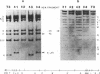
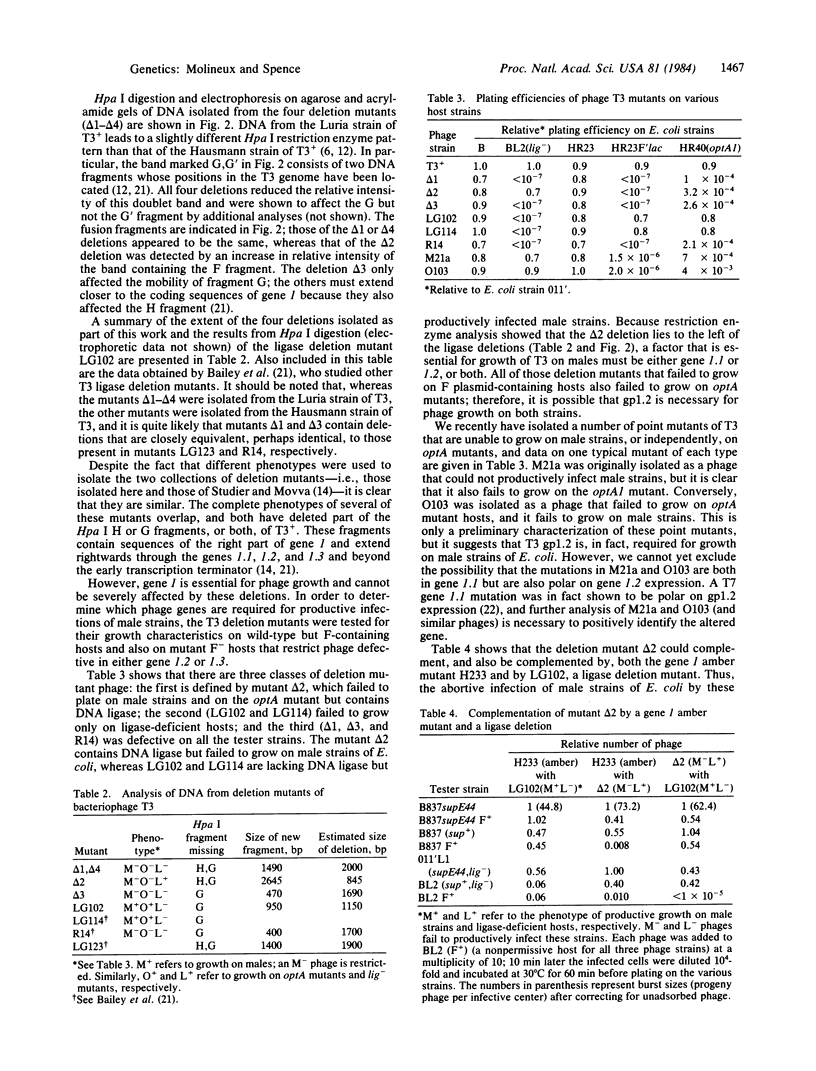
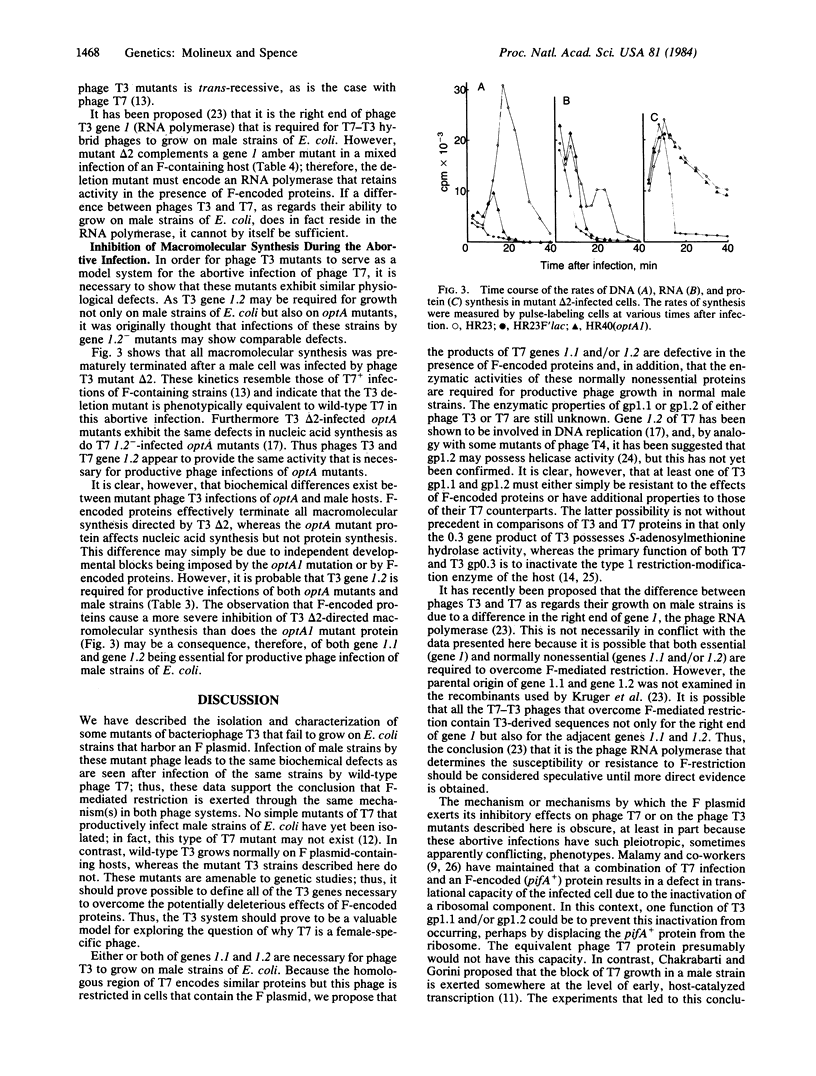
Abstract
Bacteriophage T7 and many closely related phages abortively infect plasmid F-containing (F+) strains of Escherichia coli. However phage T3, which is also closely related to T7, grows normally in F+ hosts. Mutants of phage T3 that, like T7, are subject to F-mediated restriction have been isolated. These T3 mutants lack or are defective in one or both of two genes that are nonessential for phage growth in F-, wild-type strains. Our results show that the products of phage T3 gene 1.1 or 1.2, or both, are essential for growth and suggest that the comparable phage T7 genes are naturally defective in their ability to counteract the inhibitory effects of F-encoded proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. N., Dembinski D. R., McAllister W. T. Derivation of a restriction map of bacteriophage T3 DNA and comparison with the map of bacteriophage T7 DNA. J Virol. 1980 Jul;35(1):176–183. doi: 10.1128/jvi.35.1.176-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier H., Hausmann R. Genetic map of bacteriophage T3. J Virol. 1973 Aug;12(2):417–419. doi: 10.1128/jvi.12.2.417-419.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg D. D., Mabie C. T., Malamy M. H. T7 protein synthesis in F-factor-containing cells: evidence for an episomally induced impairment of translation and relation to an alteration in membrane permeability. J Virol. 1975 Jan;17(1):94–105. doi: 10.1128/jvi.17.1.94-105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. R., Haselkorn R. Permeability lesions in male Escherichia coli infected with bacteriophage T7. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2222–2226. doi: 10.1073/pnas.72.6.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S. L., Gorini L. A link between streptomycin and rifampicin mutation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2084–2087. doi: 10.1073/pnas.72.6.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. Nucleotide sequence from the genetic left end of bacteriophage T7 DNA to the beginning of gene 4. J Mol Biol. 1981 Jun 5;148(4):303–330. doi: 10.1016/0022-2836(81)90178-9. [DOI] [PubMed] [Google Scholar]
- Gauss P., Doherty D. H., Gold L. Bacterial and phage mutations that reveal helix-unwinding activities required for bacteriophage T4 DNA replication. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1669–1673. doi: 10.1073/pnas.80.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Hansen S., Chernin L. S. Abortive infection of F-plasmid-containing Escherichia coli cells by bacterial virus T7 is determined by the right end of T7 gene 1. J Virol. 1983 Apr;46(1):293–296. doi: 10.1128/jvi.46.1.293-296.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol Rev. 1981 Mar;45(1):9–51. doi: 10.1128/mr.45.1.9-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux I. J., Mooney P. Q., Spence J. L. Recombinants between bacteriophages T7 and T3 which productively infect F-plasmid-containing strains of Escherichia coli. J Virol. 1983 Jun;46(3):881–894. doi: 10.1128/jvi.46.3.881-894.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., Malamy M. H. T7 translational control mechanisms and their inhibiton by F factors. Nat New Biol. 1971 May 12;231(19):37–41. doi: 10.1038/newbio231037a0. [DOI] [PubMed] [Google Scholar]
- Rotman G. S., Cooney R., Malamy M. H. Cloning of the pif region of the F sex factor and identification of a pif protein product. J Bacteriol. 1983 Jul;155(1):254–264. doi: 10.1128/jb.155.1.254-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Genetic analysis of gene 1.2 of bacteriophage T7: isolation of a mutant of Escherichia coli unable to support the growth of T7 gene 1.2 mutants. J Virol. 1981 Jan;37(1):343–351. doi: 10.1128/jvi.37.1.343-351.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Richardson C. C. Processing of mRNA by ribonuclease III regulates expression of gene 1.2 of bacteriophage T7. Cell. 1981 Dec;27(3 Pt 2):533–542. doi: 10.1016/0092-8674(81)90395-0. [DOI] [PubMed] [Google Scholar]
- Saito H., Tabor S., Tamanoi F., Richardson C. C. Nucleotide sequence of the primary origin of bacteriophage T7 DNA replication: relationship to adjacent genes and regulatory elements. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3917–3921. doi: 10.1073/pnas.77.7.3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. L., Mooney P. Q., Molineux I. J. Physiological properties of a T7-T3 recombinant bacteriophage that productively infects strains of Escherichia coli that harbor the F plasmid. J Virol. 1983 Jun;46(3):895–900. doi: 10.1128/jvi.46.3.895-900.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Gene 0.3 of bacteriophage T7 acts to overcome the DNA restriction system of the host. J Mol Biol. 1975 May 15;94(2):283–295. doi: 10.1016/0022-2836(75)90083-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Genetic analysis of non-essential bacteriophage T7 genes. J Mol Biol. 1973 Sep 15;79(2):227–236. doi: 10.1016/0022-2836(73)90002-8. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Movva N. R. SAMase gene of bacteriophage T3 is responsible for overcoming host restriction. J Virol. 1976 Jul;19(1):136–145. doi: 10.1128/jvi.19.1.136-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Wood W. B. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966 Mar;16(1):118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]