Abstract
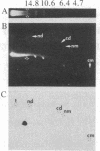
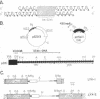
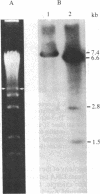
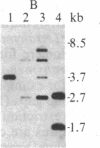
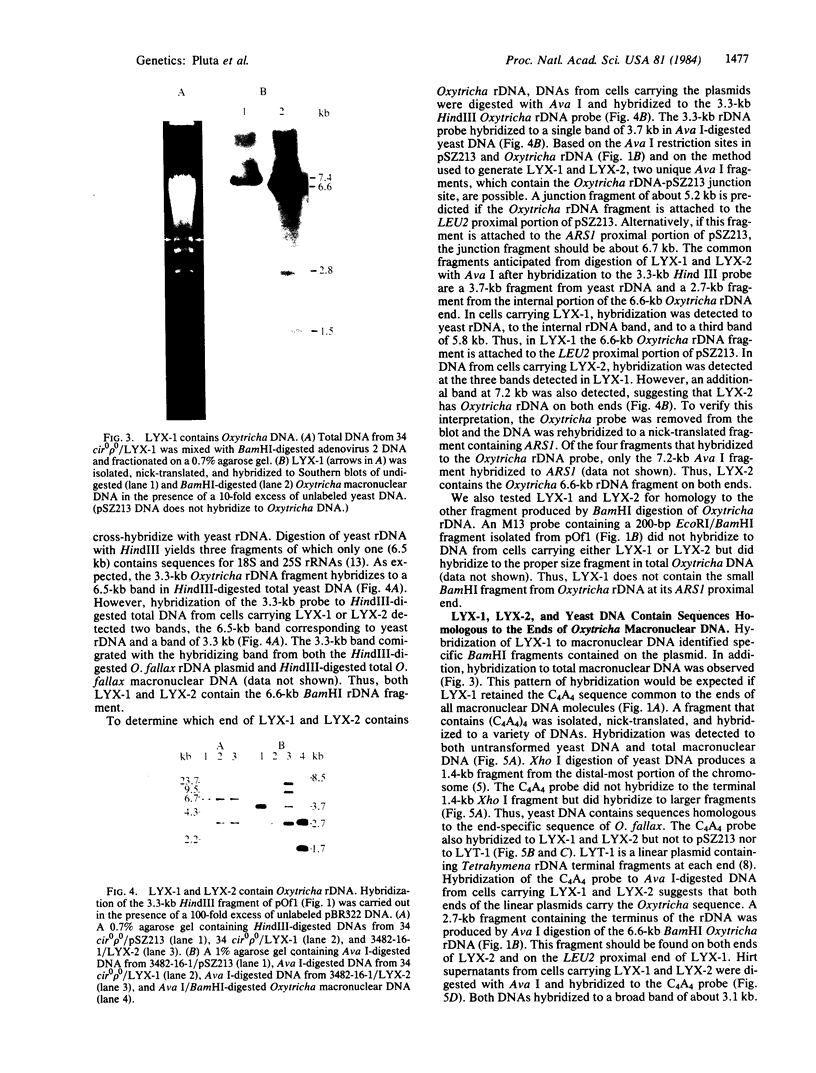
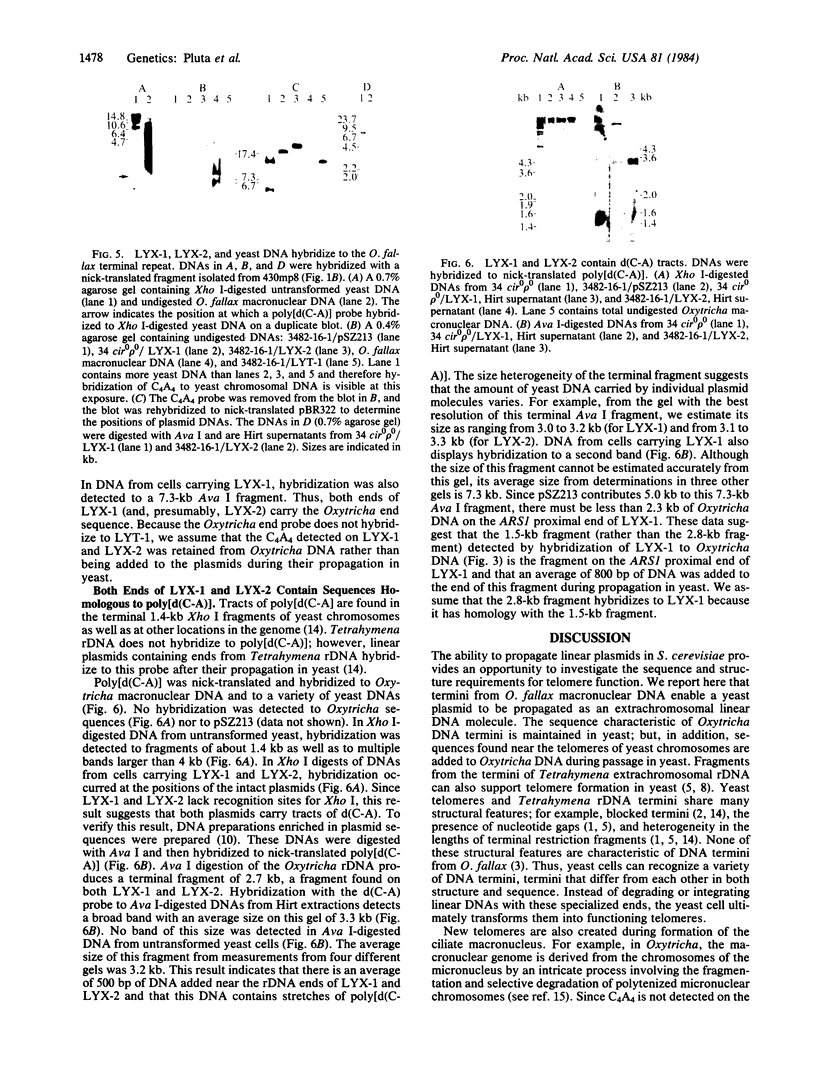
The termini of macronuclear DNA molecules from the protozoan Oxytricha fallax share a common sequence and structure, both of which differ markedly from those deduced for yeast telomeres. Despite these differences, terminal restriction fragments from O. fallax macronuclear DNA can support telomere formation in yeasts. Two linear plasmids (LYX-1 and LYX-2) constructed by ligating BamHI-digested total Oxytricha macronuclear DNA to a yeast vector were analyzed. One end of LYX-1 and both ends of LYX-2 are derived from the Oxytricha DNA that encodes rRNA (rDNA) whereas the other end of LYX-1 is from an Oxytricha fragment other than rDNA. After propagation in yeast, both ends of LYX-1 and LYX-2 retain the C4A4 repeat characteristic of the O. fallax terminal sequence. In addition, both ends of both plasmids acquire 300-1000 base pairs of DNA containing the sequence (C-A)n, a sequence found near the termini of yeast chromosomes. Thus, at least two different Oxytricha termini display distinctive properties in yeast cells in that linear plasmids containing them are not degraded nor are they integrated into chromosomal DNA. These Oxytricha termini may act directly as telomeres in yeast; alternatively, the Oxytricha DNA may serve as a signal that results in the elaboration of a yeast telomere on the ciliate DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., DeGennaro L. J., Gelfand D. H., Bishop R. J., Valenzuela P., Rutter W. J. Ribosomal RNA genes of Saccharomyces cerevisiae. I. Physical map of the repeating unit and location of the regions coding for 5 S, 5.8 S, 18 S, and 25 S ribosomal RNAs. J Biol Chem. 1977 Nov 25;252(22):8118–8125. [PubMed] [Google Scholar]
- Blackburn E. H., Budarf M. L., Challoner P. B., Cherry J. M., Howard E. A., Katzen A. L., Pan W. C., Ryan T. DNA termini in ciliate macronuclei. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1195–1207. doi: 10.1101/sqb.1983.047.01.135. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H., Gall J. G. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- Boswell R. E., Klobutcher L. A., Prescott D. M. Inverted terminal repeats are added to genes during macronuclear development in Oxytricha nova. Proc Natl Acad Sci U S A. 1982 May;79(10):3255–3259. doi: 10.1073/pnas.79.10.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Organization of DNA sequences and replication origins at yeast telomeres. Cell. 1983 Jun;33(2):563–573. doi: 10.1016/0092-8674(83)90437-3. [DOI] [PubMed] [Google Scholar]
- Dani G. M., Zakian V. A. Mitotic and meiotic stability of linear plasmids in yeast. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3406–3410. doi: 10.1073/pnas.80.11.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Spear B. B. Nucleotide sequence of a macronuclear gene for actin in Oxytricha fallax. Nature. 1982 Feb 4;295(5848):430–432. doi: 10.1038/295430a0. [DOI] [PubMed] [Google Scholar]
- Kaine B. P., Spear B. B. Putative actin genes in the macronucleus of Oxytricha fallax. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5336–5340. doi: 10.1073/pnas.77.9.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B. O., Yao M. C. Tandemly repeated hexanucleotide at Tetrahymena rDNA free end is generated from a single copy during development. Cell. 1982 Nov;31(1):177–182. doi: 10.1016/0092-8674(82)90417-2. [DOI] [PubMed] [Google Scholar]
- Kiss G. B., Amin A. A., Pearlman R. E. Two separate regions of the extrachromosomal ribosomal deoxyribonucleic acid of Tetrahymena thermophila enable autonomous replication of plasmids in Saccharomyces cerevisiae. Mol Cell Biol. 1981 Jun;1(6):535–543. doi: 10.1128/mcb.1.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauth M. R., Spear B. B., Heumann J., Prescott D. M. DNA of ciliated protozoa: DNA sequence diminution during macronuclear development of Oxytricha. Cell. 1976 Jan;7(1):67–74. doi: 10.1016/0092-8674(76)90256-7. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Kupfer D. M. Control of Saccharomyces cerevisiae 2microN DNA replication by cell division cycle genes that control nuclear DNA replication. J Mol Biol. 1977 Oct 25;116(2):249–260. doi: 10.1016/0022-2836(77)90215-7. [DOI] [PubMed] [Google Scholar]
- Pluta A. F., Kaine B. P., Spear B. B. The terminal organization of macronuclear DNA in Oxytricha fallax. Nucleic Acids Res. 1982 Dec 20;10(24):8145–8154. doi: 10.1093/nar/10.24.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae P. M., Spear B. B. Macronuclear DNA of the hypotrichous ciliate Oxytricha fallax. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4992–4996. doi: 10.1073/pnas.75.10.4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear B. B. Isolation and mapping of the rRNA genes in the macronucleus of Oxytricha fallax. Chromosoma. 1980;77(2):193–202. doi: 10.1007/BF00329544. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Blackburn E. H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982 May;29(1):245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- Walmsley R. M., Szostak J. W., Petes T. D. Is there left-handed DNA at the ends of yeast chromosomes? Nature. 1983 Mar 3;302(5903):84–86. doi: 10.1038/302084a0. [DOI] [PubMed] [Google Scholar]
- Zakian V. A., Kupfer D. M. Replication and segregation of an unstable plasmid in yeast. Plasmid. 1982 Jul;8(1):15–28. doi: 10.1016/0147-619x(82)90037-3. [DOI] [PubMed] [Google Scholar]