Abstract
Background
Land plants (embryophytes) are monophyletic and encompass four major clades: liverworts, mosses, hornworts and polysporangiophytes. The liverworts are resolved as the earliest divergent lineage and the mosses as sister to a crown clade formed by the hornworts and polysporangiophytes (lycophytes, monilophytes and seed plants). Alternative topologies resolving the hornworts as sister to mosses plus polysporangiophytes are less well supported. Sporophyte development in liverworts depends only on embryonic formative cell divisions. A transient basal meristem contributes part of the sporophyte in mosses. The sporophyte body in hornworts and polysporangiophytes develops predominantly by post-embryonic meristematic activity.
Scope
This paper explores the origin of the sporophyte shoot in terms of changes in embryo organization. Pressure towards amplification of the sporangium-associated photosynthetic apparatus was a major driver of sporophyte evolution. Starting from a putative ancestral condition in which a transient basal meristem produced a sporangium-supporting seta, we postulate that in the hornwort–polysporangiophyte lineage the basal meristem acquired indeterminate meristematic activity and ectopically expressed the sporangium morphogenetic programme. The resulting sporophyte body plan remained substantially unaltered in hornworts, whereas in polysporangiophytes the persistent meristem shifted from a mid-embryo to a superficial position and was converted into an ancestral shoot apical meristem with the evolution of sequential vegetative and reproductive growth.
Conclusions
The sporophyte shoot is interpreted as a sterilized sporangial axis interpolated between the embryo and the fertile sporangium. With reference to the putatively ancestral condition found in mosses, the sporophyte body plans in hornworts and polysporangiophytes are viewed as the product of opposite heterochronic events, i.e. an anticipation and a delay, respectively, in the development of the sporangium. In either case the result was a pedomorphic sporophyte permanently retaining juvenile characters.
Keywords: bryophytes, embryo, development, meristems, plant evolution, sporophyte shoot, stomata
INTRODUCTION
Molecular phylogenies have resolved land plants (embryophytes) as monophyletic with charophytic ancestry. Living land plants encompass four major clades: liverworts, mosses, hornworts and tracheophytes (lycophytes, monilophytes and seed plants). The liverworts are resolved as the earliest divergent lineage and the mosses as the sister group to a crown clade formed by the hornworts and tracheophytes. Alternative topologies resolving the mosses as the sister group to tracheophytes are less well supported (Qiu et al., 2006, 2007; Qiu, 2008; Chang and Graham, 2011).
Characterized by a dominant gametophyte and uniaxial sporophyte permanently associated with the gametophyte, liverworts, mosses and hornworts are traditionally referred to as ‘bryophytes’, a taxonomic assemblage now considered paraphyletic. Bryophytes produce sporophytes with a single sporangium or capsule, hence the designation as monosporangiates. The tracheophytes are markedly different from bryophytes in that after an initial embryonic phase the sporophyte becomes autonomous, ramifies and produces multiple sporangia. Extant and extinct tracheophytes, plus some fossil relatives with branched sporophytes but possibly lacking lignified vascular tissue, are collectively referred to as the polysporangiophytes (Kenrick and Crane, 1997a, b; Kenrick, 2000; Gerrienne and Gonez, 2011).
Microfossil evidence indicates that land plants with bryophytic affinities appeared in the Ordovician at least 470 million years ago. This is consistent with an estimation of the divergence time of liverworts, the earliest extant land plant lineage, suggesting a Late-Ordovician origin (Heinrichs et al., 2007). The oldest accepted land plant macrofossils are from Mid-Silurian rocks with an age of about 425 million years and have been described as isotomously branched sporophyte axes bearing terminal Cooksonia-type sporangia (Edwards and Feehan, 1980). Macrofossils recognized as zosterophylls (e.g. Bathurstia and Zosterophyllum; Kotyk et al., 2002) or basal lycopsids (eg Baragwanathia; Richards, 2000), both relatively advanced members of the tracheophyte lineage (Kenrick and Crane, 1997a, b), have been described from Late-Silurian compressions about 410 million year old. Thus, although sparse and somewhat controversial, the paleobotanical evidence indicates that the transition from the bryophyte grade to the polysporangiate grade took place during a 45 million year interval between the Mid Ordovician and Mid Silurian. Molecular clock analyses suggest earlier origins for major embryophyte clades (e.g. 568–815 million years ago in Clarke et al., 2011), possibly implying a longer time for the bryophyte to polysporangiophyte transition.
The mature sporophyte of liverworts consists of a sporangium or capsule, containing the spore-forming apparatus, a seta elongating solely by cell expansion (Thomas, 1980), and an absorptive foot. This body plan is established by formative cell divisions (Gunning et al., 1978) at an early stage of development; subsequent sporophyte growth depends on proliferative cell division and cell expansion in the absence of any localized area of cell division recognizable as a meristem (Cooke et al., 2004). The mature sporophyte in mosses has a similar anatomy to that in liverworts, but the seta and a part of the foot arise from a transient meristem developing in the middle of the spindle-shaped embryo. The mature sporophyte of hornworts lacks a seta and consists of a foot and a sporangial axis, the latter growing from a basal meristem that remains active throughout sporophyte life (Cooke et al., 2004; Sakakibara et al., 2008; Ligrone et al., 2012). The sporophyte body plan of polysporangiophytes, in its basic form epitomized by leafless rhyniopsid plants (Taylor et al., 2009), is a free-living branched axial body (the sporophyte shoot) developing from a persistent apical meristem (the shoot apical meristem, or SAM) and eventually producing multiple terminal sporangia. In the following discussion, the term sporophyte shoot will be used to indicate the vegetative part of the sporophyte in polysporangiophytes, independent of the presence of leaves. We define the embryo as the post-fertilization developmental stage during which the sporophyte body plan is established. In liverworts this coincides with the phase of formative cell division. The same does not hold true for mosses, hornworts and polysporangiophytes because here, owing to the development of a meristematic area, formative cell divisions persist after the establishment of the sporophyte body plan. For these three groups, we view the appearance of a meristem (either basal or apical) as the event marking the transition from embryonic to post-embryonic sporophyte development.
Despite profound differences in ontogeny, the sporophyte shoot in polysporangiophytes has generally been assumed to have evolved from the seta of mosses (Smith, 1955; Mishler and Churchill, 1985). Rejecting this view, Kato and Akiyama (2005) interpreted the seta as part of the bryophyte sporangium, and the sporophyte shoot as a novel structure interpolated between the embryo and sporogenesis. This appears to overlook the fact that evolution is a contingent process that produces innovations by modifying already existing structures or mechanisms rather than creating new ones (Jacob, 1977). Indeed, a growing body of evidence points to a substantial and, until recently, unrecognized continuity in anatomy, biochemistry, physiology and genetics between the bryophyte and polysporangiophyte grade (Cooke et al., 2004; Raven and Edwards, 2004; Floyd and Bowman, 2007; Ligrone et al., 2012). We infer that the sporophyte shoot probably evolved by modification of an ancestral bryophytic pattern of embryo development. The present paper explores the origin of the sporophyte shoot in the putative bryophytic ancestor of polysporangiophytes and its possible evolutionary relationship with the sporophyte in mosses and hornworts.
STOMATA AS A GUIDELINE FOR ANALYSING THE ORIGIN OF THE SPOROPHYTE SHOOT
Stomata are one of the most distinctive features of the sporophyte shoot. By adjusting their aperture in response to multiple external and internal signals, stomata work to maintain a favourable balance of water loss and CO2 uptake (Brodribb et al., 2009; Lawson, 2009; Brodribb and McAdam, 2011). Stomata also occur in the sporophyte of mosses and hornworts (Paton and Pearce, 1957), and molecular evidence (Bergmann and Sack, 2007; Peterson et al., 2010; Rychel and Peterson, 2010; Chater et al., 2011) supports a monophyletic origin of stomata in the putative common ancestor of the three lineages. Because of this, mosses, hornworts and polysporangiophytes have been collectively referred to as the stomatophytes (Ligrone et al., 2012).
As an adaptation pivotal to the evolution of homeohydry and of large-sized land plants (reviewed in Raven, 2002; Proctor, 2007), stomata have had a tremendous impact on the biology and geochemistry of the planet (for reviews, see Beerling, 2007; Berry et al., 2010). However, the existence of stomata in poikilohydric bryophytes indicates that these structures might not have been associated with homeohydry since their origin. It has been suggested that stomata evolved in the sporophyte of the ancestral stomatophyte as a means to divert water and solutes from the parental gametophyte and, at maturity, also facilitate spore dispersal (Ligrone et al., 2012).
The ancestral stomata were most probably simple apertures lacking an opening/closing mechanism, and arguably more advanced functions evolved in parallel with an increasingly complex sporophyte body. Unfortunately, the current understanding of the evolution of stomatal responsiveness is incomplete, due in part to conflicting data in bryophytes and early diverging tracheophytes (Brodribb and McAdam, 2011; Chater et al., 2011; Ruszala et al., 2011; McAdam and Brodribb, 2012).
More informative in the context of the present analysis is stomatal distribution. In mosses, stomata are localized in the sporangium, and the same has been assumed for the putative ancestral stomatophytes; stomata are expressed throughout the fertile sporophyte axis in hornworts, whereas in polysporangiophytes they are found in the sporophyte shoot (Ligrone et al., 2012). Stomata also occurred in the sporangia of Early Devonian polysporangiophytes such as Aglaophyton major, Nothia aphylla and Cooksonia pertoni (Edwards et al., 1998); hence, the lack of stomata in the sporangia of extant polysporangiophytes is probably a loss (Ligrone et al., 2012). We conclude that (1) the ancestral stomatous part of the stomatophyte sporophyte is the sporangium; and (2) stomatal distribution is a phylogenetic trait holding essential clues on the origin of the sporophyte shoot and its relationship with the sporophyte in mosses and hornworts.
EVOLUTION OF SPOROPHYTE BODY PLANS IN STOMATOPHYTES AND ORIGIN OF THE SPOROPHYTE SHOOT
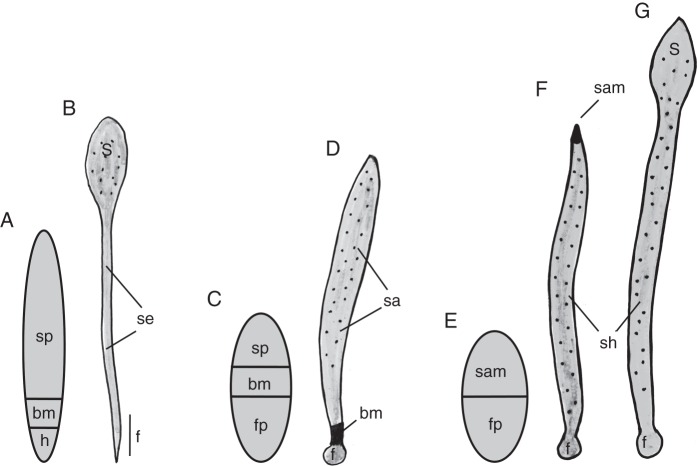
In a previous analysis of land plant evolution, we argued that the sporophyte of ancestral stomatophytes had a vascularized seta and a sporangium with stomata, air spaces and chlorenchyma (Ligrone et al., 2012). This pattern of sporophyte anatomy was assumed to be plesiomorphic in mosses, and the lack of one or more of the above characters in early diverging or advanced moss lineages was considered to be the result of independent loss (Ligrone et al., 2012). In line with the above considerations, here we assume that the sporophyte of the ancestral stomatophyte developed from a moss-like spindle-shaped embryo (Cooke et al., 2004; Sakakibara et al., 2008; Uzawa and Higuchi, 2010; Ligrone et al., 2012) consisting of a sporangium primordium, a haustorium of hypobasal derivation and, between these, a unifacial basal meristem (Fig. 1A, B).
Fig. 1.
Embryo and mature sporophyte in stomatophytes. (A, B) A putative embryo and sporophyte in ancestral stomatophytes; the embryo is assumed to consist, as in present-day mosses, of three organogenetic areas: a sporangium primordium (sp), a basal meristem (bm) and a haustorium (h); these generated, respectively, a sporangium (s), a seta (se) plus the upper part of the foot (f), and the lower part of the foot in the mature sporophyte. (C, D) An embryo and mature sporophyte in hornworts. (C) An embryo consisting of a sporangium primordium (sp), a basal meristem and a foot primordium (fp). (D) The basal meristem (bm) is active for sporophyte life and produces an indeterminate sporangial axis (sa). (E–G) A putative embryo, immature and mature sporophyte in an ancestral polysporangiophyte. The embryo is assumed to consist of an ancestral shoot apical meristem (sam) and a foot primordium (fp). Post-embryonic growth from the ancestral SAM produced a leafless, uniaxial indeterminate sporophyte shoot (sh) and eventually a terminal sporangium (s). f, sporophyte foot. Stippling indicates stomata. The embryo stages are not to scale with the other stages.
A major character distinguishing the hornworts and polysporangiophytes from mosses is an amplification of the part of the sporophyte body expressing stomata and photosynthetic tissue. This coincides with the whole sporangium in the hornworts and the sporophyte shoot in polysporangiophytes.
The early embryo of hornworts consists of two tiers of four cells, with eight cells in total. This octant stage is sharply different from the spindle-shaped embryo of mosses but is found in most pteridophyte embryos during initial development (Johnson and Renzaglia, 2008, 2009). The upper and lower tier of the 8-celled hornwort embryo are a sporangium primordium and a foot primordium, respectively (Renzaglia, 1978). As in mosses, a meristematic area develops at the base of the sporangium primordium after delineation of an endothecium and amphithecium (regions restricted to the sporangium of mosses and hornworts) but before the sporogenous tissue (archesporium) is defined (Renzaglia, 1978; Fig. 1C). As in mosses, this meristem is unifacial and produces new tissue acropetally; hence, it is referred to as the basal meristem. Unlike mosses, however, the basal meristem in hornworts reproduces the developmental pattern of the sporangium primordium and remains active for the life of the sporophyte. Consequently, the sporangial axis of the hornwort sporophyte is an indeterminate structure that arises almost entirely from the basal meristem, with a very minor contribution from the sporangium primordium (Fig. 1D).
With reference to the putative moss-like ancestral embryo, we suggest that the hornwort sporophyte developmental pattern evolved through ectopic expression of the morphogenetic programme of the sporangium in the meristematic area ancestrally deputed to producing the seta. Having lost the tissue contribution from the basal meristem, the foot acquired a bulbous shape and the seta was suppressed (Fig. 1D).
The key event marking the divergence of the polysporangiophyte lineage was the evolution of a SAM, a bifunctional apical meristem performing two alternative morphogenetic programmes. In the vegetative mode, the SAM produces an indeterminate sterile axis, i.e. the sporophyte shoot (Fig. 1F), and in the reproductive mode a determinate sporangial axis (Fig. 1G), the transition from vegetative to reproductive growth involving the loss of the initial cell(s) and consequent end of indeterminate growth (reviewed by Stahl and Simon, 2010). An apical meristem either evolved de novo from the sporangium primordium or, more simply, the meristematic area shifted from an intercalary to a superficial location. Based on the common octant stage of present-day hornwort and polysporangiophyte embryos, we speculate that the latter is the likely scenario and that this transition, involving the loss of the ancestral sporangium primordium, took place after the basal meristem had acquired an indeterminate character and had been co-opted into the production of sporangial tissue. The conversion of the newly evolved apical meristem into an ancestral SAM entailed two further key changes. One was the interpolation of a phase of vegetative growth preceding the development of the archesporium; the other an inversion in the polarity of formative cell divisions. These were no longer integrated between already determined tissues as in hornworts but rather were positioned in such a way that the initial cell(s) could maintain an apical position and thus ‘lead the way’ for iterative growth. We postulate that, although lacking the ability to produce leaf primordia, the apical meristem of the leafless unbranched sporophyte in ancestral polysporangiophytes possessed the fundamental properties of a SAM, i.e. an indeterminate meristematic activity and delayed sporogenesis (Fig. 1E).
DISCUSSION AND CONCLUSIONS
Appearing ancestrally in a poikilohydric sporophyte and most probably originally lacking a functional link with photosynthesis (Ligrone et al., 2012), stomata have turned out to be a key tool affording a better control of water balance and more efficient photosynthetic activity. If terrestrialization gave plants access to greater relative amounts of carbon dioxide, the evolution of stomata and air spaces was the innovation that enabled them to exploit this opportunity to its full extent (Raven, 1996, 2002; Raven and Edwards, 2004). Arguably, the conversion of ancestral stomata into the multisensing ‘smart’ valves of present-day angiosperms was gradual (Franks and Farquhar, 2007; Brodribb et al., 2009). Recent studies suggest that the stomata in hornworts (J. G. Duckett, Natural History Museum, London, UK, unpubl. res.), lycophytes and ferns (Brodribb and McAdam, 2011; McAdam and Brodribb, 2012) lack key responses to abscisic acid; moreover, hornwort stomata exhibit only partial closure under water stress and remain open when dead (J. G. Duckett, Natural History Museum, London, UK, unpubl. res.). It is nevertheless significant that in mosses and hornworts stomata are associated with well-defined chlorenchyma with schizogenous air spaces (Raven, 1996; J. G. Duckett, Natural History Museum, London, UK, unpubl. res.) and, hence, most probably the anatomical organization was in place in the ancestral stomatophytes to support the evolution of opening and closing cycles.
Once they appeared, stomata were the major driver of sporophyte evolution. We suggest that the ancestral role of the basal meristem in stomatophytes was to push the expanding sporangium out of gametophytic tissues, thereby permitting the stomata and underlying air spaces to become functional in gas exchange as early as possible. This is the condition typically observed in extant mosses (Uzawa and Higuchi, 2010; Ligrone et al., 2012). The conversion of the basal meristem into an indeterminate meristem producing sporangial tissue instead of a seta entailed a significant amplification of the stomata–air space–chlorenchyma system in ancestral hornworts and polysporangiophytes. It is parsimonious to assume that this condition evolved before the two lineages diverged. Possibly, a pre-adaptation leading the way to the loss of the seta and diversion of the basal meristem towards the production of sporangial tissue was a reduction of mechanical constriction from the gametophytic tissue surrounding the young sporophyte. The resulting sporophyte body plan remained substantially unaltered in the hornwort lineage, except in the genus Notothylas which has a reduced sporophyte (Renzaglia et al., 2009). The longitudinal division of the zygote is a hornwort apomorphy possibly related to the sunken nature of the archegonium (Renzaglia et al., 2009). The same innovation appeared independently in eusporangiate ferns (Johnson and Renzaglia, 2009).
In the scenario proposed here, further elaboration of the sporophyte body plan and underpinning embryo organization in the polysporangiophyte lineage involved suppression of the sporangium primordium, meristem apicalization and a temporal splitting of the developmental programmes for the vegetative (epidermis with stomata and photosynthetic tissue) and reproductive part of the sporangium (archesporium and associated tissues). The key agent of positional/temporal shifting and silencing of developmental programmes is homeotic mutation, a class of mutations affecting regulatory genes and/or their targets, and recognized as a major driver of body plan change in plants and animals (Vinicius, 2010; Pires and Dolan, 2012). In a broad sense, homeotic genes also embrace genes encoding small RNAs associated with the timing of developmental transitions (Moss, 2007; Poethig, 2009).
The hypothesis that the ancestral SAM arose from an embryo area pre-determined to produce sporangial tissue implies interpretation of the sporophyte shoot as a sterilized sporangial axis intercalated between the early embryo and the fertile sporangium. Sterilization and diverted development of reproductive structures is a recurrent mechanism of morphological innovation in plants. Striking examples are the modification of marginal flowers in the inflorescences of Asteraceae into sterile structures for attraction of pollinators, the conversion of stamens into staminodes and even the origin of petals from parts of the androecium (reviewed by Crane and Kenrick, 1997). Other likely examples are microphylls in lycophytes and interseminal scales in Bennettitales, both suggested to be derived from sterilized sporangia, for the former as an alternative to the enation model (Crane and Kenrick, 1997). Indeed, partial sterilization of the sporangium is also the likely mechanism at the origin of the capsule neck or apophysis in peristomate mosses, that is a specialized sporangium segment containing stomata and chlorenchyma but lacking archesporial tissue (Goffinet et al., 2009). It may also be observed that sterilization of potential sporogenous cells is at the very origin of the sporophyte vegetative tissue in embryophytes (Hemsley, 1994).
With reference to the putatively ancestral condition found in extant mosses, the sporophyte body plans in hornworts and polysporangiophytes may be viewed as the expression of opposite heterochronic events, i.e. an anticipation (progenesis) and a delay (neoteny), respectively, in the development of the sporangium (Gould, 1977; Alberch et al., 1979; Ridley, 2004). In hornworts, the sporophyte starts producing archesporial tissue in the embryo and young sporophyte (Renzaglia et al., 2009), whereas in polysporangiophytes the sporophyte vegetative body grows for a relatively long time before sporangial development initiates. In either case, the result is a pedomorphic sporophyte permanently retaining juvenile characters: an active meristem and the potential to produce spores.
The evolutionary model presented here assumes that a moss-like spindle-shaped embryo is plesiomorphic in stomatophytes and that the embryo and sporophyte body plans in hornworts and polysporangiophytes arose by sequential elaboration of this ancestral pattern. This model is consistent with molecular phylogenies as well as with substantial similarity in the sporophyte body plan of mosses and liverworts, the latter being the earliest diverging extant embryophyte lineage (Qiu et al., 2006, 2007; Qiu, 2008; Chang and Graham, 2011). An alternative scenario assuming the ancestral stomatophyte embryo to be similar to the globular embryo of hornworts and the spindle-shaped embryo to be an apomorphy of mosses would be less consistent with phylogenetic evidence but would still be compatible with our model, its main implication being, in our opinion, that the seta of mosses should be interpreted as an innovation derived from a sterilized sporangial segment. An independent origin of the moss seta by partial sterilization of the sporangial axis appears to be a plausible hypothesis, although less parsimonious than our model because it implies two separate events of sporangial sterilization in stomatophytes and rules out homology with the liverwort seta. A polysporangiophyte-like plesiomorphic embryo would not only be at sharp variance with phylogeny but would also imply a sequence of events far less parsimonious than the scenarios outlined above.
Unlike determinate sporophyte development in liverworts and mosses, sporophyte development in hornworts and polysporangiophytes is essentially a stochastic process involving an unpredictable number of cell divisions although producing highly regular forms. Arguably, the evolution of an indeterminate body increased the photosynthetic and reproductive potential of the sporophyte but possibly also amplified a conflict of interest with the parental gametophyte, at least in terms of allocation of water and mineral nutrients (Haig and Wilczek, 2006). Probably this was the major factor driving sporophyte evolution towards autonomy in polysporangiophytes. Because of the absence of vascular tissue, stomatal transpiration in the hornwort sporophyte presumably is lower than might be expected in a comparable vascularized system, and this may have worked in reducing potential conflict with the gametophyte; moreover, the basal position of the meristem in the hornwort sporophyte is probably incompatible with branching, which presumably was an essential prerequisite for evolving root-like structures and gaining autonomy (Ligrone et al., 2012). Taken together, these two factors may account for the retention of a bryophytic life cycle in hornworts.
The root apical meristem (RAM) and leaf primordium present in the embryo of extant polysporangiophytes (Johnson and Renzaglia, 2008, 2009) are additions that followed the evolution of branching and were associated with roots and leaves; each appeared at least twice independently, in lycophytes and euphyllophytes (Raven and Edwards, 2001; Pires and Dolan, 2012). The leaf primordium most probably resulted from further segregation from the SAM (Johnson and Renzaglia, 2009), whereas the origin of the root is more uncertain (Raven and Edwards, 2001; Pires and Dolan, 2012). Several regulatory genes that in angiosperms control SAM functioning have homologues expressed in the RAM (Stahl and Simon, 2010), suggesting that the RAM originated by duplication of the SAM.
In the last two decades, molecular research has identified several classes of genes involved in the control of the SAM. Major examples include Class III HD-Zip and Class 1 KNOX transcription factors, both essential for the initiation and maintenance of the SAM in angiosperms, gymnosperms and ferns, and KANADI genes whose ectopic expression in the SAM causes terminal differentiation in arabidopsis (Emery et al., 2003; Floyd et al., 2006; reviewed by Floyd and Bowman, 2007). The functioning of the SAM in arabidopsis and other angiosperms also depends on a regulatory loop between clavata and wuschel genes (Schoof et al., 2000; Taguchi-Shiobara et al., 2001; Suzaki et al., 2006). MIKC MADS-box genes control flower development and include the ABC homeotic genes (Soltis et al., 2007). The LEAFY gene is involved in the control of the transition from vegetative to reproductive growth in angiosperms by regulating the transcription of ABC genes; AP2 genes, a gene family associated with floral development in angiosperms, include sequences involved in stem cell maintenance and transition from vegetative to reproductive growth in both early diverging and more derived tracheophytes (reviewed by Floyd and Bowman, 2007).
The recent addition of the near complete genome sequence of the moss Physcomitrella patens (Rensing et al., 2008) has permitted the identification of moss homologues for most of the above-mentioned genes (Tanahashi et al., 2005; Singer and Ashton, 2007; Sakakibara et al., 2008; see also reviews by Floyd and Bowman, 2007; Shaw et al., 2011; Pires and Dolan, 2012).
The overall evidence indicates that the evolution of the complex gene networks underpinning sporophyte development in angiosperms entailed repeated events of duplication, functional co-option and neo-functionalization of genes already present in the ancestral genotype. A similar picture is produced by comparative analysis of growth-promoting hormones in early diverging and more derived land plants (Ross and Reid, 2010). So, it is increasingly evident that understanding the molecular bases of sporophyte evolution and development requires filling the knowledge gap between angiosperms and bryophytes. Essential to this purpose is nuclear genome sequencing in liverworts and hornworts.
It is anticipated that the present analysis will inspire future research and provide a framework for data testing and interpretation. Even within the boundaries of limited current knowledge, the hypotheses presented are amenable to experimental testing. Research on shared genetic signatures of sporophyte meristems in mosses, hornworts and polysporangiophytes not only might permit assessment of homology but also is a promising line of enquiry for genetic markers of the transition from embryonic to post-embryonic development. The same applies for testing the hypothesis of a common origin of indeterminate sporophyte development in hornworts and polysporangiophytes. Likely candidates include homologues of Class 1 Knox and Class III HD-Zip genes. In Physcomitrella the former control formative cell division in the sporangium primordium and basal meristem whereas they are not expressed in the gametophyte (Sakakibara et al., 2008). Class III HD-Zip homologues are expressed in the haploid bodies of Chara, the three bryophytic lineages, and the fern Ceratopteris (Floyd et al., 2006), and expression has also been reported in the sporophyte in the hornwort Phaeoceros and several tracheophytes including Ceratopteris (Floyd et al., 2006). Our model points to differences in the timing of spore production as a major character distinguishing mosses, hornworts and polysporangiophytes. Expression and functional analysis of genes controlling the transition from vegetative to reproductive growth, notably EP2 genes (Floyd and Bowman, 2007), is likely to produce data useful to test our model and, in particular, our heterochronic interpretation of sporophyte evolution in hornworts and polysporangiophytes.
ACKNOWLEDGEMENTS
Funding for this work was provided by a grant (Orto Botanico) from the Provincia di Caserta (Italy). We thank Dr Marco Vigliotti (Dipartimento di Scienze ambientali, SUN, Italy) for assistance in the preparation of the figure.
LITERATURE CITED
- Alberch P, Gould SJ, Oster GF, Wake DB. Size and shape in ontogeny and phylogeny. Paleobiology. 1979;5:296–317. [Google Scholar]
- Beerling D. The emerald planet. How plants changed Earth's history. Oxford: Oxford University Press; 2007. [Google Scholar]
- Bergmann FC, Sack FD. Stomatal development. Annual Review of Plant Biology. 2007;58:163–181. doi: 10.1146/annurev.arplant.58.032806.104023. [DOI] [PubMed] [Google Scholar]
- Berry JA, Beerling DJ, Franks PJ. Stomata: key players in the Earth system, past and present. Current Opinion in Plant Biology. 2010;13:233–240. doi: 10.1016/j.pbi.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. Passive origins of stomatal control in vascular plants. Science. 2011;331:582–585. doi: 10.1126/science.1197985. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Jordan GJ, Field TS. Evolution of stomata responsiveness to CO2 and optimization of water-use efficiency among land plants. New Phytologist. 2009;183:839–847. doi: 10.1111/j.1469-8137.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- Chang Y, Graham SW. Inferring the higher-order phylogeny of mosses (Bryophyta) and relatives using a large, multigene plastid data set. American Journal of Botany. 2011;98:839–849. doi: 10.3732/ajb.0900384. [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, et al. Regulatory mechanisms controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology. 2011;21:1025–1029. doi: 10.1016/j.cub.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Clarke JT, Warnock RCM, Donogue PCJ. Establishing a time-scale for plant evolution. New Phytologist. 2011;192:266–301. doi: 10.1111/j.1469-8137.2011.03794.x. [DOI] [PubMed] [Google Scholar]
- Cooke TJ, Poli DB, Cohen JD. Did auxin play a crucial role in the evolution of novel body plans during the Late-Silurian–Early Devonian radiation of land plants? In: Hemsley AR, Poole I, editors. The evolution of plant physiology. Linnean Society Symposium Series 21. London: Academic Press; 2004. pp. 85–107. [Google Scholar]
- Crane PR, Kenrick P. Diverted development of reproductive organs: a source of morphological innovation in land plants. Plant Systematics and Evolution. 1997;206:161–174. [Google Scholar]
- Cronk QCB. The molecular organography of plants. Oxford: Oxford University Press; 2009. [Google Scholar]
- Crum H. Structural diversity of bryophytes. Ann Harbor, MI: University of Michigan Herbarium; 2001. [Google Scholar]
- Edwards D, Feehan J. Records of Cooksonia-type sporangia from late Wenlock strata in Ireland. Nature. 1980;287:41–42. [Google Scholar]
- Edwards D, Kerp H, Hass H. Stomata in early land plants: an anatomical and ecophysiological approach. Journal of Experimental Botany. 1998;49:255–278. [Google Scholar]
- Emery JF, Floyd SK, Alvarez J, et al. Radial patterning of Arabidopsis shoots by Class III HD-Zip and KANADI genes. Current Biology. 2003;13:1768–1774. doi: 10.1016/j.cub.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Floyd S, Bowman JL. The ancestral developmental tool kit of land plants. International Journal of Plant Sciences. 2007;168:1–35. [Google Scholar]
- Floyd SK, Zalewski CS, Bowman JL. Evolution of Class III homeodomain leucine zipper genes in streptophytes. Genetics. 2006;173:373–388. doi: 10.1534/genetics.105.054239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Farquhar GD. The mechanical diversity of stomata and its significance in gas-exchange control. Plant Physiology. 2007;143:78–87. doi: 10.1104/pp.106.089367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrienne LE, Gonez P. Early evolution of life cycles in embryophytes: a focus on the fossil evidence of gametophyte/sporophyte size and morphological complexity. Journal of Systematics and Evolution. 2011;49:1–16. [Google Scholar]
- Goffinet B, Buck W, Shaw J. Bryophyte biology. Cambridge: Cambridge University Press. In: Goffinet B, Shaw J; 2009. Morphology, anatomy and classification of the Bryophyta; pp. 55–138. [Google Scholar]
- Gould SJ. Ontogeny and phylogeny. Cambridge, MA: Belknap Press of Harvard University Press; 1977. [Google Scholar]
- Gunning BES, Hughes JE, Hardham AR. Formative and proliferative cell divisions, cell differentiation, and developmental changes in the meristem of Azolla roots. Planta. 1978;143:121–144. doi: 10.1007/BF00387786. [DOI] [PubMed] [Google Scholar]
- Haig D, Wilczek A. Sexual conflict and the alternation of haploid and diploid generations. Philosophical Transactions of the Royal Society B: Biological Sciences. 2006;361:335–343. doi: 10.1098/rstb.2005.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemsley AR. The origin of the land plant sporophyte: an interpolation scenario. Biological Reviews. 1994;69:263–273. [Google Scholar]
- Heinrichs J, Hentschel J, Wilson R, Feldberg K, Schneider H. Evolution of leafy liverworts (Jungermanniidae, Marchantiophyta): estimating divergence times from chloroplast DNA sequences using penalized likelihood with integrated fossil evidence. Taxon. 2007;56:31–44. [Google Scholar]
- Jacob F. Evolution and tinkering. Science. 1977;196:1161–1166. doi: 10.1126/science.860134. [DOI] [PubMed] [Google Scholar]
- Johnson GP, Renzaglia KS. Embryology of Ceratopteris richardii (Pteridaceae, tribe Ceratoptericeae), with emphasis on placental development. Journal of Plant Research. 2008;121:581–592. doi: 10.1007/s10265-008-0187-3. [DOI] [PubMed] [Google Scholar]
- Johnson GP, Renzaglia KS. Evaluating the diversity of pteridophyte embryology in the light of recent phylogenetic analyses leads to new inferences on character evolution. Plant Systematics and Evolution. 2009;283:149–164. [Google Scholar]
- Kato M, Akiyama H. Interpolation hypothesis for origin of the vegetative sporophyte of land plants. Taxon. 2005;54:443–450. [Google Scholar]
- Kenrick P. The relationships of vascular plants. Philosophical Transactions of the Royal Society B: Biological Sciences. 2000;355:847–855. doi: 10.1098/rstb.2000.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. The origin and early evolution of plants on land. Nature. 1997a;389:33–39. [Google Scholar]
- Kenrick P, Crane PR. The origin and early diversification of land plants. A cladistic study. Washington: Smithsonian Institution Press; 1997b. [Google Scholar]
- Kotyk ME, Basinger JF, Gensel PG, Defreitas TA. Morphologically complex plant macrofossils from the Late Silurian of Arctic Canada. American Journal of Botany. 2002;89:1004–1013. doi: 10.3732/ajb.89.6.1004. [DOI] [PubMed] [Google Scholar]
- Lawson T. Guard cell photosynthesis and stomatal function. New Phytologist. 2009;181:13–34. doi: 10.1111/j.1469-8137.2008.02685.x. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. Major transitions in the evolution of early land plants: a bryological perspective. Annals of Botany. 2012;109:851–871. doi: 10.1093/aob/mcs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ. Fern and lycophyte guard cells do not respond to endogenous abscisic acid. The Plant Cell. 2012;24:1510–1521. doi: 10.1105/tpc.112.096404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss EG. Heterochronic genes and the nature of developmental time. Current Biology. 2007;17:R425–R434. doi: 10.1016/j.cub.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Mishler BD, Churchill SP. Transition to a land flora: phylogenetic relationships of the green algae and bryophytes. Cladistics. 1985;1:305–328. doi: 10.1111/j.1096-0031.1985.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Paton JA, Pearce JV. The occurrence, structure and functions of the stomata in British bryophytes. Transactions of the British Bryological Society. 1957;3:228–259. [Google Scholar]
- Peterson KM, Rychel AL, Torii KU. Out of the mouths of plants: the molecular basis of the evolution and diversity of stomatal development. The Plant Cell. 2010;22:296–306. doi: 10.1105/tpc.109.072777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires N, Dolan L. Morphological evolution in land plants: new design with old genes. Philosophical Transactions of the Royal Society B: Biological Sciences. 2012;367:508–518. doi: 10.1098/rstb.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poethig RS. Small RNAs and developmental timing in plants. Current Opinion in Genetics and Development. 2009;19:374–378. doi: 10.1016/j.gde.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF. Ferns, evolution, scale and intellectual impedimenta. New Phytologist. 2007;176:504–506. doi: 10.1111/j.1469-8137.2007.02232.x. [DOI] [PubMed] [Google Scholar]
- Qiu Y-L. Phylogeny and evolution of charophytic algae and land plants. Journal of Systematics and Evolution. 2008;46:287–306. [Google Scholar]
- Qiu Y-L, Li L, Wang B, et al. The deepest divergences in land plants inferred from phylogenomic evidence. Proceedings of the National Academy of Sciences, USA. 2006;103:15511–15516. doi: 10.1073/pnas.0603335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y-L, Li L, Wang B, et al. A nonflowering land plant phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. International Journal of Plant Sciences. 2007;168:691–708. [Google Scholar]
- Raven JA. Into the voids: the distribution, function, development and maintenance of gas spaces in plants. Annals of Botany. 1996;78:137–142. [Google Scholar]
- Raven JA. Selection pressures on stomatal evolution. New Phytologist. 2002;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Raven JA, Edwards D. Roots: evolutionary origins and biogeochemical evidence. Journal of Experimental Botany. 2001;52:381–401. doi: 10.1093/jexbot/52.suppl_1.381. [DOI] [PubMed] [Google Scholar]
- Raven JA, Edwards D. The evolution of plant physiology. Linnean Society Symposium Series 21. London: Academic Press; 2004. Physiological evolution of lower embryophytes: adaptations to the terrestrial environment; pp. 17–41. Hemsley AR, Poole I. [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- Renzaglia KS. A comparative morphology and developmental anatomy of the Anthocerotophyta. Journal of the Hattori Botanical Laboratory. 1978;44:31–90. [Google Scholar]
- Renzaglia KS, Villareal JC, Duff RJ. Bryophyte biology. Cambridge: Cambridge University Press; 2009. New insights into morphology, anatomy, and systematics of hornworts; pp. 139–171. Goffinet B, Shaw J. [Google Scholar]
- Richards RB. The age of the earliest club mosses: the Silurian Baragwanathia flora in Victoria, Australia. Geological Magazine. 2000;137:207–209. [Google Scholar]
- Ridley M. Evolution. Malden, MA: Blackwell Publishing; 2004. [Google Scholar]
- Ross JJ, Reid JB. Evolution of growth-promoting plant hormones. Functional Plant Biology. 2010;37:795–805. [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, et al. Land plants acquired stomatal control early in their evolutionary history. Current Biology. 2011;21:1030–1035. doi: 10.1016/j.cub.2011.04.044. [DOI] [PubMed] [Google Scholar]
- Rychel AL, Peterson KM. Plant twitter: ligands under 140 amino acids enforcing stomatal patterning. Journal of Plant Research. 2010;123:275–280. doi: 10.1007/s10265-010-0330-9. [DOI] [PubMed] [Google Scholar]
- Sakakibara K, Nishiyama T, Deguchi H, Hasebe M. Class 1 KNOX genes are not involved in shoot development in the moss Physcomitrella but do function in sporophyte development. Evolution and Development. 2008;10:555–566. doi: 10.1111/j.1525-142X.2008.00271.x. [DOI] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haeckel A, et al. The stem cell population in Arabidopsis shoot meristems is maintained by a regulatory loop between CLAVATA and WUSCHEL genes. Cell. 2000;100:635–644. doi: 10.1016/s0092-8674(00)80700-x. [DOI] [PubMed] [Google Scholar]
- Shaw AJ, Szövényi P, Shaw B. Bryophyte diversity and evolution: windows into early evolution of land plants. American Journal of Botany. 2011;98:352–369. doi: 10.3732/ajb.1000316. [DOI] [PubMed] [Google Scholar]
- Singer SD, Ashton NW. Revelation of ancestral roles of KNOX genes by a functional analysis of Physcomitrella homologues. Plant Cell Reports. 2007;26:2039–2054. doi: 10.1007/s00299-007-0409-5. [DOI] [PubMed] [Google Scholar]
- Smith GM. Cryptogamic botany, vol. 2. Bryophytes and Pteridophytes. New York: McGraw-Hill; 1955. [Google Scholar]
- Soltis DE, Chanderbali AS, Kim S, Buzco M, Soltis PS. The ABC model and its applicability to basal angiosperms. Annals of Botany. 2007;100:155–163. doi: 10.1093/aob/mcm117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl Y, Simon R. Plant primary meristems: shared functions and regulatory mechanisms. Current Opinion in Plant Biology. 2010;13:53–58. doi: 10.1016/j.pbi.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Suzaki T, Toriba T, Fujimoto M, Tsutsumi N, Kitano H, Hirano HY. Conservation and diversification of meristem maintenance mechanism in Oryza sativa: function of the FLORAL ORGAN NUMBER2 gene. Plant and Cell Physiology. 2006;47:1591–1602. doi: 10.1093/pcp/pcl025. [DOI] [PubMed] [Google Scholar]
- Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D. The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes and Development. 2001;15:2755–2766. doi: 10.1101/gad.208501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanahashi T, Sumikawa N, Kato M, Hasebe M. Diversification of gene function: homologs of the floral regulator FLO/LFY control the first zygotic cell division in the moss Physcomitrella patens. Development. 2005;132:1727–1736. doi: 10.1242/dev.01709. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Taylor EL, Krings M. Paleobotany. The biology and evolution of fossil plants. Amsterdam: Academic Press; 2009. [Google Scholar]
- Thomas RJ. Cell elongation in hepatics: the seta system. Bulletin of the Torrey Botanical Club. 1980;107:339–345. [Google Scholar]
- Uzawa M, Higuchi M. Comparative development of the sporophyte–gametophyte junction in six mosses. Journal of Plant Resaearch. 2010;123:777–787. doi: 10.1007/s10265-010-0339-0. [DOI] [PubMed] [Google Scholar]
- Vinicius L. Modular evolution. How natural evolution produces biological complexity. Cambridge: Cambridge University Press; 2010. [Google Scholar]