Abstract
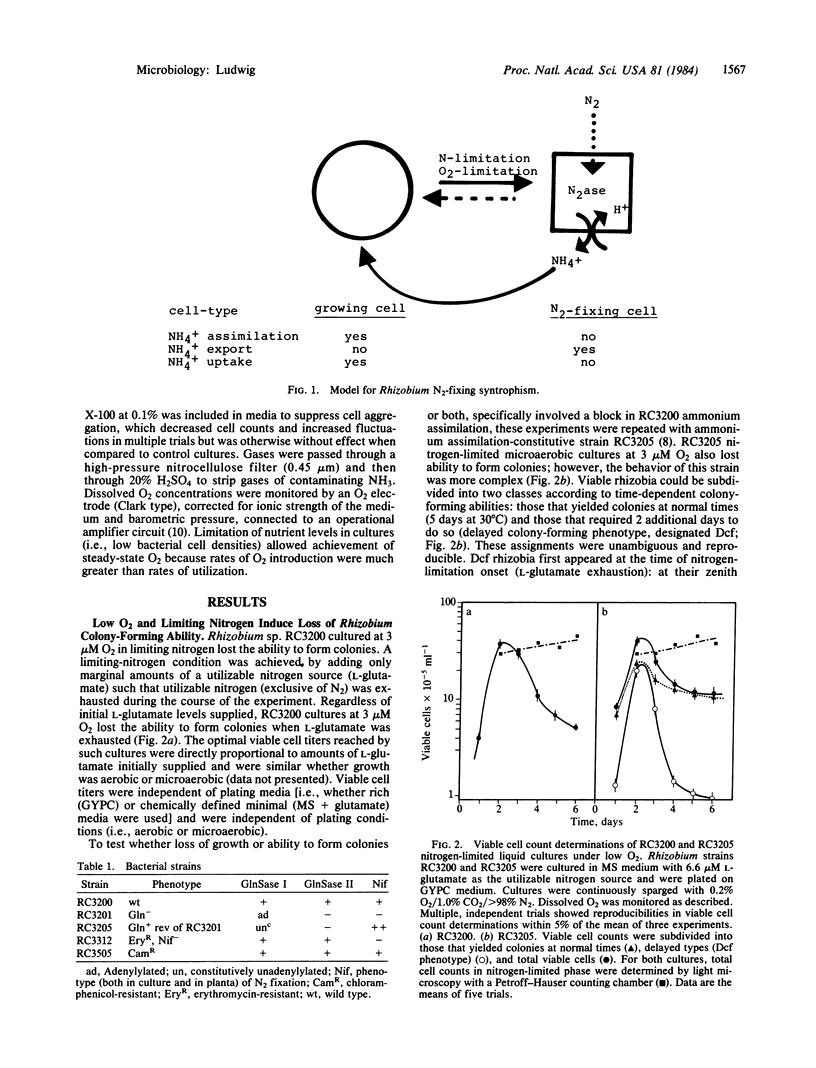
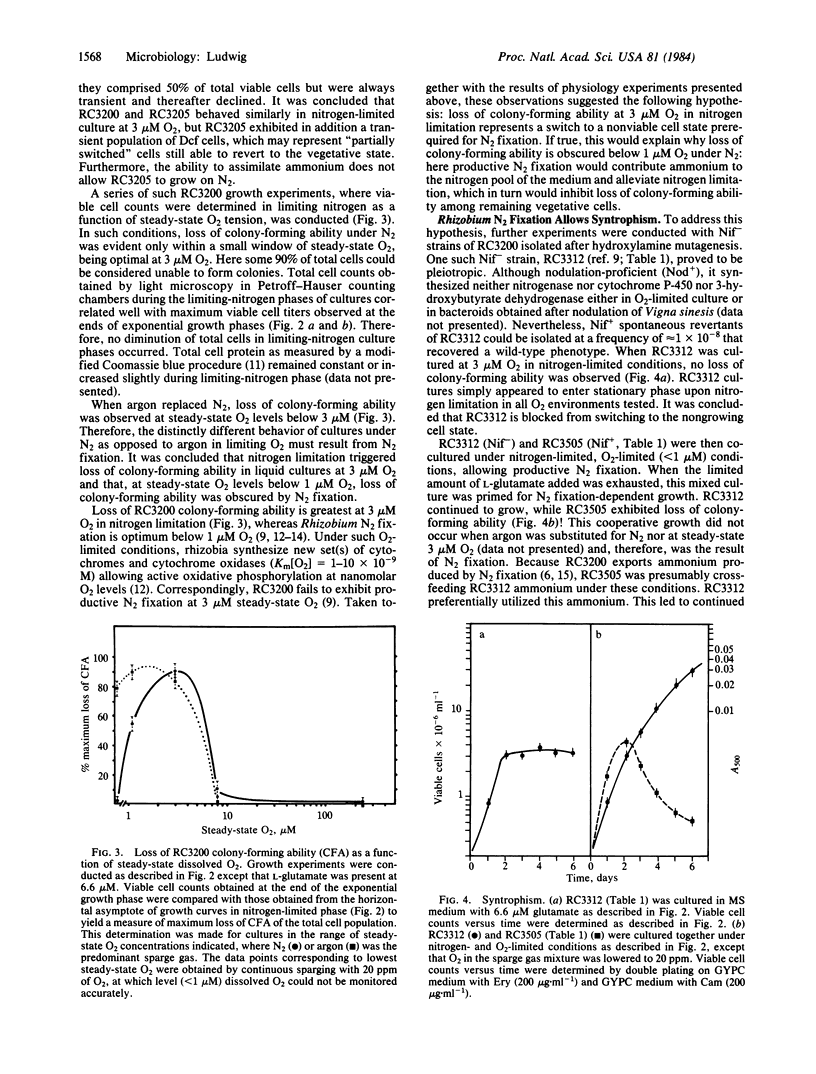
A model for free-living N2 fixation by Rhizobium sp. RC3200 is presented that asserts that this process occurs in nongrowing cells. Cultures containing mixed populations of cell types, N2-fixing and vegetative, grow cooperatively. In nitrogen-limited liquid suspension cultures, cooperative growth occurs by means of ammonium that is produced and exported by nongrowing, N2-fixing cells and transported to vegetative cells. This model implies prokaryotic differentiation: the creation of metabolically specialized cells, terminally nonviable, that functionally cooperate in a higher cell order. Here, the switch to a Rhizobium N2-fixing cell state is regulated by both O2 and utilizable nitrogen.
Keywords: prokaryotic differentiation, syntrophism
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Turner G. L. Systems utilizing oxygenated leghemoglobin and myoglobin as sources of free dissolved O2 at low concentrations for experiments with bacteria. Anal Biochem. 1979 Jul 1;96(1):165–174. doi: 10.1016/0003-2697(79)90569-4. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Dilworth M. J. Ammonia assimilation by rhizobium cultures and bacteroids. J Gen Microbiol. 1975 Jan;86(1):39–48. doi: 10.1099/00221287-86-1-39. [DOI] [PubMed] [Google Scholar]
- Gober J. W., Kashket E. R. Methylammonium uptake by Rhizobium sp. strain 32H1. J Bacteriol. 1983 Mar;153(3):1196–1201. doi: 10.1128/jb.153.3.1196-1201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. L. Acetylene reduction by pure cultures of Rhizobia. J Bacteriol. 1975 Sep;123(3):1265–1268. doi: 10.1128/jb.123.3.1265-1268.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Physiological roles of glutamine synthetases I and II in ammonium assimilation in Rhizobium sp. 32H1. J Bacteriol. 1980 Mar;141(3):1209–1216. doi: 10.1128/jb.141.3.1209-1216.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A. Regulation of Rhizobium nitrogen fixation by the unadenylylated glutamine synthetase I system. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5817–5821. doi: 10.1073/pnas.77.10.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig R. A., Signer E. R. Glutamine synthetase and control of nitrogen fixation in Rhizobium. Nature. 1977 May 19;267(5608):245–248. doi: 10.1038/267245a0. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Sweet W. J., Houchins J. P., Rosen P. R., Arp D. J. Polarographic measurement of H2 in aqueous solutions. Anal Biochem. 1980 Sep 15;107(2):337–340. doi: 10.1016/0003-2697(80)90393-0. [DOI] [PubMed] [Google Scholar]
- Tjepkema J., Evans H. J. Nitrogen fixation by free-living Rhizobium in a defined liquid medium. Biochem Biophys Res Commun. 1975 Jul 22;65(2):625–628. doi: 10.1016/s0006-291x(75)80192-6. [DOI] [PubMed] [Google Scholar]


