1126/science.1058289. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki S, Rashel M, Uchiyama J, Sakurai S, Ujihara T, Kuroda M, et al. Bacteriophage therapy: a revitalized therapy against bacterial infectious diseases. J Infect Chemother. 2005;11:211–9. doi: 10.1007/s10156-005-0408-9. [DOI] [PubMed] [Google Scholar]
- 7.Nelson D, Loomis L, Fischetti VA. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci USA. 2001;98:4107–12. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuch R, Nelson D, Fischetti VA. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–9. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 9.Loeffler JM, Djurkovic S, Fischetti VA. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect Immun. 2003;71:6199–204. doi: 10.1128/IAI.71.11.6199-6204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai MJ, Lin NT, Hu A, Soo PC, Chen LK, Chen LH, et al. Antibacterial activity of Acinetobacter baumannii phage varphiAB2 endolysin (LysAB2) against both gram-positive and gram-negative bacteria. Appl Microbiol Biotechnol. 2011;90:529–39. doi: 10.1007/s00253-011-3104-y. [DOI] [PubMed] [Google Scholar]
- 11.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphlococcus aureus bacteremia. N Engl J Med. 2001;344:11–6. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 12.Coello R, Jimenez J, García M, Arroyo P, Minguez D, Fernandez C, et al. Prospective study of infection, colonization and carriage of methicillin-resistant Staphylococcus aureus in an outbreak affecting 990 patients. Eur J Clin Microbiol Infect Dis. 1994;13:74–81. doi: 10.1007/BF02026130. [DOI] [PubMed] [Google Scholar]
- 13.De Lencastre H, Kristinsson KG, Brito-Avo A, Sanches IS, Sa-Leao R, Saldanha J, et al. Carriage of respiratory tract pathogens and molecular epidemiology of Streptococcus pneumoniae colonization in healthy children attending day acre centers in lisbon, portugal. Microb Drug Resist. 1999;5:19–29. doi: 10.1089/mdr.1999.5.19. [DOI] [PubMed] [Google Scholar]
- 14.Hudson L, Hay FC. Practical Immunology. Oxford: Blackwell Scientific Publ., 1976. [Google Scholar]
- 15.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–81. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeffler JM, Nelson D, Fischetti VA. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 17.Nelson D, Chahalis P, Zhu S, Fischetti VA, Ply C. The first multimeric bacteriophage lysin. Proc Natl Acad Sci USA. 2006;103:10765–70. doi: 10.1073/pnas.0604521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Díaz E, López R, García JL. Chimeric phage-bacterial enzymes: a clue to the modular evolution of genes. Proc Natl Acad Sci USA. 1990;87:8125–9. doi: 10.1073/pnas.87.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García P, García JL, García E, Sánchez-Puelles JM, López R. Modular organization of the lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Gene. 1990;86:81–8. doi: 10.1016/0378-1119(90)90116-9. [DOI] [PubMed] [Google Scholar]
- 20.Loessner MJ. Bacteriophage endolysins- current state of research and applications. Curr Opin Microbiol. 2005;8:480–7. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl Environ Microbiol. 2007;73:7150–4. doi: 10.1128/AEM.01783-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarre WW, Ton-That H, Faull KF, Schneewind O. Multiple enzymatic activities of the murein hydrolase from staphylococcal phage phi11. Identification of a D-alanyl-glycine endopeptidase activity. J Biol Chem. 1999;274:15847–56. doi: 10.1074/jbc.274.22.15847. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Q, Nelson D, Zhu S, Fischetti VA. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother. 2005;49:111–7. doi: 10.1128/AAC.49.1.111-117.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López R, García E, García P, García JL. The pneumococcal cell wall degrading enzymes: a modular design to create new lysins? Microb Drug Resist. 1997;3:199–211. doi: 10.1089/mdr.1997.3.199. [DOI] [PubMed] [Google Scholar]
- 25.López R, García JL, García E, Ronda C, García P. Structural analysis and biological significance of the cell wall lytic enzymes of Streptococcus pneumoniae and its bacteriophage. FEMS Microbiol Lett. 1992;79:439–47. doi: 10.1111/j.1574-6968.1992.tb14074.x. [DOI] [PubMed] [Google Scholar]
- 26.García E, García JL, Arrarás A, Sánchez-Puelles JM, López R. Molecular evolution of lytic enzymes of Streptococcus pneumoniae and its bacteriophages. Proc Natl Acad Sci USA. 1988;85:914–8. doi: 10.1073/pnas.85.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermoso JA, Monterroso B, Albert A, Galán B, Ahrazem O, García P, et al. Structural basis for selective recognition of pneumococcal cell wall by modular endolysin from phage Cp-1. Structure. 2003;11:1239–49. doi: 10.1016/j.str.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 28.Korndörfer IP, Danzer J, Schmelcher M, Zimmer M, Skerra A, Loessner MJ. The crystal structure of the bacteriophage PSA endolysin reveals a unique fold responsible for specific recognition of Listeria cell walls. J Mol Biol. 2006;364:678–89. doi: 10.1016/j.jmb.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 29.Porter CJ, Schuch R, Pelzek AJ, Buckle AM, McGowan S, Wilce MC, et al. The 1.6 A crystal structure of the catalytic domain of PlyB, a bacteriophage lysin active against Bacillus anthracis. J Mol Biol. 2007;366:540–50. doi: 10.1016/j.jmb.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 30.Low LY, Yang C, Perego M, Osterman A, Liddington RC. Structure and lytic activity of a Bacillus anthracis prophage endolysin. J Biol Chem. 2005;280:35433–9. doi: 10.1074/jbc.M502723200. [DOI] [PubMed] [Google Scholar]
- 31.Loessner MJ, Kramer K, Ebel F, Scherer S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol. 2002;44:335–49. doi: 10.1046/j.1365-2958.2002.02889.x. [DOI] [PubMed] [Google Scholar]
- 32.Weiss K, Lavardiere M, Lovgren M, Delorme J, Poirier L, Beliveau C. Group A streptococcus carriage among close contacts of patients with invasive infections. Am J Epidemiol. 1999;149:863–8. doi: 10.1093/oxfordjournals.aje.a009902. [DOI] [PubMed] [Google Scholar]
- 33.Donovan DM, Dong S, Garrett W, Rousseau GM, Moineau S, Pritchard DG. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl Environ Microbiol. 2006;72:2988–96. doi: 10.1128/AEM.72.4.2988-2996.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daniel A, Euler C, Collin M, Chahales P, Gorelick KJ, Fischetti VA. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2010;54:1603–12. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jervis EJ, Haynes CA, Kilburn DG. Surface diffusion of cellulases and their isolated binding domains on cellulose. J Biol Chem. 1997;272:24016–23. doi: 10.1074/jbc.272.38.24016. [DOI] [PubMed] [Google Scholar]
- 36.Yoong P, Nelson D, Schuch R, Fischetti VA. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J Bacteriol. 2004;186:4808–12. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, et al. Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage phi MR11. J Infect Dis. 2007;196:1237–47. doi: 10.1086/521305. [DOI] [PubMed] [Google Scholar]
- 38.Sass P, Bierbaum G. Lytic activity of recombinant bacteriophage phi11 and phi12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl Environ Microbiol. 2007;73:347–52. doi: 10.1128/AEM.01616-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Flaherty S, Coffey A, Meaney W, Fitzgerald GF, Ross RP. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J Bacteriol. 2005;187:7161–4. doi: 10.1128/JB.187.20.7161-7164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clyne M, Birkbeck TH, Arbuthnott JP. Characterization of staphylococcal Y-lysin. J Gen Microbiol. 1992;138:923–30. doi: 10.1099/00221287-138-5-923. [DOI] [PubMed] [Google Scholar]
- 41.Sonstein SA, Hammel JM, Bondi A. Staphylococcal bacteriophage-associated lysin: a lytic agent active against Staphylococcus aureus. J Bacteriol. 1971;107:499–504. doi: 10.1128/jb.107.2.499-504.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu J, Xu W, Lei L, Huang J, Feng X, Sun C, et al. LysGH15, a novel bacteriophage lysin, protects a murine bacteremia model efficiently against lethal methicillin-resistant Staphylococcus aureus infection. J Clin Microbiol. 2011;49:111–7. doi: 10.1128/JCM.01144-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeffler JM, Fischetti VA. Synergistic lethal effect of a combination of phage lytic enzymes with different activities on penicillin-sensitive and -resistant Streptococcus pneumoniae strains. Antimicrob Agents Chemother. 2003;47:375–7. doi: 10.1128/AAC.47.1.375-377.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jado I, López R, García E, Fenoll A, Casal J, García P. Phage lytic enzymes as therapy for antiobiotic-resistant Streptococcus pneumoniae infection in a murine sepsis model. J Antimicrob Chemother. 2003;52:967–73. doi: 10.1093/jac/dkg485. [DOI] [PubMed] [Google Scholar]
- 45.Djurkovic S, Loeffler JM, Fischetti VA. Synergistic killing of Streptococcus pneumoniae with the bacteriophage lytic enzyme Cpl-1 and penicillin or gentamicin depends on the level of penicillin resistance. Antimicrob Agents Chemother. 2005;49:1225–8. doi: 10.1128/AAC.49.3.1225-1228.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morens DM, Taubenberger JK, Folkers GK, Fauci AS. An historical antecedent of modern guidelines for community pandemic influenza mitigation. Public Health Rep. 2009;124:22–5. doi: 10.1177/003335490912400105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brundage JF, Shanks GD. What really happened during the 1918 influenza pandemic? The importance of bacterial secondary infections. J Infect Dis. 2007;196:1717–8. doi: 10.1086/522355. [DOI] [PubMed] [Google Scholar]
- 48.Tsukioka Y, Yamashita Y, Oho T, Nakano Y, Koga T. Biological function of the dTDP-rhamnose synthesis pathway in Streptococcus mutans. J Bacteriol. 1997;179:1126–34. doi: 10.1128/jb.179.4.1126-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Witzenrath M, Schmeck B, Doehn JM, Tschernig T, Zahlten J, Loeffler JM, et al. Systemic use of the endolysin Cpl-1 rescues mice with fatal pneumococcal pneumonia`. Crit Care Med. 2009;37:642–9. doi: 10.1097/CCM.0b013e31819586a6. [DOI] [PubMed] [Google Scholar]
- 50.Walsh S, Shah A, Mond J. Improved Pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob Agents Chemother. 2003;47:554–8. doi: 10.1128/AAC.47.2.554-558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grandgirard D, Loeffler JM, Fischetti VA, Leib SL. Phage lytic enzyme cpl-1 for antibacterial therapy in experimental pneumococcal meningitis. J Infect Dis. 2008;197:1519–22. doi: 10.1086/587942. [DOI] [PubMed] [Google Scholar]
- 52.Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae Endocarditis in rats. Antimicrob Agents Chemother. 2005;49:4789–92. doi: 10.1128/AAC.49.11.4789-4792.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García P, García E, Ronda C, Tomasz A, López R. Inhibition of lysis by antibody aginst phage-associated lysin and requirement of choline residues in the cell wall for progeny phage release in Streptococcus pneumoniae. Curr Microbiol. 1983;8:137–40. doi: 10.1007/BF01568846. [DOI] [Google Scholar]
- 54.Schmitz JE, Daniel A, Collin M, Schuch R, Fischetti VA. Rapid DNA library construction for functional genomic and metagenomic screening. Appl Environ Microbiol. 2008;74:1649–52. doi: 10.1128/AEM.01864-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitz JE, Ossiprandi MC, Rumah KR, Fischetti VA. Lytic enzyme discovery through multigenomic sequence analysis in Clostridium perfringens. Appl Microbiol Biotechnol. 2011;89:1783–95. doi: 10.1007/s00253-010-2982-8. [DOI] [PMC free article] [PubMed] [Google Scholar]