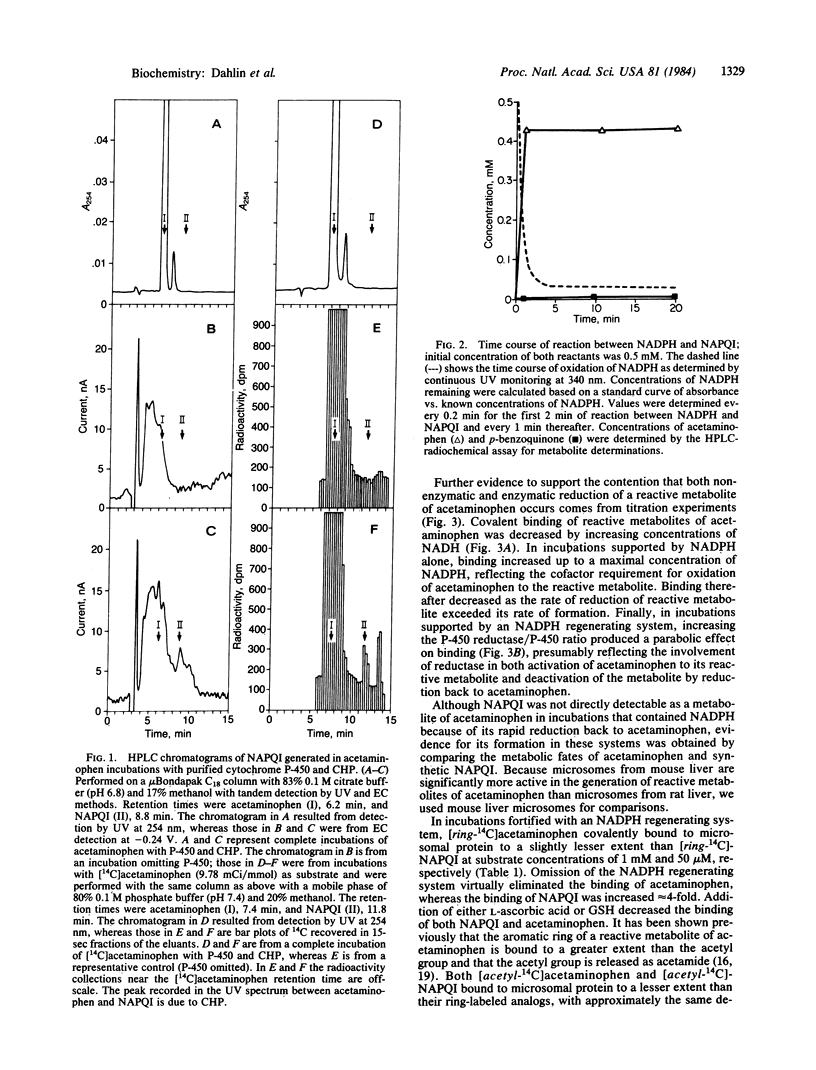
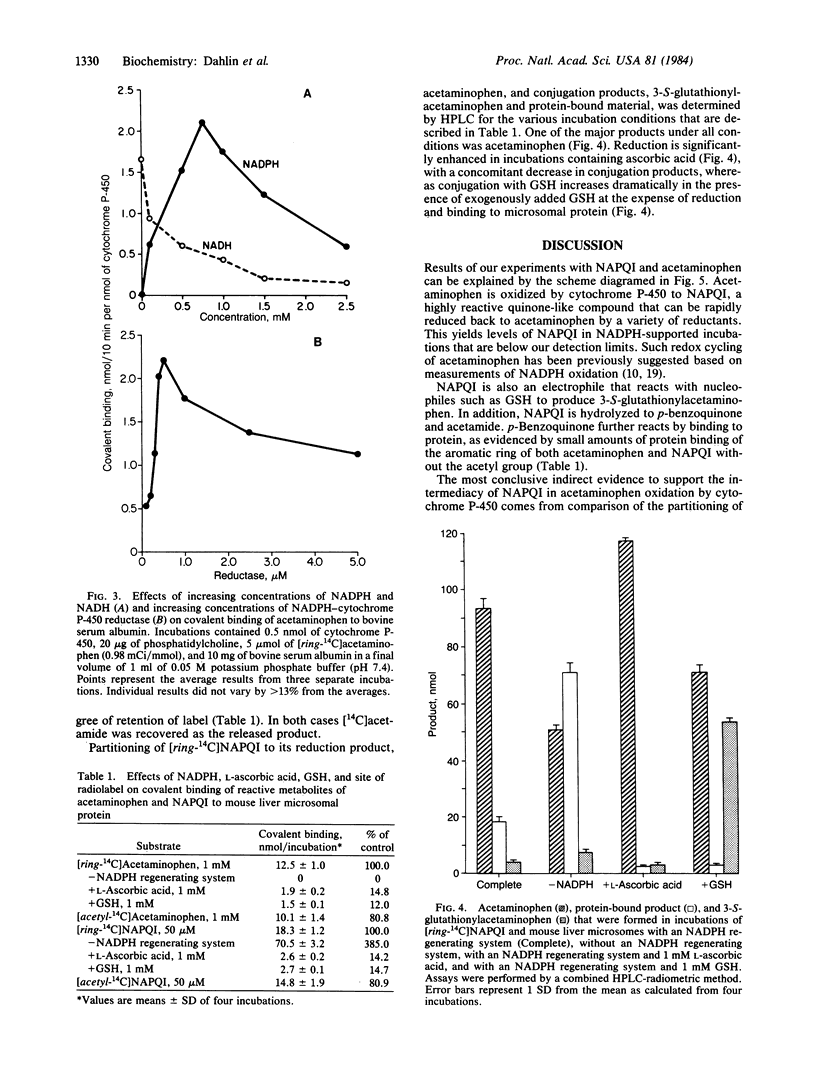
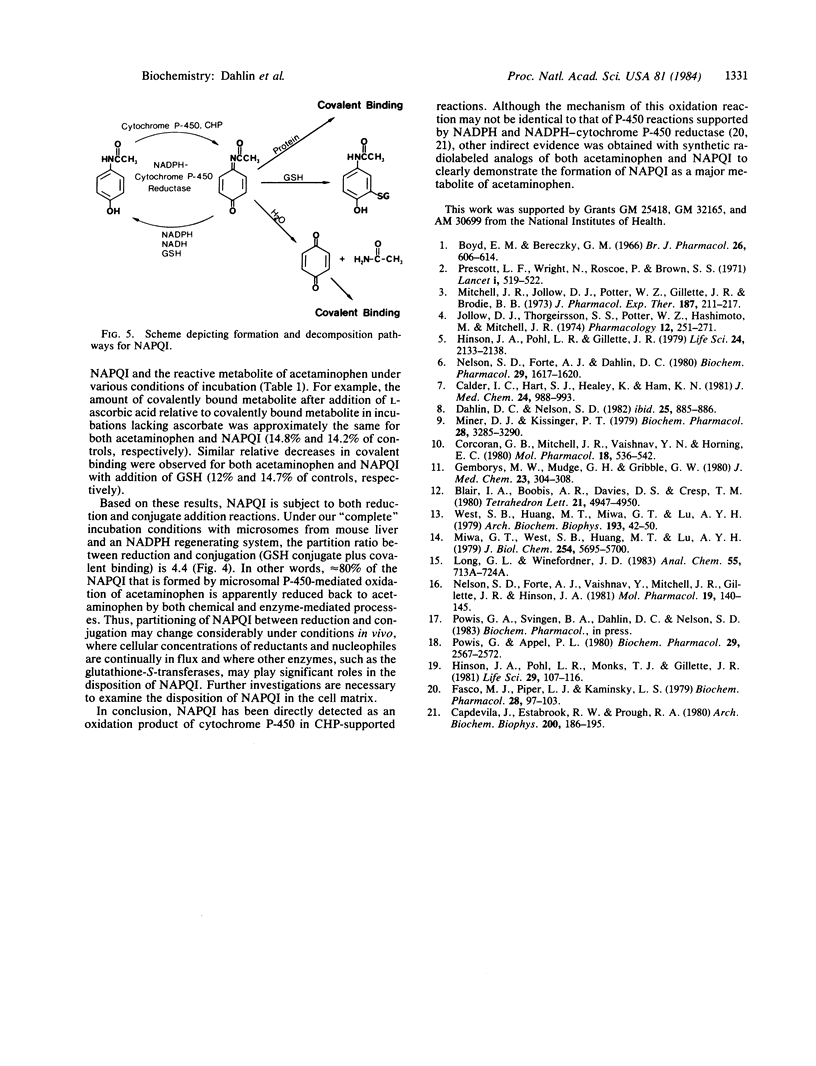
Abstract
N-acetyl-p-benzoquinone imine (NAPQI) has been proposed as the toxic metabolite of acetaminophen for the past 10 years, although it has never been detected as an enzymatic oxidation product of acetaminophen. We report (i) direct detection of NAPQI formed as an oxidation product of acetaminophen by cytochrome P-450 and cumene hydroperoxide and (ii) indirect evidence that is compelling for NAPQI formation from acetaminophen by cytochrome P-450, NADPH, and NADPH-cytochrome P-450 reductase. Evidence is provided for the rapid reduction of NAPQI back to acetaminophen by NADPH and NADPH-cytochrome P-450 reductase such that steady-state levels of NAPQI were below our detection limits of 6.7 X 10(-8) M. In mouse liver microsomal incubations, radiolabeled analogs of both NAPQI and acetaminophen bound covalently to microsomal protein with the loss of approximately equal to 20% of the acetyl group as acetamide. The binding in each case was decreased by glutathione with concomitant formation of 3-S-glutathionylacetaminophen. The binding also was decreased by L-ascorbic acid, NADPH, and NADH with reduction of NAPQI to acetaminophen. Results of partitioning experiments indicate that NAPQI is a major metabolite of acetaminophen, a considerable fraction of which is rapidly reduced back to acetaminophen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calder I. C., Hart S. J., Healey K., Ham K. N. N-hydroxyacetaminophen: a postulated toxic metabolite of acetaminophen. J Med Chem. 1981 Aug;24(8):988–993. doi: 10.1021/jm00140a014. [DOI] [PubMed] [Google Scholar]
- Capdevila J., Estabrook R. W., Prough R. A. Differences in the mechanism of NADPH- and cumene hydroperoxide-supported reactions of cytochrome P-450. Arch Biochem Biophys. 1980 Mar;200(1):186–195. doi: 10.1016/0003-9861(80)90345-8. [DOI] [PubMed] [Google Scholar]
- Corcoran G. B., Mitchell J. R., Vaishnav Y. N., Horning E. C. Evidence that acetaminophen and N-hydroxyacetaminophen form a common arylating intermediate, N-acetyl-p-benzoquinoneimine. Mol Pharmacol. 1980 Nov;18(3):536–542. [PubMed] [Google Scholar]
- Fasco M. J., Piper L. J., Kaminsky L. S. Cumene hydroperoxide-supported microsomal hydroxylations of warfarin--a probe of cytochrome P-450 multiplicity and specificity. Biochem Pharmacol. 1979;28(1):97–103. doi: 10.1016/0006-2952(79)90276-4. [DOI] [PubMed] [Google Scholar]
- Gemborys M. W., Mudge G. H., Gribble G. W. Mechanism of decomposition of N-hydroxyacetaminophen, a postulated toxic metabolite of acetaminophen. J Med Chem. 1980 Mar;23(3):304–308. doi: 10.1021/jm00177a019. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Pohl L. R., Gillette J. R. N-Hydroxyacetaminophen: a microsomal metabolite of N-hydroxyphenacetin but apparently not of acetaminophen. Life Sci. 1979 Jun 4;24(23):2133–2138. doi: 10.1016/0024-3205(79)90111-5. [DOI] [PubMed] [Google Scholar]
- Hinson J. A., Pohl L. R., Monks T. J., Gillette J. R. Acetaminophen-induced hepatotoxicity. Life Sci. 1981 Jul 13;29(2):107–116. doi: 10.1016/0024-3205(81)90278-2. [DOI] [PubMed] [Google Scholar]
- Jollow D. J., Thorgeirsson S. S., Potter W. Z., Hashimoto M., Mitchell J. R. Acetaminophen-induced hepatic necrosis. VI. Metabolic disposition of toxic and nontoxic doses of acetaminophen. Pharmacology. 1974;12(4-5):251–271. doi: 10.1159/000136547. [DOI] [PubMed] [Google Scholar]
- Miner D. J., Kissinger P. T. Evidence for the involvement of N-acetyl-p- quinoneimine in acetaminophen metabolism. Biochem Pharmacol. 1979 Nov 15;28(22):3285–3290. doi: 10.1016/0006-2952(79)90123-0. [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Jollow D. J., Potter W. Z., Gillette J. R., Brodie B. B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973 Oct;187(1):211–217. [PubMed] [Google Scholar]
- Miwa G. T., West S. B., Huang M. T., Lu A. Y. Studies on the association of cytochrome P-450 and NADPH-cytochrome c reductase during catalysis in a reconstituted hydroxylating system. J Biol Chem. 1979 Jul 10;254(13):5695–5700. [PubMed] [Google Scholar]
- Nelson S. D., Forte A. J., Dahlin D. C. Lack of evidence for N-hydroxyacetaminophen as a reactive metabolite of acetaminophen in vitro. Biochem Pharmacol. 1980 Jun 1;29(11):1617–1620. doi: 10.1016/0006-2952(80)90622-x. [DOI] [PubMed] [Google Scholar]
- Nelson S. D., Forte A. J., Vaishnav Y., Mitchell J. R., Gillette J. R., Hinson J. A. The formation of arylating and alkylating metabolites of phenacetin in hamsters and hamster liver microsomes. Mol Pharmacol. 1981 Jan;19(1):140–145. [PubMed] [Google Scholar]
- Powis G., Appel P. L. Relationship of the single-electron reduction potential of quinones to their reduction by flavoproteins. Biochem Pharmacol. 1980 Oct 1;29(19):2567–2572. doi: 10.1016/0006-2952(80)90068-4. [DOI] [PubMed] [Google Scholar]
- Prescott L. F., Roscoe P., Wright N., Brown S. S. Plasma-paracetamol half-life and hepatic necrosis in patients with paracetamol overdosage. Lancet. 1971 Mar 13;1(7698):519–522. doi: 10.1016/s0140-6736(71)91125-1. [DOI] [PubMed] [Google Scholar]
- West S. B., Huang M. T., Miwa G. T., Lu A. Y. A simple and rapid procedure for the purification of phenobarbital-inducible cytochrome P-450 from rat liver microsomes. Arch Biochem Biophys. 1979 Mar;193(1):42–50. doi: 10.1016/0003-9861(79)90006-7. [DOI] [PubMed] [Google Scholar]


