Abstract
Ceruloplasmin (ferroxidase) is a copper-binding protein known to promote Fe2+ oxidation in plasma of mammals. Besides its classical ferroxidase activity, ceruloplasmin is known to catalyze the oxidation of various substrates, such as amines and catechols. Assays based on cyclic hydroxylamine oxidation are used to quantify and detect free radicals in biological samples ex vivo and in vitro. We show here that human ceruloplasmin promotes the oxidation of the cyclic hydroxylamine 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride (CPH) and related probes in Chelex-treated phosphate buffer and rat serum. The reaction is suppressed by the metal chelators DTPA, EDTA and Desferal, while heparin and bathocuproine have no effect. Catalase or SOD additions do not interfere with the CPH-oxidation yield, demonstrating that free radicals are not involved in the CPH oxidation mediated by ceruloplasmin. Plasma samples immunodepleted of ceruloplasmin have lower levels of CPH oxidation, which confirms the role of ceruloplasmin (ferroxidase) as a biological oxidizing agent of cyclic hydroxylamines. In conclusion, we show that the ferroxidase activity of ceruloplasmin is a possible biological source of artifacts in the cyclic hydroxylamine-oxidation assay used for ROS detection and quantification.
Keywords: Ceruloplasmin, Free radicals, Electron Spin and Paramagnetic Resonance, Spin probes
INTRODUCTION
Free radicals and reactive oxygen species (ROS) are involved in physiological and pathological responses of living organisms [1]; various strategies have been employed in attempts to detect and quantify these species [2–4]. However, this task has been shown to be far from simple because of the low steady-state concentrations and short half-lives of free radicals in vivo [5].
Electron paramagnetic resonance (EPR) is the primary and most unambiguous technique for detection of free radicals, although it has low sensitivity relative to the reactivity of most free radicals. Recently, diamagnetic cyclic hydroxylamines, which can be oxidized by ROS to paramagnetic nitroxides, have been proposed as probes suitable to quantify free radicals [3]. It is known that this approach lacks specificity regarding the oxidant/free radical that oxidizes the probe and the chemical identity of the possible free radical [4]; thus, additional experiments must be carried out to validate these measurements [3]. One typical example is the use of the cyclic hydroxylamine oxidation assay in cellular systems in vitro for superoxide detection. Those studies use the total nitroxide formation in the presence of DTPA minus that in samples containing SOD to estimate specifically the level of superoxide production [6–8].
Although the use of spectroscopic probes seems to be a simple means for the detection and quantification of ROS in cellular systems, there are other inherent limitations of this methodology and many sources of artifacts, such as: (i) accessibility and adequate concentration of the probes at cellular sites of ROS production; (ii) perturbation of the system studied by the probes; (iii) production of ROS by the probes themselves; (iv) low stability of some probes in the intracellular milieu and/or products formed that may be additionally metabolized in cellular systems; and (v) undesired probe reactions in complex biological systems. A point of considerable concern is the potential reduction or oxidation of spectroscopic probes by redox-active components of biological systems without ROS involvement [4].
Ceruloplasmin (ferroxidase, EC 1.16.3.1) is a blue-copper plasma glycoprotein that binds up to 7 copper atoms. This protein is a member of the multicopper oxidase family, such as the well-known laccase and ascorbate oxidase. Although it exhibits structural homology to the copper oxidases, it was first described because it binds approximately 95% of the total circulating copper and is strongly related to metal homeostasis of iron and copper [9]. Among the different functions of ceruloplasmin [10], the most important in vivo is its ferroxidase activity that promotes Fe2+ one-electron oxidation coupled to the reduction of molecular oxygen directly to water [10, 11]. This activity is known to be fundamental for iron incorporation into transferrin and, therefore, for iron homeostasis [9]. Patients diagnosed with aceruloplasminemia, a rare genetic disease that is characterized by a 50% reduction of ceruloplasmin levels in plasma, have higher intracellular-hepatic levels of iron and generally develop neurological degenerative diseases due to iron deprivation in the nervous-system cells [9, 12]. It is generally known that ceruloplasmin also exhibits other oxidase activities directed to a wide range of substrates such as aromatic amines, phenols and catecholamines [13–15]. Enzymatic competition kinetic experiments [14, 15] and recent X-ray crystallographic studies [16] showed that there are different binding sites in ceruloplasmin for different categories of compounds.
Ceruloplasmin is a positive acute-phase protein, which means that its level in plasma is elevated in disorders accompanied by inflammation [17, 18]. Higher serum levels of ceruloplasmin are associated with different pathological and physiological states (Table 1), such as during viral infections [19], rheumatoid arthritis [20], alcoholic liver steatosis [21] and nonalcoholic steatohepatitis [22], cardiovascular diseases [23–28], cancer [29–31], diabetes type 1 and 2 [32–34], other diseases [35–37] and pregnancy [38]. It is noteworthy that the pathological conditions are associated with higher ROS formation [39–45].
Table 1.
Human physiological or pathological states associated with increased levels of ceruloplasmin.
| Physiological/ Pathological state | Fold increase in serum levels of ceruloplasmin |
Ref. |
|---|---|---|
| Rheumatoid arthritis | 1.62 | [20] |
| Alcoholic liver steatosis | 1.39 | [21] |
| Non-alcoholic steatohepatitis | 1.30 | [22] |
| Coronary artery disease | 1.61 | [23] |
| Cardiovascular disease | 1.83 | [28] |
| Lymphocytic leukemia | 2.25 | [31] |
| Diabetes | Without diabetic complications: 1.37 | [32] |
| With diabetic complications: 1.66 | ||
| Schizophrenia | 1.19 | [35] |
| Lupus | 1.51 | [36] |
| Metabolic syndrome, insulin resistance | 1.17 | [37] |
| Pregnancy | 3.3 | [38] |
In this work, we have investigated the ability of ceruloplasmin (ferroxidase) to catalyze the oxidation of cyclic hydroxylamine probes, with the aim of unmasking possible artifacts occurring during ROS measurements using this methodology. Here we show that ceruloplasmin (ferroxidase) mediates the CPH-nitroxide formation in serum or Chelex-treated phosphate buffer samples in a non-free radical-mediated process through the recycling of contaminant trace iron present ubiquitously in the solutions; thus, we reveal a possible relevant source of artifacts in the hydroxylamine-oxidation assays used for ROS detection.
MATERIALS AND METHODS
Chemicals
The spin probes 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride (CPH), 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride (CMH), 1-hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine hydrochloride (PPH), 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine hydrochloride (TEMPONE-H), 1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium chloride hydrochloride (CAT1-H), 1-hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine hydrochloride (TMH), and N-(1-hydroxy-2,2,6,6-tetramethylpiperidin-4-yl)-2-methylpropanamide hydrochloride (TMTH) were obtained from Alexis Biochemicals (San Francisco, CA, USA). Human ceruloplasmin (EC 1.16.3.1), diethylenetriaminepentaacetic acid (DTPA), deferoxamine mesylate salt (Desferal), ethylenediaminetetraacetic acid (EDTA), heparin sodium salt, bathocuproine disulfonic acid, ammonium iron(II) sulfate, and o-dianisidine dihydrochloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). Catalase from beef liver, 65,000 U/mg crystalline suspension in water (EC 1.11.1.6) and SOD from bovine erythrocytes, 5000 U/mg lyophilized (EC 1.15.1.1), were obtained from Roche Molecular Biochemicals (Indianapolis, IN, USA). Polyclonal antibody antihuman ceruloplasmin, affinity purified, from goat was obtained from Bethyl Laboratories (Montgomery, TX, USA). A coimmuniprecipitation kit and polyacrylamide desalting columns were obtained from Pierce®;, Thermo Scientific (Rockford, IL, USA). Chelex 100 resin was purchased from Bio-Rad Laboratories (Hercules, CA, USA). All the reactions were carried out in 100 mM sodium phosphate buffer, pH 7.4. The buffer was treated with Chelex 100 resin to diminish the concentration of transition metal ions and thereby minimize trace-metal interference.
Preparation of CPH, CMH, PPH, TEMPONE-H, CAT1-H, TMH, TMTH stock solutions
Stock solutions of CPH, CMH, PPH, TEMPONE-H, CAT1-H, TMH, TMTH (2 mM) in Chelex-treated 100 mM phosphate buffer, pH 7.4, were prepared daily shortly before use.
Animals
CD male rats weighing 200–250 g (Charles River Breeding Laboratories, Raleigh, NC, USA) were used. Rats were housed in a room with air conditioning and a 12/12h light/dark cycle, fed a standard rat chow (NIH open formula, Ziegler Brothers, Gardner, PA, USA), and had access to water ad libitum. All studies were approved by the institutional review board and adhered to NIH guidelines for the care and handling of experimental animals.
Ex vivo studies with rat serum samples
Animals were deep-anesthetized using pentobarbital injected ip (50–75 mg/kg). After confirmation of a deep anesthetic state, the heart was exposed and blood was drawn with a needle and syringe. The blood was allowed to clot in silicone-coated vacutainers for 30 min at 37°C followed by 3h at 4°C. Vacutainers were centrifuged (2000 rpm for 10 min at 4°C) and the serum was collected and frozen for later analysis.
Measurement of rat plasma ceruloplasmin levels
Plasma activity of ceruloplasmin was measured by the method of Ravin [46] modified by Schosinsky et al. [47]. Briefly, ceruloplasmin activity was measured using the difference in the absorbance of the oxidized o-dianisidine in samples incubated for 5 and 20 min (Є540nm = 9.6 mM−1cm−1, after acid addition).
Ceruloplasmin serum immunodepletion
Serum samples were depleted of ceruloplasmin using a polyclonal antibody antihuman ceruloplasmin, affinity-purified, produced in goats (Bethyl Laboratories, Montgomery, TX, USA) and a commercial kit for coimmunoprecipitation according to the instructions of the manufacturer (Pierce®, Thermo Scientific, Rockford, IL, USA). Briefly, immunoaffinity columns for ceruloplasmin were prepared by covalent immobilization of the purified antibody onto a funtionalized-agarose support (AminoLink® Plus). Control samples were prepared in parallel using a control-agarose resin provided in the kit. Columns prepared with 50 µL of resin were incubated for 24h with 200 µL of serum diluted 1:1 (V/V) with water. The flow-through was collected by centrifugation (2000 g for 30 s) and used for ceruloplasmin activity measurements and the cyclic hydroxylamine oxidation assay.
Electron paramagnetic resonance experiments
EPR spectra were recorded on a Bruker EMX EPR spectrometer equipped with an ER 4122 SHQ cavity (Billerica, MA, USA). The following settings were used: microwave power, 20 mW; modulation amplitude, 1 G; magnetic field modulation, 100 kHz; conversion time, 163.84 ms; and time constant, 40.96 ms. Relative EPR signal intensity was measured from the first integral area of the first line of the recorded nitroxide spectra. All experiments were incubated for 10 min at 25°C.
Statistical analysis
All experiments were carried out in duplicate or triplicate each day and repeated twice on different days. Data were means ± SEM. Data were analyzed using a Student's t-test. Differences were considered significant when P < 0.05.
RESULTS
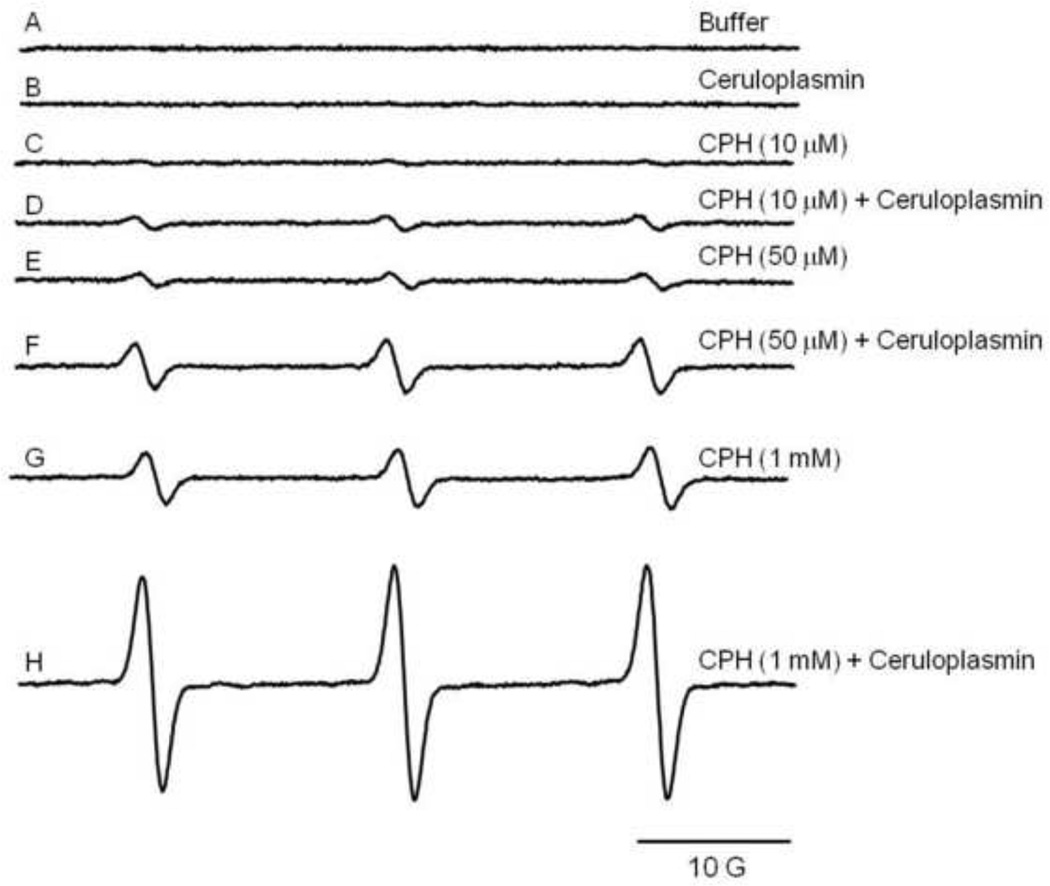
Samples containing ceruloplasmin (1 µM) and the hydroxylamine CPH (10 µM, 50 µM or 1 mM) produced EPR signals (Fig. 1D, F, H) in a CPH concentration-dependent manner. Control studies demonstrated that a much weaker signal was observed in the absence of ceruloplasmin (Fig. 1C, E, G). The signal observed when no ceruloplasmin was added to the incubation mixture was probably the result of trace quantities of CPH nitroxide present in the stock solution or due to the oxidation of the probe by trace metals still present in the Chelex-treated buffer. In fact, autoxidation of the hydroxylamine stock solution prepared in Chelex-treated buffer and incubated in ice resulted in an increase of 10 ± 2% in the area of the nitroxide every 3h. All the spectra shown in all the figures were recorded using the same probe solution, and samples were prepared in time to have all the samples scanned in less than 2h. Because spectra with good signal-to-noise were obtained with 1 mM CPH, the investigation was pursued using this concentration [48, 49].
Figure 1.
Ceruloplasmin catalyzes CPH-nitroxide formation in Chelex-treated phosphate buffer. Shown are representative EPR spectra of samples containing (A) buffer, (B) 1 µM ceruloplasmin, (C) 10 µM CPH and (D) in the presence of 1 µM ceruloplasmin, (E) 50 µM CPH and (F) in the presence of 1 µM ceruloplasmin, (G) 1 mM CPH and (H) in the presence of 1 µM ceruloplasmin.
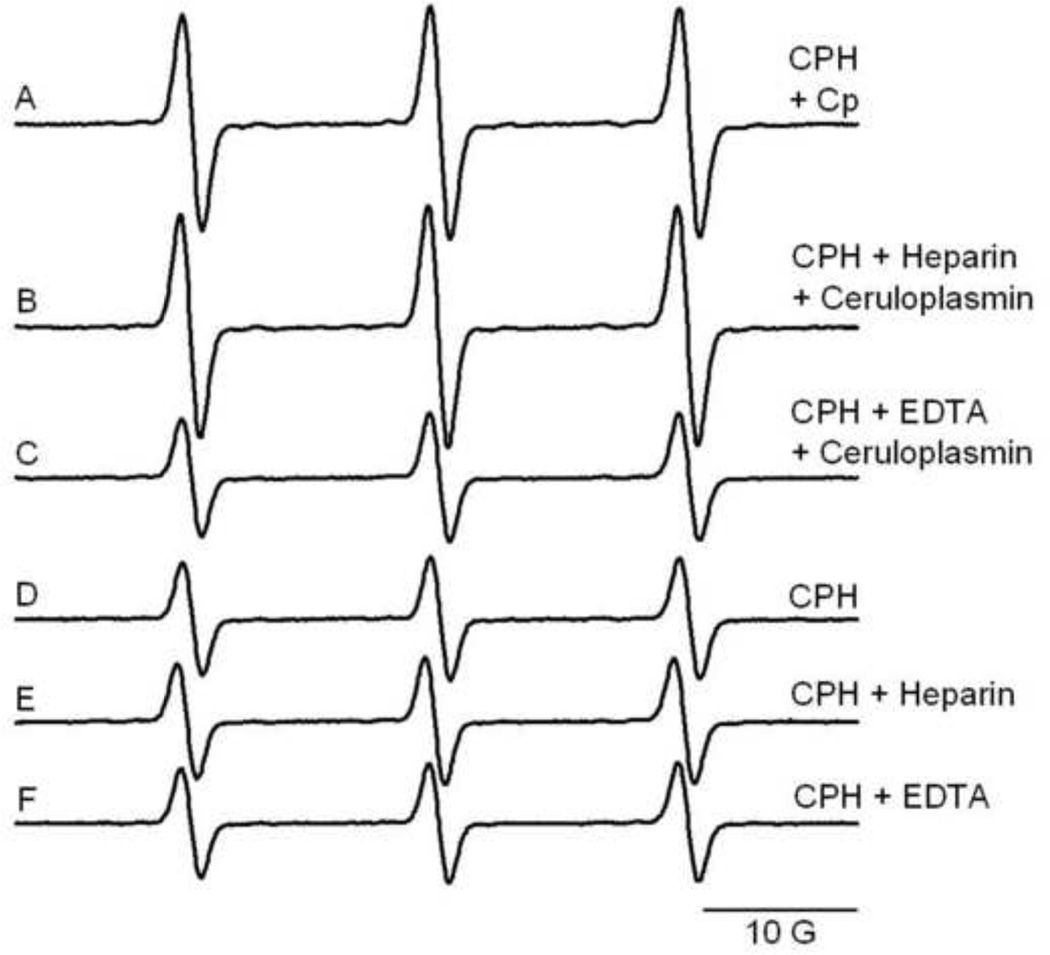
In the literature, the use of CPH as a spin probe for free radical quantification is most often used with plasma and blood samples. The possible interference of heparin and EDTA, the most common additives used for plasma collection, were evaluated in samples of CPH (1 mM) and ceruloplasmin (1 µM) (Fig. 2). The ceruloplasmin-dependent signal was not affected by heparin addition (Fig. 2B), in contrast to the effect of EDTA which inhibited the reaction (Fig. 2C). In the absence of ceruloplasmin, neither heparin (Fig. 2E) nor EDTA (Fig. 2F) had any effect.
Figure 2.
Effect of heparin and EDTA on CPH-nitroxide formation catalyzed by ceruloplasmin in samples prepared with Chelex-treated phosphate buffer. Representative EPR spectra of samples containing (A) 1 mM CPH and 1 µM ceruloplasmin, and in the presence of (B) Heparin (50 U/mL) or (C) EDTA (200 µM) are shown. Control samples in the absence of ceruloplasmin (D, E and F) are shown.
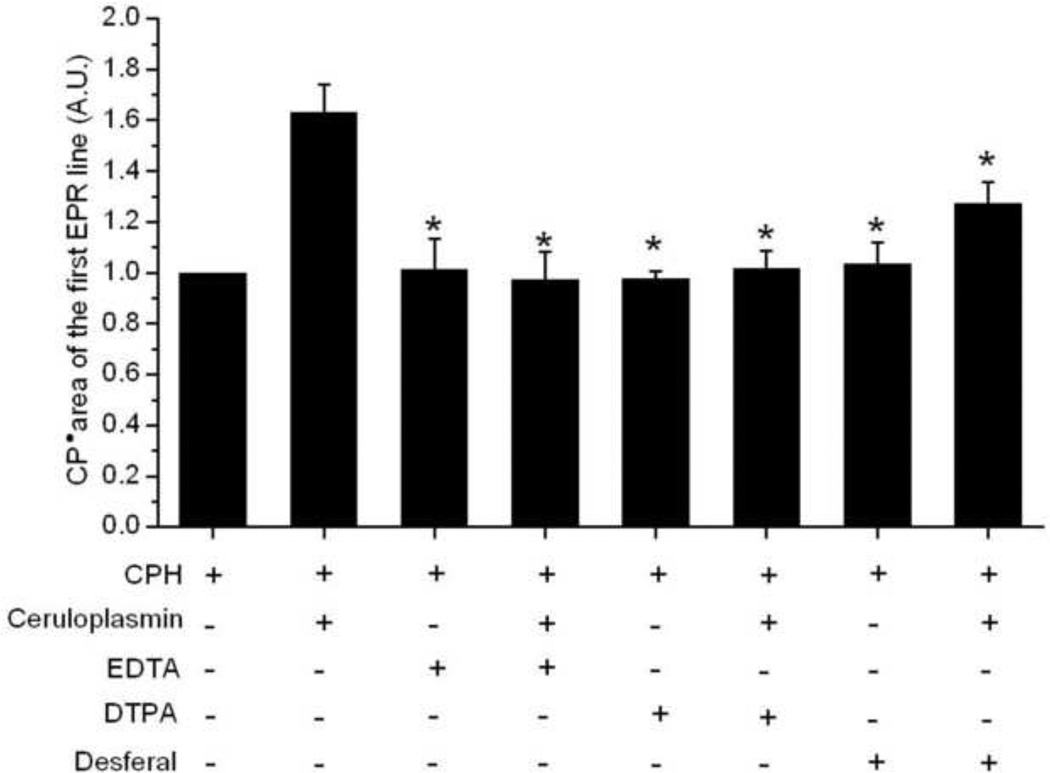
Besides EDTA, other metal chelators, DTPA and Desferal, significantly inhibited the CPH-nitroxide formation mediated by ceruloplasmin (Fig. 3) but, again, had no effect on CPH nitroxide formation in samples with CPH alone. The same behavior was observed for the other cyclic hydroxylamines tested: CMH, PPH, TEMPONE-H, CAT1-H, TMH and TMTH (1 mM) (data not shown).
Figure 3.
Effect of different transition metal chelators on the CPH-nitroxide formation catalyzed by ceruloplasmin in samples prepared with Chelex-treated phosphate buffer. Spectra were taken for reaction mixtures containing CPH (1 mM), or CPH (1 mM) and ceruloplasmin (1 µM) in the presence of the transition metal chelators EDTA, DTPA and Desferal (200 µM). The areas of the first EPR line were normalized to the area of the sample containing only 1 mM CPH. * P < 0.05, compared to the area of the sample CPH + ceruloplasmin.
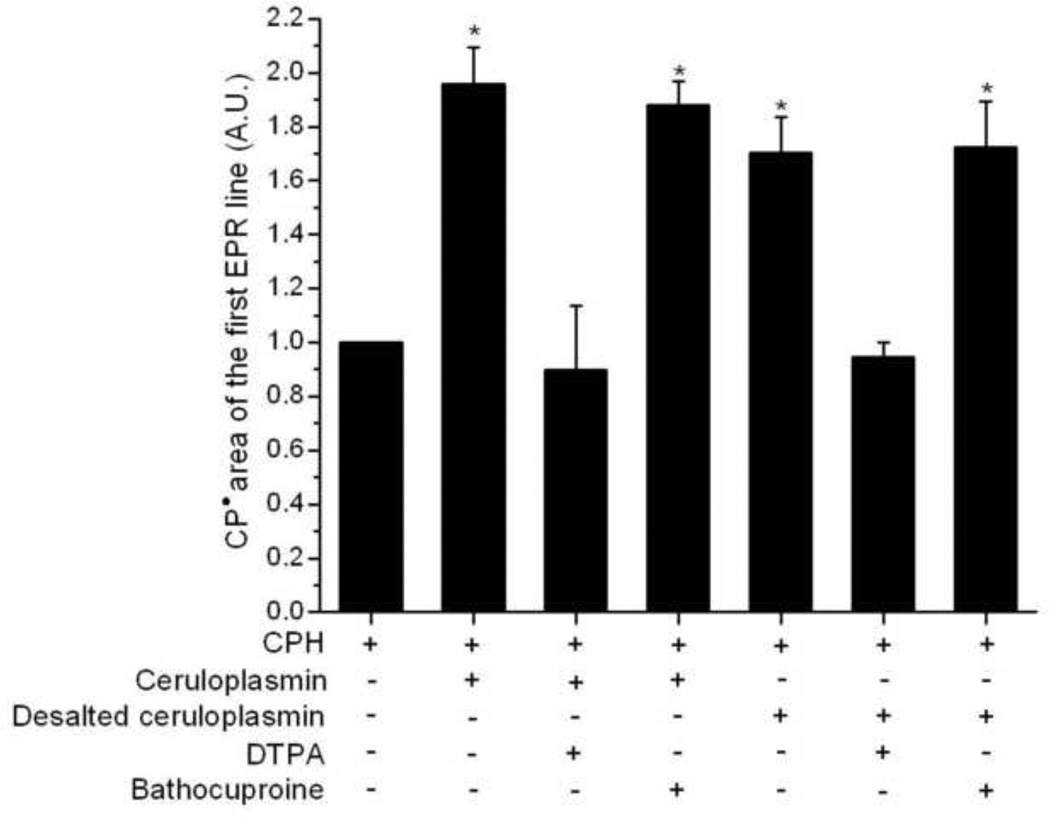
The possible contamination of copper in our ceruloplasmin solution was investigated using bathocuproine, a chelator with high affinity for Cu(I) [50]. We also prepared a desalted solution of ceruloplasmin to investigate the possible interference of unbound and loosely bound copper, or loosely bound iron, in the ceruloplasmin used. Reactions carried out with the desalted ceruloplasmin or prepared with bathocuproine showed no inhibition in ceruloplasmin-dependent CPH-nitroxide formation, in contrast to the effect of DTPA addition (Fig. 4).
Figure 4.
Effect of transition metal ion chelators in the CPH-nitroxide formation catalyzed by ceruloplasmin and desalted ceruloplasmin in samples prepared with Chelex-treated phosphate buffer. Spectra were taken for reaction mixtures containing CPH (1 mM) and ceruloplasmin (2 µM) in the presence of DTPA (200 µM) or bathocuproine (200 µM). The areas of the first EPR line were normalized to the area of the sample containing only 1 mM CPH. Statistically significant differences compared to the sample of only CPH are shown (* P < 0.05).
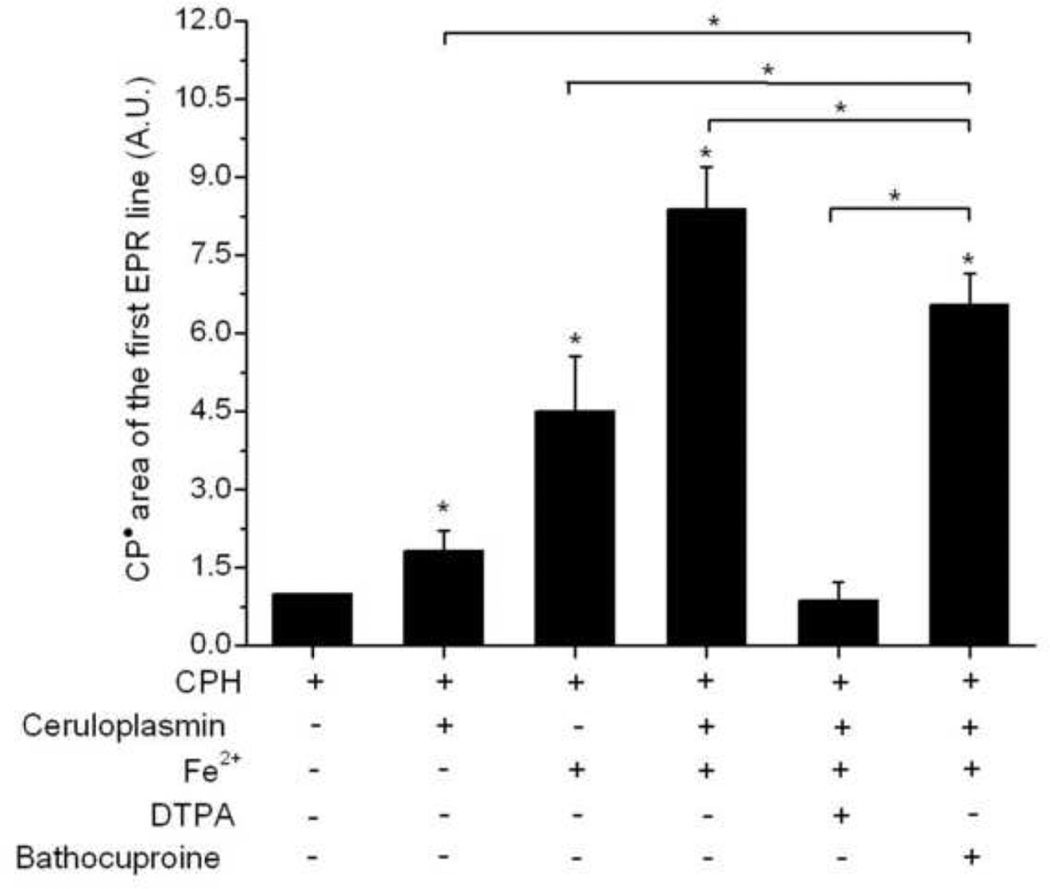
The effect of iron in the ceruloplasmin (ferroxidase)-catalyzed oxidation of CPH was studied in samples with and without DTPA and bathocuproine (Fig. 5). This metal induced significantly higher levels of CPH-nitroxide formation in samples without ceruloplasmin (4.5 times higher than the control), but this effect was significantly higher in the presence of the ferroxidase (8.5 times compared to the control). The ceruloplasmin-dependent enhancement of the iron-mediated CPH oxidation was completely blocked by DTPA (200 µM), in contrast to the modest effect of bathocuproine (200 µM). Indeed, this chelator is well-known for its high affinity to Cu(I), but has a weak complexation with different metals, such as iron [50, 51].
Figure 5.
The effect of iron (Fe2+), DTPA and bathocuproine in the CPH-nitroxide formation in samples of ceruloplasmin. Spectra were taken for reaction mixtures containing CPH (1 mM) and ceruloplasmin (2 µM) in the presence of Fe2+ (0.1 µM), with or without DTPA (200 µM) or bathocuproine (200 µM). The areas of the first EPR line were normalized to the area of the sample containing only 1 mM CPH. Asterisks on the top of the bars represent statistically significant differences compared to the sample of only CPH, whereas brackets show statistically significant differences between the groups pointed out (* P < 0.05).
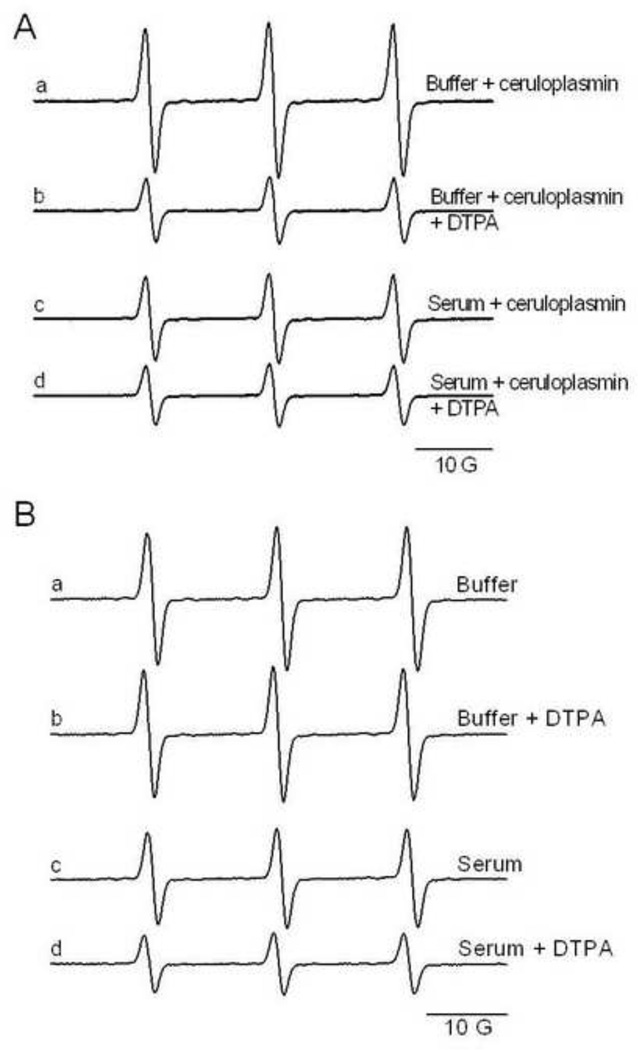
Samples prepared with serum showed the same behavior as samples prepared with Chelex-treated buffer, in which CPH oxidation was increased by ceruloplasmin (0.25–4 µM, Supp. Fig. 1). As in Chelex-treated phosphate buffer, the increased CPH-nitroxide formation in serum samples with added ceruloplasmin was blocked by the metal chelator DTPA (200 µM) (Fig. 6A, lines b and d). At the same time, it is obvious that serum samples with added ceruloplasmin had smaller signals than samples prepared with Chelex-treated buffer (Fig 6A lines a and c, Supp. Fig. 1), perhaps due to lower concentrations of nonchelated trace metals present in the serum.
Figure 6.
Effect of ceruloplasmin and DTPA in the CPH-nitroxide formation in Chelex-treated phosphate buffer or rat serum. In (A) reaction mixtures were composed of 1 µM ceruloplasmin and1 mM CPH prepared with Chelex-treated phosphate buffer or rat serum, with and without DTPA (200 µM). In (B) samples were prepared as in (A) but without ceruloplasmin. The spectra were recorded after incubation for 10 min at 25°C.
Catalase (650 U/mL) and/or SOD (50 U/mL) did not affect the formation of CPH nitroxide mediated by ceruloplasmin in Chelex-treated buffer or rat serum (Supp. Fig. 2). It proves that neither H2O2 nor superoxide anion is involved in the oxidation of CPH mediated by ceruloplasmin.
As already mentioned, the basal level for CPH oxidation (samples without ceruloplasmin addition) was not changed by the addition of metal chelators in the samples prepared in Chelex-treated buffer (Fig. 6B, lines a and b), but it was indeed inhibited in samples of serum in which ceruloplasmin was already present (Fig. 6B, lines c and d) and is consistent with CPH oxidation by the endogenous ferroxidase.
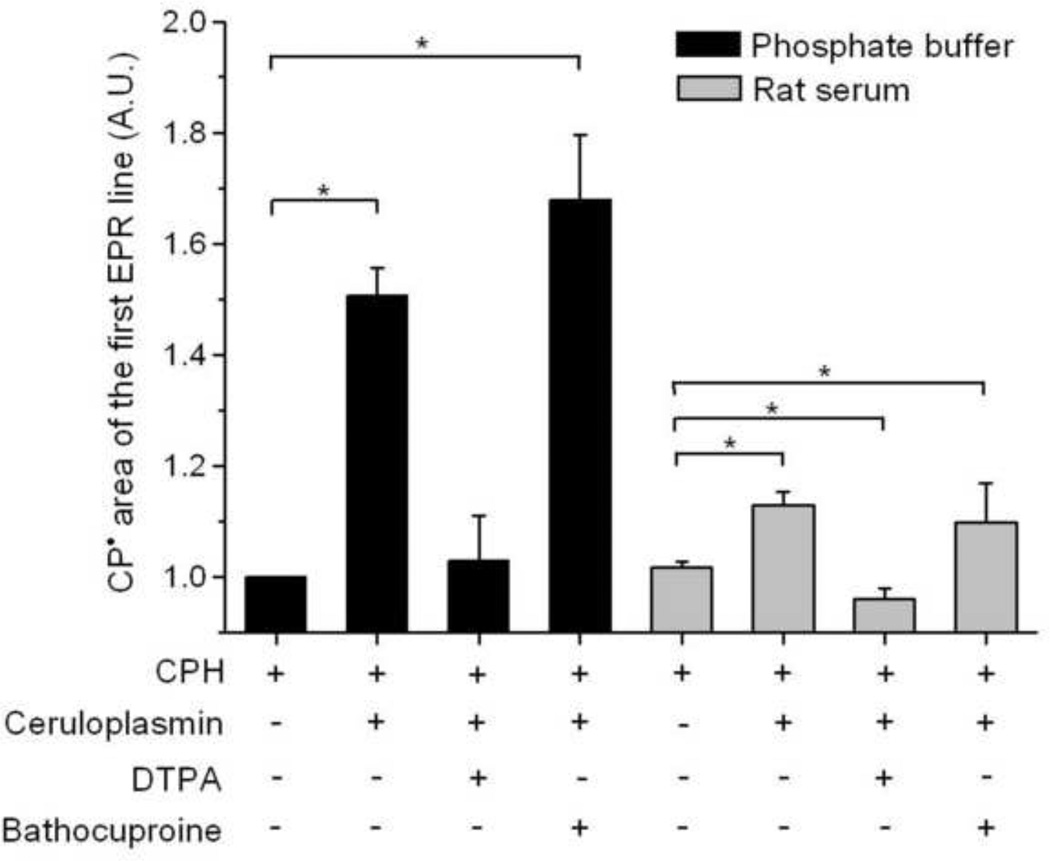
Samples prepared with Chelex-treated buffer or rat serum were compared for the effect of DTPA and bathocuproine on ceruloplasmin-mediated CPH-nitroxide formation (Fig. 7). As expected from the previous experiments, bathocuproine did not inhibit cyclic hydroxylamine oxidation in samples prepared with rat serum or Chelex-treated buffer. Ceruloplasmin-mediated CPH-nitroxide formation in samples prepared with Chelex-treated buffer or rat serum was totally blocked by DTPA.
Figure 7.
Effect of bathocuproine and DTPA on CPH-nitroxide formation in samples of ceruloplasmin prepared with Chelex-treated phosphate buffer or rat serum. Reaction mixtures were composed of 1 mM CPH and 1 µM ceruloplasmin prepared with Chelex-treated phosphate buffer or rat serum with and without DTPA or bathocuproine (200 µM). Statistically significant differences are shown (* P < 0.05).
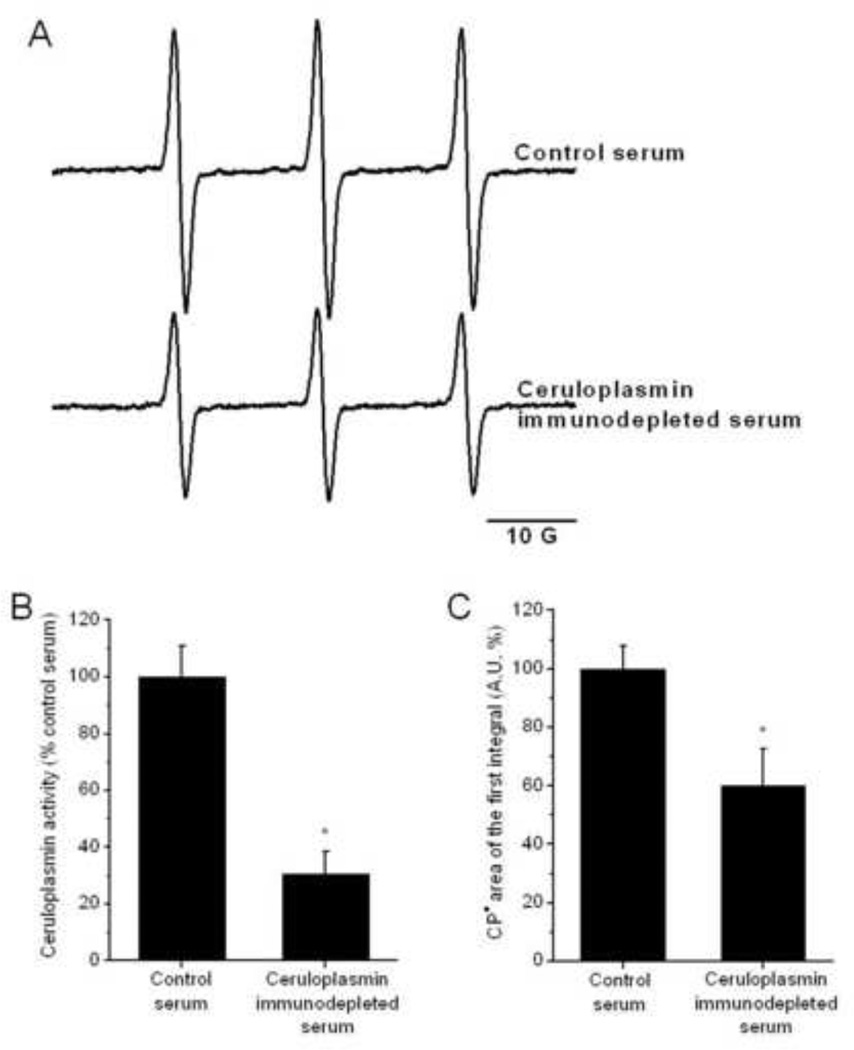
To further demonstrate the role of ceruloplasmin in the ex vivo oxidation of cyclic hydroxylamines, we prepared serum samples that were immunodepleted of ceruloplasmin (Fig. 8). The ceruloplasmin immunodepletion procedure resulted in a reduction of 69 ± 10% in the ceruloplasmin serum activity (n = 6) (Fig. 8B). CPH nitroxide formation in the ceruloplasmin-immunodepleted samples was significantly lower by 43 ± 9% (Fig. 8A, C).
Figure 8.
CPH-nitroxide yield in samples prepared with ceruloplasmin-immunodepleted rat serum. Panel (A) shows the EPR-spectra of CPH-nitroxide signals detected in samples of control and ceruloplasmin-immunodepleted rat serum with 1 mM CPH. The spectra were recorded after incubation for 10 min at 25°C. The activity of ceruloplasmin (B) was determined in the control and immunodepleted rat serum samples. The calculated areas of the CPH-nitroxide signals are shown in (C). The data in panels (B) and (C) are expressed as percentage of the average control rat serum sample measured. Statistically significant differences are shown (* P < 0.05).
DISCUSSION
ROS are known to be involved in various pathologies [39–45], and the majority of these disorders have an inflammatory basis that is known to be associated with high ceruloplasmin levels in serum (Table 1). Here we investigated the hypothesis that ceruloplasmin, a major copper-carrying protein and a ferroxidase enzyme, might be able to oxidize cyclic hydroxylamine probes, leading to possible misinterpretation of in vivo free radical formation.
Among the cyclic hydroxylamines, 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride (CPH) is easily the most popular probe for in vivo and in vitro free radical quantification [3, 48, 49, 52–55]. In fact, our in vitro studies showed increasing EPR signals in the presence of increasing concentrations of CPH and ceruloplasmin. The weaker signal observed when no ceruloplasmin was present in the system is not surprising, because it is known that even trace amounts of transition metal ions are able to catalyze the oxidation of hydroxylamines [56].
We found that H2O2 and superoxide anion are not involved in the CPH oxidation mediated by ceruloplasmin in Chelex-treated buffer or serum. It has been shown that the oxidation of different ceruloplasmin substrates, such as 6-hydroxydopamine [57] and iron [15], is coupled to the direct reduction of O2 to water.
Ceruloplasmin (ferroxidase) is unique among the multicopper oxidases because it contains three Type 1 copper sites, as well as a Type 2 copper and a Type 3 copper pair in close proximity that can constitute a ‘trinuclear’ copper cluster [58]. It is supposed that substrates are oxidized at the first center, a reaction mediated by a one-electron reduction of Cu(II). The electron is then transferred to the closely associated tricluster, which coordinates oxygen until enough electrons are present to reduce the oxygen to water [59, 60]. In contrast to other multicopper oxidases, for which the canonical substrate is well established, ceruloplasmin has the peculiar ability to utilize a number of structurally unrelated molecules as direct electron donors, including Fe(II), aromatic amines, catechols [61], and even nitric oxide [62, 63]. Employing partial removal of copper from ceruloplasmin, Musci et al. [64] showed that the three ceruloplasmin sites may be functionally active independently.
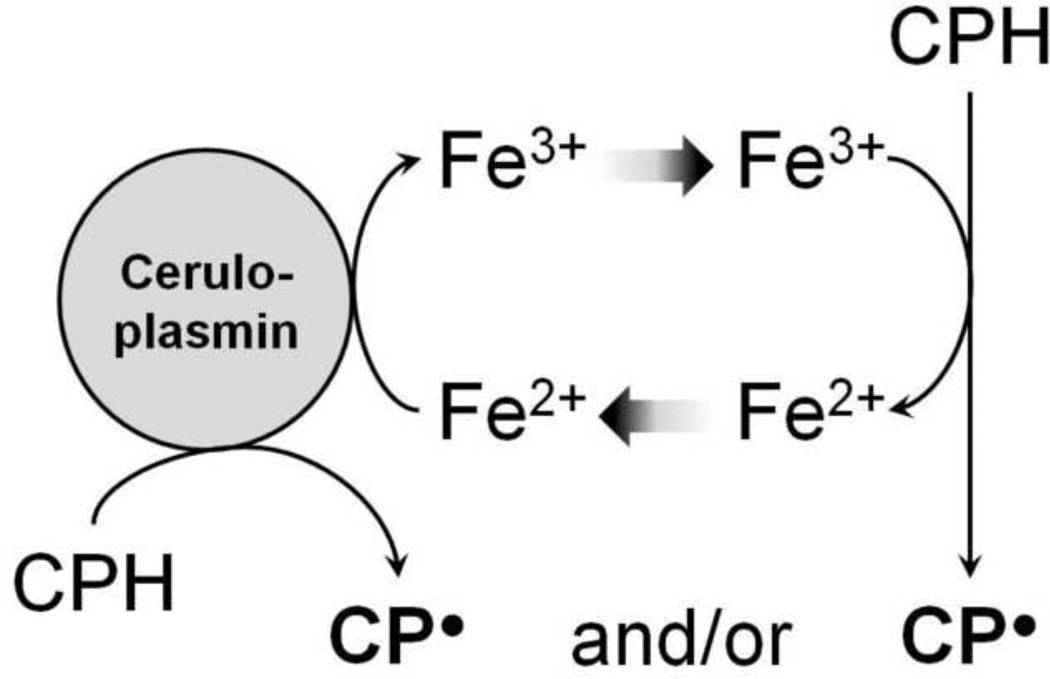
However, our results show that the CPH oxidation by ceruloplasmin is inhibited by different metal chelators (DTPA, EDTA and Desferal), demonstrating the involvement of metals in the hydroxylamine oxidation mediated by ceruloplasmin (ferroxidase). Since addition of the Cu(I) chelator bathocuproine or the use of a desalted ceruloplasmin solution did not cause inhibition in CPH-nitroxide formation in samples prepared in Chelex-treated buffer or rat serum, we conclude that our ceruloplasmin solution is essentially free of unbound or loosely bound copper. Therefore, we propose that CPH-nitroxide formation is increased through ferroxidase activity by catalyzing the oxidation of ferrous iron (Fe2+) to ferric iron (Fe3+), which ultimately oxidizes the hydroxylamine to its nitroxide (Scheme 1). Furthermore, iron addition to samples containing ceruloplasmin showed higher CPH-nitroxide yields that could be totally blocked by DTPA but not by bathocuproine. These results are corroborated by previous data of Curzon et al. [65] and McDermott et al. [15], who reported ceruloplasmin acting as a nonspecific oxidase towards various phenols, catechols, and biogenic amines.
Scheme 1.
Serum samples that were submitted to an immunodepletion procedure for ceruloplasmin showed significantly lower CPH-nitroxide formation than control samples, indicating that ceruloplasmin is one species in the serum responsible for oxidizing the hydroxylamine. This result is also supported by the inhibition of the CPH-nitroxide formation in neat serum samples with added DTPA.
Blood collection for plasma separation involves the addition of additives, such as heparin or the metal chelator EDTA. This transition metal chelator inhibited CPH oxidation, but the addition of heparin to samples with ceruloplasmin did not interfere with the CPH-nitroxide yield.
In agreement with our conclusions, previous work by Erel and co-workers [66, 67] showed that the ferroxidase activity of ceruloplasmin is highly correlated to the oxidation of alchilamine, a chromogenic amine compound used in the commercially available oxidative stress assay “d-ROM test”.
The cyclic hydroxylamine-oxidation approach to measure free radicals in serum has inherent limitations. In serum samples, ceruloplasmin (ferroxidase) can contribute to the oxidation of CPH in a non-free radical-mediated process. Since many physiological and pathological states are associated with increased serum levels of ceruloplasmin, such as those listed in Table 1, our results call into question a significant body of work where increases in the hydroxylamine-oxidation yield in the serum of patients has been interpreted as an increase in the ROS formation.
Supplementary Material
HIGHLIGHTS.
Artifactual oxidation of hydroxylamines mediated by ceruloplasmin
Trace metals are involved in the hydroxylamine oxidation mediated by ceruloplasmin
Ceruloplasmin in serum is a possible source of artifactual hydroxylamine oxidation
ACKNOWLEDGMENTS
The authors acknowledge Dr. Kalina Ranguelova and Dr. Olivier M. Lardinois for helpful discussions and Ms. Jean Corbett, Dr. Ann Motten and Ms. Mary Mason for their valuable assistance in the preparation of this manuscript. Supported by the intramural research program of the NIH/NIEHS.
LIST OF ABBREVIATIONS
- ROS
Reactive Oxygen Species
- EPR
Electron Spin and Paramagnetic Resonance
- CPH
1-Hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine hydrochloride
- CMH
1-Hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine hydrochloride
- PPH
1-Hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine hydrochloride
- TEMPONE-H
1-Hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine hydrochloride
- CAT1-H
1-Hydroxy-2,2,6,6-tetramethylpiperidin-4-yl-trimethylammonium chloride hydrochloride
- TMH
1-Hydroxy-4-methoxy-2,2,6,6-tetramethylpiperidine hydrochloride
- TMTH
N-(1-Hydroxy-2,2,6,6-tetramethylpiperidin-4-yl)-2-methylpropanamide hydrochloride
- DTPA
Diethylenetriaminepentaacetic acid
- Desferal
Deferoxamine mesylate salt
- EDTA
Ethylenediaminetetraacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Commoner B, Townsend J, Pake GE. Free radicals in biological materials. Nature. 1954;174:689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- 2.Shi H, Timmins G, Monske M, Burdick A, Kalyanaraman B, Liu Y, Clement JL, Burchiel S, Liu KJ. Evaluation of spin trapping agents and trapping conditions for detection of cell-generated reactive oxygen species. Arch. Biochem. Biophys. 2005;437:59–68. doi: 10.1016/j.abb.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 3.Dikalov SI, Dikalova AE, Mason RP. Noninvasive diagnostic tool for inflammation-induced oxidative stress using electron spin resonance spectroscopy and an extracellular cyclic hydroxylamine. Arch. Biochem. Biophys. 2002;402:218–226. doi: 10.1016/S0003-9861(02)00064-4. [DOI] [PubMed] [Google Scholar]
- 4.Bartosz G. Use of spectroscopic probes for detection of reactive oxygen species. Clin. Chim. Acta. 2006;368:53–76. doi: 10.1016/j.cca.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Mason RP, Hanna PM, Burkitt MJ, Kadiiska MB. Detection of oxygen-derived radicals in biological-systems using electron spin resonance. Environ. Health. Persp. 1994;102:33–36. doi: 10.1289/ehp.94102s1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dikalov SI, Kirilyuk IA, Voinov M, Grigor'ev IA. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic. Res. 2011;45:417–430. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dikalov SI, Li W, Mehranpour P, Wang SS, Zafari AM. Production of extracellular superoxide by human lymphoblast cell lines: Comparison of electron spin resonance techniques and cytochrome C reduction assay. Biochem. Pharmacol. 2007;73:972–980. doi: 10.1016/j.bcp.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dikalov SI, Vitek MP, Mason RP. Cupric-amyloid β peptide complex stimulates oxidation of ascorbate and generation of hydroxyl radical. Free Radic. Biol. Med. 2004;36:340–347. doi: 10.1016/j.freeradbiomed.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Hellman NE, Gitlin JD. Ceruloplasmin metabolism and function. Annu. Rev. Nutr. 2002;22:439–458. doi: 10.1146/annurev.nutr.22.012502.114457. [DOI] [PubMed] [Google Scholar]
- 10.Osaki S, Walaas O. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase. II. Rate constants at various steps and formation of a possible enzyme-substrate complex. J. Biol. Chem. 1967;242:2653–2657. [PubMed] [Google Scholar]
- 11.Gutteridge JMC. Antioxidant properties of caeruloplasmin towards iron- and copper-dependent oxygen radical formation. Febs Lett. 1983;157:37–40. doi: 10.1016/0014-5793(83)81111-9. [DOI] [PubMed] [Google Scholar]
- 12.Harris ZL, Takahashi Y, Miyajima H, Serizawa M, Macgillivray RTA, Gitlin JD. Aceruloplasminemia: Molecular characterization of this disorder of iron metabolism. P. Natl. Acad. Sci. USA. 1995;92:2539–2543. doi: 10.1073/pnas.92.7.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Løvstad RA. A kinetic study on the phenothiazine dependent oxidation of NADH by bovine ceruloplasmin. Biometals. 2006;19:1–5. doi: 10.1007/s10534-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 14.Osaki S. Kinetic studies of ferrous ion oxidation with crystalline human ferroxidase (ceruloplasmin) J. Biol. Chem. 1966;241:5053–5059. [PubMed] [Google Scholar]
- 15.Mcdermot JA, Huber CT, Osaki S, Frieden E. Role of iron in oxidase activity of ceruloplasmin. Biochim. Biophys. Acta. 1968;151:541–557. doi: 10.1016/0005-2744(68)90001-6. [DOI] [PubMed] [Google Scholar]
- 16.Zaitsev VN, Zaitseva I, Papiz M, Lindley PF. An X-ray crystallographic study of the binding sites of the azide inhibitor and organic substrates to ceruloplasmin, a multi copper oxidase in the plasma. J. Biol. Inorg. Chem. 1999;4:579–587. doi: 10.1007/s007750050380. [DOI] [PubMed] [Google Scholar]
- 17.Rice EW. Evaluation of role of ceruloplasmin as an acute-phase reactant. Clin. Chim. Acta. 1961;6:652–655. doi: 10.1016/0009-8981(61)90110-3. [DOI] [PubMed] [Google Scholar]
- 18.Gruys E, Toussaint MJ, Niewold TA, Koopmans SJ. Acute phase reaction and acute phase proteins. J. Zhejiang Univ. Sci. B. 2005;6:1045–1056. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Novikova I, Zlotnikova M. Ceruloplasmin plasma levels in patients with severe forms of herpes infection. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2011;155:361–366. doi: 10.5507/bp.2011.051. [DOI] [PubMed] [Google Scholar]
- 20.Li TW, Zheng BR, Huang ZX, Lin Q, Zhao LK, Liao ZT, Zhao JJ, Lin ZM, Gu JR. Screening disease-associated proteins from sera of patients with rheumatoid arthritis: a comparative proteomic study. Chin. Med. J. (Engl) 2010;123:537–543. [PubMed] [Google Scholar]
- 21.Uhlikova E, Kupcova V, Szantova M, Turecky L. Plasma copper and ceruloplasmin in patients with alcoholic liver steatosis. Bratisl. Lek. Listy. 2008;109:431–433. [PubMed] [Google Scholar]
- 22.Koruk M, Tayşi S, Savaş MC, Yilmaz O, Akçay F, Karakök M. Serum levels of acute phase proteins in patients with nonalcoholic steatohepatitis. Turk. J. Gastroenterol. 2003;14:12–17. [PubMed] [Google Scholar]
- 23.Engström G, Lind P, Hedblad B, Stavenow L, Janzon L, Lindgärde F. Effects of cholesterol and inflammation-sensitive plasma proteins on incidence of myocardial infarction and stroke in men. Circulation. 2002;105:2632–2637. doi: 10.1161/01.cir.0000017327.69909.ff. [DOI] [PubMed] [Google Scholar]
- 24.Engström G, Stavenow L, Hedblad B, Lind P, Eriksson KF, Janzon L, Lindgärde F. Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke: a population-based study. Diabetes. 2003;52:442–447. doi: 10.2337/diabetes.52.2.442. [DOI] [PubMed] [Google Scholar]
- 25.Lind P, Hedblad B, Stavenow L, Janzon L, Eriksson KF, Lindgärde F. Influence of plasma fibrinogen levels on the incidence of myocardial infarction and death is modified by other inflammation-sensitive proteins: a long-term cohort study. Arterioscler. Thromb. Vasc. Biol. 2001;21:452–458. doi: 10.1161/01.atv.21.3.452. [DOI] [PubMed] [Google Scholar]
- 26.Fox PL, Mazumder B, Ehrenwald E, Mukhopadhyay CK. Ceruloplasmin and cardiovascular disease. Free Radic. Biol. Med. 2000;28:1735–1744. doi: 10.1016/s0891-5849(00)00231-8. [DOI] [PubMed] [Google Scholar]
- 27.Göçmen AY, Şahin E, Semiz E, Gümüşlü S. Is elevated serum ceruloplasmin level associated with increased risk of coronary artery disease? Can. J. Cardiol. 2008;24:209–212. doi: 10.1016/s0828-282x(08)70586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awadallah SM, Hamad M, Jbarah I, Salem NM, Mubarak MS. Autoantibodies against oxidized LDL correlate with serum concentrations of ceruloplasmin in patients with cardiovascular disease. Clin. Chim. Acta. 2006;365:330–336. doi: 10.1016/j.cca.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Varela AS, Saez JJBL, Senra DQ. Serum ceruloplasmin as a diagnostic marker of cancer. Cancer Lett. 1997;121:139–145. doi: 10.1016/s0304-3835(97)00340-6. [DOI] [PubMed] [Google Scholar]
- 30.Wang KK, Liu N, Radulovich N, Wigle DA, Johnston MR, Shepherd FA, Minden MD, Tsao MS. Novel candidate tumor marker genes for lung adenocarcinoma. Oncogene. 2002;21:7598–7604. doi: 10.1038/sj.onc.1205953. [DOI] [PubMed] [Google Scholar]
- 31.Gundogdu M, Kaya H, Gulcin I, Erdem F, Cayir K, Keles M, Yilmaz A. Oxidase activity of ceruloplasmin and some acute phase reactant and trace element concentrations in serum of patients with chronic lymphocytic leukemia. Scot. Med. J. 2007;52:24–27. doi: 10.1258/rsmsmj.52.1.24. [DOI] [PubMed] [Google Scholar]
- 32.Cunningham J, Leffell M, Mearkle P, Harmatz P. Elevated plasma ceruloplasmin in insulin-dependent diabetes mellitus: evidence for increased oxidative stress as a variable complication. Metabolism. 1995;44:996–999. doi: 10.1016/0026-0495(95)90095-0. [DOI] [PubMed] [Google Scholar]
- 33.Daimon M, Susa S, Yamatani K, Manaka H, Hama K, Kimura M, Ohnuma H, Kato T. Hyperglycemia is a factor for an increase in serum ceruloplasmin in type 2 diabetes. Diabetes Care. 1998;21:1525–1528. doi: 10.2337/diacare.21.9.1525. [DOI] [PubMed] [Google Scholar]
- 34.Memişoğullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J. Diabetes Complicat. 2004;18:193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 35.Wolf TL, Kotun J, Meador-Woodruff JH. Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia. Schizophr. Res. 2006;86:167–171. doi: 10.1016/j.schres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz A, Sari RA, Gundogdu M, Kose N, Dag E. Trace elements and some extracellular antioxidant proteins levels in serum of patients with systemic lupus erythematosus. Clin. Rheumatol. 2005;24:331–335. doi: 10.1007/s10067-004-1028-y. [DOI] [PubMed] [Google Scholar]
- 37.Kim CH, Park JY, Kim JY, Choi CS, Kim Y, II, Chung YE, Lee MS, Hong SK, Lee KU. Elevated serum ceruloplasmin levels in subjects with metabolic syndrome: a population-based study. Metabolism. 2002;51:838–842. doi: 10.1053/meta.2002.33348. [DOI] [PubMed] [Google Scholar]
- 38.Louro MO, Cocho JA, Tutor JC. Assessment of copper status in pregnancy by means of determining the specific oxidase activity of ceruloplasmin. Clin. Chim. Acta. 2001;312:123–127. doi: 10.1016/s0009-8981(01)00607-6. [DOI] [PubMed] [Google Scholar]
- 39.Halliwell BG. J Free radicals, ageing and disease. In: Halliwell BJG, J, editors. Free radicals in biology and medicine. Oxford, UK: Clarendon Press; 1989. pp. 416–494. [Google Scholar]
- 40.Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr. Opin. Drug Discov. Devel. 2009;12:240–245. [PubMed] [Google Scholar]
- 41.Harrison DG, Gongora MC. Oxidative stress and hypertension. Med. Clin. North. Am. 2009;93:621–635. doi: 10.1016/j.mcna.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 42.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009;3:73–80. doi: 10.2174/187221309787158371. [DOI] [PubMed] [Google Scholar]
- 43.Mirshafiey A, Mohsenzadegan M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran. J. Allergy Asthma Immunol. 2008;7:195–202. [PubMed] [Google Scholar]
- 44.Praticò D. Evidence of oxidative stress in Alzheimer's disease brain and antioxidant therapy: lights and shadows. Ann. N. Y. Acad. Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 45.Ha H, Hwang IA, Park JH, Lee HB. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res. Clin. Pract. 2008;82(Suppl 1):S42–S45. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Ravin HA. An improved colorimetric enzymatic assay of ceruloplasmin. J. Lab. Clin. Med. 1961;58:161–168. [PubMed] [Google Scholar]
- 47.Schosinsky KH, Lehmann HP, Beeler MF. Automated determination of serum ceruloplasmin activity with o-dianisidine dihydrochloride as substrate. Clin. Chem. 1975;21:757–759. [PubMed] [Google Scholar]
- 48.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HHHW, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–2825. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 50.Hawkins CJ, Perrin DD. Oxidation-reduction potentials of metal complexes in water. Part II. Copper complexes with 2,9-dimethyl- and 2-chloro-1,10-phenanthroline. J Chem. Soc. 1963:2996–3002. [Google Scholar]
- 51.Pilipenko AT, Falendysh ER. Analytical chemistry of metal complexes with nitrogen-containing ligands of the 2,2'-bipyridyl type. Russian Chem. Rev. 1972;41:991–1008. [Google Scholar]
- 52.Dikalov S, Grigor'ev IA, Voinov M, Bassenge E. Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: quantification of extracellular superoxide radicals formation. Biochem. Biophys. Res. Commun. 1998;248:211–215. doi: 10.1006/bbrc.1998.8936. [DOI] [PubMed] [Google Scholar]
- 53.Valgimigli M, Valgimigli L, Trere D, Gaiani S, Pedulli GF, Gramantieri L, Bolondi L. Oxidative stress EPR measurement in human liver by radical-probe technique. Correlation with etiology, histology and cell proliferation. Free Radic. Res. 2002;36:939–948. doi: 10.1080/107156021000006653. [DOI] [PubMed] [Google Scholar]
- 54.Valgimigli L, Valgimigli M, Gaiani S, Pedulli GF, Bolondi L. Measurement of oxidative stress in human liver by EPR spin-probe technique. Free Radic. Res. 2000;33:167–178. doi: 10.1080/10715760000300721. [DOI] [PubMed] [Google Scholar]
- 55.Kozlov AV, Szalay L, Umar F, Fink B, Kropik K, Nohl H, Redl H, Bahrami S. EPR analysis reveals three tissues responding to endotoxin by increased formation of reactive oxygen and nitrogen species. Free Radic. Biol. Med. 2003;34:1555–1562. doi: 10.1016/s0891-5849(03)00179-5. [DOI] [PubMed] [Google Scholar]
- 56.Dikalov SI, Vitek MP, Maples KR, Mason RP. Amyloid β peptides do not form peptide-derived free radicals spontaneously, but can enhance metal-catalyzed oxidation of hydroxylamines to nitroxides. J. Biol. Chem. 1999;274:9392–9399. doi: 10.1074/jbc.274.14.9392. [DOI] [PubMed] [Google Scholar]
- 57.Medda R, Calabrese L, Musci G, Padiglia A, Floris G. Effect of ceruloplasmin on 6-hydroxydopamine oxidation. Biochem. Mol. Biol. Int. 1996;38:721–728. [PubMed] [Google Scholar]
- 58.Bielli P, Calabrese L. Structure to function relationships in ceruloplasmin: a 'moonlighting' protein. Cell Mol. Life. Sci. 2002;59:1413–1427. doi: 10.1007/s00018-002-8519-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Musci G, Polticelli F, Calabrese L. Structure/function relationships in ceruloplasmin. Adv. Exp. Med. Biol. 1999;448:175–182. doi: 10.1007/978-1-4615-4859-1_15. [DOI] [PubMed] [Google Scholar]
- 60.Malmström BG. Enzymology of oxygen. Annu. Rev. Biochem. 1982;51:21–59. doi: 10.1146/annurev.bi.51.070182.000321. [DOI] [PubMed] [Google Scholar]
- 61.Frieden EH, Hsieh HS. Ceruloplasmin: the copper transport protein with oxidase activity. In: Nriagu JO, editor. Copper in the environment, Part II. New York: John Wiley; 1979. pp. 241–284. [Google Scholar]
- 62.Inoue K, Akaike T, Miyamoto Y, Okamoto T, Sawa T, Otagiri M, Suzuki S, Yoshimura T, Maeda H. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J. Biol. Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 63.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat. Chem. Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 64.Musci G, Fraterrigo TZ, Calabrese L, McMillin DR. On the lability and functional significance of the type 1 copper pool in ceruloplasmin. J. Biol. Inorg. Chem. 1999;4:441–446. doi: 10.1007/s007750050330. [DOI] [PubMed] [Google Scholar]
- 65.Curzon G. The effects of some ions and chelating agents on the oxidase activity of caeruloplasmin. Biochem. J. 1960;77:66–73. doi: 10.1042/bj0770066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 67.Harma MI, Harma M, Erel O. d-ROMs test detects ceruloplasmin, not oxidative stress. Chest. 2006;130:1276. doi: 10.1378/chest.130.4.1276. author reply 1276–1277; [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.