Abstract
Background and Aims
The aquatic moss Fontinalis antipyretica requires a slow rate of dehydration to survive a desiccation event. The present work examined whether differences in the dehydration rate resulted in corresponding differences in the production of reactive oxygen species (ROS) and therefore in the amount of cell damage.
Methods
Intracellular ROS production by the aquatic moss was assessed with confocal laser microscopy and the ROS-specific chemical probe 2,7-dichlorodihydrofluorescein diacetate. The production of hydrogen peroxide was also quantified and its cellular location was assessed.
Key Results
The rehydration of slowly dried cells was associated with lower ROS production, thereby reducing the amount of cellular damage and increasing cell survival. A high oxygen consumption burst accompanied the initial stages of rehydration, perhaps due to the burst of ROS production.
Conclusions
A slow dehydration rate may induce cell protection mechanisms that serve to limit ROS production and reduce the oxidative burst, decreasing the number of damaged and dead cells due upon rehydration.
Keywords: Cell survival, confocal microscopy, dehydration rate, desiccation, diaminobenzidine, dichlorodihydrofluorescein diacetate, Fontinalis antipyretica, oxygen consumption, reactive oxygen species, ROS
INTRODUCTION
Mosses are poikilohydric organisms lacking the structures needed to prevent water loss. Some mosses can tolerate as little as 0·08 g H2O (g dry mass)−1 and recover fully on rehydration (Alpert and Oliver, 2002). Among the factors that influence desiccation tolerance are the dehydration rate and duration, temperature and light (Alpert and Oliver, 2002). Desiccation-tolerant mosses differ in their abilities to withstand drying and to recover from desiccation (Abel, 1956). Recovery depends, to some degree, on the habitat, i.e. on the circadian/seasonal variations in water availability (Tuba et al., 1998). Even the semi-aquatic bryophytes such as Cinclidotus fontinaloides and Leskea polycarpa can behave like terrestrial mosses displaying desiccation tolerance (Dyer and Duckett, 1984). Thus, in aquatic mosses of the Mediterranean region, seasonal variations may have a greater influence than the daily variations to which terrestrial mosses are subjected. In species belonging to the latter, such as Syntrichia ruralis, constitutive protection mechanisms are thought to confer desiccation tolerance, while inducible repair mechanisms come into play during rehydration (Bewley et al., 1978, 1993; Oliver, 1991). However, Beckett (1999) showed that the mesic Atrichum androgynum was able to tolerate desiccation if there was a period of partial dehydration prior to a desiccation event or if the moss was subjected to abscisic acid (ABA) treatment. Werner et al. (1991) also showed in Funaria hygrometrica that ABA increased during slow dehydration. These works indicate that an induced protection system operates to develop desiccation tolerance, under certain circumstances. Therefore, most mosses of dry, sun-exposed sites that have constitutive desiccation tolerance can withstand fast dehydration rates. On the other hand, mosses of moist or shady habitats, usually more sensitive to fast dehydration, require a period of sub-critical water stress during which metabolic changes occur (commonly associated with sugar metabolism and/or synthesis of specific proteins) which confer desiccation tolerance, similar to the induced mechanisms observed in the desiccation-tolerant vascular plants (Farrant et al., 1999; Bartels and Salamini, 2001). Recent molecular–cladistic research shows that bryophytes have continued to evolve in response to the evolving environment during the Mesozoic and Cenozoic (Goffinet et al., 2004). As the development of desiccation tolerance has inherent energetic and metabolic costs, during evolution, mesic/aquatic bryophytes might have foregone the constitutive cellular protection in favour of an inducible system allowing them to compete better in a mesic habitat (Oliver, 2008).
Aquatic mosses are not usually the subject of desiccation studies, although they are clearly thought to be desiccation sensitive (Brown and Buck, 1979; Seel et al., 1992a; Franks and Bergstrom, 2000; Robinson et al., 2000). This finding is consistent with the expectation that desiccation protection mechanisms are unnecessary and therefore absent or less well developed in desiccation-sensitive mosses of humid/aquatic habitats. However, Cruz de Carvalho et al. (2011) recently showed that the aquatic moss Fontinalis antipyretica, previously described as desiccation intolerant (Brown and Buck, 1979), is indeed able to tolerate desiccation if it dehydrates at a slow rate. A similar effect was observed in the lichen photobiont Trebouxia erici (Gasulla et al., 2009). Moreover, a slow dehydration rate was reported to be crucial in desiccation-tolerant vascular plants, such as Xerophyta humilis and Myrothamnus flabellifolius, in which tolerance is induced through slow drying (Farrant et al., 1999).
Desiccation induces an increase in oxidative stress (Smirnoff, 1993), such that as cellular water content decreases, organelles with high rates of electron flow, i.e. chloroplasts, mitochondria and peroxisomes, together with membrane oxidases and peroxidases (Mittler, 2002; Scheibe and Beck, 2011) upregulate the production of superoxide (O2·−), hydroxyl (OH·) and hydrogen peroxide (H2O2). These reactive oxygen species (ROS) react with proteins, lipids and nucleic acids, thereby causing damage to enzymes (Wolff et al., 1986; Halliwell and Gutteridge, 1999), membranes (Senaratna and McKersie, 1983; McKersie et al., 1989; Halliwell and Gutteridge, 1999; Leprince et al., 2000) and chromosomes (Dizdaroglu, 1994). Proteomic studies carried out in resurrection plants have shown that during dehydration there may be an increase in ROS-scavenging enzymes (Ingle et al., 2007; Jiang et al., 2007). On the other hand, since the photosynthetic system is blocked during desiccation, there is generally a decrease in proteins related to photosynthetic activity to avoid ROS formation (Ingle et al., 2007; Oliver et al., 2010). The decrease in the fluidity of membranes (McKersie et al., 1989) caused by lipid peroxidation leads to their fusion and interferes with their permeability upon rehydration. In contrast, in vascular plants, extracellular ROS production has been shown to play an important defensive role, acting directly on pathogenic bacteria and fungi at the site of infection (Wojtaszek, 1997; Murphy et al., 1998), or by stimulating defence mechanisms in neighbouring cells (Miller et al., 2008). In terrestrial bryophytes, the H2O2 burst has different functions, acting as: (1) a toxic compound leading to cell death (Apel and Hirt, 2004); (2) a signal that induces the expression of protective genes; (3) a cell to cell communication system, inducing protection in neighbouring cells (Apel and Hirt, 2004; Miller et al., 2008); and (4) a defence against fungi and bacteria (Mayaba et al., 2002) especially in the moist environments where F. antipyretica grows.
Although ROS production in response to desiccation/rehydration has been determined in terrestrial mosses (Minibayeva and Beckett, 2001; Mayaba et al., 2002; Beckett et al., 2004), to our knowledge it has not been investigated in aquatic mosses. In addition, there are no published studies examining the impact of dehydration rate on ROS production in mosses previously subjected to desiccation, although pools of the antioxidants ascorbate and glutathione were shown to decrease sharply in Syntrichia ruraliformis (Seel et al., 1992b) and S. ruralis (Dhindsa, 1987) under conditions of rapid dehydration. Therefore, the aim of the present work was to investigate the response to dehydration of F. antipyretica. Specifically we asked whether this response includes an oxidative burst that is sensitive to the dehydration rate in terms of intensity and tissue location. We hypothesized that ROS production – and thus oxidative damage – is greater in mosses subjected to a fast vs. a slow drying rate.
MATERIALS AND METHODS
Plant material and culture conditions
Samples of the moss Fontinalis antipyretica L. ex Hedw. were collected at the natural park of Serra de S. Mamede, central Portugal, from a natural and well preserved stream with no observable human impact. Samples were transported under cooling conditions (about 5 °C) to the laboratory, where they were cleaned of debris and sediments in distilled water. The mosses were grown in culture medium (Traubenberg and Ah-Peng, 2004) under controlled conditions [17 °C day/13 °C night, photosynthetic active radiation (PAR) of 20–30 µmol m−2 s−1 and a photoperiod of 16 h] and maintained under the same conditions for no more than 60 d before being used in the analyses. Five replicates of ten shoot tips (1 cm) were selected for each treatment. The relative water content (RWC) was calculated according to Deltoro et al. (1998) using the procedures described in Cruz de Carvalho et al. (2011). After the external water had been blotted from the tips using lab paper, the samples were first weighed to determine the full turgor weight. They were subsequently dried, and the fresh weight (stress weight) was determined. At the end of the assays, the samples were dried at 80 °C for 48 h and then weighed to determine the dry weight.
Dehydration induction and recovery
Dehydration was carried out by placing samples in small containers over saturated salt solutions. In the slow dehydration experiments, the mosses were incubated for 24 h over a saturated solution of K2SO4 [corresponding to 95 % relative humidity (RH), and a dehydration rate of 0·12 ± 0·03 mg H2O h−1]; for fast dehydration, the mosses were incubated for 3 h over a saturated solution of NH4NO3 in the confocal laser microscopy assays (65 % RH) and over saturated Ca(NO3)2·4H2O in the remaining assays (50 % RH) (a dehydration rate of 0·69 ± 0·09 mg H2O h−1), in both cases under ambient temperature (20–23 °C) and at low PAR (2–5 µmol m−2 s−1). Under the described conditions, the RWC of the four replicates at the end of the treatment was 13 ± 3 % and 18 ± 3 % for the fast and slow dehydration samples, respectively. The rates and patterns of dehydration in F. antipyretica are described in detail in Cruz de Carvalho et al. (2011). For confocal microscopy, the mosses were rehydrated directly on the microscopy slides. For oxygen exchange measurements, the mosses were subjected to fast and slow dehydration for different lengths of time (0·5, 1, 2, 24, 168, 336 and 960 h) and then rehydrated through immersion in the oxygen electrode solution (0·1 mm KHCO3).
Epifluorescence probes and confocal microscopy imaging analysis
The epifluorescent probe 2,7-dichlorodihydrofluorescein diacetate (DCFH2-DA) was used to detect intracellular ROS production. DCFH2-DA easily penetrates the cell membrane and is then hydrolysed by cellular esterases to dichlorodihydrofluorescein (DCFH2), which is trapped within the cell. In the presence of cellular free radicals, DCFH2 is oxidized to fluorescent 2′,7′-dichlorofluorescein (DCF), which is observable by confocal microscopy (DCF, λexc = 504 nm, λem = 524 nm). The oxidative air pollutant cumene hydroperoxide (CHP; 10 µm) and the antioxidant ascorbic acid (10 mm) were used as controls to stimulate (Catalá et al., 2010) and counteract ROS production, respectively. For confocal laser microscopy assays, the leaves of the moss were detached from the stem after slow (95 % RH) and fast (65 % RH) dehydration, mounted individually on slides and rehydrated for 1 h according to Table 1. To evaluate the RWC of these samples, they were compared with other leaves submitted to the same dehydration conditions and used for dry weight determination. Samples were observed by confocal laser scanning microscopy (TCS Leica SP confocal laser scanner microscope, Leica, Heidelberg, Germany) at the Servei Central de Suport a la Investigació Experimental (Universitat de València, Valencia) using an Ar excitation laser (λexc = 488 nm) for DCFH2-DA (λem = 543 nm). Chlorophyll autofluorescence was also evaluated (λem = 633 nm). The magnification is indicated in each figure. For each treatment, four leaves were examined, resulting in four images. Both positive ROS and chlorophyll autofluorescence signals were measured by quantifying the amount of green and red signal, respectively. Thus, in the 8-bit images of the DCFH2-DA treatments, the number of pixels in the intensity range of 44–255, corresponding to the green signal, was quantified using ImageJ 1·43 (available at http://rsbweb.nih.gov/ij/; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD, USA). Pixels in the 0–43 range were considered to indicate the absence of signal, based on the observation that 95–99 % of the pixels of images from unstressed and untreated samples were mostly in this range.
Table 1.
Rehydration treatments after slow (95 % RH) and fast (65 % RH) dehydration of individual leaves of the aquatic moss Fontinalis antipyretica mounted on slides for confocal laser scanning microscopy
| 1 | Deionized water |
| 2 | 50 µm 2′,7′-dichlorodihydrofluorescein diacetate (DCFH2-DA) |
| 3 | 10 µm cumene hydroperoxide (CHP) + 50 µm DCFH2-DA |
| 4 | 10 mm ascorbic acid (Asc) + 50 µm DCFH2-DA |
| 5 | 10 mm Asc + 10 µm CHP + 50 µm DCFH2-DA |
Hydrogen peroxide production and localization
Four replicates of the aquatic moss were submitted to either slow (95 % RH) dehydration for 24 h or fast (50 % RH) dehydration for 3 h, followed by a 24 h recovery in culture medium. The samples were then boiled in ethanol for 10 min, left overnight in cold ethanol and observed using an optical microscope (Leica DM2500, Leica Microsystems, Wetzlar, Germany). Images were acquired with a digital camera (Leica DFC500, Leica Microsystems). In two independent assays (three replicates each), one for measuring and the other for localizing H2O2 production, the samples were submitted to the same slow and fast dehydration treatments. Hydrogen peroxide production was quantified using the xylenol orange assay of Gay and Gebicki (2000) while the cellular location of H2O2 was determined using diaminobenzidine (DAB) staining (5 mm, 3 h) before and after dehydration and 24 h after recovery.
Oxygen consumption analysis
Samples of the aquatic moss were submitted to either slow (95 % RH, 24 replicates) or fast (50 % RH, 18 replicates) dehydration for 0·5, 1, 2, 24, 168, 336 and 960 h. Oxygen consumption was measured in each sample before and after dehydration using a Clark-type liquid phase oxygen electrode (DW2/2 electrode chamber, Hansatech Instruments Ltd, Norfolk, UK) in a 0·1 mm KHCO3 solution. Respiration was measured for 10 min in the dark. Rehydration was achieved by immersing the samples in the oxygen electrode solution. Another set of three samples each was submitted to either non-stressed conditions, slow dehydration (95 % RH) for 24 h or fast dehydration (50 % RH) for 3 h, and then assayed to determine the effects of the inhibitors potassium cyanide (KCN; 1 mm) and salicylhydroxamic acid (SHAM; 5 mm) on oxygen consumption in the first 5–7 min following rehydration. KCN inhibits cytochrome c oxidase in the mitochondria electron transport chain as well as metalloenzymes, such as catalases (Allen and Whatley, 1978), peroxidases (Choi et al., 2007) and superoxide dismutases (Chen et al., 2001), while SHAM inhibits the mitochondrial alternative oxidase (Vanlerberghe and McIntosh, 1997).
Statistical analysis
Relationships between variables/parameters and RWC were investigated using linear regression analysis. Pearson correlation coefficients (r) and the degrees of freedom (d.f.) were used to determine the levels of significance (P) between observed vs. predicted data. A pool of 174 replicate samples from controls corresponding to four different collection periods was used to establish the control value for oxygen consumption. All previous values of all the assays were used to create box-and-whiskers plots, in which the horizontal line represented the median, the boxes the 25 % and 75 % quartiles, and the whiskers the 5 % and 95 % quantiles. Whenever necessary, significant differences between groups were determined through analysis of variance (ANOVA) with a Tukey post-hoc test (significance level α = 0·05). All statistical analyses were performed with GraphPad Prism 5·03 for Windows (2009) (GraphPad Software, San Diego CA, USA).
RESULTS
ROS production depends on the dehydration rate
Intracellular ROS production during the rehydration of F. antipyretica tips was determined by immersing the dehydrated samples in deionized water containing the probe DFCH2-DA (50 µm) and then measuring the amount of the green fluorescent oxidation product DCF, which reflects the reaction of the probe with intracellular free radicals.
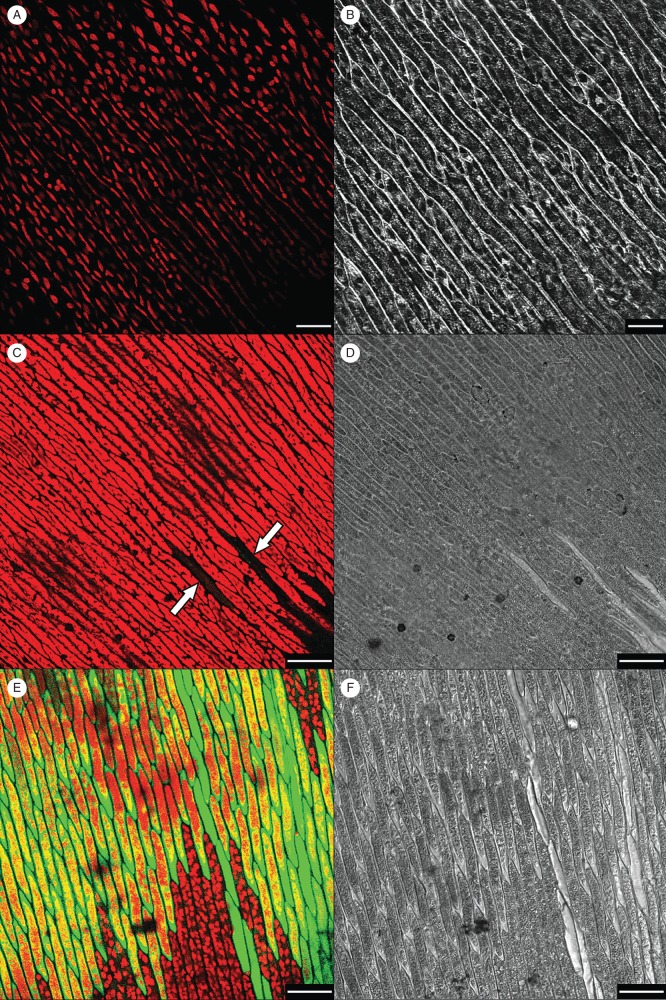
In the control hydrated leaves, elongated cells containing peripheral elongated chloroplasts were observed (Fig. 1A, red autofluorescence). Green autofluorescence that could interfere with DCF localization was absent. Following slow dehydration, there was a remarkable morphological change in the leaves, in that the cell walls and cytoplasm took on a condensed appearance and chloroplasts or other structures were no longer visible (Fig. 1C, D). Confocal images showed a generalized red autofluorescence not due to chlorophyll because the location did not match that of chloroplasts (Fig. 1C). Moreover, the red fluorescence appeared first at one site in the cell and then spread across the cytoplasm. In addition, some cells showed a very uniform and light inner space, as if they were empty, but they also emitted a weak DFC green fluorescence, presumably due to free radical production (Fig. 1C, arrowheads). This pattern was not related to the position of these cells in the leaves, as they were scattered individually rather than forming clusters.
Fig. 1.
Leaves of the aquatic moss Fontinalis antipyretica incubated with 50 µm (final concentration) of the probe DFCH2-DA before dehydration (A, B) and 1 h after rehydration (C–F). Samples were dehydrated either slowly (95 % RH; C, D) or rapidly (65 % RH; E, F). Green ROS fluorescence and red autofluorescence are shown. Arrows indicate the only cells with ROS fluorescence in slowly dried leaves. White scale bars: (A, B) = 25 µm, (C–F) = 50 µm. (A, C, E) Confocal images; (B, D, F) bright-field images.
In cells subjected to fast rehydration, there was a burst of free radicals and all cells of the leaves assumed the generalized appearance of empty cells (Fig. 1E). Small rounded chloroplasts were observed in some cells, and some areas emitted red autofluorescence. None of the cells contained the condensed structures seen in the slowly dehydrated leaves. However, the photosynthetic tissues of rapidly dehydrated leaves and, to a lesser extent, those of slowly dehydrated leaves were brownish in colour (data not shown).
In cells rehydrated with the oxidant CHP, high-level ROS production was determined in the rapidly dehydrated leaves whereas there was no detectable production in slowly dehydrated samples (data not shown). The appearance of rapidly dehydrated leaves rehydrated with the antioxidant ascorbic acid (Asc) was similar to that of slowly dehydrated cells with no detectable ROS production. This pattern also occurred in leaves rehydrated with both Asc and CHP (data not shown).
As shown in Fig. 2, the number of green pixels was much higher in rapidly dehydrated than in slowly dehydrated leaves. In a semi-quantitative analysis based on the number of pixels, the addition of CHP increased the number of green pixels in slowly dehydrated leaves but did not cause a further increment in rapidly dehydrated leaves. In contrast, Asc treatment strongly decreased the number of green pixels in rapidly dehydrated leaves, an effect that was maintained following the combined treatment with Asc and CHP, as no green pixels were present during rehydration.
Fig. 2.
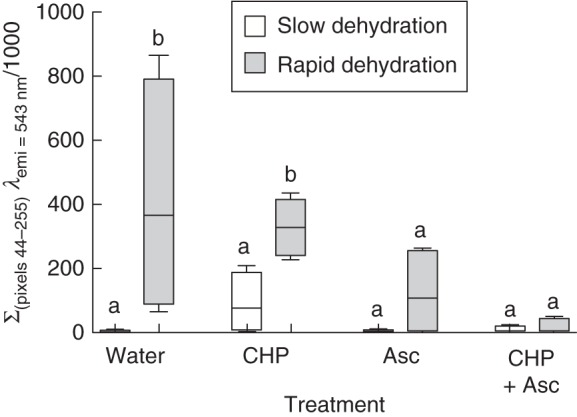
Confocal microscopy image analysis based on the quantification of pixels with intensities between 44 and 255 as seen on 8-bit images viewed at 543 nm. Slowly (95 % RH) and rapidly (65 % RH) dehydrated leaves of the aquatic moss F. antipyretica rehydrated with different treatments in the presence of the ROS-specific probe DFCH2-DA (for details see Table 1). In the box-and-whiskers plots, the horizontal line represents the median, the boxes the 25 % and 75 % quartiles, and whiskers the minimum and maximum values of four independent leaves. Values with different letters in the rehydration treatments between slow and fast dehydration are statistically different.
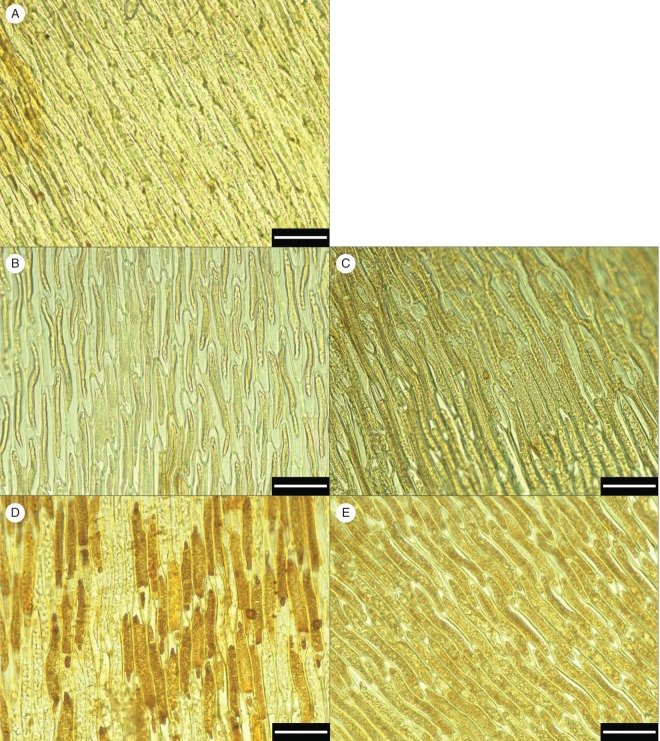
Intra- and extracellular H2O2 production was localized based on the dark brown product generated by DAB staining (Fig. 3). In slowly dehydrated samples (Fig. 3B, C), H2O2 production was minimal, similar to that of non-stressed control samples (Fig. 3A), whereas in rapidly dehydrated samples H2O2 production was marked (Fig. 3D) and continued during recovery (Fig. 3E).
Fig. 3.
Hydrogen peroxide (H2O2) localization by diaminobenzidine (DAB) staining (5 mm, 3 h) in the non-stressed control (A), after slow (95 % RH, B) and fast (50 % RH, D) dehydration, and after a 24 h recovery from slow (C) and fast (E) dehydration, in leaves of the aquatic moss F. antipyretica. White scale bars = 50 µm.
The H2O2 production was monitored throughout the first 30 min of rehydration (Fig. 4). While slow drying resulted in an increase in H2O2 as early as after 5 min of rehydration [33 ± 12 µmol H2O2 (g d. wt)−1 h−1], the amount was not statistically different from that of the non-stressed control [17 ± 9 µmol H2O2 (g d. wt)−1 h−1]. However, rapid drying resulted in a large peak of H2O2 production [73 ± 11 µmol H2O2 (g d. wt)−1 h−1], 4-fold higher than control values, 5 min after rehydration. In fact, H2O2 levels were always higher in rapidly dried moss than in slowly dried moss, although, over time, the rate of H2O2 production decreased in both treatments.
Fig. 4.
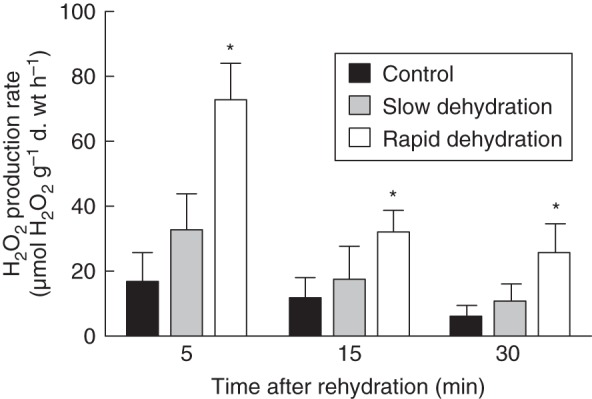
Extracellular hydrogen peroxide (H2O2) production rate in non-stressed control and in slowly (95 % RH) and rapidly (50 % RH) dehydrated leaves of F. antipyretica after 5, 15 and 30 min of rehydration. Columns indicate the mean and the bars the standard deviation of four replicates. An asterisk indicates a column value statistically different from the control value.
Oxygen consumption peaks in the early stages of rehydration
Prior to dehydration, oxygen consumption was 33 ± 11 µmol O2 (g d. wt)−1 h−1. In non-stressed samples, the addition of KCN greatly (70 %) inhibited oxygen consumption, probably by inhibiting cytochrome c oxidase. Under the same conditions, the alternative oxidase pathway accounts for approx. 16 % of the respiratory electron flux. The residual oxygen consumption is relatively high (approx. 14 %). Rehydration induced a high rate of oxygen consumption in the first 5–7 min: up to 15-fold higher than the pre-desiccation levels in slowly dehydrated leaves but 40 times higher in rapidly dehydrated leaves. This result suggested that the amount of oxygen consumption during rehydration was dependent on the drying rate (Fig. 5). However, regardless of the drying rate, oxygen consumption was fully inhibited by KCN but not by SHAM (Table 2), suggesting the involvement of the cytochrome c pathway but not the oxidase pathway in the recovery from dehydration.
Fig. 5.
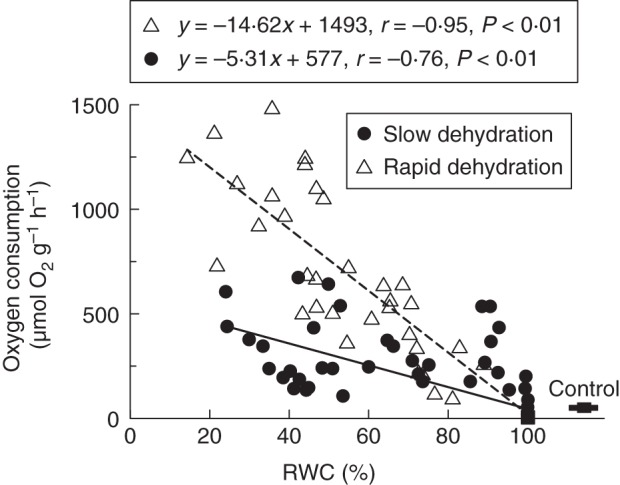
Oxygen consumption rate in the first 5–7 min of rehydration in samples dehydrated to different relative water contents (RWCs) at slow (95 % RH, solid line) and fast (50 % RH, dashed line) dehydration rates, in leaves of the aquatic moss F. antipyretica.
Table 2.
Oxygen consumption and inhibition by KCN and SHAM in leaves of the aquatic moss Fontinalis antipyretica rehydrated from 20 % RWC
| Inhibition (%) |
||||
|---|---|---|---|---|
| Treatment | Oxygen consumption rate (μmol O2 g−1 d. wt h−1) | + KCN (1 mm) | + SHAM (5 mm) | + KCN (1 mm) + SHAM (5 mm) |
| Control | 17 ± 1 | 70 ± 1 | 16 ± 2 | 77 ± 7 |
| Slow dehydration | 192 ± 14 | 100 ± 0 | 0 ± 0 | 100 ± 0 |
| Fast dehydration | 859 ± 58 | 100 ± 0 | 0 ± 0 | 100 ± 0 |
Values are mean ± s.d. (n = 3).
DISCUSSION
ROS production and damaged cells
In this work, ROS production in cells of the aquatic moss F. antipyretica was shown to be related to the dehydration rate, with low levels of ROS production determined in leaves that were slowly dehydrated and significantly higher levels in those that were rapidly dehydrated. The 70-fold difference in the production of ROS between rapidly and slowly dehydrated samples is clearly relevant to the impact of oxidative damage on cell survival, since these compounds are lethal to mosses.
While there are no previous reports showing a relationship in mosses between the rate of water loss and ROS production, similar results were obtained with S. ruralis with respect to the activity of glutathione reductase (GR), an antioxidant enzyme (Dhindsa, 1987). In that study, slow dehydration of the moss correlated with an increase in GR and thus with low cellular levels of oxidized glutathione (GSSG), whereas during fast dehydration there was no change in GR activity and GSSG levels were higher (Dhindsa, 1987). In a later study, these high levels of GSSG were shown to account for the inhibition of protein synthesis observed immediately after rehydration (Dhindsa, 1991), an effect that was suggested to be a carry-over from the post-desiccation repair mechanisms. According to Proctor and co-workers, the lack of recovery from rapid dehydration occurring in the light may be due to photodamage since in mosses and liverworts the recovery after desiccation was shown to be independent of de novo protein synthesis in the dark, suggesting that organelles need time to resume their original shapes and functions (Proctor, 2000; Proctor and Smirnoff, 2000; Proctor et al., 2007a, b; Pressel et al., 2009). Oxidative stress increased directly with the dehydration rate in S. ruralis (Dhindsa, 1987), as was also the case in F. antipyretica. Thus, despite their completely different ecological preferences, the former occurring in the desert and the latter in streams, the physiological responses of these two species to desiccation are similar. Moreover, in both species, slowly dried samples were shown to recover completely after a desiccation event (Dhindsa, 1987; Cruz de Carvalho et al., 2011). However, in rapidly dehydrated samples, photosynthesis and respiration returned to control levels after a few hours in S. ruralis (Schonbeck and Bewley, 1981) whereas recovery was shown to be only partial (25–50 %) in F. antipyretica, perhaps due to the death of some of the cells (Cruz de Carvalho et al., 2011). This conclusion is supported by the present work, in which some of the dehydrated cells were observed to be completely empty, especially those subjected to fast dehydration.
The death of some rapidly dehydrated cells and the disrupted aspect of others were probably related to the high-level production of ROS, as determined using the DCFH2-DA probe and confocal microscopy. These morphological changes were also seen in Fontinalis hypnoides, in which exposure to pollution and other stresses (Glime and Keen, 1984; Gimeno and Puche, 1999) ultimately led to cell death. Our finding that empty cells also produced high levels of ROS suggested that these compounds are involved in the cell-damaging effects. The increase in red fluorescence following rehydration, with its spread from one point in the cell across the cytoplasm, was probably due to the leakage of phenolic compounds from membrane-damaged vacuoles (Hura et al., 2009).
Regarding the spatial pattern of ROS production with respect to cell injury, the marginal parts of the leaves would seem to be more susceptible to the negative effects of drying since they are in closer contact with the dry atmosphere; however, this was not the case. In slowly dried leaves, the cells that produced ROS and showed evidence of injury were scattered across the leaves rather than clustered along the margins. This pattern resembles that of plant signalling cells in response to pathogenic infection (Morel and Dangl, 1997). In rapidly dried leaves, the majority of the cells produced ROS and were injured.
Among the ROS produced in F. antipyretica is H2O2. Indeed, there was a large burst in H2O2 production immediately after rehydration, analogous to what occurs in desiccation-tolerant Atrichum androgynum (Mayaba et al., 2002) and in the less tolerant Dumortiera hirsuta (Beckett et al., 2004). As shown by the DAB assay, H2O2 production is associated not only with chloroplasts but also with plasma membranes, especially in rapidly dried cells. However, since H2O2 is able to permeate membranes and aquaporins (Henzler and Steudle, 2000), it was difficult to determine its relative proportions in the different cellular fractions.
Mosses subjected to slow drying had sufficient time (>3 h) to adapt to the desiccation event, and their limited damage compared with rapidly dried samples suggests the induction of protection mechanisms involving protein synthesis. According to this scenario, mosses subjected to fast dehydration (3 h) would lack the time needed to induce protein synthesis and cellular antioxidative systems, resulting in enhanced ROS production and cell death. Mosses were shown to have both constitutive and inducible mechanisms to cope with desiccation (Oliver and Bewley, 1997; Mayaba et al., 2001). Since rapidly dehydrated samples of S. ruralis but not of F. antipyretica recover after a few hours, the relative importance of such protection mechanisms would seem to differ in the two species. Alternatively, their temporal regulation may be different, with F. antipyretica more dependent on long-term inducible protection mechanisms. In desiccation-tolerant vascular plants such as Craterostigma plantagineum, a desiccation event that allows a slow rate of dehydration will lead to the induction of desiccation tolerance mechanisms allowing survival. These mechanisms involve sucrose and late embryogenesis abundant (LEA) proteins (Bianchi et al., 1991; Bartels and Salamini, 2001), both of which are also present in mosses (Oliver et al., 2004).
In most studies in which mosses were subjected to desiccation, neither the rate of water loss nor the drying method used was reported (e.g. Sun, 2002). For example, in the various studies, drying has been carried out at constant RH in silica gel, or at the same RH but at different temperatures (10 and 22 °C), presumably resulting in different rates of water loss that make it difficult to compare species performance. In mosses grown in the field, morphological features and habitat variations can be expected to alter water loss conditions dramatically and specifically. Our results indicate the need to consider the rate of water loss at the cellular level if mosses are to be correctly classified in terms of their physiological tolerance of desiccation.
The oxygen consumption burst during rehydration
Oxygen consumption and inhibitor effects in control samples of F. antipyretica were similar (slightly less KCN inhibition) to those reported by other authors (Maberly, 1985; Azcón-Bieto et al., 1987). However, in this work, despite a similar RWC, oxygen consumption was lower in slowly than in rapidly dehydrated samples, suggesting that there is a critical limit to the water loss rate above which a large burst in oxygen consumption occurs. Yet, regardless of the dehydration rate, the oxygen consumption burst was insensitive to SHAM, an inhibitor of the mitochondrial alternative oxidase, but was fully inhibited by KCN, an inhibitor of the mitochondrial cytochrome c oxidase, as well as several metalloenzymes, such as catalases (Allen and Whatley, 1978), peroxidases (Choi et al., 2007) and superoxide dismutases (Chen et al., 2001) involved in ROS detoxification. A burst in oxygen consumption has also been observed in other mosses (Dilks and Proctor, 1974; Krochko et al., 1979) and lichens (Smith and Molesworth, 1973; Farrar and Smith, 1976), in which an uncoupling of mitochondrial respiration (Krochko et al., 1979) and a breakdown of cell compartmentalization (Farrar and Smith, 1976) were, respectively, proposed as the underlying mechanisms. However, the magnitude of oxygen consumption is not compatible with the amount of mitochondrial respiration, suggesting the additional involvement of superoxide (O2·−) production, with the consumption of molecular oxygen, in a reaction catalysed by KCN-sensitive extracellular peroxidases (Bestwick et al., 1997). The loss of tonoplast integrity may also explain the high level of oxygen consumption, since increased ROS production can account for the burst in oxygen consumption and may bring the vacuolar content, enriched in phenols, in contact with cytosolic polyphenol oxidases (Thipyapong et al., 2004) that catalyse the oxidation of these compounds. An increase in red fluorescence under conditions of dehydration stress has been correlated with a high content of phenolic compounds (Hura et al., 2009). In F. antipyretica, with its high phenol content (Glime, 2006), this would explain the browning of the photosynthetic tissues.
The slow dehydration rate in F. antipyretica seems crucial for the establishment of desiccation tolerance, as demonstrated by Cruz de Carvalho et al. (2011). This may allow the induction of cell protection mechanisms similar to what happens to mesic bryophytes in contrast to the highly desiccation-tolerant terrestrial bryophytes. These protection mechanisms limit ROS production and reduce the oxidative burst, increasing the survival rate of cells upon rehydration.
ACKNOWLEDGEMENTS
This work was supported in Portugal by Fundação para a Ciência e Tecnologia (FCT) PhD grant SFRH/BD/31424/2006 and FEDER POCI 2010: POCI/AMB/63160/2004, PPCDT/AMB/63160/2004, Lisbon. In Spain, this study was funded by the Spanish Ministry of Science and Innovation (CGL2009-13429-C02-01/02), the AECID (PCI_A_l024755/09) and the Generalitat Valenciana (PROMETEO 174/2008 GVA). We thank Sonia Priego (SCSIE, Universitat de València) and Professor Francisco García-Breijo (Universidad Politécnica de Valencia) for their technical assistance with confocal laser microscopy imaging, and Professor Lia Ascensão (Universidade de Lisboa) for her technical assistance in optical microscopy imaging.
LITERATURE CITED
- Abel WO. Die Austrocknungsresistenz der Laubmoose. Österreichische Akademie der Wissenschaften, Mathematisch-Naturwissenschaftliche Klasse, Sitzungsberichte, Abteilung 1. 1956;165:619–707. [Google Scholar]
- Allen JF, Whatley FR. Effects of inhibitors of catalase on photosynthesis and on catalase activity in unwashed preparations of intact chloroplasts. Plant Physiology. 1978;61:957–960. doi: 10.1104/pp.61.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert P, Oliver MJ. Drying without dying. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing; 2002. pp. 3–43. [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Physiology. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Azcón-Bieto J, Murillo J, Peñuelas J. Cyanide-resistant respiration in photosynthetic organs of freshwater aquatic plants. Plant Physiology. 1987;84:701–706. doi: 10.1104/pp.84.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels D, Salamini F. Desiccation tolerance in the resurrection plant Craterostigma plantagineum. A contribution to the study of drought tolerance at the molecular level. Plant Physiology. 2001;127:1346–1353. [PMC free article] [PubMed] [Google Scholar]
- Beckett RP. Partial dehydration and ABA induce tolerance to desiccation-induced ion leakage in the moss Atrichum androgynum. South African Journal of Botany. 1999;65:1–6. [Google Scholar]
- Beckett RP, Minibayeva FV, Lüthjec S, Böttger M. Reactive oxygen species metabolism in desiccation-stressed thalli of the liverwort Dumortiera hirsuta. Physiologia Plantarum. 2004;122:3–10. [Google Scholar]
- Bestwick CS, Brown IR, Bennett MHR, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. The Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Halmer P, Krochko JE, Winner WE. Metabolism of a drought-tolerant and a drought-sensitive moss: respiration, ATP synthesis and carbohydrate status. In: Crowe JH, Clegg JS, editors. Dry biological systems. New York: Academic Press; 1978. pp. 185–203. [Google Scholar]
- Bewley JD, Reynolds TL, Oliver MJ. Evolving strategies in the adaptation to desiccation. In: Close TJ, Bray EA, editors. Plant responses to cellular dehydration during environmental stress. Current topics in plant physiology. Vol. 10. Rockville, MD: American Society of Plant Physiologists; 1993. pp. 193–201. [Google Scholar]
- Bianchi G, Gamba A, Murelli C, Salamini F, Bartels D. Novel carbohydrate metabolism in the resurrection plant Craterostigma plantagineum. The Plant Journal. 1991;1:355–359. doi: 10.1046/j.1365-313X.1991.t01-11-00999.x. [DOI] [PubMed] [Google Scholar]
- Brown DH, Buck GW. Desiccation effects and cation distribution in bryophytes. New Phytologist. 1979;82:115–125. [Google Scholar]
- Catalá M, Gasulla F, Real AEP, García-Breijo F, Reig-Armiñana J, Barreno E. Fungal-associated NO is involved in the regulation of oxidative stress during rehydration in lichen symbiosis. BMC Microbiology. 2010;10:297. doi: 10.1186/1471-2180-10-297. http://dx.doi.org/10.1186/1471-2180-10-297 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-R, Liao C-W, Mao SJT, Chen T-H, Weng C-N. A simple technique for the simultaneous determination of molecular weight and activity of superoxide dismutase using SDS–PAGE. Journal of Biochemical and Biophysical Methods. 2001;47:233–237. doi: 10.1016/s0165-022x(00)00162-7. [DOI] [PubMed] [Google Scholar]
- Choi HW, Kim YJ, Lee SC, Hong JK, Hwang BK. Hydrogen peroxide generation by the pepper extracellular peroxidase CaPO2 activates local and systemic cell death and defense response to bacterial pathogens. Plant Physiology. 2007;145:890–904. doi: 10.1104/pp.107.103325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho R, Branquinho C, Marques da Silva J. Physiological consequences of desiccation in the aquatic bryophyte Fontinalis antipyretica. Planta. 2011;234:195–205. doi: 10.1007/s00425-011-1388-x. [DOI] [PubMed] [Google Scholar]
- Deltoro VI, Catalaynd A, Gimeno C, Abadía A, Barreno E. Changes in chlorophyll a fluorescence, photosynthetic CO2 assimilation and xanthophyll cycle interconversions during dehydration in desiccation-tolerant and intolerant liverworts. Planta. 1998;207:224–228. [Google Scholar]
- Dhindsa RS. Glutathione status and protein synthesis during drought and subsequent rehydration of Tortula ruralis. Plant Physiology. 1987;83:816–819. doi: 10.1104/pp.83.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhindsa RS. Drought stress, enzymes of glutathione metabolism, oxidation injury, and protein synthesis in Tortula ruralis. Plant Physiology. 1991;95:648–651. doi: 10.1104/pp.95.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks TJK, Proctor MCF. The pattern of recovery of bryophytes after desiccation. Journal of Bryology. 1974;8:97–115. [Google Scholar]
- Dizdaroglu M. Chemical determination of oxidative DNA-damage by gas-chromatography mass-spectrometry. Methods in Enzymology. 1994;234:3–16. doi: 10.1016/0076-6879(94)34072-2. [DOI] [PubMed] [Google Scholar]
- Dyer AF, Duckett JG. The experimental biology of Bryophytes. London: Academic Press; 1984. [Google Scholar]
- Farrant JM, Cooper K, Kruger LA, Sherwin HW. The effect of drying rate on the survival of three desiccation-tolerant angiosperm species. Annals of Botany. 1999;84:371–379. [Google Scholar]
- Farrar JF, Smith DC. Ecological physiology of the lichen Hypogymnia physodes. III. The importance of the rewetting phase. New Phytologist. 1976;77:115–125. [Google Scholar]
- Franks AJ, Bergstrom DM. Corticolous bryophytes in microphyll fern forests of south-east Queensland: distribution on Antarctic beech (Nothofagus moorei) Austral Ecology. 2000;25:386–393. [Google Scholar]
- Gasulla F, Gómez de Nova P, Esteban-Carrasco A, Zapata JM, Barreno E, Guéra A. Dehydration rate and time of desiccation affect recovery of the lichenic algae Trebouxia erici: alternative and classical protective mechanisms. Planta. 2009;231:195–208. doi: 10.1007/s00425-009-1019-y. [DOI] [PubMed] [Google Scholar]
- Gay C, Gebicki JM. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Analytical Biochemistry. 2000;284:217–220. doi: 10.1006/abio.2000.4696. [DOI] [PubMed] [Google Scholar]
- Gimeno C, Puche F. Chlorophyll content and morphological changes in cellular structure of Rhynchostegium riparioides (Hedw.) Card. (Brachytheciaceae, Musci) and Fontinalis hypnoides Hartm. (Fontinalaceae, Musci) in response to water pollution and transplant containers on Palancia river (East, Spain) Nova Hedwigia. 1999;68:197–216. [Google Scholar]
- Glime JM. Bryophytes and herbivory. Cryptogamie Bryologie. 2006;27:191–203. [Google Scholar]
- Glime JM, Keen RE. The importance of bryophytes in a man-centered world. Journal of the Hattori Botanical Laboratory. 1984;55:133–146. [Google Scholar]
- Glime JM, Vitt DH. The physiological adaptations of aquatic Musci. Lindbergia. 1984;10:41–52. [Google Scholar]
- Goffinet B, Hollowell V, Magill R. St Louis, MO: Missouri Botanical Garden Press; 2004. Molecular systematics of bryophytes. [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3rd edn. Oxford: Oxford University Press; 1999. [Google Scholar]
- Henzler T, Steudle E. Transport and metabolic degradation of hydrogen peroxide in Chara coralline: model calculations and measurements with the pressure probe suggest transport of H2O2 across water channels. Journal of Experimental Botany. 2000;51:2053–2066. doi: 10.1093/jexbot/51.353.2053. [DOI] [PubMed] [Google Scholar]
- Hura T, Hura K, Grzesiak S. Leaf dehydration induces different content of phenolics and ferulic acid in drought-resistant and -sensitive genotypes of spring triticale. Zeitschrift für Naturforschung C. 2009;64:85–95. doi: 10.1515/znc-2009-1-215. [DOI] [PubMed] [Google Scholar]
- Ingle RA, Schmidt UG, Farrant JM, Thomson JA, Mundree SG. Proteomic analysis of leaf proteins during dehydration of the resurrection plant Xerophyta viscosa. Plant, Cell and Environment. 2007;30:435–446. doi: 10.1111/j.1365-3040.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- Jiang G, Wang Z, Shang H, Yang W, Hu Z, Phillips J, Deng X. Proteome analysis of leaves from the resurrection plant Boea hygrometrica in response to dehydration and rehydration. Planta. 2007;225:1405–1420. doi: 10.1007/s00425-006-0449-z. [DOI] [PubMed] [Google Scholar]
- Krochko JE, Winner WE, Bewley JD. Respiration in relation to adenosine triphosphate content during desiccation and rehydration of a desiccation-tolerant and a desiccation-intolerant moss. Plant Physiology. 1979;64:13–17. doi: 10.1104/pp.64.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leprince O, Harren FJM, Buitink J, Alberda M, Hoekstra FA. Metabolic dysfunction and unabated respiration precede the loss of membrane integrity during dehydration of germinating radicles. Plant Physiology. 2000;122:597–608. doi: 10.1104/pp.122.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maberly SC. Photosynthesis by Fontinalis antipyretica: I. Interaction between photon irradiance, concentration of carbon dioxide and temperature. New Phytologist. 1985;100:127–140. [Google Scholar]
- Marusek CM, Trobaugh NM, Flurkey WH, Inlow JK. Comparative analysis of polyphenol oxidase from plant and fungal species. Journal of Inorganic Biochemistry. 2006;100:108–123. doi: 10.1016/j.jinorgbio.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Mayaba N, Beckett RP, Csintalan Z, Tuba Z. ABA increases the desiccation tolerance of photosynthesis in the Afromontane understory moss Atrichum androgynum. Annals of Botany. 2001;86:1093–1100. [Google Scholar]
- Mayaba N, Minibayeva F, Beckett RP. An oxidative burst of hydrogen peroxide during rehydration following desiccation in the moss Atrichum androgynum. New Phytologist. 2002;155:275–283. [Google Scholar]
- McKersie BD, Crowe JH, Crowe LM. Free fatty acid effects on leakage, phase properties and fusion of fully hydrated model membranes. Biochimica et Biophysica Acta. 1989;982:156–160. [Google Scholar]
- Miller G, Shulaev V, Mittler R. Reactive oxygen signalling and abiotic stress. Physiologia Plantarum. 2008;133:481–489. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- Minibayeva F, Beckett RP. High rates of extracellular superoxide production in bryophytes and lichens, and an oxidative burst in response to rehydration following desiccation. New Phytologist. 2001;152:333–343. [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Morel J-B, Dangl JL. The hypersensitive response and the induction of cell death in plants. Cell Death and Differentiation. 1997;4:671–683. doi: 10.1038/sj.cdd.4400309. [DOI] [PubMed] [Google Scholar]
- Murphy TM, Asard H, Cross AR. Possible sources of reactive oxygen during the oxidative burst in plants. In: Asard H, Bérczi A, Cauberg RJ, editors. Plasma membrane redox systems and their role in biological stress and disease. Dordrecht. The Netherlands: Kluwer Academic Publishers; 1998. pp. 215–246. [Google Scholar]
- Oliver MJ. Influence of protoplasmic water loss on the control of protein synthesis in the desiccation-tolerant moss Tortula ruralis: ramifications for a repair-based mechanism of desiccation tolerance. Plant Physiology. 1991;97:1501–1511. doi: 10.1104/pp.97.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver MJ. Biochemical and molecular mechanisms of desiccation tolerance in bryophytes. In: Goffinet B, Shaw AJ, editors. Bryophyte biology. 2nd edn. Cambridge, UK: Cambridge University Press; 2008. pp. 269–297. [Google Scholar]
- Oliver MJ, Bewley JD. Desiccation-tolerance of plant tissues: a mechanistic overview. Horticultural Reviews. 1997;18:171–213. [Google Scholar]
- Oliver MJ, Dowd SE, Zaragoza J, Mauget SA, Payton PR. The rehydration transcriptome of the desiccation-tolerant bryophyte Tortula ruralis: transcript classification and analysis. BMC Genomics. 2004;5:89. doi: 10.1186/1471-2164-5-89. http://dx.doi.org/10.1186/1471-2164-5-89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver MJ, Jain R, Balbuena TS, Agrawal G, Gasulla F, Thelen JJ. Proteome analysis of leaves of the desiccation-tolerant grass. Sporobolus stapfianus, in response to dehydration. Phytochemistry. 2010;72:1273–1284. doi: 10.1016/j.phytochem.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Pressel S, Duckett JG, Ligrone R, Proctor MCF. Effects of de- and rehydration in desiccation-tolerant liverworts: a cytological and physiological study. International Journal of Plant Science. 2009;170:182–199. [Google Scholar]
- Proctor MCF. The bryophyte paradox: tolerance to desiccation, evasion of drought. Plant Ecology. 2000;151:41–49. [Google Scholar]
- Proctor MCF, Smirnoff N. Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments. Journal of Experimental Botany. 2000;51:1695–1704. doi: 10.1093/jexbot/51.351.1695. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Ligrone L, Duckett JG. Desiccation tolerance in the moss Polytrichum formosum: physiological and fine-structural changes during desiccation and recovery. Annals of Botany. 2007a;99:75–93. doi: 10.1093/aob/mcl246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF, Oliver MJ, Wood AJ, et al. Desiccation-tolerance in bryophytes: a review. Bryologist. 2007b;110:595–621. [Google Scholar]
- Robinson SA, Wasley J, Popp M, Lovelock CE. Desiccation tolerance of three moss species from continental Antarctica. Australian Journal of Plant Physiology. 2000;27:379–388. [Google Scholar]
- Scheibe R, Beck E. Drought, desiccation, and oxidative stress. In: Lüttge U, Beck E, Bartels D, editors. Plant desiccation tolerance, Ecological Studies 215. Berlin: Springer; 2011. pp. 209–231. [Google Scholar]
- Schonbeck MW, Bewley JD. Responses of the moss Tortula ruralis to desiccation treatments. I. Effects of minimum water content and rates of dehydration and rehydration. Canadian Journal of Botany. 1981;59:2698–2706. [Google Scholar]
- Seel WE, Baker NR, Lee JA. Analysis of the decrease in photosynthesis on desiccation of mosses from xeric and hydric environments. Physiologia Plantarum. 1992a;86:451–458. [Google Scholar]
- Seel WE, Hendry GAF, Lee JA. Effects of desiccation on some activated oxygen processing enzymes and antioxidants in mosses. Journal of Experimental Botany. 1992b;43:1031–1037. [Google Scholar]
- Senaratna T, McKersie BD. Dehydration injury in germinating soybean (Glycine max L. merr) seeds. Plant Physiology. 1983;72:620–624. doi: 10.1104/pp.72.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist. 1993;125:27–58. doi: 10.1111/j.1469-8137.1993.tb03863.x. [DOI] [PubMed] [Google Scholar]
- Smith DC, Molesworth S. Lichen physiology XIII. Effects of rewetting dry lichens. New Phytologist. 1973;72:525–533. [Google Scholar]
- Sun WQ. Methods for the study of water relations under desiccation stress. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CABI Publishing; 2002. pp. 47–92. [Google Scholar]
- Thipyapong P, Melkonian J, Wolfe DW, Steffens JC. Suppression of polyphenol oxidases increases stress tolerance in tomato. Plant Science. 2004;167:693–703. [Google Scholar]
- Traubenberg RC, Ah-Peng C. A procedure to purify and culture a clonal strain of the aquatic moss Fontinalis antipyretica for use as a bioindicator of heavy metals. Archives of Environmental Contamination and Toxicology. 2004;46:289–295. doi: 10.1007/s00244-003-3040-7. [DOI] [PubMed] [Google Scholar]
- Tuba Z, Proctor MCF, Csintalan Z. Ecophysiological responses of homoiochlorophyllous and poikilochlorophyllous desiccation tolerant plants: a comparison and ecological perspective. Plant Growth Regulation. 1998;24:211–217. [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Werner O, Espin RMR, Bopp M, Atzorn R. Abscisic acid-induced drought tolerance in Funaria hygrometrica Hedw. Planta. 1991;186:99–103. doi: 10.1007/BF00201503. [DOI] [PubMed] [Google Scholar]
- Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochemical Journal. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SP, Garner A, Dean RT. Free radicals, lipids and protein degradation. Trends in Biochemical Sciences. 1986;11:27–31. [Google Scholar]