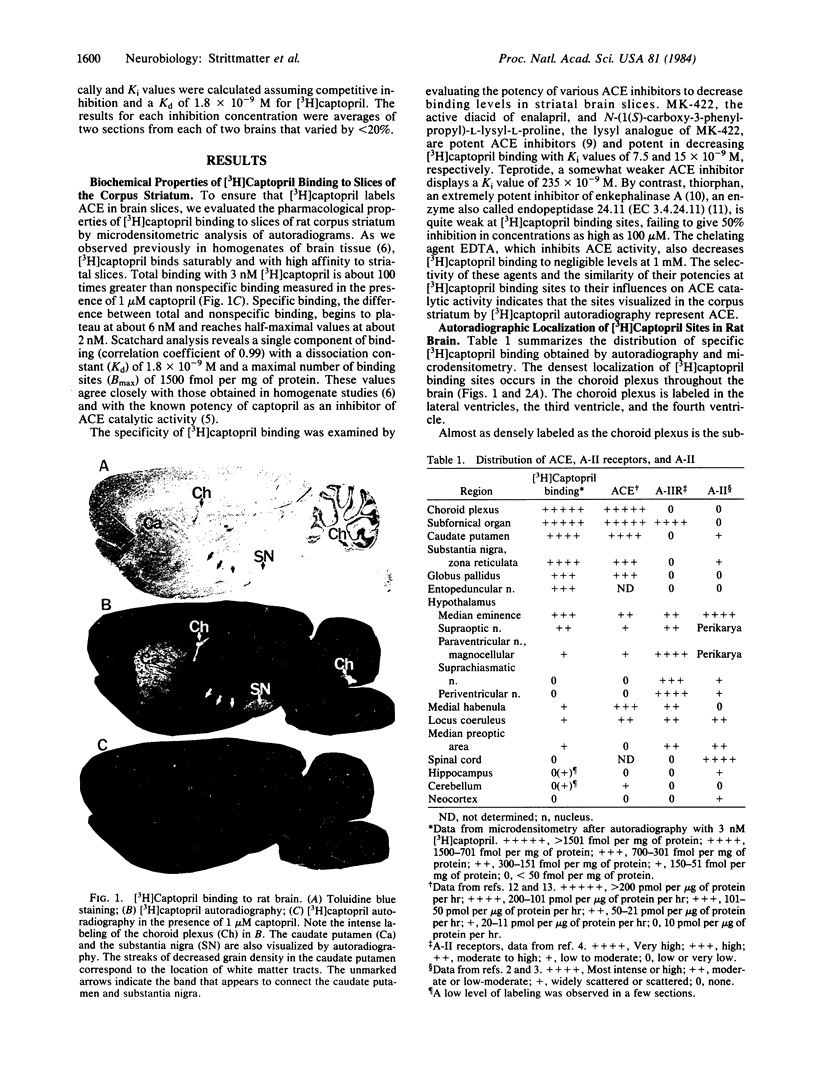
Abstract
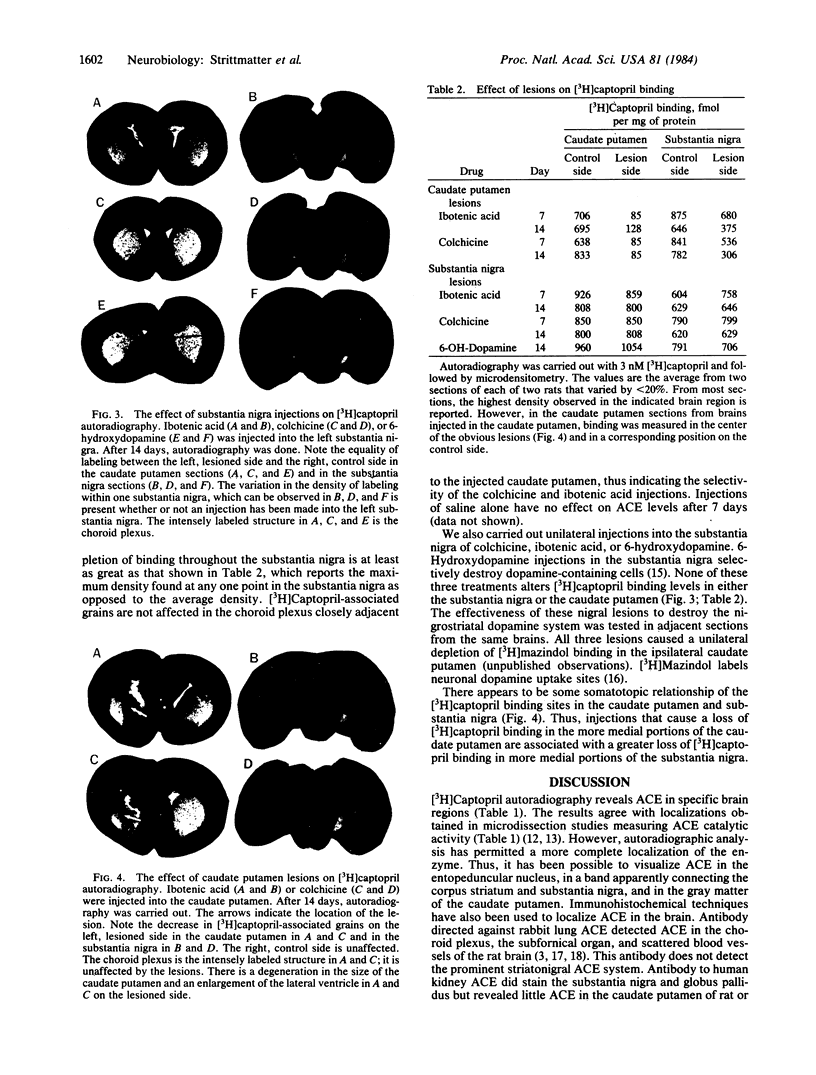
We have visualized angiotensin-converting enzyme (ACE; dipeptidyl carboxypeptidase, peptidylpeptide hydrolase, EC 3.4.15.1) in rat brain by in vitro [3H]captopril autoradiography. [3H]Captopril binding to brain slices displays a high affinity (Kd = 1.8 X 10(-9) M) and a pharmacological profile similar to that of ACE activity. Very high densities of [3H]captopril binding were found in the choroid plexus and the subfornical organ. High densities were present in the caudate putamen and substantia nigra, zona reticulata. Moderate levels were found in the entopeduncular nucleus, globus pallidus, and median eminence of the hypothalamus. Lower levels were detectable in the supraoptic and paraventricular nuclei of the hypothalamus, the medial habenula, the median preoptic area, and the locus coeruleus. Injection of ibotenic acid or colchicine into the caudate putamen decreased [3H]captopril-associated autoradiographic grains by 85% in the ipsilateral caudate putamen and by greater than 50% in the ipsilateral substantia nigra. Thus, ACE in the substantia nigra is located on presynaptic terminals of axons originating from the caudate putamen, and ACE in the caudate putamen is situated in neuronal perikarya or at the terminals of striatal interneurons. The lack of effect of similar injections into the substantia nigra confirmed that the caudate putamen injections did not cause trans-synaptic changes. The presence of [3H]captopril binding is consistent with an ACE-mediated production of angiotensin II in some brain regions. Although [3H]captopril autoradiography reveals ACE in a striatonigral pathway, there is no evidence for angiotensin II involvement in such a neuronal pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arregui A., Emson P. C., Spokes E. G. Angiotensin-converting enzyme in substantia nigra: reduction of activity in Huntington's disease and after intrastriatal kainic acid in rats. Eur J Pharmacol. 1978 Nov 1;52(1):121–124. doi: 10.1016/0014-2999(78)90029-8. [DOI] [PubMed] [Google Scholar]
- Arregui A., Iversen L. L. Angiotensin-converting enzyme: presence of high activity in choroid plexus of mammalian brain. Eur J Pharmacol. 1978 Nov 1;52(1):147–150. doi: 10.1016/0014-2999(78)90035-3. [DOI] [PubMed] [Google Scholar]
- Brownfield M. S., Reid I. A., Ganten D., Ganong W. F. Differential distribution of immunoreactive angiotensin and angiotensin-converting enzyme in rat brain. Neuroscience. 1982 Jul;7(7):1759–1769. doi: 10.1016/0306-4522(82)90033-1. [DOI] [PubMed] [Google Scholar]
- Chevillard C., Saavedra J. M. Distribution of angiotensin-converting enzyme activity in specific areas of the rat brain stem. J Neurochem. 1982 Jan;38(1):281–284. doi: 10.1111/j.1471-4159.1982.tb10883.x. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Ondetti M. A. Inhibitors of angiotensin-converting enzyme for treatment of hypertension. Biochem Pharmacol. 1980 Jul 1;29(13):1871–1877. doi: 10.1016/0006-2952(80)90096-9. [DOI] [PubMed] [Google Scholar]
- Defendini R., Zimmerman E. A., Weare J. A., Alhenc-Gelas F., Erdös E. G. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983 Jul;37(1):32–40. doi: 10.1159/000123512. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Matsas R., Turner A. J., Kenny A. J. Kidney neutral endopeptidase and the hydrolysis of enkephalin by synaptic membranes show similar sensitivity to inhibitors. Biochem J. 1982 May 1;203(2):519–522. doi: 10.1042/bj2030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Ganten D., Köhler C., Schüll B., Speck G. Evidence for differential localization of angiotensin-I converting enzyme and renin in the corpus striatum of rat. Acta Physiol Scand. 1980 Nov;110(3):321–323. doi: 10.1111/j.1748-1716.1980.tb06670.x. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., Blaustein R. O., Snyder S. H. [3H]mazindol binding associated with neuronal dopamine uptake sites in corpus striatum membranes. Eur J Pharmacol. 1983 Jun 17;90(4):461–462. doi: 10.1016/0014-2999(83)90574-5. [DOI] [PubMed] [Google Scholar]
- Kilcoyne M. M., Hoffman D. L., Zimmerman E. A. Immunocytochemical localization of angiotensin II and vasopressin in rat hypothalamus: evidence for production in the same neuron. Clin Sci (Lond) 1980 Dec;59 (Suppl 6):57s–60s. doi: 10.1042/cs059057s. [DOI] [PubMed] [Google Scholar]
- Krutzsch H. C. Determination of polypeptide amino acid sequences from the carboxyl terminus using angiotensin I converting enzyme. Biochemistry. 1980 Nov 11;19(23):5290–5296. doi: 10.1021/bi00564a022. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F. A., Quirion R., Saavedra J. M., Aguilera G., Catt K. J. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett A. A., Harris E., Tristram E. W., Wyvratt M. J., Wu M. T., Taub D., Peterson E. R., Ikeler T. J., ten Broeke J., Payne L. G. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980 Nov 20;288(5788):280–283. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- Rix E., Ganten D., Schüll B., Unger T., Taugner R. Converting-enzyme in the choroid plexus, brain, and kidney: immunocytochemical and biochemical studies in rats. Neurosci Lett. 1981 Mar 10;22(2):125–130. doi: 10.1016/0304-3940(81)90075-6. [DOI] [PubMed] [Google Scholar]
- Roques B. P., Fournié-Zaluski M. C., Soroca E., Lecomte J. M., Malfroy B., Llorens C., Schwartz J. C. The enkephalinase inhibitor thiorphan shows antinociceptive activity in mice. Nature. 1980 Nov 20;288(5788):286–288. doi: 10.1038/288286a0. [DOI] [PubMed] [Google Scholar]
- Saavedra J. M., Fernandez-Pardal J., Chevillard C. Angiotensin-converting enzyme in discrete areas of the rat forebrain and pituitary gland. Brain Res. 1982 Aug 12;245(2):317–325. doi: 10.1016/0006-8993(82)90814-9. [DOI] [PubMed] [Google Scholar]
- Schwarcz R., Hökfelt T., Fuxe K., Jonsson G., Goldstein M., Terenius L. Ibotenic acid-induced neuronal degeneration: a morphological and neurochemical study. Exp Brain Res. 1979 Oct;37(2):199–216. doi: 10.1007/BF00237708. [DOI] [PubMed] [Google Scholar]
- Singh E. A., McGeer E. G. Angiotensin converting enzyme in kainic acid--injected striata. Ann Neurol. 1978 Jul;4(1):85–86. doi: 10.1002/ana.410040116. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Kapiloff M. S., Snyder S. H. [3H]Captopril binding to membrane associated angiotensin converting enzyme. Biochem Biophys Res Commun. 1983 May 16;112(3):1027–1033. doi: 10.1016/0006-291x(83)91721-7. [DOI] [PubMed] [Google Scholar]
- Unnerstall J. R., Niehoff D. L., Kuhar M. J., Palacios J. M. Quantitative receptor autoradiography using [3H]ultrofilm: application to multiple benzodiazepine receptors. J Neurosci Methods. 1982 Jul;6(1-2):59–73. doi: 10.1016/0165-0270(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Wigger H. J., Stalcup S. A. Distribution and development of angiotensin converting enzyme in the fetal and newborn rabbit. An immunofluorescence study. Lab Invest. 1978 May;38(5):581–585. [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. A new method for receptor autoradiography: [3H]opioid receptors in rat brain. Brain Res. 1979 Dec 28;179(2):255–270. doi: 10.1016/0006-8993(79)90442-6. [DOI] [PubMed] [Google Scholar]