Abstract
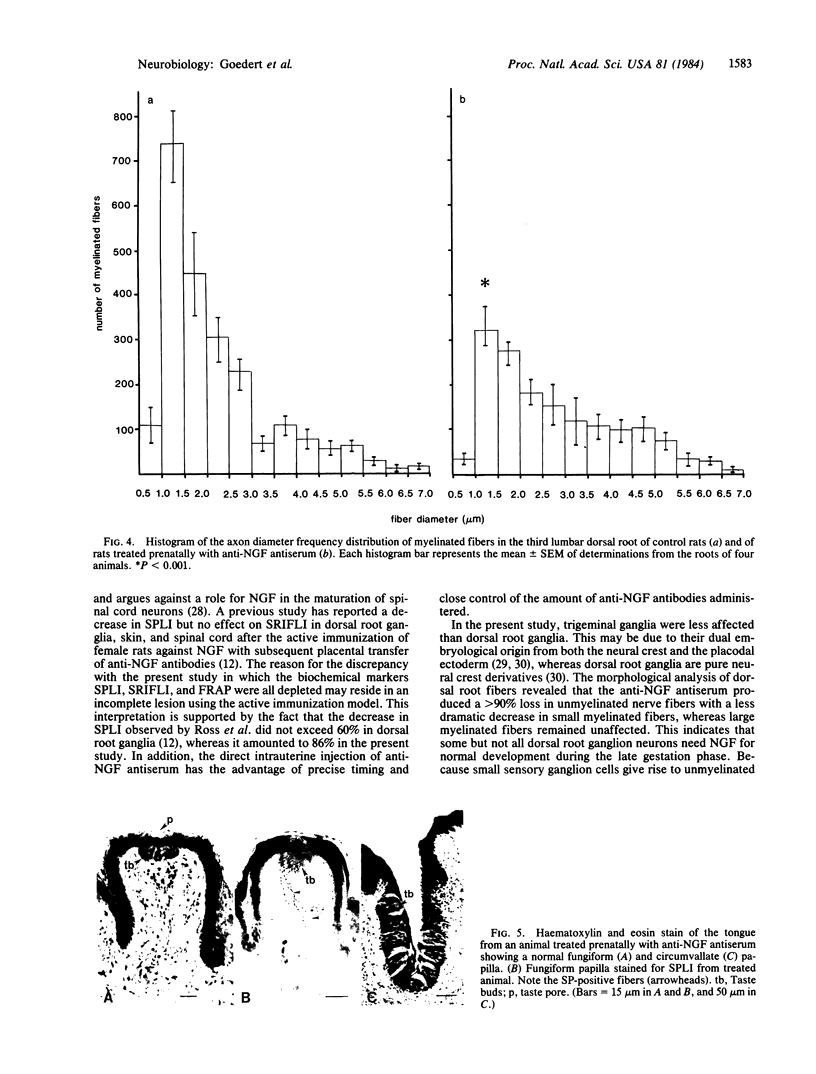
The importance of nerve growth factor (NGF) for the development of sensory ganglia was investigated by injecting rat fetuses (16.50 days of gestation) with a single dose of anti-NGF antiserum. Four months later the treated animals showed a very large decrease in substance P- and somatostatin-like immunoreactivities in dorsal root ganglia and skin with a lesser decrease in trigeminal ganglia. Fluoride-resistant acid phosphatase, substance P-, and somatostatin-like immunoreactivities were greatly decreased in the dorsal horn of the spinal cord. No change in neurotensin- and [Met]enkephalin-like immunoreactivities was observed. The anti-NGF antiserum treatment produced a greater than 90% decrease in the number of unmyelinated dorsal root fibers and a 35% decrease in the total number of myelinated fibers. The loss in myelinated fibers was restricted to small-diameter fibers with no change in large-diameter fibers. No change in taste bud morphology was noted, thereby refuting the proposal that anti-NGF antiserum treatment may represent an animal model for familial dysautonomia. The present results indicate that NGF is a necessary requirement for the normal development of a significant population of prenatal rat dorsal root ganglion cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloe L., Cozzari C., Calissano P., Levi-Montalcini R. Somatic and behavioral postnatal effects of fetal injections of nerve growth factor antibodies in the rat. Nature. 1981 Jun 4;291(5814):413–415. doi: 10.1038/291413a0. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Sensory neurons in culture: changing requirements for survival factors during embryonic development. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1199–1203. doi: 10.1073/pnas.77.2.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis M., Pearce F. L., Vernon C. A. Some studies on the mechanism of action of antibodies to nerve growth factor. Neuroscience. 1979;4(9):1391–1398. doi: 10.1016/0306-4522(79)90166-0. [DOI] [PubMed] [Google Scholar]
- Fenton E. L. Tissue culture assay of nerve growth factor and of the specific antiserum. Exp Cell Res. 1970 Mar;59(3):383–392. doi: 10.1016/0014-4827(70)90645-2. [DOI] [PubMed] [Google Scholar]
- Goedert M., Otten U., Schäfer T., Schwab M., Thoenen H. Immunosympathectomy: lack of evidence for a complement-mediated cytotoxic mechanism. Brain Res. 1980 Nov 17;201(2):399–409. doi: 10.1016/0006-8993(80)91043-4. [DOI] [PubMed] [Google Scholar]
- Goedert M., Otten U., Thoenen H. Biochemical effects of antibodies against nerve growth factor on developing and differentiated sympathetic ganglia. Brain Res. 1978 Jun 9;148(1):264–268. doi: 10.1016/0006-8993(78)90401-8. [DOI] [PubMed] [Google Scholar]
- Gorin P. D., Johnson E. M. Experimental autoimmune model of nerve growth factor deprivation: effects on developing peripheral sympathetic and sensory neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5382–5386. doi: 10.1073/pnas.76.10.5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMBURGER V. Experimental analysis of the dual origin of the trigeminal ganglion in the chick embryo. J Exp Zool. 1961 Nov;148:91–123. doi: 10.1002/jez.1401480202. [DOI] [PubMed] [Google Scholar]
- Hendry I. A. Cell division in the developing sympathetic nervous system. J Neurocytol. 1977 Jun;6(3):299–309. doi: 10.1007/BF01175193. [DOI] [PubMed] [Google Scholar]
- Hunt S. P., Kelly J. S., Emson P. C., Kimmel J. R., Miller R. J., Wu J. Y. An immunohistochemical study of neuronal populations containing neuropeptides or gamma-aminobutyrate within the superficial layers of the rat dorsal horn. Neuroscience. 1981;6(10):1883–1898. doi: 10.1016/0306-4522(81)90029-4. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- IRIUCHIJIMA J., ZOTTERMAN Y. Conduction rates of afferent fibres to the anterior tongue of the dog. Acta Physiol Scand. 1961 Feb-Mar;51:283–289. doi: 10.1111/j.1748-1716.1961.tb02138.x. [DOI] [PubMed] [Google Scholar]
- Jancsó G., Kiraly E., Jancsó-Gábor A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977 Dec 22;270(5639):741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Gorin P. D., Brandeis L. D., Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980 Nov 21;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Kanazawa I., Jessell T. Post mortem changes and regional distribution of substance P in the rat and mouse nervous system. Brain Res. 1976 Nov 26;117(2):362–367. doi: 10.1016/0006-8993(76)90748-4. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Nerve growth factor stimulates development of substance P in the embryonic spinal cord. Brain Res. 1981 Mar 9;208(1):135–145. doi: 10.1016/0006-8993(81)90626-0. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Similarities in development of substance P and somatostatin in peripheral sensory neurons: effects of capsaicin and nerve growth factor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4644–4647. doi: 10.1073/pnas.78.7.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson S. N., Biscoe T. J. Development of mouse dorsal root ganglia: an autoradiographic and quantitative study. J Neurocytol. 1979 Jun;8(3):265–274. doi: 10.1007/BF01236122. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. DESTRUCTION OF THE SYMPATHETIC GANGLIA IN MAMMALS BY AN ANTISERUM TO A NERVE-GROWTH PROTEIN. Proc Natl Acad Sci U S A. 1960 Mar;46(3):384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer N., Lembeck F., Goedert M., Otten U. Effects of antibodies against nerve growth factor on the postnatal development of substance P-containing sensory neurons. Neurosci Lett. 1982 Mar 17;29(1):47–52. doi: 10.1016/0304-3940(82)90362-7. [DOI] [PubMed] [Google Scholar]
- Miller M. S., Buck S. H., Sipes I. G., Yamamura H. I., Burks T. F. Regulation of substance P by nerve growth factor: disruption by capsaicin. Brain Res. 1982 Oct 28;250(1):193–196. doi: 10.1016/0006-8993(82)90969-6. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Goedert M., Hunt S. P., Bond A. The nature of the substance P-containing nerve fibres in taste papillae of the rat tongue. Neuroscience. 1982;7(12):3137–3151. doi: 10.1016/0306-4522(82)90236-6. [DOI] [PubMed] [Google Scholar]
- Nagy J. I., Hunt S. P. Fluoride-resistant acid phosphatase-containing neurones in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience. 1982 Jan;7(1):89–97. doi: 10.1016/0306-4522(82)90155-5. [DOI] [PubMed] [Google Scholar]
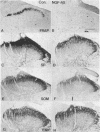
- Nagy J. I., Iversen L. L., Goedert M., Chapman D., Hunt S. P. Dose-dependent effects of capsaicin on primary sensory neurons in the neonatal rat. J Neurosci. 1983 Feb;3(2):399–406. doi: 10.1523/JNEUROSCI.03-02-00399.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten U., Goedert M., Mayer N., Lembeck F. Requirement of nerve growth factor for development of substance P-containing sensory neurones. Nature. 1980 Sep 11;287(5778):158–159. doi: 10.1038/287158a0. [DOI] [PubMed] [Google Scholar]
- Otten U., Lorez H. P., Businger F. Nerve growth factor antagonizes the neurotoxic action of capsaicin on primary sensory neurones. Nature. 1983 Feb 10;301(5900):515–517. doi: 10.1038/301515a0. [DOI] [PubMed] [Google Scholar]
- Otten U., Peck M., Businger F., Schlumpf M., Lichtensteiger W. Role of nerve growth factor in the development of peptide-containing primary afferent neurons. Monogr Neural Sci. 1983;9:29–35. doi: 10.1159/000406875. [DOI] [PubMed] [Google Scholar]
- Pearson J. Familial dysautonomia (a brief review). J Auton Nerv Syst. 1979 Dec;1(2):119–126. doi: 10.1016/0165-1838(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Ross M., Löfstrandh S., Gorin P. D., Johnson E. M., Schwartz J. P. Use of an experimental autoimmune model to define nerve growth factor dependency of peripheral and central substance P-containing neurons in the rat. J Neurosci. 1981 Nov;1(11):1304–1311. doi: 10.1523/JNEUROSCI.01-11-01304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
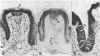
- Yankner B. A., Shooter E. M. The biology and mechanism of action of nerve growth factor. Annu Rev Biochem. 1982;51:845–868. doi: 10.1146/annurev.bi.51.070182.004213. [DOI] [PubMed] [Google Scholar]