Abstract
We sought to explore the imidazo[1,2-a]pyridin-3-amines for TLR7 (or 8)-modulatory activities. This chemotype, readily accessed via the Groebke-Blackburn-Bienaymé multi-component reaction, resulted in compounds that were TLR7/8-inactive, but exhibited bacteriostatic activity against Gram-positive bacteria, including methicillin-resistant S. aureus (MRSA). To investigate the mechanism of antibacterial activity of this new chemotype, a resistant strain of S. aureus was generated by serially passaging the organism in escalating doses of the most active analogue. A comparison of minimum inhibitory concentrations (MICs) of known bacteriostatic agents in wild-type and resistant strains indicates a novel mechanism of action. Structure-activity relationship studies have led to the identification of positions on the scaffold for additional structural modifications that should allow for the introduction of probes designed to examine cognate binding partners and molecular targets, while not significantly compromising antibacterial potency.
Keywords: Antibacterials, Imidazopyridines, Antibiotics, MRSA, Bacteriostatic, Multicomponent reaction, Groebke reaction
Introduction
The introduction of antibiotics into the therapeutic armamentarium in the early 20th century revolutionized the management of microbial infections. Once considered ‘wonder drugs’, antibiotics have perhaps become victims of their own success, and resistance to these drugs have almost invariably followed on the heels of their widespread use (and misuse). The incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections continues to increase alarmingly not only in hospital-associated settings (nosocomial infections), but in recent times, also in community settings in the United States, and throughout the globe. The increase in morbidity and mortality due to S. aureus infections [1] is a reflection of increased invasive procedures, indwelling devices, older age, and comorbidities, as well as the acquisition of resistance to commonly-used antimicrobial agents. Of particular concern is the emergence of multidrug-resistant strains of Gram-positive bacteria, with the loss of susceptibility to a wide range of reserve antibiotics such as vancomycin [2]. The need for the development of effective antibiotics is urgent, especially in the face of a diminishing pipeline of drugs for antimicrobial chemotherapy.
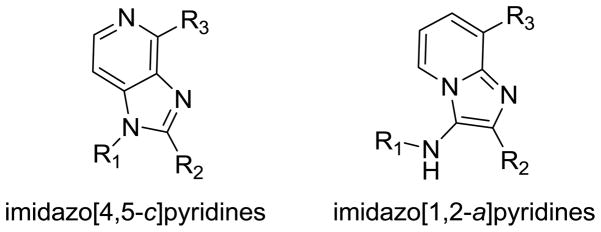
Our work in the recent past has focused on the discovery and development of vaccine adjuvants. Of particular interest to us are small molecule agonists of toll-like receptor-7 (TLR7). Given that the known small molecule agonistic chemotypes are limited to imidazoquinolines [3–5] and oxoadenines [6–10], we are actively exploring related structures. We have recently noted that 1H-imidazo[4,5-c]pyridin-4-amines are TLR7-agonistic (E. Yoo et al., manuscript submitted, Fig. 1). We therefore asked whether the structurally-related imidazo[1,2-a]pyridin-3-amines (Fig. 1) would possess TLR7-modulatory properties. This chemotype is readily amenable to a rapid elaboration of combinatorial libraries using the one-pot multi-component Groebke-Blackburn-Bienaymé reaction [11–13]. An initial test-library of 24 compounds proved to be neither agonistic nor antagonistic at TLR7. However, some of the initial compounds were found to have antibacterial activity against S. aureus with a clear indication of possible structure-activity relationships. Subsequent focused libraries of compounds were synthesized. These latter compounds, too, were not active in TLR7 screens, but displayed prominent bacteriostatic activity against several Gram-positive bacteria, including methicillin-resistant S. aureus (MRSA). To investigate the mechanism of antibacterial activity of this new chemotype, a resistant strain of S. aureus was generated by serially passaging the organism in escalating doses of the most active analogue. A comparison of minimum inhibitory concentrations (MICs) of known bacteriostatic agents in wild-type and resistant strains indicate a novel mechanism of action. These findings served as a point of departure for further exploration of SAR and mechanisms of bacteriostatic activity in this chemotype.
Fig. 1.
Structures of 1H-imidazo[4,5-c]pyridine and 1H-imidazo[1,2-a]pyridine scaffolds.
Results and Discussion
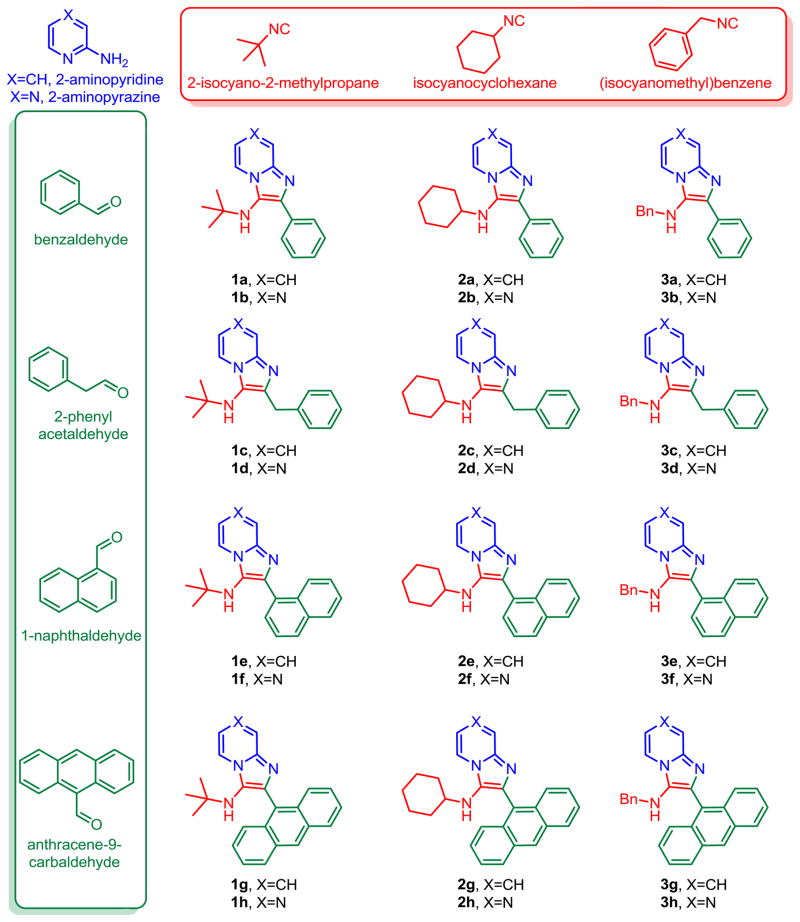
Our initial test-library comprising of twenty-four compounds was synthesized (Scheme 1) using two amidines (2-aminopyridine and 2-aminopyrazine), three isonitriles (2-isocyano-2-methylpropane, isocyanocyclohexane, (isocyanomethyl)benzene), and four aldehydes (benzaldehyde, 2-phenylacetaldehyde, 1-naphthaldehyde, anthracene-9-carbaldehyde). The syntheses of 1a–3h (Scheme 1) proceeded smoothly. All compounds were tested in TLR7 agonism and antagonism assays using specific reporter gene-based cellular assays as described earlier [14–16]; to our disappointment, none of the compounds displayed any activity in these assays up to concentrations of 250 μM (data not shown). The assay plates were stored in the autoclave room at room temperature prior to disposal, and, quite by accident, we observed a dose-dependent inhibition of a bacterial contaminant in such plates. We therefore decided to examine the antibacterial activities of these compounds in antibacterial screens (E. coli ATCC 9637 and S. aureus ATCC 13709) routinely employed in our laboratory [17,18]. Four compounds were identified to be inhibitory to S. aureus ATCC 13709 but not E. coli ATCC 9637. Maximal antibacterial activity resided in compounds derived from 2-aminopyridine, bearing either a bulky N-tert-butyl or cyclohexyl groups at C3, and a large aromatic pendant group (naphthyl or anthracenyl) at C2 (1e, 1g, 2e, 2g, Scheme 1, Table 1). The MICs of both 1g and 2g for S. aureus ATCC 13709 and MRSA ATCC 33591 were 3.91 μg/mL, (Table 1), and 7.81 μg/mL for coagulase-negative S. epidermidis ATCC 35983 (Supplementary data). We examined the MIC values of representative leads (1e, 1g and 2g) against a variety of other Gram-positive organisms (E. fecalis, clinical isolates of S. pneumoniae, and S. pyogenes); significant MICs (<10 μg/mL) were observed in all of the leads only against S. pyogenes, indicating a narrow spectrum of activity (data not shown).
Scheme 1.
Synthesis of a library of compounds using Groebke multicomponent reaction using 2 different amidines, 3 different isonitriles and 4 different aldehydes.
Reagents and conditions: MW, 400W, 110 °C, 20 min, HCl/dioxane, CH3CN.
Table 1.
Minimum Inhibitory Concentration values (μg/mL) of compounds.a
| Structure | Compound Number | MIC (μg/mL) | |
|---|---|---|---|
| S. aureus | MRSA | ||
|
1a | ND | NT |
|
1b | ND | NT |
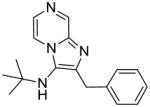
|
1c | ND | NT |
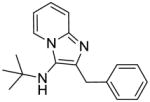
|
1d | ND | NT |
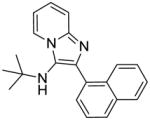
|
1e | 62.5 | 31.25 |
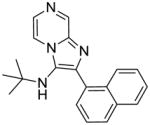
|
1f | ND | NT |
|
1g | 3.91 | 3.91 |
|
1h | ND | NT |
|
2a | ND | NT |
|
2b | ND | NT |
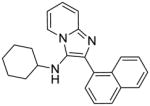
|
2c | ND | NT |
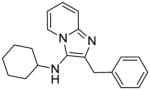
|
2d | ND | NT |
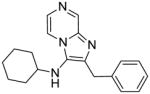
|
2e | 7.81 | 15.62 |
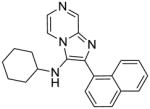
|
2f | ND | NT |
|
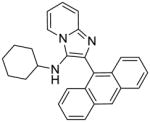
2g | 3.91 | 3.91 |
|
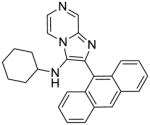
2h | 7.81 | NT |
|
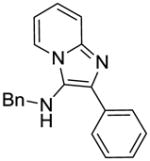
3a | ND | NT |
|
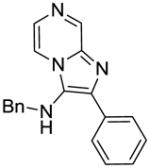
3b | ND | NT |
|
3c | ND | NT |
|
3d | ND | NT |
|
3e | 15.62 | NT |
|
3f | ND | NT |
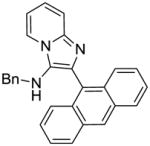
|
3g | ND | NT |
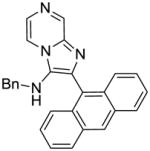
|
3h | ND | NT |
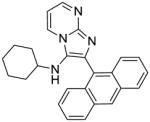
|
4a | 15.62 | NT |
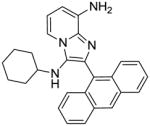
|
4b | 15.62 | NT |
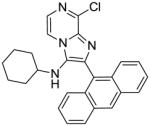
|
4c | ND | NT |
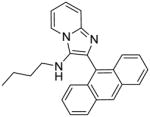
|
5a | 3.91 | 7.81 |
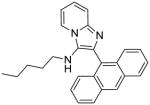
|
5b | 3.91 | 7.81 |
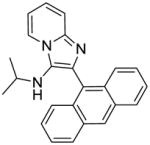
|
5c | 15.62 | 15.62 |
|
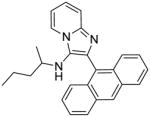
5d | 7.81 | 3.91 |
|
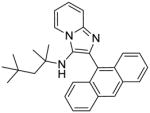
5e | 3.91 | 3.91 |
|
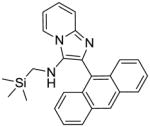
5f | 15.62 | NT |
|
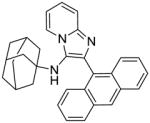
5g | 15.62 | NT |
|
5h | ND | NT |
|
5i | ND | NT |
|
6 | ND | NT |
|
7a | 7.81 | NT |
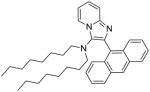
|
7b | 31.25 | NT |
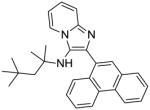
|
7c | 62.5 | NT |
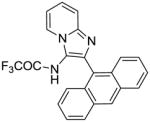
|
8a | ND | NT |
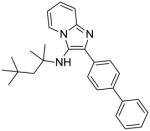
|
8b | 7.81 | NT |
|
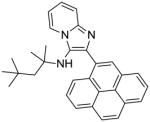
8c | 15.62 | NT |
|
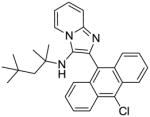
9 | 7.81 | NT |
|
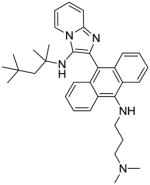
10 | 3.91 | NT |
|
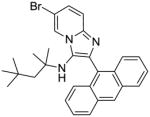
11 | 15.62 | NT |
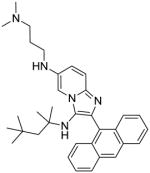
|
12 | 3.91 | NT |
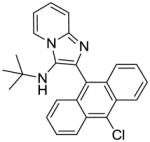
|
13 | 7.81 | NT |
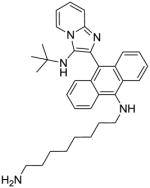
|
14 | 7.81 | 15.62 |
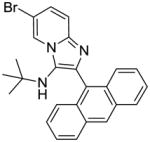
|
15 | 15.62 | NT |
|
16 | 3.91 | 7.81 |
ND denotes no activity detected at 100 μg/mL and NT denotes not tested.
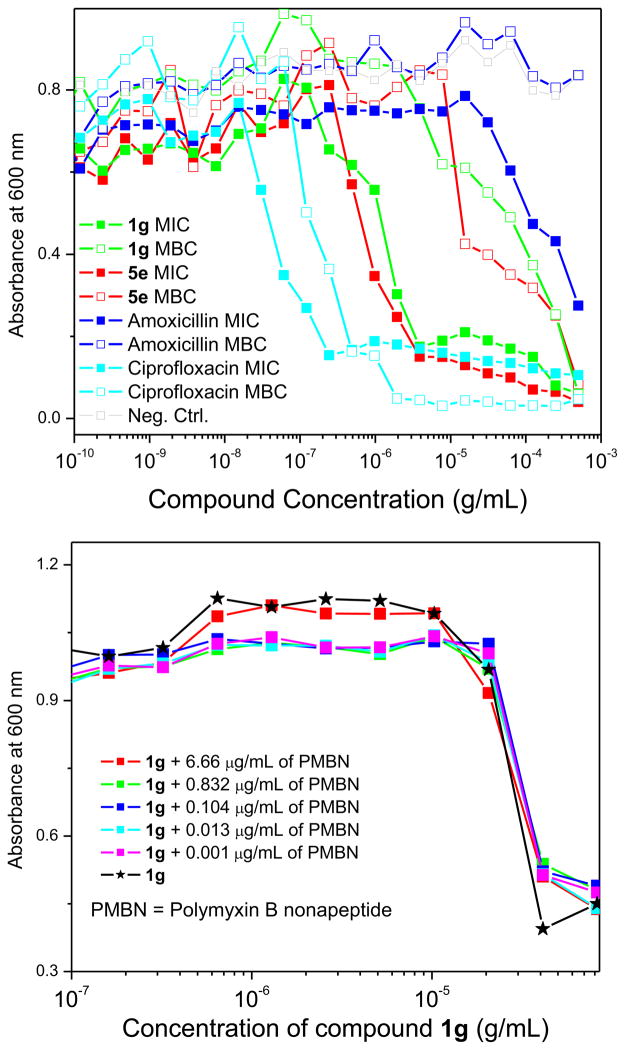
The active compounds listed above were all determined to be bacteriostatic rather than bactericidal by conventional microplate MBC assays (Fig. 2) [19]. The outer membrane of Gram-negative bacteria serves as a permeability barrier for hydrophobic antimicrobials [20,21], and we wondered if the lack of activity of these compounds against Gram-negative bacteria could be attributable to its bulky, nonpolar nature. We therefore performed additional assays using polymyxin B nonapeptide [22–24], which is commonly used to permeabilize the outer-membranes of Gram-negative bacteria, rendering the organisms susceptible to otherwise impermeable antimicrobials [17]; however, the imidazopyridines did not exert any significant antibacterial effect even at high concentrations of polymyxin B nonapeptide (Fig. 2), confirming that the antibacterial spectrum of these compounds is specific to Gram-positive organisms.
Fig. 2.
Top: Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) data of active 1H-imidazo[1,2-a]pyridine compounds. MICs were determined using MRSA (ATCC strain 33591) by broth microdilution method in Mueller-Hinton broth (MHB). Ciprofloxacin and amoxicillin were used as positive and negative controls, respectively. For MBC determinations, 0.5 μL of each of the 384 wells in the parent MIC plate was diluted into 80 μL of fresh MHB. Bottom: Lack of sensitization in E. coli (ATCC strain 9637) by polymyxin B nonapeptide to 1g.
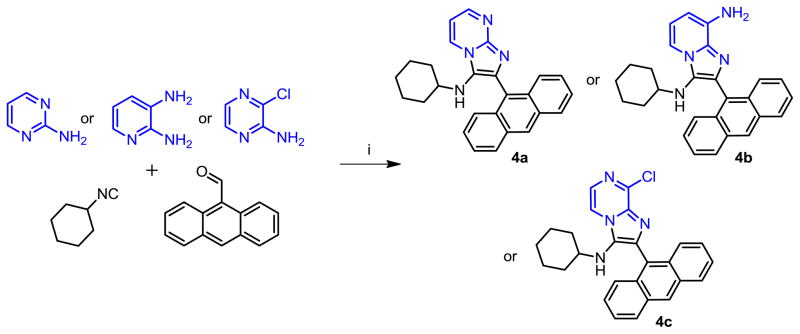
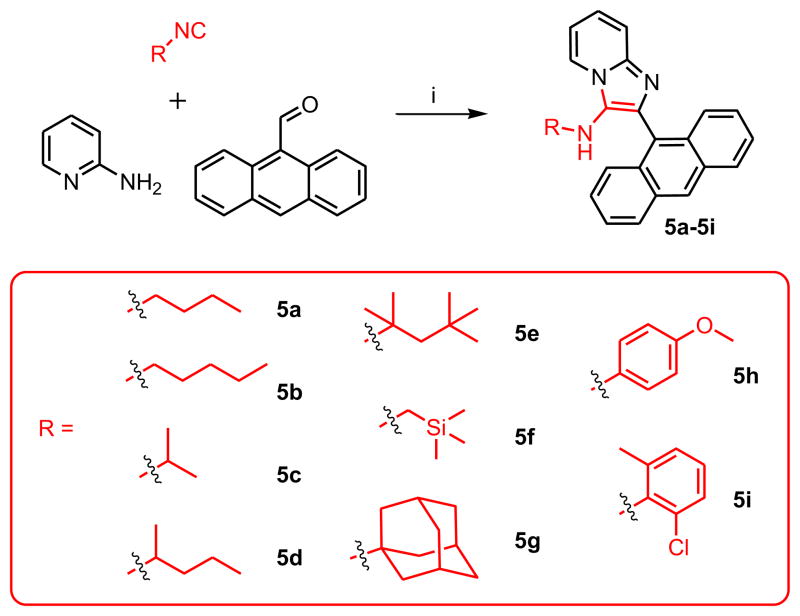
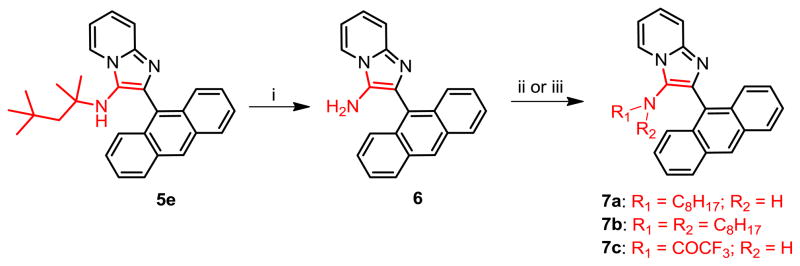
Given that 1g and 2g demonstrated identical potencies, we arbitrarily selected isocyanocyclohexane and anthracene-9-carbaldehyde as the invariant components, and varied the amidine component; we chose 2-aminopyrimidine, 2,3-diaminopyridine, and 2-amino-3-chloropyrazine (Scheme 2). The 2-amino-3-chloropyrazine-derived compound 4c was inactive, while 4a and 4b exhibited lower activities than 1g (Table 1). Next, we varied the isonitrile component. We explored nine different isonitriles (Scheme 3) chosen to include linear aliphatic (as in 5a and 5b), branched aliphatic (5c–5e), silyl-containing (5f), adamantyl (5g), and aromatic substituents (5h, 5i). We observed that the N-2,4,4-trimethylpentan-2-yl group of 5e, could be dealkylated under strongly acidic conditions as has been reported recently [25], affording the possibility of introducing alkyl or acyl substituents (represented by 7a–7c, Scheme 4) that were not accessible through commercially available isonitriles. Optimal activity profiles appeared to correspond to compounds with branched-chain substituents (5d, 5e), while compounds with aromatic substituents (5h, 5i) were bereft of antibacterial activity (Table 1).
Scheme 2.
Groebke reaction using different amidines.
Reagents and conditions: i. MW, 400W, 110 °C, 30 min, HCl/dioxane, CH3CN.
Scheme 3.
Groebke reaction using various isonitriles.
Reagents and conditions: i. MW, 400W, 110 °C, 20 min, HCl/dioxane, CH3CN.
Scheme 4.
Derivatization of the C3-amine.
Reagents and conditions: i. HCl/dioxane. For compounds 7a and 7b: ii. C7H15CHO, CH3COOH, NaCNBH3, MeOH. For compound 7c: iii. (CF3CO)2O, CH2Cl2.
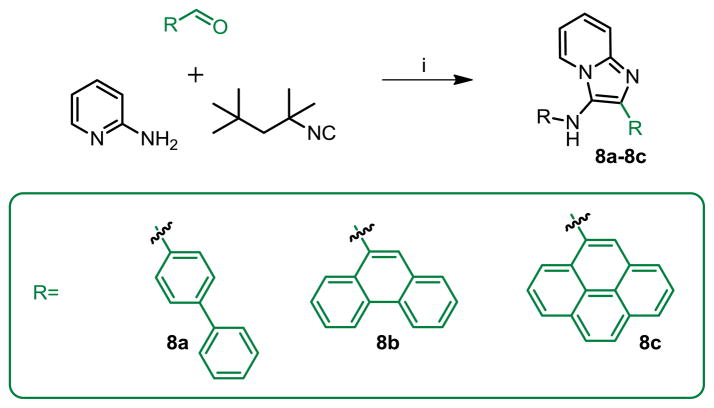
Noting that bulky, aldehyde-derived aromatic substituents at C2 corresponded to maximal activity (exemplified by the anthracenyl group in 1g, 2g, 5a–5g), it was of interest to explore other similar functional groups. Compounds with biphenyl (8a), phenanthrenyl (8b), and pyrenyl (8c) substituents were therefore synthesized and evaluated (Scheme 5). Compounds 8b and 8c exhibited lower activity than the anthracenyl-bearing compound, while the biphenyl compound 8a was inactive, pointing to the necessity of a large polycyclic, aromatic group at C2; the higher activity of 5e with its bulky anthracenyl group would appear to suggest that the polycyclic group of a particular topology is a determinant of activity.
Scheme 5.
Groebke reaction using various aldehydes.
Reagents and conditions: i. MW, 400W, 110 °C, 20 min, HCl/dioxane, CH3CN.
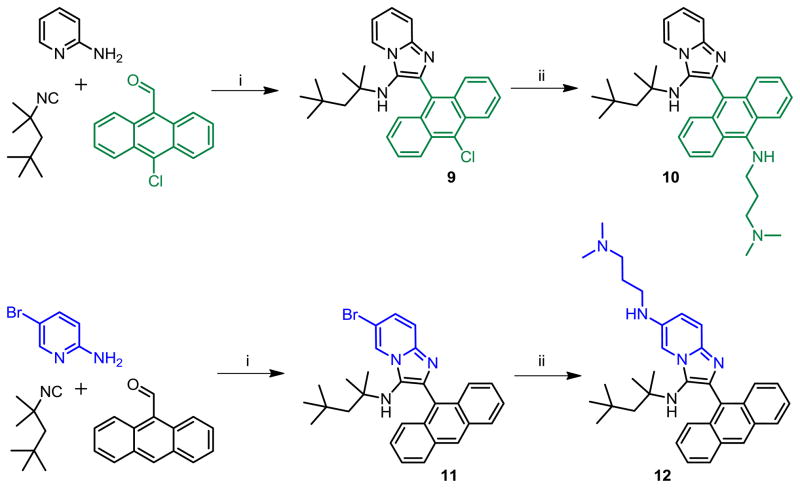
The compound that showed maximal activity thus far was the very lipophilic 5e, and it was desirable to explore more polar analogues for future evaluation in in vivo models. We therefore attempted to introduce N,N-dimethylaminopropylamino groups on both the amidine- and aldehyde-derived portions of 5e. This was achieved in a straightforward manner using appropriate halo-substituted components, followed by Buchwald-Hartwig coupling as depicted in Scheme 6. The antibacterial activities of 10 and 12 were found to be identical to that of 5e (Table 1).
Scheme 6.
Syntheses of polar derivatives of 5e.
Reagents and conditions: i. MW, 400W, 110 °C, 30 min, HCl/dioxane, CH3CN. ii. K(O-tBu), Pd2(dba)3, DavePhos, NH2-(CH2)3-N(CH3)2, dioxane, 80°C.
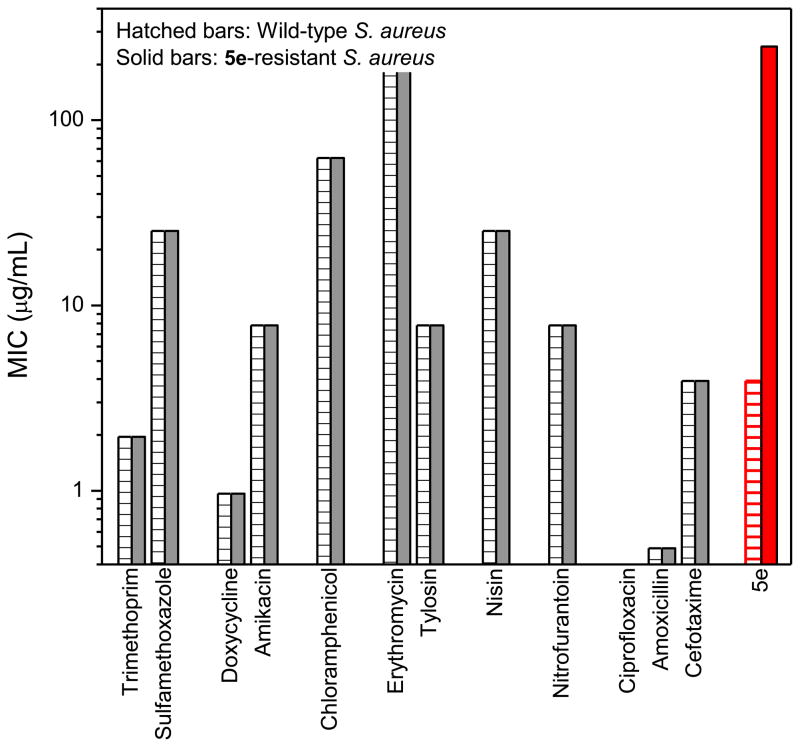
Although the lead compounds of this chemotype exhibit narrow-spectrum bacteriostatic activity against Gram-positive organisms, the substantial potency against MRSA warranted an attempt at understanding the mechanism of action. Our preliminary studies have been to examine the antibiograms of wild-type and 5e-resistant S. aureus organisms, comparing a variety of antimicrobials with known mechanisms of action. 5e-resistant S. aureus organisms were generated by exposing the bacterium to escalating doses of the compound. Within about 10 serial passages, organisms that withstood 5e up to concentrations of 100 μg/mL emerged. The MICs of various classes of bacteriostatic and bactericidal antibiotics were found to be identical within experimental error for both the wild-type and 5e-resistant S. aureus, suggesting that the molecular target of 5e and related compounds were distinct and unique (Fig. 3).
Fig. 3.
Comparison of antimicrobial susceptibility of wild-type S. aureus ATCC 13709 (hatched bars) and 5e-resistant organism (solid bars) to a range of bacteriostatic antibiotics with known mechanisms of action, and 5e. Bacteriostatics include trimethoprim and sulfamethoxazole (dihydrofolate reductase pathway), doxycycline and amikacin (30S ribosomal subunit), chloramphenicol (23S ribosomal subunit), erythromycin and tylosin (50S ribosomal subunit), nisin (lipid II), nitrofurantoin (bacterial DNA); also included were the bactericidal controls, ciprofloxacin, amoxicillin, and cefotaxime.
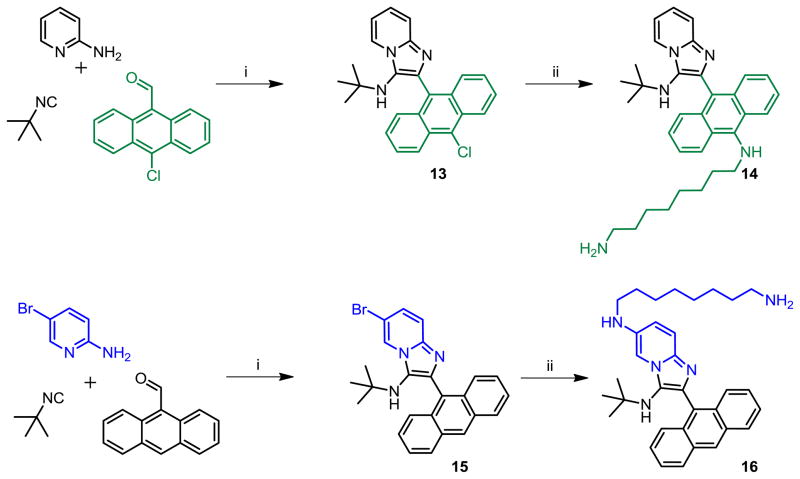
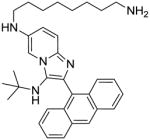
We noticed, however, that upon prolonged storage (~ 2 weeks), aqueous solutions of hydrochloride salts of both 10 and 12 were degrading gradually, giving rise to N-dealkylated products as was discussed earlier (LC-MS data in Supporting Information, Page S136). Furthermore, we were desirous of introducing a functional group that would permit facile coupling of probes such as fluorophores or biotin in order to identify the possible molecular target(s) of 5e. We elected to replace the N-2,4,4-trimethylpentan-2-yl group with the more stable tert-butyl group, and the N,N-dimethylaminopropylamino group with a 1,8-diaminooctane, using the Buchwald-Hartwig coupling strategy employed earlier (Scheme 7). The introduction of the primary amine-bearing 1,8-diaminooctanyl group on either the anthracenyl or pyridinyl portions of the molecule do not result in significant attenuation of antibacterial activity relative to 1g (Table 1), allowing the possibility of exploring the binding partners for these compounds.
Scheme 7.
Syntheses of 1g derivatives bearing terminal primary amines.
Reagents and conditions: i. MW, 400W, 110 °C, 30 min, HCl/dioxane, CH3CN. ii. K(O-tBu), Pd2(dba)3, DavePhos, NH2-(CH2)8-NHBoc, dioxane, 80°C; (b) 4M HCl/dioxane.
In conclusion, our attempts at exploring imidazo[1,2-a]pyridin-3-amines for TLR7 (or 8)-modulatory activities have unexpectedly led to the identification of a novel chemotype with substantial bacteriostatic activity against Gram-positive bacteria, including methicillin-resistant S. aureus (MRSA). We note that antibacterial activities of the imidazo[1,2-a]pyridines have been reported earlier; the compounds reported herein show narrow-spectrum selectivity, only against Gram-positive organisms [26,27]. Our preliminary results suggest that the mechanism of action may be distinct from known bacteriostatics. Structure-activity relationship studies have led to the identification of positions on the scaffold for additional structural modifications that should allow for the introduction of probes designed to examine cognate binding partners and molecular targets, while not significantly compromising antibacterial potency.
Experimental Section
Chemistry
All of the solvents and reagents used were obtained commercially and used as such unless noted otherwise. Moisture- or air-sensitive reactions were conducted under nitrogen atmosphere in oven-dried (120 °C) glass apparatus. The solvents were removed under reduced pressure using standard rotary evaporators. Flash column chromatography was carried out using RediSep Rf ‘Gold’ high performance silica columns on CombiFlash Rf instrument unless otherwise mentioned, while thin-layer chromatography was carried out on silica gel CCM pre-coated aluminum sheets. Microwave reactions were done in Synthos 3000 instrument (Anton Paar). IR spectra were recorded on Shimadzu 8400 series FTIR instrument and values are reported in cm−1. Purity for all final compounds was confirmed to be greater than 95% by LC-MS using a Zorbax Eclipse Plus 4.6 mm × 150 mm, 5 μm analytical reverse phase C18 column with H2O-isopropanol or H2O-CH3CN gradients and an Agilent ESI-TOF mass spectrometer (mass accuracy of 3 ppm) operating in the positive ion acquisition mode. Unless otherwise mentioned, the compounds synthesized were obtained as yellow solids.
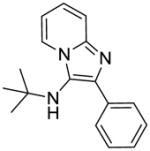
Synthesis of Compound 1a: N-(tert-butyl)-2-phenylimidazo[1,2-a]pyridin-3-amine
To the solution of 2-aminopyridine (24 mg, 0.25 mmol) in anhydrous acetonitrile, were added benzaldehyde (28 μL, 0.28 mmol), a catalytic amount of 4N HCl/dioxane (10 μL) and tert-butyl isonitrile (27 μL, 0.24 mmol). The reaction mixture was then heated under microwave conditions (400 W, 110 °C, 20 min). After the reaction mixture was cooled to room temperature, solvent was removed and the residue was purified using column chromatography to obtain the compound 1a as white solid (44 mg, 69%). 1H NMR (400 MHz, CDCl3) δ 8.23 (dt, J = 6.9, 1.2 Hz, 1H), 7.90 (dt, J = 8.1, 1.6 Hz, 2H), 7.55 (dt, J = 9.0, 1.0 Hz, 1H), 7.43 (t, J = 7.6 Hz, 2H), 7.31 (t, J = 7.4 Hz, 1H), 7.13 (ddd, J = 9.0, 6.6, 1.3 Hz, 1H), 6.77 (td, J = 6.8, 1.1 Hz, 1H), 3.12 (s, 1H), 1.04 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 135.42, 128.43, 128.32, 127.52, 124.17, 123.64, 117.48, 111.46, 101.91, 56.59, 30.43. MS (ESI) calculated for C17H19N3, m/z 265.16, found 266.17 (M + H)+.
Compounds 1b-1f, 2a-2f and 3a-3f were synthesized similarly as compound 1a
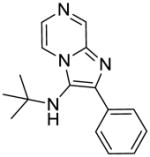
1b: N-(tert-butyl)-2-phenylimidazo[1,2-a]pyrazin-3-amine (45 mg, 62%)
1H NMR (400 MHz, CDCl3) δ 9.00 (d, J = 1.4 Hz, 1H), 8.14 (dd, J = 4.6, 1.5 Hz, 1H), 7.91 (dd, J = 8.3, 1.3 Hz, 2H), 7.86 (d, J = 4.6 Hz, 1H), 7.46 (t, J = 7.5 Hz, 2H), 7.37 (t, J = 7.4 Hz, 1H), 3.19 (s, 1H), 1.05 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 143.54, 142.40, 137.43, 134.38, 129.04, 128.66, 128.38, 128.33, 125.17, 116.49, 100.13, 57.11, 30.45. MS (ESI) calculated for C16H18N4, m/z 266.15, found 267.16 (M + H)+.
1c: 2-benzyl-N-(tert-butyl)imidazo[1,2-a]pyridin-3-amine (41 mg, 61%)
1H NMR (400 MHz, CDCl3) δ 8.16 (dt, J = 6.9, 1.1 Hz, 1H), 7.47 (dt, J = 9.0, 1.0 Hz, 1H), 7.28 – 7.23 (m, 4H), 7.21 – 7.14 (m, 1H), 7.08 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.72 (td, J = 6.8, 1.1 Hz, 1H), 4.20 (s, 2H), 2.57 (s, 1H), 1.18 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 139.83, 139.62, 128.68, 128.48, 126.06, 123.41, 123.35, 117.06, 110.93, 101.78, 98.20, 55.71, 34.36, 30.45. MS (ESI) calculated for C18H21N3, m/z 279.17, found 280.19 (M + H)+.
1d: 2-benzyl-N-(tert-butyl)imidazo[1,2-a]pyrazin-3-amine (31 mg, 43%)
1H NMR (400 MHz, CDCl3) δ 8.92 (d, J = 1.4 Hz, 1H), 8.06 (dd, J = 4.6, 1.5 Hz, 1H), 7.81 (d, J = 4.6 Hz, 1H), 7.32 – 7.27 (m, 2H), 7.25 – 7.18 (m, 3H), 4.24 (s, 2H), 2.62 (s, 1H), 1.20 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 142.95, 142.78, 138.88, 137.32, 128.72, 128.67, 126.45, 116.28, 56.29, 34.39, 30.48. MS (ESI) calculated for C17H20N4, m/z 280.17, found 281.18 (M + H)+.
1e: N-(tert-butyl)-2-(naphthalen-1-yl)imidazo[1,2-a]pyridin-3-amine (56 mg, 74%)
1H NMR (400 MHz, CDCl3) δ 8.34 (dt, J = 6.9, 1.1 Hz, 1H), 8.02 – 7.85 (m, 3H), 7.68 – 7.44 (m, 5H), 7.19 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.83 (td, J = 6.8, 1.1 Hz, 1H), 3.00 (s, 1H), 0.78 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 133.83, 132.94, 131.98, 128.48, 128.25, 128.15, 126.57, 125.73, 125.70, 125.34, 123.97, 123.64, 117.45, 111.37, 101.79, 100.00, 55.95, 29.76. MS (ESI) calculated for C21H21N3, m/z 315.17, found 316.19 (M + H)+.
1f: N-(tert-butyl)-2-(naphthalen-1-yl)imidazo[1,2-a]pyrazin-3-amine (29 mg, 38%)
1H NMR (400 MHz, CDCl3) δ 9.07 (d, J = 1.4 Hz, 1H), 8.24 (dd, J = 4.6, 1.5 Hz, 1H), 7.97 – 7.87 (m, 4H), 7.67 – 7.42 (m, 4H), 3.08 (s, 1H), 0.80 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 143.57, 141.83, 133.85, 131.76, 131.71, 128.98, 128.95, 128.67, 128.27, 126.94, 126.02, 125.31, 125.19, 116.54, 105.36, 98.21, 56.51, 29.81. MS (ESI) calculated for C20H20N4, m/z 316.17, found 317.17 (M + H)+.
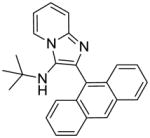
1g: 2-(anthracen-9-yl)-N-(tert-butyl)imidazo[1,2-a]pyridin-3-amine (55 mg, 63%)
IR (CHCl3) νmax (cm−1): 2970, 1500, 1473, 1342, 1311. 1H NMR (400 MHz, CDCl3) δ 8.52 (s, 1H), 8.40 (dt, J = 6.9, 1.2 Hz, 1H), 8.09 – 8.02 (m, 2H), 7.93 – 7.86 (m, 2H), 7.66 (dt, J = 9.0, 1.1 Hz, 1H), 7.51 – 7.38 (m, 4H), 7.29 – 7.20 (m, 1H), 6.89 (td, J = 6.8, 1.1 Hz, 1H), 2.69 (s, 1H), 0.64 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 142.71, 131.45, 130.94, 128.75, 127.60, 126.80, 126.34, 126.19, 125.06, 124.00, 123.62, 117.57, 111.40, 101.78, 97.01, 55.59, 29.72. MS (ESI) calculated for C25H23N3, m/z 365.19, found 366.20 (M + H)+.
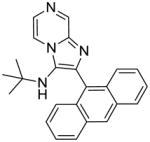
1h: 2-(anthracen-9-yl)-N-(tert-butyl)imidazo[1,2-a]pyrazin-3-amine (50 mg, 57%)
1H NMR (400 MHz, CDCl3) δ 9.14 (d, J = 1.5 Hz, 1H), 8.57 (s, 1H), 8.31 (dd, J = 4.6, 1.5 Hz, 1H), 8.12 – 8.05 (m, 2H), 7.98 (d, J = 4.6 Hz, 1H), 7.83 – 7.75 (m, 2H), 7.53 – 7.42 (m, 4H), 2.75 (s, 1H), 0.65 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 143.65, 139.88, 138.08, 131.37, 130.86, 129.05, 128.96, 128.35, 127.48, 126.81, 125.52, 125.24, 116.55, 101.78, 56.14, 29.77. MS (ESI) calculated for C24H22N4, m/z 366.18, found 367.19 (M + H)+ and 389.18 (M + Na)+.
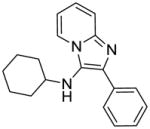
2a: N-cyclohexyl-2-phenylimidazo[1,2-a]pyridin-3-amine. White solid (51 mg, 73%)
1H NMR (500 MHz, CDCl3) δ 8.16 (d, J = 6.8 Hz, 1H), 8.05 (dd, J = 8.3, 1.2 Hz, 2H), 7.62 (d, J = 9.0 Hz, 1H), 7.45 (t, J = 7.8 Hz, 2H), 7.32 (t, J = 7.4 Hz, 1H), 7.17 (ddd, J = 8.9, 6.7, 1.2 Hz, 1H), 6.82 (td, J = 6.8, 0.9 Hz, 1H), 3.32 (s, 1H), 3.07 – 2.85 (m, 1H), 1.81 (d, J = 13.1 Hz, 2H), 1.68 (dd, J = 9.1, 3.6 Hz, 2H), 1.60 – 1.54 (m, 1H), 1.28 – 1.12 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 141.02, 135.43, 133.45, 128.74, 127.74, 127.20, 125.17, 125.04, 123.07, 116.94, 112.30, 57.02, 34.27, 25.81, 24.94. MS (ESI) calculated for C19H21N3, m/z 291.17, found 292.18 (M + H)+.
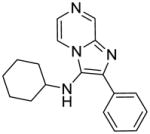
2b: N-cyclohexyl-2-phenylimidazo[1,2-a]pyrazin-3-amine (25 mg, 36%)
1H NMR (500 MHz, CDCl3) δ 8.99 (d, J = 1.4 Hz, 1H), 8.01 (ddd, J = 4.6, 3.2, 1.8 Hz, 3H), 7.85 (d, J = 4.6 Hz, 1H), 7.48 (t, J = 7.7 Hz, 2H), 7.38 (t, J = 7.4 Hz, 1H), 3.26 (s, 1H), 3.00 (s, 1H), 1.82 (dd, J = 6.6, 5.4 Hz, 2H), 1.70 (dd, J = 9.3, 3.3 Hz, 2H), 1.62 – 1.55 (m, 1H), 1.34 – 1.07 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 143.37, 139.08, 136.82, 133.62, 129.01, 128.90, 128.30, 127.42, 126.70, 115.73, 57.02, 34.41, 25.69, 24.91. MS (ESI) calculated for C18H20N4, m/z 292.17, found 293.18 (M + H)+.
2c: 2-benzyl-N-cyclohexylimidazo[1,2-a]pyridin-3-amine (51 mg, 70%)
1H NMR (500 MHz, CDCl3) δ 8.04 (dt, J = 6.8, 1.1 Hz, 1H), 7.52 – 7.46 (m, 1H), 7.31 – 7.26 (m, 4H), 7.19 (qd, J = 5.3, 2.7 Hz, 1H), 7.10 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.76 (td, J = 6.8, 1.1 Hz, 1H), 4.16 (s, 2H), 2.77 (s, 1H), 2.60 (s, 1H), 1.79 – 1.67 (m, 4H), 1.59 (s, 1H), 1.32 – 1.04 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 141.11, 139.62, 137.49, 128.68, 128.53, 126.20, 125.44, 123.43, 122.59, 116.93, 111.41, 57.32, 34.17, 34.11, 25.74, 24.85. MS (ESI) calculated for C20H23N3, m/z 305.19, found 306.20 (M + H)+.
2d: 2-benzyl-N-cyclohexylimidazo[1,2-a]pyrazin-3-amine (37 mg, 50%)
1H NMR (500 MHz, CDCl3) δ 8.92 (d, J = 1.4 Hz, 1H), 7.93 (dd, J = 4.6, 1.5 Hz, 1H), 7.81 (d, J = 4.6 Hz, 1H), 7.33 – 7.25 (m, 4H), 7.22 (ddd, J = 6.2, 3.3, 1.6 Hz, 1H), 4.20 (s, 2H), 2.81 (s, 1H), 2.68 (s, 1H), 1.84 – 1.65 (m, 4H), 1.60 (d, J = 6.1 Hz, 1H), 1.24 – 0.95 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 141.19, 139.22, 137.30, 135.01, 127.27, 127.20, 127.17, 125.71, 125.04, 114.00, 55.65, 32.84, 32.76, 24.09, 23.31. MS (ESI) calculated for C19H22N4, m/z 306.18, found 307.20 (M + H)+.
2e: N-cyclohexyl-2-(naphthalen-1-yl)imidazo[1,2-a]pyridin-3-amine (24 mg, 29%)
1H NMR (500 MHz, CDCl3) δ 8.20 (d, J = 6.8 Hz, 1H), 7.95 – 7.88 (m, 3H), 7.72 – 7.64 (m, 2H), 7.59 – 7.54 (m, 1H), 7.53 – 7.45 (m, 2H), 7.25 – 7.20 (m, 1H), 6.90 (t, J = 6.8 Hz, 1H), 3.15 (s, 1H), 2.63 (t, J = 9.6 Hz, 1H), 1.58 (d, J = 12.6 Hz, 2H), 1.50 – 1.36 (m, 3H), 1.04 – 0.77 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 141.06, 133.76, 131.95, 128.57, 128.49, 128.15, 126.76, 126.65, 125.86, 125.50, 125.36, 124.30, 122.89, 117.21, 112.14, 56.32, 33.70, 25.47, 24.51. MS (ESI) calculated for C23H23N3, m/z 341.19, found 342.20 (M + H)+.
2f: N-cyclohexyl-2-(naphthalen-1-yl)imidazo[1,2-a]pyrazin-3-amine (50 mg, 61%)
1H NMR (500 MHz, CDCl3) δ 9.05 (d, J = 1.5 Hz, 1H), 8.05 (dd, J = 4.6, 1.5 Hz, 1H), 7.96 – 7.93 (m, 2H), 7.92 – 7.89 (m, 2H), 7.61 (ddd, J = 15.2, 7.5, 4.2 Hz, 4H), 7.55 – 7.48 (m, 1H), 3.27 (s, 1H), 2.73 (s, 1H), 1.61 (dd, J = 9.5, 3.2 Hz, 2H), 1.44 (ddd, J = 14.5, 11.6, 4.6 Hz, 3H), 1.07 – 0.82 (m, 5H). 13C NMR (126 MHz, CDCl3) δ 143.40, 138.38, 136.73, 133.80, 131.90, 130.80, 129.03, 128.84, 128.58, 128.53, 128.07, 126.87, 126.08, 125.30, 125.27, 115.67, 56.12, 33.85, 25.35, 24.50. MS (ESI) calculated for C22H22N4, m/z 342.18, found 343.19 (M + H)+ and 365.17 (M + Na)+.
2g: 2-(anthracen-9-yl)-N-cyclohexylimidazo[1,2-a]pyridin-3-amine (83 mg, 88%)
1H NMR (500 MHz, CDCl3) δ 8.54 (s, 1H), 8.23 (dt, J = 6.8, 1.2 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.83 (dd, J = 8.7, 0.8 Hz, 2H), 7.66 (dt, J = 9.0, 1.0 Hz, 1H), 7.49 – 7.44 (m, 2H), 7.40 (ddd, J = 8.6, 6.5, 1.3 Hz, 2H), 7.23 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.90 (td, J = 6.8, 1.1 Hz, 1H), 2.80 (d, J = 7.4 Hz, 1H), 2.62 – 2.49 (m, 1H), 1.47 (d, J = 12.6 Hz, 2H), 1.35 – 1.24 (m, 3H), 0.89 – 0.77 (m, 3H), 0.68 (dt, J = 12.8, 6.6 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 142.02, 134.01, 131.38, 131.20, 128.59, 128.34, 128.10, 127.64, 126.25, 126.12, 125.10, 123.40, 122.81, 117.72, 111.60, 56.18, 33.62, 25.39, 24.38. MS (ESI) calculated for C27H25N3, m/z 391.20, found 392.22 (M + H)+.
2h: 2-(anthracen-9-yl)-N-cyclohexylimidazo[1,2-a]pyrazin-3-amine (17 mg, 18%)
1H NMR (500 MHz, CDCl3) δ 9.12 (d, J = 1.4 Hz, 1H), 8.59 (s, 1H), 8.13 – 8.06 (m, 3H), 7.97 (d, J = 4.6 Hz, 1H), 7.72 (dd, J = 8.7, 0.6 Hz, 2H), 7.46 (dddd, J = 10.0, 7.8, 6.5, 1.1 Hz, 4H), 2.91 (d, J = 6.2 Hz, 1H), 2.62 (s, 1H), 1.48 (d, J = 12.6 Hz, 2H), 1.29 (dd, J = 24.7, 8.3 Hz, 3H), 0.91 – 0.76 (m, 3H), 0.76 – 0.63 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 142.63, 136.28, 135.36, 130.29, 130.12, 128.74, 128.01, 127.76, 127.36, 125.75, 125.54, 124.62, 124.25, 114.68, 54.95, 32.71, 24.20, 23.32. MS (ESI) calculated for C26H24N4, m/z 392.20, found 393.20 (M + H)+.
3a: N-benzyl-2-phenylimidazo[1,2-a]pyridin-3-amine
White solid (62 mg, 86%). 1H NMR (500 MHz, CDCl3) δ 7.98 (ddt, J = 3.7, 3.0, 1.6 Hz, 3H), 7.57 (dt, J = 9.0, 1.0 Hz, 1H), 7.45 (t, J = 7.7 Hz, 2H), 7.39 – 7.26 (m, 6H), 7.13 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.74 (td, J = 6.8, 1.1 Hz, 1H), 4.20 (d, J = 6.1 Hz, 2H), 3.52 (t, J = 6.0 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 141.57, 139.06, 136.04, 134.13, 128.84, 128.30, 127.82, 127.65, 127.16, 125.77, 124.32, 122.50, 117.52, 111.94, 52.57. MS (ESI) calculated for C20H17N3, m/z 299.14, found 300.15 (M + H)+.
3b: N-benzyl-2-phenylimidazo[1,2-a]pyrazin-3-amine (45 mg, 62%)
1H NMR (500 MHz, CDCl3) δ 8.98 (d, J = 1.3 Hz, 1H), 7.94 (dt, J = 8.1, 1.6 Hz, 2H), 7.82 (dd, J = 4.6, 1.4 Hz, 1H), 7.77 (d, J = 4.6 Hz, 1H), 7.47 (t, J = 7.6 Hz, 2H), 7.39 (t, J = 7.4 Hz, 1H), 7.34 – 7.27 (m, 5H), 4.23 (d, J = 2.4 Hz, 2H), 3.66 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 143.46, 138.76, 138.56, 136.79, 133.34, 129.06, 129.03, 128.99, 128.46, 128.24, 128.08, 127.42, 127.18, 115.38, 52.39. MS (ESI) calculated for C19H16N4, m/z 300.14, found 301.15 (M + H)+.
3c: N,2-dibenzylimidazo[1,2-a]pyridin-3-amine (33 mg, 44%)
1H NMR (500 MHz, CDCl3) δ 7.95 (dt, J = 6.8, 1.1 Hz, 1H), 7.49 (dt, J = 9.1, 0.9 Hz, 1H), 7.38 – 7.15 (m, 10H), 7.09 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.72 (td, J = 6.7, 1.1 Hz, 1H), 4.01 (s, 2H), 3.94 (d, J = 5.5 Hz, 2H), 2.99 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 141.18, 139.68, 139.24, 137.57, 128.69, 128.62, 128.57, 128.28, 127.59, 126.25, 125.93, 123.36, 122.14, 117.15, 111.44, 52.83, 34.13. MS (ESI) calculated for C21H19N3, m/z 313.16, found 314.18 (M + H)+.
3d: N,2-dibenzylimidazo[1,2-a]pyrazin-3-amine (19 mg, 25%)
1H NMR (500 MHz, CDCl3) δ 7.83 – 7.72 (m, 2H), 7.35 – 7.25 (m, 5H), 7.25 – 7.18 (m, 4H), 7.16 (dd, J = 7.0, 2.2 Hz, 2H), 4.03 (s, 2H), 3.96 (d, J = 3.9 Hz, 2H), 3.15 – 3.05 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 141.87, 139.52, 137.76, 137.68, 135.44, 128.01, 127.89, 127.75, 127.71, 127.66, 127.63, 127.59, 127.56, 127.26, 127.19, 126.97, 126.84, 126.75, 125.57, 114.09, 51.49, 51.46, 33.19. MS (ESI) calculated for C20H18N4, m/z 314.15, found 315.17 (M + H)+.
3e: N-benzyl-2-(naphthalen-1-yl)imidazo[1,2-a]pyridin-3-amine (42 mg, 50%)
1H NMR (500 MHz, CDCl3) δ 8.15 – 8.10 (m, 1H), 8.01 (d, J = 8.3 Hz, 1H), 7.94 – 7.86 (m, 2H), 7.61 (d, J = 9.0 Hz, 1H), 7.55–7.44 (m, 4H), 7.18 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 7.15 – 7.11 (m, 3H), 7.02 (dd, J = 6.5, 3.0 Hz, 2H), 6.83 (td, J = 6.8, 1.0 Hz, 1H), 3.92 (d, J = 4.9 Hz, 2H), 3.53 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 141.35, 138.75, 135.57, 133.79, 132.14, 131.50, 128.45, 128.37, 128.33, 127.88, 127.34, 127.31, 126.49, 125.88, 125.82, 125.27, 123.69, 122.50, 117.60, 111.80, 52.42. MS (ESI) calculated for C24H19N3, m/z 349.16, found 350.18 (M + H)+.
3f: N-benzyl-2-(naphthalen-1-yl)imidazo[1,2-a]pyrazin-3-amine (12 mg, 14%)
1H NMR (500 MHz, CDCl3) δ 9.01 (d, J = 1.5 Hz, 1H), 7.97 – 7.89 (m, 4H), 7.83 (d, J = 4.6 Hz, 1H), 7.49 (tddd, J = 10.0, 6.8, 3.6, 1.4 Hz, 4H), 7.17 – 7.08 (m, 3H), 7.01 – 6.94 (m, 2H), 3.96 (d, J = 5.8 Hz, 2H), 3.78 (t, J = 5.8 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 143.50, 138.21, 137.83, 136.53, 133.78, 131.90, 130.44, 129.08, 128.95, 128.89, 128.49, 128.46, 128.02, 127.77, 127.73, 127.58, 126.78, 126.06, 125.45, 125.21, 115.35, 51.83. MS (ESI) calculated for C23H18N4, m/z 350.15, found 351.18 (M + H)+.
3g: 2-(anthracen-9-yl)-N-benzylimidazo[1,2-a]pyridin-3-amine (47 mg, 49%)
1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.18 (dt, J = 6.9, 1.2 Hz, 1H), 8.07 (d, J = 8.5 Hz, 2H), 7.82 (dd, J = 8.8, 0.9 Hz, 2H), 7.66 (dt, J = 9.0, 1.1 Hz, 1H), 7.50 – 7.43 (m, 2H), 7.39 (ddd, J = 8.7, 6.5, 1.3 Hz, 2H), 7.22 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 7.05 – 6.95 (m, 3H), 6.90 – 6.82 (m, 3H), 3.74 (d, J = 6.3 Hz, 2H), 3.25 (t, J = 6.3 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 140.88, 137.71, 132.57, 130.36, 130.23, 127.77, 127.54, 127.15, 127.02, 126.76, 126.63, 126.10, 125.31, 125.10, 124.09, 122.54, 121.46, 116.74, 110.71, 51.19. MS (ESI) calculated for C28H21N3, m/z 399.17, found 400.20 (M + H)+.
3h: 2-(anthracen-9-yl)-N-benzylimidazo[1,2-a]pyrazin-3-amine (8 mg, 8%)
1H NMR (500 MHz, CDCl3) δ 9.09 (d, J = 1.4 Hz, 1H), 8.59 (s, 1H), 8.08 (d, J = 8.5 Hz, 2H), 8.01 (dd, J = 4.6, 1.5 Hz, 1H), 7.91 (d, J = 4.6 Hz, 1H), 7.69 (dd, J = 8.8, 0.7 Hz, 2H), 7.52 – 7.45 (m, 2H), 7.41 (ddd, J = 8.6, 6.5, 1.2 Hz, 2H), 7.06 – 6.94 (m, 3H), 6.83 – 6.77 (m, 2H), 3.78 (d, J = 5.3 Hz, 2H), 3.44 (s, 1H). 13C NMR (126 MHz, CDCl3) δ 143.66, 138.12, 137.10, 135.78, 131.32, 131.19, 130.28, 129.06, 128.75, 128.50, 128.33, 127.55, 127.41, 126.56, 126.53, 125.74, 125.28, 115.37, 51.61. MS (ESI) calculated for C27H20N4, m/z 400.17, found 401.19 (M + H)+.
Compounds 4a–4c were synthesized similarly as compound 1a
4a: 2-(anthracen-9-yl)-N-cyclohexylimidazo[1,2-a]pyrimidin-3-amine (40 mg, 43%)
1H NMR (500 MHz, CDCl3) δ 8.61 (dd, J = 4.1, 2.1 Hz, 1H), 8.56 (s, 1H), 8.54 (dd, J = 6.8, 2.1 Hz, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.83 (dd, J = 8.7, 0.8 Hz, 2H), 7.49 – 7.44 (m, 2H), 7.41 (ddd, J = 8.6, 6.5, 1.3 Hz, 2H), 6.97 (dd, J = 6.8, 4.1 Hz, 1H), 2.83 (s, 1H), 2.47 (s, 1H), 1.43 (d, J = 12.9 Hz, 2H), 1.29 (d, J = 7.4 Hz, 3H), 0.82 (t, J = 6.6 Hz, 3H), 0.75 – 0.62 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 148.91, 144.93, 135.89, 131.34, 131.05, 130.37, 128.66, 128.12, 127.26, 126.67, 126.29, 126.07, 125.18, 108.17, 56.46, 33.55, 25.28, 24.28. MS (ESI) calculated for C26H24N4, m/z 392.20, found 393.22 (M + H)+.
4b: 2-(anthracen-9-yl)-N3-cyclohexylimidazo[1,2-a]pyridine-3,8-diamine (15 mg, 15%)
1H NMR (500 MHz, CDCl3) δ 8.54 (s, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.86 (dd, J = 8.7, 0.7 Hz, 2H), 7.70 (dd, J = 6.7, 0.9 Hz, 1H), 7.49 – 7.44 (m, 2H), 7.43 – 7.37 (m, 2H), 6.74 (t, J = 7.0 Hz, 1H), 6.40 (dd, J = 7.3, 0.9 Hz, 1H), 4.57 (s, 2H), 2.77 (s, 1H), 2.56 (s, 1H), 1.49 (d, J = 12.4 Hz, 2H), 1.29 (d, J = 5.7 Hz, 3H), 0.94 – 0.75 (m, 3H), 0.75 – 0.61 (m, 2H). 13C NMR (126 MHz, CDCl3) δ 135.97, 135.69, 132.06, 131.45, 131.37, 129.03, 128.57, 128.36, 127.62, 126.37, 126.06, 125.12, 113.22, 112.69, 101.65, 56.09, 33.63, 25.41, 24.41. MS (ESI) calculated for C27H26N4, m/z 406.22, found 407.23 (M + H)+.
4c: 2-(anthracen-9-yl)-8-chloro-N-cyclohexylimidazo[1,2-a]pyrazin-3-amine (20 mg, 20%)
1H NMR (500 MHz, CDCl3) δ 8.59 (s, 1H), 8.10 – 8.06 (m, 3H), 7.77 (d, J = 4.5 Hz, 1H), 7.70 (dd, J = 8.7, 0.7 Hz, 2H), 7.52 – 7.47 (m, 2H), 7.46 – 7.41 (m, 2H), 2.93 (d, J = 6.8 Hz, 1H), 2.66 – 2.58 (m, 1H), 1.48 (d, J = 12.4 Hz, 2H), 1.32 (d, J = 6.9 Hz, 3H), 0.92 – 0.79 (m, 3H), 0.69 (dd, J = 22.4, 11.8 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ 143.61, 136.76, 134.02, 131.59, 131.27, 131.26, 128.77, 128.58, 127.43, 126.61, 126.17, 125.63, 125.29, 115.63, 56.13, 33.74, 25.16, 24.33. MS (ESI) calculated for C26H23ClN4, m/z 426.16, found 427.17 (M + H)+.
Compounds 5a–5i were synthesized similarly as compound 1a
5a: 2-(anthracen-9-yl)-N-butylimidazo[1,2-a]pyridin-3-amine (22 mg, 25%)
1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.19 (dt, J = 6.8, 1.2 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.84 (dd, J = 8.7, 0.8 Hz, 2H), 7.67 (dt, J = 9.1, 1.0 Hz, 1H), 7.48 – 7.44 (m, 2H), 7.40 (ddd, J = 8.6, 6.5, 1.3 Hz, 2H), 7.22 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.90 (td, J = 6.8, 1.1 Hz, 1H), 2.89 (t, J = 6.1 Hz, 1H), 2.64 (q, J = 6.6 Hz, 2H), 1.09 – 0.97 (m, 2H), 0.86 – 0.72 (m, 2H), 0.43 (t, J = 7.4 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 141.97, 133.07, 131.52, 131.47, 129.40, 128.68, 128.39, 127.80, 126.46, 126.21, 125.25, 123.46, 122.61, 117.92, 111.80, 47.92, 32.18, 19.67, 13.53. MS (ESI) calculated for C25H23N3, m/z 365.19, found 366.20 (M + H)+.
5b: 2-(anthracen-9-yl)-N-pentylimidazo[1,2-a]pyridin-3-amine (35 mg, 38%)
1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.19 (dt, J = 6.8, 1.2 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.84 (dd, J = 8.7, 0.8 Hz, 2H), 7.67 (dt, J = 9.0, 1.1 Hz, 1H), 7.49 – 7.44 (m, 2H), 7.40 (ddd, J = 8.6, 6.5, 1.3 Hz, 2H), 7.22 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.90 (td, J = 6.8, 1.1 Hz, 1H), 2.91 (t, J = 6.4 Hz, 1H), 2.65 (q, J = 6.7 Hz, 2H), 1.02 (dt, J = 14.3, 7.0 Hz, 2H), 0.82 – 0.65 (m, 4H), 0.52 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 141.85, 132.95, 131.40, 131.34, 129.24, 128.56, 128.26, 127.67, 126.32, 126.10, 125.13, 123.31, 122.48, 117.81, 111.67, 48.05, 29.68, 28.58, 22.07, 13.70. MS (ESI) calculated for C26H25N3, m/z 379.20, found 380.22 (M + H)+.
5c: 2-(anthracen-9-yl)-N-isopropylimidazo[1,2-a]pyridin-3-amine (15 mg, 18%)
1H NMR (500 MHz, CDCl3) δ 8.54 (s, 1H), 8.25 (dt, J = 6.8, 1.2 Hz, 1H), 8.08 – 8.04 (m, 2H), 7.84 (dd, J = 8.7, 0.9 Hz, 2H), 7.66 (dt, J = 9.0, 1.1 Hz, 1H), 7.49 – 7.44 (m, 2H), 7.41 (ddd, J = 8.6, 6.5, 1.3 Hz, 2H), 7.23 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.90 (td, J = 6.8, 1.1 Hz, 1H), 2.86 (dq, J = 12.5, 6.3 Hz, 1H), 2.71 (d, J = 6.4 Hz, 1H), 0.69 (d, J = 6.3 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 142.10, 134.36, 131.39, 131.13, 128.63, 128.31, 128.29, 127.69, 126.23, 126.17, 125.11, 123.56, 122.78, 117.69, 111.64, 49.20, 23.15. MS (ESI) calculated for C24H21N3, m/z 351.17, found 352.19 (M + H)+.
5d: 2-(anthracen-9-yl)-N-isopropylimidazo[1,2-a]pyridin-3-amine (37 mg, 41%)
1H NMR (500 MHz, CDCl3) δ 8.54 (s, 1H), 8.23 (dd, J = 6.8, 1.0 Hz, 1H), 8.06 (d, J = 8.3 Hz, 2H), 7.89 – 7.83 (m, 2H), 7.67 (dt, J = 9.0, 1.0 Hz, 1H), 7.50 – 7.37 (m, 4H), 7.23 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.90 (td, J = 6.8, 1.1 Hz, 1H), 2.79 – 2.61 (m, 2H), 1.12 – 0.95 (m, 2H), 0.92 – 0.82 (m, 2H), 0.79 – 0.67 (m, 1H), 0.63 (d, J = 6.2 Hz, 2H), 0.39 (t, J = 7.1 Hz, 2H), 0.29 (t, J = 7.4 Hz, 1H). 13C NMR (126 MHz, CDCl3) δ 142.03, 134.21, 131.41, 131.39, 131.36, 131.19, 131.13, 128.62, 128.61, 128.59, 128.36, 128.34, 127.64, 127.61, 126.26, 126.15, 126.13, 125.09, 123.45, 123.34, 122.68, 122.60, 117.74, 117.71, 111.62, 59.63, 53.06, 39.14, 26.01, 20.85, 18.53, 13.58, 9.01. MS (ESI) calculated for C26H25N3, m/z 379.20, found 380.22 (M + H)+.
5e: 2-(anthracen-9-yl)-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridin-3-amine (51 mg, 50%)
IR (CHCl3) νmax (cm−1): 2956, 1519, 1481, 1365, 1340. 1H NMR (500 MHz, CDCl3) δ 8.53 (s, 1H), 8.41 (d, J = 6.9 Hz, 1H), 8.05 (d, J = 7.7 Hz, 2H), 7.88 (d, J = 8.5 Hz, 2H), 7.66 (d, J = 9.0 Hz, 1H), 7.50 – 7.38 (m, 4H), 7.24 (ddd, J = 8.9, 6.7, 1.2 Hz, 1H), 6.88 (td, J = 6.8, 1.0 Hz, 1H), 2.89 (s, 1H), 0.97 (s, 2H), 0.68 (s, 6H), 0.52 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 142.71, 137.08, 131.44, 131.01, 129.27, 128.71, 127.53, 126.78, 126.32, 126.24, 125.05, 123.94, 123.70, 117.51, 111.35, 59.61, 55.82, 31.20, 30.95, 28.61. MS (ESI) calculated for C29H31N3, m/z 421.25, found 422.27 (M + H)+.
5f: 2-(anthracen-9-yl)-N-((trimethylsilyl)methyl)imidazo[1,2-a]pyridin-3-amine (33 mg, 35%)
1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.15 (dt, J = 6.8, 1.1 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.89 (dd, J = 8.7, 0.7 Hz, 2H), 7.69 – 7.65 (m, 1H), 7.49 – 7.44 (m, 2H), 7.43–7.38 (m, 2H), 7.22 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.91 (td, J = 6.8, 1.0 Hz, 1H), 2.60 (s, 1H), 2.15 (s, 2H), −0.31 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 141.55, 132.06, 131.73, 131.42, 131.23, 128.58, 128.10, 127.65, 126.45, 126.02, 125.11, 123.20, 122.44, 117.79, 111.61, 38.69, −3.37. MS (ESI) calculated for C25H25N3Si, m/z 395.18, found 396.20 (M + H)+.
5g: N-(adamantan-1-yl)-2-(anthracen-9-yl)imidazo[1,2-a]pyridin-3-amine (46 mg, 43%)
1H NMR (500 MHz, CDCl3) δ 8.52 (s, 1H), 8.45 (dt, J = 6.9, 1.1 Hz, 1H), 8.08 – 8.03 (m, 2H), 7.87 (d, J = 8.8 Hz, 2H), 7.65 (dt, J = 9.0, 1.0 Hz, 1H), 7.44 (dddd, J = 10.0, 7.9, 6.5, 1.3 Hz, 4H), 7.23 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.88 (td, J = 6.8, 1.1 Hz, 1H), 2.66 (s, 1H), 1.65 (s, 3H), 1.34 (d, J = 12.1 Hz, 3H), 1.19 (d, J = 11.4 Hz, 3H), 1.13 (d, J = 2.5 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 142.80, 137.17, 131.51, 131.15, 129.27, 128.82, 127.68, 126.39, 126.35, 125.99, 125.17, 123.99, 123.87, 117.61, 111.43, 55.78, 43.30, 36.03, 29.50. MS (ESI) calculated for C31H29N3, m/z 443.24, found 444.26 (M + H)+.
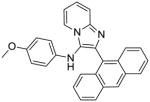
5h: 2-(anthracen-9-yl)-N-(4-methoxyphenyl)imidazo[1,2-a]pyridin-3-amine (36 mg, 36%)
1H NMR (500 MHz, CDCl3) δ 8.50 (s, 1H), 8.01 (d, J = 8.5 Hz, 2H), 7.90 – 7.83 (m, 3H), 7.74 (d, J = 9.1 Hz, 1H), 7.46 – 7.39 (m, 2H), 7.38 – 7.28 (m, 3H), 6.87 (td, J = 6.8, 1.0 Hz, 1H), 6.61 – 6.56 (m, 2H), 6.37 – 6.29 (m, 2H), 5.29 (s, 1H), 3.63 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 153.58, 142.85, 137.63, 136.25, 131.45, 131.34, 128.69, 128.04, 127.61, 126.28, 126.18, 125.19, 124.47, 123.39, 123.21, 118.11, 115.45, 114.84, 112.22, 55.69. MS (ESI) calculated for C28H21N3O, m/z 415.17, found 416.19 (M + H)+.
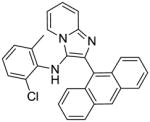
5i: 2-(anthracen-9-yl)-N-(2-chloro-6-methylphenyl)imidazo[1,2-a]pyridin-3-amine (11 mg, 11%)
1H NMR (500 MHz, CDCl3) δ8.38 (s, 1H), 8.21 (dt, J = 6.8, 1.1 Hz, 1H), 7.94 (d, J = 8.4 Hz, 2H), 7.75 (ddd, J = 15.6, 8.3, 4.8 Hz, 3H), 7.41 – 7.35 (m, 2H), 7.34 – 7.28 (m, 3H), 6.97 (td, J = 6.8, 1.1 Hz, 1H), 6.61 (dd, J = 7.9, 1.0 Hz, 1H), 6.41 – 6.35 (m, 1H), 6.29 (t, J = 7.7 Hz, 1H), 5.43 (s, 1H), 1.48 (s, 3H). 13C NMR (126 MHz, CDCl3) δ 142.20, 139.32, 137.26, 131.22, 129.58, 128.96, 128.51, 127.69, 127.36, 126.96, 126.32, 125.86, 124.98, 124.43, 124.33, 122.77, 121.59, 118.07, 112.53, 18.14. MS (ESI) calculated for C28H20ClN3, m/z 433.13, found 434.15 (M + H)+.
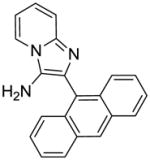
Synthesis of Compound 6: 2-(anthracen-9-yl)imidazo[1,2-a]pyridin-3-amine
Compound 5e (50 mg, 0.12 mmol) was stirred in a solution of 4M HCl/dioxane for 3 h, followed by removing the solvent under vacuum. The residue was then purified using column chromatography to obtain the compound 6 in quantitative yield. 1H NMR (500 MHz, CDCl3) δ 8.55 (s, 1H), 8.12 (d, J = 6.8 Hz, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.87 (d, J = 8.8 Hz, 2H), 7.66 (d, J = 9.1 Hz, 1H), 7.49 – 7.45 (m, 2H), 7.44 – 7.38 (m, 2H), 7.23 – 7.17 (m, 1H), 6.91 (td, J = 6.8, 0.9 Hz, 1H), 3.07 (s, 2H). 13C NMR (126 MHz, CDCl3) δ 141.49, 131.62, 131.32, 130.44, 128.77, 127.97, 127.79, 126.54, 126.32, 125.88, 125.33, 123.00, 122.10, 117.82, 111.89. MS (ESI) calculated for C21H15N3, m/z 309.13, found 310.14 (M + H)+.
Syntheses of Compounds 7a and 7b
To a solution of compound 6 (20 mg, 0.065 mmol) in anhydrous methanol, were added octyl aldehyde (15 μL, 0.098 mmol), 4 drops of acetic acid and sodium cyanoborohydride (6 mg, 0.098 mmol). The reaction mixture was stirred for 18h and the solvent was then removed under vacuum. The residue was purified using column chromatography to obtain compounds 7a (3% methanol/CH2Cl2) and 7b (2% methanol/CH2Cl2).
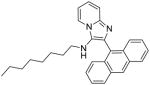
7a: 2-(anthracen-9-yl)-N-octylimidazo[1,2-a]pyridin-3-amine (10 mg, 36%)
1H NMR (500 MHz, CDCl3) δ 8.54 (s, 1H), 8.20 (dt, J = 6.8, 1.1 Hz, 1H), 8.06 (d, J = 8.4 Hz, 2H), 7.84 (dd, J = 8.7, 0.7 Hz, 2H), 7.67 (d, J = 9.0 Hz, 1H), 7.50 – 7.43 (m, 2H), 7.42 – 7.37 (m, 2H), 7.23 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.91 (td, J = 6.8, 1.1 Hz, 1H), 2.92 (s, 1H), 2.70 – 2.61 (m, 2H), 1.21 – 1.11 (m, 2H), 0.99 (ddd, J = 15.6, 8.8, 7.1 Hz, 4H), 0.90 – 0.84 (m, 2H), 0.82 (t, J = 7.3 Hz, 3H), 0.73 – 0.66 (m, 4H). 13C NMR (126 MHz, CDCl3) δ 141.95, 132.96, 131.54, 131.48, 129.37, 128.73, 128.28, 127.86, 126.44, 126.28, 125.28, 123.54, 122.65, 117.92, 111.86, 48.16, 31.79, 30.06, 29.11, 29.10, 26.56, 22.70, 14.22. MS (ESI) calculated for C29H31N3, m/z 421.25, found 422.27 (M + H)+.
7b: 2-(anthracen-9-yl)-N,N-dioctylimidazo[1,2-a]pyridin-3-amine (7 mg, 20%)
1H NMR (500 MHz, CDCl3) δ 8.54 (s, 1H), 8.30 (d, J = 6.8 Hz, 1H), 8.04 (d, J = 8.5 Hz, 2H), 7.63 (d, J = 8.7 Hz, 3H), 7.46 – 7.40 (m, 2H), 7.31 (ddd, J = 8.6, 6.5, 1.1 Hz, 2H), 7.26 – 7.21 (m, 1H), 6.90 (td, J = 6.8, 1.0 Hz, 1H), 2.51 – 2.45 (m, 4H), 1.32 – 1.19 (m, 8H), 1.19 – 1.08 (m, 8H), 1.08 – 0.93 (m, 8H), 0.85 (t, J = 7.2 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 142.02, 135.49, 132.12, 131.84, 131.45, 129.90, 128.41, 127.72, 127.10, 125.49, 125.20, 123.93, 123.19, 117.77, 111.74, 54.71, 31.92, 29.51, 29.38, 29.16, 27.09, 22.75, 14.25. MS (ESI) calculated for C37H47N3, m/z 533.38, found 534.38 (M + H)+.
Synthesis of compound 7c: N-(2-(anthracen-9-yl)imidazo[1,2-a]pyridin-3-yl)-2,2,2-trifluoro acetamide
To a solution of compound 6 (10 mg, 0.024 mmol) in anhydrous CH2Cl2, was added trifluoroacetic anhydride (4 μL, 0.03 mmol) and the reaction mixture was stirred for 12 h. The solvent was then removed under vacuum and the residue was purified using column chromatography (3% methanol/CH2Cl2) to obtain the compound 7c (10 mg, 73%). IR (CHCl3) νmax (cm−1): 1730, 1494, 1355, 1315, 1240, 1195, 1157. 1H NMR (500 MHz, MeOD) δ 8.89 (s, 1H), 8.76 (d, J = 6.8 Hz, 1H), 8.28–8.14 (m, 3H), 8.10 (d, J = 9.0 Hz, 1H), 7.77 (d, J = 8.5 Hz, 2H), 7.72 (dd, J = 10.0, 4.0 Hz, 1H), 7.59 (ddt, J = 9.9, 6.6, 3.9 Hz, 4H). 13C NMR (126 MHz, MeOD) δ 161.71, 161.41, 161.11, 160.82, 159.55, 159.23, 158.92, 158.61, 140.74, 135.66, 132.62, 132.61, 132.47, 130.22, 129.81, 129.06, 127.34, 127.00, 125.58, 118.91, 118.54, 113.88. MS (ESI) calculated for C23H14F3N3O, m/z 405.11, found 406.12 (M + H)+.
Compound 8a–8c were synthesized similarly as compound 1a
8a: 2-([1,1′-biphenyl]-4-yl)-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridin-3-amine (60 mg, 63%)
1H NMR (500 MHz, CDCl3) δ 8.27 (d, J = 6.9 Hz, 1H), 7.99 – 7.95 (m, 2H), 7.71 – 7.65 (m, 4H), 7.61 (d, J = 9.0 Hz, 1H), 7.46 (dd, J = 10.5, 4.8 Hz, 2H), 7.38 – 7.33 (m, 1H), 7.21 – 7.15 (m, 1H), 6.82 (dd, J = 6.6, 6.2 Hz, 1H), 3.29 (s, 1H), 1.61 (s, 2H), 1.05 (s, 9H), 0.99 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 140.75, 140.14, 128.92, 128.78, 128.70, 127.31, 127.27, 127.26, 127.00, 126.96, 124.63, 123.66, 123.48, 117.04, 111.69, 60.86, 57.09, 31.86, 31.76, 29.02. MS (ESI) calculated for C27H31N3, m/z 397.25, found 398.25 (M + H)+.
8b: 2-(phenanthren-9-yl)-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridin-3-amine (79 mg, 78%)
1H NMR (500 MHz, CDCl3) δ 8.78 (d, J = 8.2 Hz, 1H), 8.74 (d, J = 8.1 Hz, 1H), 8.37 (dt, J = 6.9, 1.1 Hz, 1H), 8.04 (dd, J = 8.2, 1.0 Hz, 1H), 7.92 (dd, J = 7.9, 1.2 Hz, 1H), 7.90 (s, 1H), 7.68 (dddd, J = 8.3, 6.9, 4.2, 1.4 Hz, 2H), 7.65 – 7.56 (m, 3H), 7.21 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.86 (td, J = 6.8, 1.1 Hz, 1H), 3.21 (s, 1H), 1.26 (s, 2H), 0.78 (s, 6H), 0.73 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 142.35, 139.07, 131.88, 131.67, 131.27, 130.82, 130.44, 129.27, 128.91, 127.17, 126.89, 126.83, 126.72, 126.63, 125.55, 124.24, 123.90, 123.18, 122.72, 117.53, 111.59, 60.24, 56.39, 31.63, 31.45, 28.78. MS (ESI) calculated for C29H31N3, m/z 421.25, found 422.26 (M + H)+ and 444.25 (M + Na)+.
8c: 2-(pyren-4-yl)-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridin-3-amine (47 mg, 44%)
1H NMR (500 MHz, CDCl3) δ 8.40 (dt, J = 6.9, 1.1 Hz, 1H), 8.28 (d, J = 7.8 Hz, 1H), 8.23 – 8.16 (m, 4H), 8.11 (dd, J = 12.1, 5.0 Hz, 3H), 8.02 (t, J = 7.6 Hz, 1H), 7.67 (dt, J = 9.0, 0.9 Hz, 1H), 7.23 (ddd, J = 9.0, 6.7, 1.3 Hz, 1H), 6.87 (td, J = 6.8, 1.1 Hz, 1H), 3.24 (s, 1H), 1.20 (s, 2H), 0.73 (s, 9H), 0.67 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 142.45, 139.49, 131.39, 131.00, 130.94, 130.62, 129.17, 128.39, 128.17, 127.55, 127.46, 125.92, 125.48, 125.20, 125.05, 125.01, 124.95, 124.79, 124.06, 123.77, 117.46, 111.40, 60.22, 56.27, 31.54, 31.32, 28.53. MS (ESI) calculated for C31H31N3, m/z 445.25, found 446.26 (M + H)+ and 468.25 (M + Na)+.
Compound 9 was synthesized similarly as compound 1a
9: 2-(10-chloroanthracen-9-yl)-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridin-3-amine (128 mg, 59%)
1H NMR (500 MHz, CDCl3) δ 8.60 (d, J = 8.8 Hz, 2H), 8.41 (d, J = 6.9 Hz, 1H), 7.90 (d, J = 8.8 Hz, 2H), 7.67 (d, J = 9.0 Hz, 1H), 7.62 – 7.57 (m, 2H), 7.50 – 7.45 (m, 2H), 7.28 – 7.25 (m, 1H), 6.90 (t, J = 6.8 Hz, 1H), 2.83 (s, 1H), 0.96 (s, 2H), 0.68 (s, 6H), 0.52 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 142.73, 136.53, 131.45, 129.61, 129.14, 128.64, 127.24, 127.01, 126.69, 126.58, 126.55, 125.21, 124.28, 123.73, 117.53, 111.57, 59.62, 55.90, 31.20, 31.00, 28.67. MS (ESI) calculated for C29H30ClN3, m/z 455.21, found 456.23 (M + H)+.
Synthesis of Compound 10: N1,N1-dimethyl-N3-(10-(3-((2,4,4-trimethylpentan-2-yl)amino)imidazo[1,2-a]pyridin-2-yl)anthracen-9-yl)propane-1,3-diamine
To a solution of compound 9 (50 mg, 0.11 mmol) in anhydrous dioxane, were added potassium tert-butoxide (38 mg, 0.34 mmol), N1,N1-dimethylpropane-1,3-diamine (69 μL, 0.55 mmol) and catalytic amounts of tris(dibenzylideneacetone)-dipalladium [Pd2(dba)3] and 2-dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl [DavePhos]. The reaction was heated in a sealed tube at 80 °C for 4 h. The solvent was then removed under vacuum and the residue was purified using column chromatography to obtain the compound 10 (15 mg, 26%). 1H NMR (500 MHz, MeOD) δ 8.53 (dt, J = 6.9, 1.0 Hz, 1H), 8.47 (d, J = 8.7 Hz, 2H), 7.74 (d, J = 8.5 Hz, 2H), 7.60 – 7.56 (m, 1H), 7.51 – 7.45 (m, 2H), 7.44 – 7.35 (m, 3H), 7.04 (td, J = 6.8, 1.1 Hz, 1H), 3.45 (t, J = 7.1 Hz, 2H), 2.57 – 2.52 (m, 2H), 2.30 (s, 6H), 2.02–1.91 (m, 2H), 0.95 (s, 2H), 0.74 (s, 6H), 0.45 (s, 9H). 13C NMR (126 MHz, MeOD) δ 144.90, 143.81, 137.83, 133.18, 128.54, 127.84, 127.32, 126.52, 126.46, 125.51, 125.31, 125.08, 124.09, 117.18, 113.25, 60.30, 58.65, 56.77, 51.56, 45.43, 44.86, 32.49, 31.81, 31.76, 29.47, 29.44. MS (ESI) calculated for C34H43N5, m/z 521.35, found 522.37 (M + H)+.
Compound 11 was synthesized similarly as compound 1a
11: 2-(anthracen-9-yl)-6-bromo-N-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridin-3-amine (150 mg, 63%)
1H NMR (500 MHz, CDCl3) δ 8.54 – 8.51 (m, 2H), 8.08 – 8.04 (m, 2H), 7.82 (dd, J = 8.6, 0.9 Hz, 2H), 7.56 (dd, J = 9.4, 0.7 Hz, 1H), 7.49 – 7.41 (m, 4H), 7.30 (dd, J = 9.4, 1.9 Hz, 1H), 2.91 (s, 1H), 0.96 (s, 2H), 0.68 (s, 6H), 0.50 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 141.22, 138.24, 131.56, 131.08, 128.94, 128.70, 127.97, 127.51, 127.30, 126.66, 126.08, 125.26, 124.04, 118.41, 106.62, 59.88, 55.93, 31.31, 31.07, 28.78. MS (ESI) calculated for C29H30BrN3, m/z 499.16, found 500.18 (M + H)+.
Compound 12 was synthesized similarly as compound 10
12: 2-(anthracen-9-yl)-N6-(3-(dimethylamino)propyl)-N3-(2,4,4-trimethylpentan-2-yl)imidazo[1,2-a]pyridine-3,6-diamine (21 mg, 40%)
1H NMR (500 MHz, MeOD) δ 8.61 (s, 1H), 8.11 (d, J = 8.2 Hz, 2H), 7.83 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 1.9 Hz, 1H), 7.52 – 7.42 (m, 4H), 7.39 (d, J = 9.5 Hz, 1H), 7.04 (dd, J = 9.5, 2.1 Hz, 1H), 3.18 (t, J = 6.8 Hz, 2H), 2.60 – 2.54 (m, 2H), 2.34 (s, 6H), 1.98 – 1.90 (m, 2H), 0.93 (s, 2H), 0.73 (s, 6H), 0.46 (s, 9H). 13C NMR (126 MHz, MeOD) δ 140.23, 138.50, 136.47, 133.04, 132.49, 130.41, 129.86, 128.71, 128.35, 127.41, 127.33, 126.29, 121.96, 117.07, 103.72, 60.35, 58.54, 56.85, 45.44, 43.56, 31.78, 31.70, 29.50, 27.45. MS (ESI) calculated for C34H43N5, m/z 521.35, found 522.36 (M + H)+.
Compound 13 was synthesized similarly as compound 1a
13: N-(tert-butyl)-2-(10-chloroanthracen-9-yl)imidazo[1,2-a]pyridin-3-amine (242 mg, 84%)
1H NMR (500 MHz, CDCl3) δ 8.60 (d, J = 8.8 Hz, 2H), 8.40 (dt, J = 6.9, 1.1 Hz, 1H), 7.91 (d, J = 8.8 Hz, 2H), 7.66 (d, J = 9.0 Hz, 1H), 7.60 (ddd, J = 8.8, 6.5, 1.1 Hz, 2H), 7.48 (ddd, J = 8.7, 6.5, 1.1 Hz, 2H), 7.28 – 7.23 (m, 1H), 6.90 (td, J = 6.8, 1.1 Hz, 1H), 2.63 (s, 1H), 0.65 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 142.88, 136.60, 131.47, 129.77, 129.12, 128.75, 127.13, 126.76, 126.72, 126.67, 125.36, 124.42, 123.78, 117.69, 111.71, 55.76, 29.90. MS (ESI) calculated for C25H22ClN3, m/z 399.15, found 400.17 (M + H)+.
Compound 14 was synthesized similarly as compound 10
14: N1-(10-(3-(tert-butylamino)imidazo[1,2-a]pyridin-2-yl)anthracen-9-yl)octane-1,8-diamine. Red solid (20 mg, 32%)
1H NMR (500 MHz, MeOD) δ 9.01 (d, J = 6.8 Hz, 1H), 8.55 (d, J = 8.8 Hz, 2H), 8.15 – 8.08 (m, 1H), 7.99 (dd, J = 13.0, 4.8 Hz, 3H), 7.85 (dd, J = 8.4, 7.0 Hz, 2H), 7.80 – 7.71 (m, 2H), 7.67 (dd, J = 10.0, 4.0 Hz, 1H), 3.79 (dd, J = 16.7, 8.6 Hz, 2H), 2.94 (dd, J = 14.0, 6.6 Hz, 2H), 2.00 – 1.91 (m, 2H), 1.72 – 1.63 (m, 2H), 1.46 – 1.38 (m, 6H), 0.81 (s, 9H). 13C NMR (126 MHz, MeOD) δ 139.74, 135.47, 135.36, 132.83, 130.52, 129.36, 129.01, 128.07, 127.63, 127.26, 127.03, 125.59, 123.03, 118.21, 113.15, 56.34, 54.29, 40.75, 30.28, 30.13, 29.99, 28.55, 27.62, 27.38, 27.35. MS (ESI) calculated for C33H41N5, m/z 507.34, found 508.35 (M + H)+.
Compound 15 was synthesized similarly as compound 1a
15: 2-(anthracen-9-yl)-6-bromo-N-(tert-butyl)imidazo[1,2-a]pyridin-3-amine (260 mg, 81%)
1H NMR (500 MHz, CDCl3) δ 8.54 – 8.52 (m, 2H), 8.08 – 8.04 (m, 2H), 7.83 (d, J = 8.6 Hz, 2H), 7.56 (dd, J = 9.4, 0.6 Hz, 1H), 7.49 – 7.42 (m, 4H), 7.30 (dd, J = 9.4, 1.9 Hz, 1H), 2.68 (s, 1H), 0.64 (s, 9H). 13C NMR (126 MHz, CDCl3) δ 141.20, 138.13, 131.52, 130.96, 128.97, 128.47, 128.04, 127.60, 127.27, 126.67, 125.99, 125.25, 123.94, 118.41, 106.67, 55.85, 29.83. MS (ESI) calculated for C25H22BrN3, m/z 443.10, found 444.10 (M + H)+.
Compound 16 was synthesized similarly as compound 10
16: N6-(8-aminooctyl)-2-(anthracen-9-yl)-N3-(tert-butyl)imidazo[1,2-a]pyridine-3,6-diamine. Brown solid (55 mg, 47%)
1H NMR (500 MHz, MeOD) δ 8.81 (s, 1H), 8.20 (dd, J = 6.5, 2.3 Hz, 2H), 7.85 – 7.80 (m, 2H), 7.72 – 7.56 (m, 7H), 3.23 (t, J = 7.0 Hz, 2H), 2.97 – 2.90 (m, 2H), 1.80 (dt, J = 14.5, 7.1 Hz, 2H), 1.72 – 1.64 (m, 2H), 1.59 – 1.51 (m, 2H), 1.45 (s, 6H), 0.78 (s, 9H). 13C NMR (126 MHz, MeOD) δ 141.50, 141.40, 134.23, 132.75, 132.52, 131.64, 130.29, 128.91, 128.20, 127.68, 126.85, 126.05, 121.64, 112.69, 104.55, 56.28, 45.40, 40.78, 30.46, 30.35, 30.30, 29.46, 28.65, 28.27, 27.52. MS (ESI) calculated for C33H41N5, m/z 507.34, found 508.35 (M + H)+.
Microbiological methods
MICs of the compounds were determined by broth microdilution method per CLSI (formerly NCCLS) guidelines as described earlier.36 Mid-log phase Mueller-Hinton broth (MHB; noncation supplemented) cultures of organisms (40 μL; optical density at 600 nm adjusted to 0.5 AU, and diluted 10-fold) were added to equal volumes of 2-fold serially diluted compounds in a 384-well microtiter plate with the help of a Biotek Precision 2000 automated microplate pipetting system. The MICs of known antibiotics were included as reference compounds for comparison of activity. The microtiter plates were sealed and incubated overnight at 37°C. The plates were read at an absorbance of 600 nm. The lowest concentration of an agent inhibiting growth of the organisms was recorded as the MIC.
For MBC determinations, conventional microdilution techniques were employed wherein 0.5 μL of each of the 384 wells in the parent MIC plate was diluted into 80 μL of fresh MHB using the Biotek Precision 2000 automated liquid handling device. The microtiter plates were incubated overnight at 37°C. The plates were read at an absorbance of 600 nm.
5e-resistant S. aureus organisms were generated by exposing S. aureus ATCC 13709 to escalating doses of the compound. Within about 10 serial passages, organisms that withstood 5e up to concentrations of 100 μg/mL emerged.
Supplementary Material
Acknowledgments
This work was supported by NIH/NIAID contract HHSN272200900033C.
Abbreviations
- ATCC
American Type Culture Collection
- DavePhos
2-Dicyclohexylphosphino-2′-(N,N-dimethylamino)biphenyl
- ESI-TOF
Electrospray ionization-time of flight
- HIV
Human immunodeficiency virus
- MBC
Minimum bactericidal concentration
- MIC
Minimum inhibitory concentration
- MRSA
Methicillin-resistant S. aureus
- MW
Microwave
- NF-κB
Nuclear factor-κB
- Pd2(dba)3
Tris(dibenzylideneacetone)dipalladium(0)
- SAR
Structure activity relationship
- ssRNA
single-stranded RNA
- TLR
Toll like receptor
Footnotes
Supporting Information: Characterization of intermediates and final compounds (1H, 13C, mass spectra). This material is available free of charge via the Internet at http://pubs.acs.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boucher HW, Corey GR. Clin Infect Dis. 2008;46(Suppl 5):S344–S349. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 2.Gould IM. J Antimicrob Chemother. 2011;66(Suppl 4):iv17–iv21. doi: 10.1093/jac/dkr073. [DOI] [PubMed] [Google Scholar]
- 3.Dockrell DH, Kinghorn GR. J Antimicrob Chemother. 2001;48:751. doi: 10.1093/jac/48.6.751. [DOI] [PubMed] [Google Scholar]
- 4.Garland SM. Curr Opin Infect Dis. 2003;16:85. doi: 10.1097/00001432-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Miller RL, Meng TC, Tomai MA. Drug News Perspect. 2008;21:69. doi: 10.1358/dnp.2008.21.2.1188193. [DOI] [PubMed] [Google Scholar]
- 6.Vultaggio A, Nencini F, Pratesi S, Maggi L, Guarna A, Annunziato F, Romagnani S, Parronchi P, Maggi EJ. Immunol. 2011;186:4707. doi: 10.4049/jimmunol.1002398. [DOI] [PubMed] [Google Scholar]
- 7.Chan M, Hayashi T, Kuy CS, Gray CS, Wu CC, Corr M, Wrasidlo W, Cottam HB, Carson DA. Bioconjug Chem. 2009;20:1194. doi: 10.1021/bc900054q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weterings JJ, Khan S, van der Heden van Noort GJ, Melief CJ, Overkleeft HS, van der Burg SH, Ossendorp F, Van der Marel GA, Filippov DV. Bioorg Med Chem Lett. 2009;19:2249. doi: 10.1016/j.bmcl.2009.02.095. [DOI] [PubMed] [Google Scholar]
- 9.Fili L, Ferri S, Guarna F, Sampognaro S, Manuelli C, Liotta F, Cosmi L, Matucci A, Vultaggio A, Annunziato F, Maggi E, Guarna A, Romagnani S, Parronchi PJ. Allergy Clin Immunol. 2006;118:511. doi: 10.1016/j.jaci.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Kurimoto A, Hashimoto K, Nakamura T, Norimura K, Ogita H, Takaku H, Bonnert R, McInally T, Wada H, Isobe YJ. Med Chem. 2010;53:2964. doi: 10.1021/jm100070n. [DOI] [PubMed] [Google Scholar]
- 11.Bienayme H, Bouzid K. Angew Chem, Int Ed. 1998;37:2234. doi: 10.1002/(SICI)1521-3773(19980904)37:16<2234::AID-ANIE2234>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 12.Groebke K, Weber L, Mehlin F. Synlett. 1998:661. [Google Scholar]
- 13.Blackburn C, Guan B, Fleming P, Shiosaki K, Tsai S. Tetrahedron Lett. 1998;39:3635. [Google Scholar]
- 14.Shukla NM, Malladi SS, Day V, David SA. Bioorg Med Chem. 2011;19:3801. doi: 10.1016/j.bmc.2011.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla NM, Malladi SS, Mutz CA, Balakrishna R, David SA. J Med Chem. 2010;53:4450. doi: 10.1021/jm100358c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla NM, Mutz CA, Malladi SS, Warshakoon HJ, Balakrishna R, David SA. J Med Chem. 2012;55:1106. doi: 10.1021/jm2010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balakrishna R, Wood SJ, Nguyen TB, Miller KA, Suresh Kumar EV, Datta A, David SA. Antimicrob Agents Chemother. 2006;50:852. doi: 10.1128/AAC.50.3.852-861.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warshakoon HJ, Burns MR, David SA. Antimicrob Agents Chemother. 2008 doi: 10.1128/AAC.00812-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson LR, Shanholtzer C. J Clin Microbiol Rev. 1992;5:420. doi: 10.1128/cmr.5.4.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikaido H, Vaara M. Microbiol Rev. 1985;49:1. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaara M, Vaara T. Antimicrobial Agents and Chemotherapy. 1983;24:107. doi: 10.1128/aac.24.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura Y, Matsunaga H, Vaara MJ. Antibiot Tokyo. 1992;45:742. doi: 10.7164/antibiotics.45.742. [DOI] [PubMed] [Google Scholar]
- 23.Vaara M. Microbiological Reviews. 1992;56:395. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viljanen P, Matsunaga H, Kimura Y, Vaara MJ. Antibiot Tokyo. 1991;44:517. doi: 10.7164/antibiotics.44.517. [DOI] [PubMed] [Google Scholar]
- 25.Baviskar AT, Madaan C, Preet R, Mohapatra P, Jain V, Agarwal A, Guchhait SK, Kundu CN, Banerjee UC, Bharatam PV. J Med Chem. 2011;54:5013. doi: 10.1021/jm200235u. [DOI] [PubMed] [Google Scholar]
- 26.Al Tel TH, Al Qawasmeh RA. Eur J Med Chem. 2010;45:5848. doi: 10.1016/j.ejmech.2010.09.049. [DOI] [PubMed] [Google Scholar]
- 27.Al Tel TH, Al Qawasmeh RA, Zaarour R. Eur J Med Chem. 2011;46:1874. doi: 10.1016/j.ejmech.2011.02.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.