Abstract
BACKGROUND AND PURPOSE
Positive allosteric modulation of α4β2 nicotinic acetylcholine (nACh) receptors could add a new dimension to the pharmacology and therapeutic approach to these receptors. The novel modulator NS9283 was therefore tested extensively.
EXPERIMENTAL APPROACH
Effects of NS9283 were evaluated in vitro using fluorescence-based Ca2+ imaging and electrophysiological voltage clamp experiments in Xenopus oocytes, mammalian cells and thalamocortical neurons. In vivo the compound was tested in models covering a range of cognitive domains in mice and rats.
KEY RESULTS
NS9283 was shown to increase agonist-evoked response amplitude of (α4)3(β2)2 nACh receptors in electrophysiology paradigms. (α2)3(β2)2, (α2)3(β4)2 and (α4)3(β4)2 were modulated to comparable extents, but no effects were detected at α3-containing or any 2α : 3β stoichiometry nACh receptors. Native nACh receptors in thalamocortical neurons similarly displayed DHβE-sensitive currents that were receptive to modulation. NS9283 had favourable effects on sensory information processing, as shown by reversal of PCP-disrupted pre-pulse inhibition. NS9283 further improved performance in a rat model of episodic memory (social recognition), a rat model of sustained attention (five-choice serial reaction time task) and a rat model of reference memory (Morris water maze). Importantly, the effects in the Morris water maze could be fully reversed with mecamylamine, a blocker of nACh receptors.
CONCLUSIONS AND IMPLICATIONS
These results provide compelling evidence that positive allosteric modulators acting at the (α4)3(β2)2 nACh receptors can augment activity across a broad range of cognitive domains, and that α4β2 nACh receptor allosteric modulation therefore constitutes a promising therapeutic approach to symptomatic treatment of cognitive impairment.
Keywords: Nicotinic acetylcholine receptors, α4 nACh receptors, β2 nACh receptors, allosteric modulation, cognitive dysfunction, therapeutics
Introduction
On consideration of results obtained with nicotinic acetylcholine (nACh) receptor agonists, there is ample evidence of pro-cognitive effects of nicotine from preclinical models as well as from clinical studies in various patient populations (Rezvani and Levin, 2001). Agonists selectively targeting either α4β2 or α7 nACh receptors, accounting for ∼80% and ∼10–15%, respectively, of all nACh receptors in the mammalian brain (Gotti et al., 2006), have been demonstrated to improve cognitive function across a range of preclinical cognition models and in smaller clinical trials (Taly et al., 2009). In addition to cognitive effects, agonists of the α4β2 nACh receptor have also been shown to possess analgesic properties in humans (Rowbotham et al., 2009). Nevertheless, inherent mechanistic aspects of nACh receptor agonist pharmacology may constrain the level of efficacy attainable with this ligand class. Firstly, the α4β2 nACh receptor has been demonstrated to undergo both short- and long-lasting/permanent forms of desensitization, the latter becoming predominant with increasing duration of agonist exposure (Fenster et al., 1999; Yu et al., 2009). Secondly, systemic administration of nACh receptor agonists would be anticipated to result in global and tonic receptor stimulation, an activation pattern, which is almost certainly different from the spatially and temporally encoded nACh receptor activation produced by endogenous cholinergic neurotransmission. The latter point is underscored by recent work from Sarter and co-workers, providing direct experimental demonstration of temporally segregated cholinergic transients being intimately involved in mediating sustained attention (Parikh et al., 2007).
From a therapeutic perspective, positive allosteric modulators (PAMs) of nACh receptors are therefore potentially of significant pharmaceutical interest. In contrast to agonists, PAMs would not lead to agonist-induced receptor desensitization and be expected to augment cholinergic signalling by increasing nACh receptor responsiveness to ACh and therefore to preserve and amplify temporal and spatial information content of endogenous receptor activation. The caveat, however, is that PAM efficacy would inherently rely upon the presence of a reasonable level of cholinergic neurotransmission, a prerequisite that may not be present in all types of cognitive disorders (e.g. severe stages of Alzheimer's disease).
Research efforts have in recent years led to the discovery of a number of small-molecule PAMs highly selective for the α7 nACh receptor and certain of these compounds have indeed been demonstrated to augment sensory gating and cognitive function in different rodent cognition models (Hurst et al., 2005; Ng et al., 2007; Timmermann et al., 2007; Faghih et al., 2009). In contrast, only a few reports on PAMs selective for the α4β2 nACh receptor have appeared (Albrecht et al., 2008; Springer et al., 2008; Weltzin and Schulte, 2010). Moreover, unequivocal evidence of pro-cognitive pharmacology of such α4β2 nACh receptor PAMs has not been reported to date.
The α4β2 nACh receptor allosteric modulator NS9283, which is a novel compound identified in a drug discovery effort by NeuroSearch A/S, was recently reported to augment analgesic properties of the α4β2 agonist ABT-594 in a range of preclinical models of various pain behaviours (Lee et al., 2011; Zhu et al., 2011). Interestingly, after administration of NS9283 alone, only minor analgesic effects were seen in one specific pain model, whereas a large leftward shift in the ABT-594 dose–response relationships was seen upon co-administrations in all the regimens tested. The overall effect of NS9283 was hence to push the analgesic efficacy window of ABT-594 towards lower doses. Importantly, the side effect profile of ABT-594 appeared unaffected by NS9283 co-administration, and since the low therapeutic window of ABT-594 has limited its use in clinical trials, this is a highly intriguing finding.
To further establish therapeutic opportunities of α4β2 PAMs, the present report provides a comprehensive in vitro characterization of NS9283 and demonstrates it to be a stoichiometry-dependent modulator of α2- and α4-containing nACh receptors that acts by increasing functional ACh potency at the receptor level. NS9283 was shown to possess pharmacokinetic properties adequate for elucidating its pharmacology after systemic administration and was found to augment cognitive function across a range of models spanning a spectrum of different cognitive sub-domains. These findings highlight the therapeutic potential of allosteric modulation of α4β2 nACh receptors in the treatment of cognitive dysfunction.
Methods
Materials
NS9283 was synthesized at NeuroSearch A/S or at Abbott Laboratories. Phencyclidine–HCl was synthesized at NeuroSearch A/S. [3H]-α-BgTx (60 Ci·mmol−1) and [3H]-cytisine (30 Ci·mmol−1) were purchased from PerkinElmer Life and Analytical Sciences (Skovlunde, Denmark). The following compounds were purchased from Sigma-Aldrich (Broenby, Denmark): mecamylamine–HCl (M9020) (–)-Nicotine (N5260), d-tubocurarine (93750), ACh (A9101), geneticin G418 (A1720), poly-d-lysine (P7405), hydroxypropyl-methyl-cellulose (H7509), 2-hydroxypropyl-β-cyclodextrin (HPβCD, 332593), kynurenic acid (K3375), GABAzine (S106), dihydro-β-erythroidine hydrobromide (DHβE), dextrose and all other salts and chemicals (of analytical grade or higher). HEK293 (CRL-1573), TE671 (CRL-8805) and GH4C1 (CCL 82.2) were from American Type Culture Collection (Manassas, VA, USA). Tissue culture flasks were from Nalge Nunc International (Fisher Scientific, Slangerup, Denmark). Ham's F-10 medium (31550-023), FBS (10270-106) and horse serum (26050-088) were from Gibco (Life Technologies, Naerum, Denmark). DMEM was from Lonza (Basel, Switzerland) (BE12-604/U1); 384-well microtitre plates were from Corning, Inc. (Fisher Scientific). LipofectAMINE PLUS™, Trypsin/EDTA, Fluo-4/AM and Zeocin (450430) were from Invitrogen (Life Technologies). Drug and molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2011).
Radioligand binding experiments
Preparation of tissue suspensions and binding to rat cortical, hippocampal or TE671 cell membranes was performed similarly to previously described procedures (Timmermann et al., 2007; Mirza et al., 2008).
[3H]-cytisine binding
In brief, the assay was performed at 1 nM [3H]-cytisine in 50 mM Tris–HCl buffer (containing in mM: 120 NaCl, 5 KCl, 1 MgCl2 and 2.5 CaCl2; pH 7.4) in a final volume of 550 µL and incubated for 90 min at 2°C in triplicate. NS9283 was tested at final concentrations ranging from 0.1 nM to 100 µM. Non-specific binding was defined using 100 µM (–)-nicotine. Saturation curves were obtained for the binding of 0.1 to 10 nM [3H]-cytisine in the absence and presence of 30 µM NS9283, and binding at each concentration was determined using three samples for total and non-specific binding, respectively. Data were fitted using a hyperbolic function assuming a single binding site for [3H]-cytisine.
[3H]-α-BgTx binding
In brief, the assay was performed at 1 nM [3H]-α-BgTx in the Tris–HCl buffer mentioned above containing 0.01% BSA in a final volume of 550 µL and incubated for 2 h at 37°C in triplicate. NS9283 was tested at final concentrations ranging from 0.1 to 100 µM. Non-specific binding was defined using 1 mM (–)-nicotine. [3H]-α-BgTx binding to cellular membrane fractions from TE671 cells was performed in a similar fashion. Non-specific binding was determined in the presence of 100 µM d-tubocurarine. NS9283 was tested at final concentrations ranging from 0.01 to 100 µM.
In all assays, binding was terminated by filtration, and the amount of radioactivity on the filters was determined by conventional liquid scintillation counting. Protein concentration was measured by the Lowry method. Data fitting was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA).
cDNA and cRNA preparation
Human cDNA clones of the α2, α3, α4, α7, β2 and β4 nACh receptor subunits were cloned and inserted into plasmid expression vectors as previously described (Timmermann et al., 2007). Cloning of cDNAs for the human α1β2γ2s GABAA receptor was as previously described (Mirza et al., 2008). For transient as well as stable expression experiments, nACh receptor cDNAs were subcloned into one of three variants of the pNS3 vector, that are NeuroSearch A/S custom-designed vectors in which the cDNA insert is linked through an IRES element to either a neomycin, hygromycin or zeocin resistance coding cassette. For preparing cRNAs, plasmids were linearized immediately downstream of the cDNA inserts and cRNA produced using the mMessage mMachine T7 Transcription kit (Ambion, Cambridgeshire, UK).
Cell culture and transfections
Cell culture and transient transfections were performed as previously described (Timmermann et al., 2007). Briefly, HEK293, TE671 and GH4C1 cells were propagated in tissue culture flasks kept at 37°C in a humidified atmosphere containing 5% CO2. The growth medium for HEK293 and TE671 consisted of DMEM supplemented with 10% FBS, whereas GH4C1 were propagated in Ham's F-10 medium supplemented with 15% horse serum and 2.5% FBS. For transient expression of hα4β2 in GH4C1, cells were seeded 1 day before their transfection in 35 mm Petri dishes containing poly-d-lysine-coated glass coverslips (Ø 3.5 mm) and transfected the following day using LipofectAMINE PLUS™ according to manufacturer's instructions. To generate clones stably expressing human α4β2 nACh receptors, HEK293 cells were seeded in T12.5 culture flasks, cultured to 50–70% confluency and transfected with a total of 1 µg of the expression plasmid mixture using LipofectAMINE PLUS™ according to manufacturer's instructions. Twenty-four hours post transfection, cells were detached using Trypsin/EDTA and seeded in T75 culture flasks with a tear-off lid. To select stably expressing cells, the culture medium was supplemented with 0.5 mg·mL−1 G418 and 0.125 mg·mL−1 Zeocin. Following selection, single clones were picked and propagated in selection media until sufficient cells for freezing were available. Once the frozen stock was secured, the cells were propagated in regular culture media.
Ca2+ imaging experiments
The Ca2+ imaging experiments were adapted to procedures previously described (Ji et al., 2007). In brief, HEK293-hα4β2 or TE671 cells were seeded in poly-d-lysine-coated 384-well microtitre plates (Corning Inc.) and allowed to proliferate for 24 h. Dye loading was performed by incubating cells with 2 µM fluo-4/AM for 1.5 h at room temperature. Dye not taken up by the cells was removed by aspiration, followed by three washing cycles with NMDG buffer (containing in mM: 140 NMDG, 5 KCl, 1 MgCl2, 10 CaCl2, 10 HEPES, pH 7.4). The microtitre plates were then placed in the FLIPR (Molecular Devices, Sunnyvale, CA, USA) and subjected to test solutions. Fitting of data was performed using GraphPad Prism.
Electrophysiology experiments
Patch clamp experiments
Electrophysiological measurements in GH4C1 cells were performed in voltage clamp using conventional whole-cell patch clamp techniques as described previously (Timmermann et al., 2007). In brief, data were obtained with an EPC-9 amplifier (HEKA) and the holding potential was −60 mV. Throughout the experiments, cells were perfused with extracellular buffer (containing in mM: 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and pH adjusted to 7.4 using NaOH). Pipettes were backfilled with intracellular buffer [containing in mM: 120 potassium gluconate, 6 KCl, 5 NaCl, 2 MgCl2, 10 HEPES, 0.5 EGTA, 2 ATP and 0.2 GTP (added immediately before use) and pH adjusted to 7.4 using KOH]. Agonist and NS9283 were delivered with an ultra fast application system using a double-barrelled application pipette (θ-tube) controlled via a piezo-ceramic device (Burleigh Instruments, Thorlabs, Newton, NJ, USA). Initial open bath pipette resistance was approximately 2 MΩ, and series resistance was compensated by 80%. Data were sampled at 20 kHz, low-pass filtered at 6.7 kHz and only accepted if the series resistance was <10 MΩ. Agonist application pulses lasted 1 s, and the stimulation frequency was 1 pulse per 30 s, to ensure full recovery of the nACh receptors from agonist-induced desensitization between pulses.
Two-electrode voltage clamp
Electrophysiological responses from Xenopus laevis oocytes were measured using the two-electrode voltage-clamp technique as described previously (Mirza et al., 2008). X. laevis frogs (Nasco, Fort Atkinson, WI, USA) were kept using standard protocols approved by NeuroSearch's Animal Care and Use Committee. Defolliculated oocytes from adult females, micro-injected with ∼25 ng cRNA 2–5 days previously were placed in custom-designed recording chambers that were continuously perfused with >2 mL·min−1 OR2 solution (containing in mM: 90 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2 and 5 HEPES; adjusted to pH 7.4). Recording electrodes were backfilled with 2 M KCl, and submerged in OR2 solution, the electrode resistances were in the range of 0.5 to 1 MΩ. Currents were amplified by Geneclamp 500B amplifiers (Molecular Devices, LLC, Sunnyvale, CA, USA), low-pass filtered at 20 Hz and digitized at 200 Hz by a Digidata 1322A (Molecular Devices). Drug solutions were applied through a capillary tube, with an inner diameter of 1.5 mm placed approximately 2 mm from the oocyte. A flow rate of 2 mL·min−1 through the capillary tube during applications ensured a rapid exchange of liquid surrounding the oocyte (in the order of a few seconds). The application length was set to last 30 s, which was sufficient to obtain peak currents. The time interval between recordings was 5 min, during which time the oocyte was perfused with OR2 in the bath and through the capillary tube. Fitting of data was performed using GraphPad Prism.
Brain slice electrophysiology
Sprague–Dawley rats postnatal age 15–23 days (2–3 weeks-old), were killed by decapitation. The brain was quickly removed and placed in ice-cold slicing solution (containing in mM: 85 NaCl, 4 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 15 glucose, 75 sucrose, adjusted to pH 7.4 with O2/CO2). Horizontal slices (300 µm) were cut using a Leica vibrotome (VT1200) and transferred into a recording solution (containing in mM: 126 NaCl, 2.5 KCl, 2 MgCl2, 2 CaCl2, 1.25 NaH2PO4, 11 glucose, 26 NaHCO3 adjusted to pH 7.4 with O2/CO2). Slices were then incubated for 30 min at 37°C and thereafter maintained oxygenated at room temperature until used. Ventrobasal thalamus was identified in slices containing the insula capsule (IC) and nuclei reticular thalami (nRT) as the light region next to the nRT was easily identified extending from the nose of hippocampus. For electrophysiological recordings, slices were transferred to a 30°C heated chamber filled with recording solution to which was added 2 mM kynurenic acid and 10 µM GABAzine. Neurons were visualised using an Olympus BX51W1 upright microscope equipped with oblique illumination, and patched on a computer monitor. Patch clamp electrodes were backfilled with an electrode solution (containing in mM: 140 CsCl, 10 HEPES, 10 EGTA, 1 CaCl2, 5 QX314-Br, 2 Mg-ATP, 0,4 Na-GTP with pH adjusted to 7.3 and osmolarity ∼300) and had serial resistance of 2–3 MΩ when submerged in recording solution. Neurons were voltage clamped to −60 mV without access resistance compensation using an EPC9 amplifier (HEKA, HEKA Elektronik GmbH, Lambrecht/Pfalz, Germany). Access resistance was approximately 4 MΩ at breakthrough and was not allowed above 12 MΩ. Recordings were sampled at 10 kHz and filtered at 3 kHz. All compounds were bath-applied by gravity flow. The maximal of current amplitudes were measured by fitting a gaussian to a point histogram of current values using clampfit. For control traces, the mean corresponded to several 20 s traces, whereas the peak current following compound additions was taken as one value fitted to one small section. Data acquisition was performed using the Patchmaster programme (HEKA). Raw data were converted to .abf file format using fitmaster (HEKA) and ABF Utility (Synaptosoft Inc., Fort Lee, NJ, USA), and analysis was done using Clampfit (Molecular Devices, LLC, Sunnyvale, CA, USA). Statistical analysis was conducted using SigmaPlot (Systat Software, Inc., San Jose, CA, USA). Data are presented as the mean ± SD.
Animals
NeuroSearch site
Male wistar rats (250–300 g; Taconic, Lille Skensved, Denmark), male Lister hooded rats (450–500 g; Charles River Laboratories, Portage, MI) and female NMRI mice (20–23 g; Harlan Laboratories, Horst, the Netherlands) were kept group housed in ventilated closed racks (Scantainer, Karlslunde, Denmark) at constant temperature (21°C) and humidity (60–70%) with a 12 h light: 12 h dark schedule (lights on at 0600 h). Food (altromin rat pellets) and water were available ad libitum except for animals used in the 5-choice serial reaction time study. These animals were on a restricted water schedule consisting of free water access for 15 min every day at least 30 min after finishing the testing. Experiments were conducted during daytime in the light phase. Animals were habituated to the experimental room approximately 24 h before the start of experiments. All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010). All experimental procedures carried out in these studies were in compliance with the European Communities Council Directive of 24. November 1986 (86/609/EEC) and approved by the Danish Animal Welfare Committee, appointed by the Danish Ministry of Justice.
Abbott site
Male Sprague–Dawley rats (Charles River Laboratories) were group-housed and acclimatized to the animal facility for at least one week before testing. Animals were tested in the light phase of a 12 h light : 12 h dark schedule (lights on at 0600 h). Animals were allowed free access to food and water except during the test period. All experimental procedures were conducted under protocols approved by Abbott Laboratories Institutional Animal Care and Use Committee (IACUC).
Pharmacokinetics
To determine the pharmacokinetic properties of NS9283, groups of naïve rats were dosed with the compound, and plasma and brain samples were collected at various time points post dosing. Animals used in i.p. injection studies were allowed free access to food, whereas animals for the p.o. administration study were fasted 16 h before dosing. NS9283 (0.1, 1 and 10 mg·kg−1) was administered to male Sprague–Dawley rats by i.p. injection. Plasma samples from three animals per time point were collected at 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h post dose; and brain samples were collected at 1, 4, 6 and 24 h post the two higher doses. Likewise, NS9283 (3 mg·kg−1) was administered i.p. to male Wistar rats; plasma samples were collected from two animals per time point at 0.5, 1, 2, 3, 4 and 6 h and brain samples at the 1 h time point. Finally, NS9283 (1 mg·kg−1) was administered p.o. to male Wistar rats; plasma samples from three animals per time point were collected at 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h and brain samples were collected at 1, 4, 6 and 24 h post dose. Plasma and brain samples were stored at −80°C until analysis. For all studies, the NS9283 formulation was prepared in 10% HPβCD resulting in a clear solution.
Sample preparation for plasma was performed by standard precipitation methods using acetonitrile or by liquid–liquid extraction using MBTE. Brain samples were homogenized using a bullet blender with zirconium beads, and the homogenates were subsequently prepared by protein precipitation using acetonitrile or by liquid–liquid extraction using MBTE. Plasma and brain samples were analysed using optimized LC-MS/MS methods, and quantification of NS9283 was performed by external calibration in blank plasma or brain matrix, respectively. The analytical methods have a calibration range of 1–1000 ng·mL−1. The pharmacokinetic parameters have been calculated using non-compartmental analysis (Pharsight WinNonLin 5.2, Munich, Germany).
Locomotor activity
Locomotor activity was evaluated in test cages (20 × 40 × 18 cm; TSE Systems GmbH, Bad Homburg, Germany) equipped with infrared sensors (6 × 2) for automatic acquisition of animal movements. Female NMRI mice were individually placed in cages and left undisturbed. After 30 min of habituation, they were administered either vehicle or NS9283, and locomotor activity was subsequently recorded via interruption of infrared sensor pairs for 120 min and processed on a computer (ActiMot software, TSE Systems). NS9283 was dissolved in 10% HPβCD and administered p.o. in a dose volume of 10 mL·kg−1. Doses correspond to the free base weight of NS9283. Statistical evaluation of drug effect on locomotor activity was performed by one-way anova with drug treatment as factor using SigmaPlot.
Rectal temperature
Core body temperature was evaluated by measuring the rectal temperature in female NMRI mice. Measurements were performed 30 min post administration of vehicle or NS9283 by inserting a lubricated thermistor probe (2 mm diameter) 20 mm into the rectum (ELLAB Instruments thermometer, Ellab A/S, Hilleroed, Denmark). The mice were hand-held at the base of the tail during this determination, and the thermistor probe was left in place until steady readings were obtained (10–15 s). NS9283 was dissolved in 10% HPβCD and administered p.o. in a dose volume of 10 mL·kg−1. Doses correspond to the free base weight of NS9283. Statistical evaluation of drug effect on core body temperature was performed by Kruskal–Wallis one-way anova on ranks with drug treatment as factor followed by Tukey's post hoc comparisons using SigmaPlot.
Social recognition
Male Sprague–Dawley adult (2–4 months; 400–450 g) and juvenile (50–60 g) rats were allowed to acclimatize to the test room for 90–120 min before starting the experiment. Following acclimatizion, adult rats were placed alone in their respective test cages and, after a brief habituation period (30 min), allowed to interact for a 5 min duration with a juvenile rat (trial; T1). During the interactive trial, the adult exhibits investigative behaviours that include close following, grooming and/or sniffing of the juvenile for as much as 40–50% of the trial duration. The time of the investigative interaction is recorded in seconds. The juvenile rat was then removed; and the adult rats were immediately administered varying doses of vehicle, NS9283 or nicotine and returned to their home cage. A second 5 min interactive trial (T2) was conducted 120 min later in the same test cage where investigative behaviour of the adult rat was again monitored and the time recorded in seconds. Recognition ratios of time spent investigating the familiar juvenile in trial two divided by time spent investigating the juvenile in trial one (T2/T1) were calculated and can be used as an index of the adult rat's recollection of the previous encounter. If recognition memory were lost over the 120 min interval between trials, the investigative behaviour would be similar for the two trials; however, if memory were retained, the ratio would be lower (<1).
NS9283 and nicotine were dissolved in 34% w v-1 HPβCD and administered i.p. at an injection volume of 1.0 mL·kg−1. Doses correspond to the free base weight of NS9283 and nicotine. Statistical differences between group means were assessed by a one-way anova with treatment as factor followed by Tukey's post hoc multiple comparison using GraphPad Prism.
Five-choice serial reaction time task
A complete test apparatus consisted of eight standard modular operant test chambers (Med Associates Inc., St. Albans, VT, USA). In each chamber, the front wall is concavely curved and contains five nose poke units with infrared detectors located across each nose poke unit and a standard yellow stimulus light mounted above each unit. On the rear wall, a dipper cup is located that can be programmed to deliver a water reward. Each chamber was contained separately in soundproof cabinets and illuminated with a standard 3 W house light positioned in the centre of the roof. MED-PC® software (SOF-700RA-8A) controlled manoeuvring of the chambers.
Water-deprived male Lister hooded rats were trained to discriminate a brief (0.5 s) visual cue randomly presented in one out of the five nose poke units. A correct response is recorded if the rat nose pokes in the unit, where the brief visual stimulus was presented, and the rat is then allowed to collect a water reward. After the reward is collected, a new trial starts with an inter-trial interval (ITI) period. If the rat pokes in another unit, or fails to respond within a 6 s hold period, an incorrect response or an omission is recorded, respectively, no reward is delivered and the house light is turned off for a 5 s time-out dark period. After this dark period, the house light is turned on again and a new trial starts with the ITI period. The following behavioural variables are recorded for each rat and further processed for statistical analysis: response accuracy: defined as % correct responses out of the total number of correct and incorrect responses; errors of omissions: defined as % omissions out of the total number of correct, incorrect and omitted responses; premature responses: defined as the number of anticipatory responses made during the ITI before the onset of the visual stimulus; response latencies: latency to respond in seconds in a unit following the onset of the visual stimulus obtained for correct and incorrect responses separately; magazine latency: defined as the latency to collect water reward in seconds following a correct response; perseverative responses: defined as the number of responses either in the same hole as the stimulus or in a different unit; head entries: defined as the number of entries in the magazine following a water reward; completed trials: defined as the total number of correct and incorrect responses. A test session lasted for 45 min and the study was conducted over four test sessions in a Latin square design with all doses represented each test day. NS9283 was dissolved in 10% HPβCD and administered i.p. 30 min before the test. (–)-Nicotine ditartrate was dissolved in 0.9% saline and administered s.c. 10 min before test start. Doses correspond to the free base weight of both compounds, and they were administered in a dose volume of 5 mL·kg−1. Testing was performed on Tuesdays and Thursdays, and the rats were allowed 2 weeks of wash-out period between testing regimes. Statistical evaluation of drug effect on behavioural variables was performed by one-way repeated-measure anova with drug treatment as factor and animal number as repeated measure followed by Fisher's LSD post hoc multiple comparisons to assess significance levels using SigmaPlot.
Morris's water maze
The study was conducted as previously described (Timmermann et al., 2007). In short, male Wistar rats were trained to locate the spatial position of a submerged platform in a water maze pool with an inner diameter of 1.6 m. The water maze was filled with water to a depth of 31 cm, and the water was kept at a constant temperature of 20 ± 1°C. The platform had a diameter of 10 cm, was made of metal wire grid and submerged 0.5–1 cm below the water surface. The room was equipped with salient visual cues that were visible in all directions as seen from the water surface of the pool. Swim paths were automatically recorded and analysed with a video tracking system (Videotrack Viewpoint, Viewpoint Life Sciences, Inc., Lyon, France). Animals were given four consecutive trials per day starting from four different positions over 4 days with a fixed platform position in the centre of the northern quadrant. For each trial, animals were placed in the water maze, with the head facing the wall of the maze, and were allowed a maximum of 60 s to locate the hidden platform. When they found the platform, they were left there for 15 s. If they did not succeed in locating the platform within 60 s, they were placed on it by the experimenter and left for 15 s. From the video recordings, the following four variables are processed for statistical analysis: distance: defined as the total distance swum in cm before reaching the platform within the time frame; % trials platform not found: defined as % of trials in which the rat did not locate the platform within the time frame; time in thigmotaxis: defined as the time spent in seconds swimming along the pool edges before reaching the platform or maximal time frame; swimming speed: defined as the average swimming speed in cm·s−1 until reaching the platform or maximal time frame. Three days before the first acquisition day, rats were divided into appropriate groups and treated once daily with V + V + V or V + P + V. For the four acquisition days, rats were in one experiment pretreated daily with: V + V + V, V + P + V, NS9283+P + V, or NS9283+P + M and in another experiment with: V + V + V, V + P + V, V + V + M or V + P + M (V: vehicle, P: PCP and M: mecamylamine). NS9283 was dissolved in 10% HPβCD, was administered p.o., whereas PCP was dissolved in 0.9% saline and administered s.c. For co-administration of PCP and mecamylamine, the compounds were dissolved in 0.9% saline, mixed and injected s.c. In all cases, a dose volume of 5 mL·kg−1 was used, and the compounds were administered 30 min before the first trial on each day. Doses correspond to the free base weight of NS9283 and salt weights of PCP and mecamylamine. Statistical evaluation of data was performed by two-way repeated-measures anova with drug treatment and days as factors followed by Fisher's LSD post hoc multiple comparisons using SigmaPlot.
Results
To identify positive allosteric modulators (PAMs) of α4β2 nACh receptors, a HEK293 cell line stably expressing human α4 and β2 nACh receptor subunits was employed in random screening of a chemical compound library. The screening assay was based on real-time Ca2+-imaging (in FLIPR) and consisted of an initial addition of test compound followed by a subsequent addition of nicotine at a low concentration (∼EC30) 3 min later. Compounds that failed to evoke a response when added alone but acted to increase the nicotine response above a pre-specified level were selected for further characterization of α4β2 PAM efficacy. One particular chemical series emerged from this screening campaign as possessing robust α4β2 PAM activity in combination with reasonable drug-like physicochemical properties and was therefore subjected to further chemical optimization. NS9283 (Figure 1A) was discovered through this effort and subjected to a comprehensive characterization.
Figure 1.
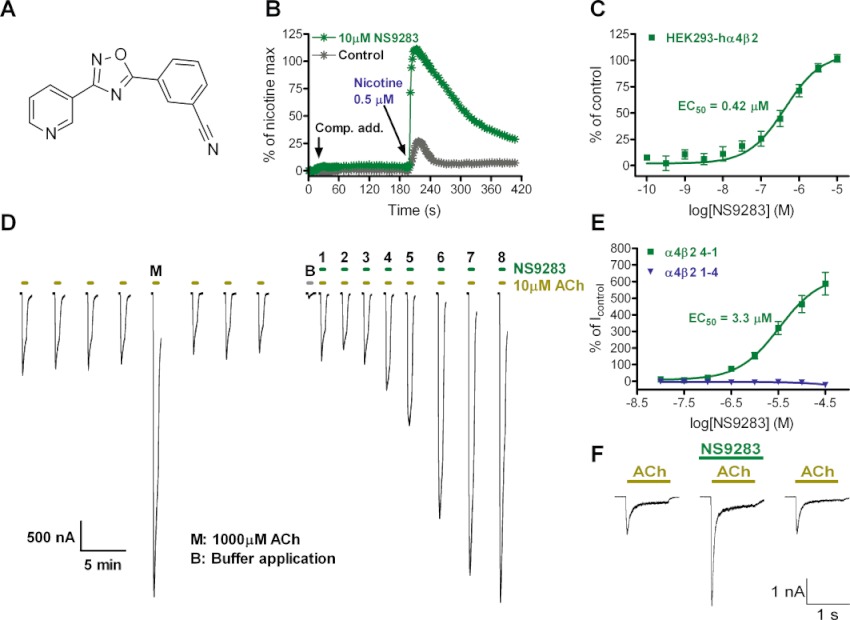
Allosteric modulation of α4β2 nACh receptors by NS9283. (A) Chemical structure of 3-[3-(3-pyridyl)-1,2,4-oxadiazol-5-yl]benzonitrile (NS9283). (B) NS9283 fails to activate α4β2 nACh receptors but increases the amplitude of nicotine-evoked Ca2+-responses. NS9283 or control buffer was added to HEK293-hα4β2 cells after a 20 s stabilization period, and a ∼EC20 concentration of nicotine was added after 200 s. Response amplitudes were background subtracted and normalized to the effect of a saturating nicotine concentration (100 µM). The traces shown represent average responses from 16 wells for each experiment. (C) Concentration–response relationship for NS9283 allosteric modulation. Nicotine-induced (0.5 µM) Ca2+-responses (baseline subtracted and normalized; presented as mean ± SEM of four experiments) were fitted to the Hill equation by non-linear regression. (D) Representative current traces of ACh and NS9283+ACh in two-electrode voltage-clamp experiments in Xenopus laevis oocytes. The oocyte was injected with α4 and β2 nACh receptor subunits in a 4:1 ratio. Application of ACh or NS9283 mixed with ACh is indicated by a bar above each trace. NS9283 concentrations were increased in half log unit increments starting with lowest concentration (10 nM) in application ‘1’ and ending with highest concentration (31.6 µM) in application ‘8’. (E) Concentration–response relationships for NS9283 modulation of ACh-evoked currents in X. laevis oocytes. Oocytes were injected with α4 and β2 nACh receptor subunits in a 1:4 or 4:1 ratio to obtain (α4)2(β2)3 or (α4)3(β2)2 receptors, respectively. Peak current amplitudes were baseline subtracted and normalized with respect to the current induced by ACh (1 µM for (α4)2(β2)3, 10 µM for (α4)3(β2)2), and are presented as mean ± SEM of 11–12 experiments. Data points for (α4)3(β2)2 were fitted to the Hill equation by non-linear regression, and the result is seen in Table 1. (F) Representative current traces from whole-cell patch-clamp experiments on human α4β2 nACh receptors transiently expressed in GH4C1 cells. Cells were clamped at −60 mV and stimulated with ACh (100 µM) in the absence, presence and after wash-out of NS9283 (10 µM), respectively.
In vitroα4β2 PAM activity
NS9283 does not activate α4β2 receptors by itself but dramatically increases the peak amplitude of the nicotine-induced Ca2+ flux in a stable HEK293-hα4β2 cell line (Figure 1B). In further experiments, this effect of NS9283 was found to be concentration-dependent, and the data could be fitted by the empirical Hill equation (Figure 1C), suggesting the presence of a specific and saturable binding site. To substantiate and characterize the α4β2 PAM effect of NS9283, the compound was tested in X. laevis oocytes using two-electrode voltage-clamp electrophysiology. It is well established that the α4β2 nACh receptor can assemble in two different subunit stoichiometries with differing functional sensitivities towards activation by ACh denoted the high-sensitivity, corresponding to (α4)2(β2)3, and the low-sensitivity, corresponding to (α4)3(β2)2, sub-forms (Nelson et al., 2003; Zhou et al., 2003). In order to test whether NS9283 displayed activity at both sub-forms, cRNA for the α4 and β2 subunits were co-expressed in 1:4 or 4:1 ratios, which has previously been shown to give uniform populations of (α4)2(β2)3 and (α4)3(β2)2 receptors, respectively (Harpsøe et al., 2011). Consistent with the results obtained using Ca2+-imaging, concentrations of NS9283 above 0.1 µM clearly increased (positively modulated) the peak current amplitudes of ACh-evoked (α4)3(β2)2 currents compared with control traces (Figure 1D). The concentration-dependence of PAM activity at this receptor could be fitted well by the Hill equation, whereas, surprisingly, there was no detectable positive modulation at the (α4)2(β2)3 receptor (Figure 1E). PAM activity of NS9283 was further confirmed in patch-clamp electrophysiology experiments on rat GH4C1 cells transiently expressing human α4β2 nACh receptors. NS9283 produced a significant increase in peak current amplitude evoked by a sub-saturating concentration of ACh (Figure 1F), without producing any detectable current activation when applied alone (data not shown).
The selectivity profile of NS9283 towards other Cys-loop receptors, in particular nACh receptors, was investigated in further oocyte electrophysiology experiments. It is presently not fully established to what extent other heteromeric (α- and β-subunit containing) nACh receptor subtypes also assemble in two equivalent stoichiometries, although there is evidence that this may be true for α2β2 and α3β2; (Khiroug et al., 2004; Briggs et al., 2006). Therefore, to accommodate this possibility, all subsequent experiments on heteromeric nACh receptor subtypes were conducted under conditions favouring either 2α : 3β or 3α : 2β receptor stoichiometries. Interestingly, NS9283 also acted as a PAM on (α2)3(β2)2, (α2)3(β4)2 and (α4)3(β4)2 receptors with roughly equivalent potency by comparison with its modulatory effect on α4β2 (Table 1). By contrast, there was no or marginal effects of NS9283 on α3-containing receptors as well as all receptor combinations expressed as 2α : 3β stoichiometries. NS9283 further failed to modulate the function of homomeric α7 nACh receptors in oocytes as well as the neuromuscular nACh receptor in Ca2+ imaging experiments on the TE671 cell line (endogenous α1β1γδ expression; data not shown) and consistent with this no significant inhibition of [3H]-α-BgTx binding to rat brain tissue or TE671 cell membranes was seen (Ki > 45 µM). Another representative member of the Cys-loop receptor family, the GABA type A receptor α1β2γ2, was also unaffected by NS9283 (Table 1). Finally, NS9283 at a concentration of 10 µM did not display detectable off-target binding affinity in a screen encompassing 30 different ion channels and GPCRs (HitProfilingScreen, MDS Pharma Services, Bothell, WA, USA).
Table 1.
Functional selectivity profile of NS9283
| Receptor1 | Ratio | EC502(µM) | Emax3(%) | n |
|---|---|---|---|---|
| α2 : β2 | 1:10 | −17 ± 2 | 12 | |
| α2 : β2 | 4:1 | 1.3 (0.84–2.1) | 1244 ± 110 | 6 |
| α2 : β4 | 1:10 | 10 ± 11 | 9 | |
| α2 : β4 | 4:1 | 1.7 (1.2–2.5) | 1678 ± 135 | 9 |
| α3 : β2 | 1:10 | −16 ± 3 | 12 | |
| α3 : β2 | 4:1 | 52 ± 19 | 6 | |
| α3 : β4 | 1:10 | −28 ± 1 | 3 | |
| α3 : β4 | 4:1 | −26 ± 4 | 9 | |
| α4 : β2 | 1:4 | −22 ± 2 | 11 | |
| α4 : β2 | 4:1 | 3.3 (2.0–5.6) | 638 ± 86 | 12 |
| α4 : β4 | 1:10 | −30 ± 4 | 6 | |
| α4 : β4 | 4:1 | 2.3 (1.8–2.8) | 460 ± 24 | 15 |
| α7 | 1 | 5 ± 5 | 6 | |
| GABAA (α1β2γ2) | 1:1:2 | 3 ± 1 | 3 |
Concentration–response relationships for NS9283 (0.01–31.6 µM) were determined for the receptor subunit combinations indicated, using Xenopus oocyte two-electrode voltage-clamp recordings. All heteromeric nACh receptor subunit combinations were expressed at two α : β ratios to yield a (possible) 3α : 2β as well as a 2α : 3β subunit stoichiometry. Test ACh-concentrations were selected to be in the vicinity of EC20, based on ACh concentration–response relationships determined for each receptor type and was: 100 µM ACh (α3β4 4:1), 31.6 µM ACh (α2β4 4:1, α3β4 1:10, α7), 10 µM ACh (α2β2 4:1, α2β4 1:10, α3β2 4:1, α4β2 4:1, α4β4 1:10, α4β4 4:1) or 1 µM ACh (α2β2 1:10, α3β2 1:10, α4β2 1:4). Test concentration for the GABAA receptor was 1 µM GABA (α1β2γ2).
Peak current modulation by NS9283 was normalized to the current response of baseline ACh-applications and baseline subtracted. Average current responses as a function of concentration were fitted to the Hill equation by non-linear regression, yielding the EC50-values with 95% confidence intervals indicated.
Maximal efficacy (Emax) of NS9283 is stated as maximal fitted efficacy ±95% confidence interval or where meaningful fitting was not possible average peak current modulation ± SEM at 31.6 µM NS9283.
Mode of action
To provide a more detailed description of its effects on α4β2 nACh receptor function, a series of additional binding, Ca2+ imaging and oocyte electrophysiology experiments were conducted with NS9283. In radioligand binding studies, NS9283 did not produce significant displacement of the nACh receptor ligand [3H]-cytisine binding to rat cortical tissue (Ki > 43 µM). Furthermore, the binding affinity (Kd) and total number of [3H]-cytisine binding sites (Bmax) were unaffected by NS9283 (control: Kd= 0.95 ± 0.15 nM and Bmax= 80 ± 9 pmol·mg−1 protein; 30 µM NS9283: Kd= 1.02 ± 0.10 nM and Bmax= 80 ± 6 pmol·mg−1 protein). These observations suggest that NS9283 interacts with a receptor site distinct from the orthosteric agonist (ACh) binding site and show that NS9283 binding does not appear to induce any measurable change in radioligand binding affinity at the orthosteric site. In Ca2+ imaging studies using the HEK293-α4β2 cell line, a robust and concentration-dependent leftward shift of the nicotine concentration–response curve was observed upon pre-incubation with increasing concentrations of NS9283 (Figure 2A). The magnitude of nicotine concentration–response EC50 left shift (the ΔpEC50 value) plotted as a function of six different NS9283 concentrations was approximated well by the Hill equation, resulting in a maximal fitted ΔpEC50= 1.7 corresponding to a ∼50-fold shift (Figure 2B). The EC50 value obtained by this method closely matches the NS9283 potency estimate derived from concentration–response testing at a fixed agonist concentration (Figure 1C). This indicates that the functional effects of NS9283 can be described as an apparent increase in agonist (nicotine) potency in these experiments, a statement that is fully consistent with the observation that NS9283 did not exert much effect on the magnitude of the maximal Ca2+ signal achievable (Figure 2A). It was next sought to corroborate these findings in the context of direct α4β2 nACh receptor current recordings in voltage-clamped oocytes with the endogenous ligand ACh. Pre-incubation of oocytes expressing (α4)3(β2)2 nACh receptors with NS9283 (31.6 µM) before agonist activation resulted in a marked leftward shift, by a factor of ∼500, in the ACh concentration–response relationship, with little change in maximal peak current amplitudes (Figure 2C). The magnitude of this agonist potency shift is roughly 10-fold greater than the shift observed in Ca2+ imaging studies for reasons that are unclear at this point; however, the functional effect of NS9283 remained consistent in both testing regimes. In agreement with the selectivity characterization described above, no effect of NS9283 was observed at (α4)2(β2)3 receptors (Figure 2D). Whereas other nicotinic receptor PAMs (e.g. α7 nACh receptor PAMs) are known to cause profound changes in receptor desensitization characteristics, inspection of current traces from patch-clamp (Figure 1F) in particular but also oocyte (Figure 1D) experiments suggest that NS9283 displays little, if any, effect on receptor desensitization kinetics. However, this property was not investigated in further detail.
Figure 2.
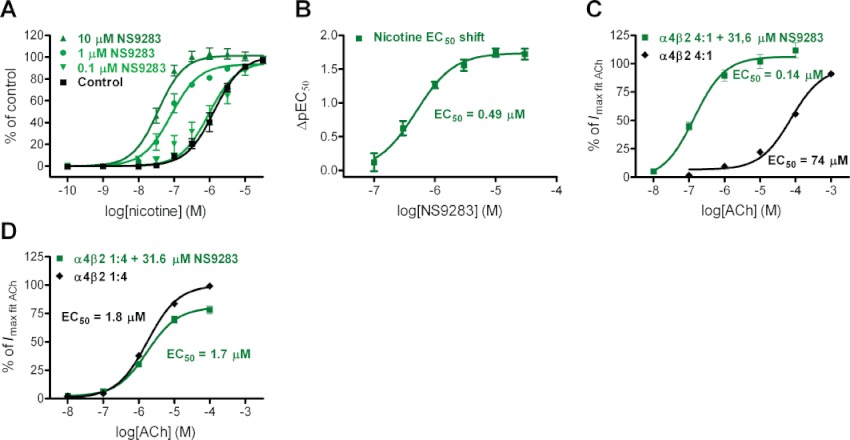
Allosteric modulation of α4β2 nACh receptors by NS9283 is conferred through increases in functional agonist potency. (A) NS9283 concentration-dependently leftward shifts the nicotine concentration–response (C–R) relationship for activating Ca2+-flux. Nicotine C-R relationships in HEK293-hα4β2 cells are shown under control conditions and at three concentrations of NS9283. Data points, baseline subtracted and normalized to the maximal nicotine (100 µM) effect, are presented as mean ± SEM of n= 4–6 experiments and are fitted to the Hill equation by non-linear regression. (B) Leftward shift in agonist C-R fully accounts for NS9283-induced modulation. The magnitude of the nicotine C–R leftward shift was quantified as the difference in log EC50 with and without NS9283 (ΔpEC50-value) and plotted as a function of NS9283 concentration. These C–R data for NS9283 were fitted well by the Hill equation. (C) NS9283 leftward-shifts the C-R relationship of ACh at the (α4)3(β2)2 nAChR in X. laevis oocytes. Oocytes were injected with α4 and β2 nAChR receptor subunits in a 4:1 ratio, and C–R relationships for ACh were determined in the absence and presence of NS9283 (31.6 µM) as data set pairs; that is, two 5-point ACh C–R relationships obtained for each oocyte, the first without and the second with NS9283. Data points, baseline subtracted and normalized to the maximal value obtained by fitting each oocyte ACh C-R to the Hill equation by non-linear regression, are presented as mean ± SEM of n= 12 experiments; and these were then fitted to the Hill equation by non-linear regression. (D) NS9283 does not alter the C–R relationship of ACh at (α4)2(β2)3 nAChR in X. laevis oocytes. Oocytes were injected with α4 and β2 nACh receptor subunits in a 1:4 ratio, and ACh C–R relationships were determined as described above. Data points were treated as described in (C) and are presented as mean ± SEM of n= 15 experiments.
Effect at native receptors
The ability of NS9283 to modulate native, neuronal rat α4β2 nACh receptors was investigated using whole-cell patch clamp recordings from thalamocortical neurons (TC) in rat brain slices containing the thalamus ventrobasal complex. Bath application of 100 µM ACh evoked an inward current as well as an increase in current noise (Figure 3A). This current was characterized by a peak within the first 70 s followed by a current decay phase that typically lasted 60–100 s. Co-application of NS9283 evoked further current and current noise and a new set of peak/decay phases. Quantified, ACh evoked a peak current increase of ΔImax=−165 ± 99 pA and subsequent co-application of NS9283 further increased the holding current to a peak level of ΔImax=−392 ± 171 pA (Figure 3C), corresponding to an average 2.6-fold increase, relative to the ACh response (P= 0.001, Student's t-test). Applying NS9283 and ACh in the reverse order (i.e. NS9283 before ACh) revealed that NS9283 alone does not induce a detectable current, consistent with the experiments using recombinant receptors (Figure 3B and D). Upon subsequent ACh co-application, a large current of ΔImax=−878 ± 192 pA was evoked (Figure 3D). The current rise time from baseline to peak was significantly faster in the presence of NS9283, compared with its absence (ACh: 54 ± 15 s; ACh + NS9283: 23 ± 11 s; P= 0.006, Student's t-test). To ascertain the contribution of α4β2 nACh receptors to the observed effects of NS9283, DHβE (10 µM), a β2*-selective antagonist, was co-applied and observed to abolish ACh-induced currents entirely, regardless of the order of ACh and NS9283 application (Figure 3A, B and D).
Figure 3.
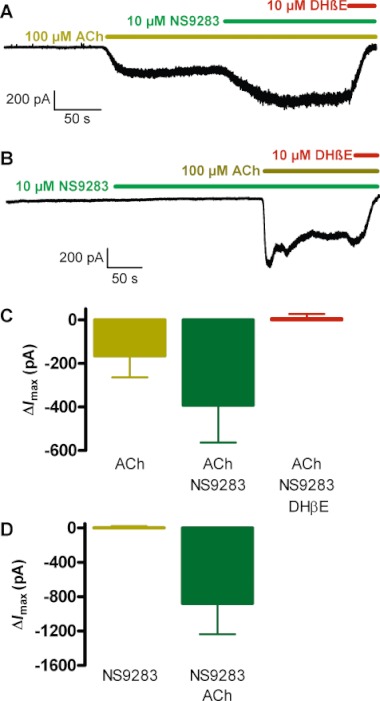
NS9283 modulates ACh-evoked currents in thalamocortical (TC) neurons. (A) Representative current trace of whole-cell patch clamp recording of a TC neuron in the presence of Cs (140 mM in the pipette), kynurenic acid and GABAzine. The experiment was initiated after the cell reached a steady-state holding current level. ACh (100 µM) was bath-applied and following current stabilization NS9283 (10 µM) and finally DHβE (10 µM) was further applied as indicated by the horizontal lines. (B) Representative current trace for an experiment similar to that in (A) except for the order of ACh and NS9283 applications. (C) NS9283 positively modulates ACh-evoked currents in TC neurons. Maximal change in current (ΔImax) evoked by application of ACh (left), ACh+NS9283 (middle) or ACh + NS9283+DHβE (right) versus control current are presented as mean ± SD of six experiments. (D) NS9283 does not by itself evoke currents in TC neurons. Maximal current (ΔImax) evoked by NS9283 (left) or NS9283 + ACh (right) are presented as mean ± SD of four experiments.
Pharmacokinetic properties
To further address its potential drug-like properties, and not least to gauge the utility of NS9283 as a probe for the in vivo pharmacology of allosteric α4β2 nACh receptor modulation, systemic exposure of NS9283 was determined after its administration to male Sprague–Dawley or Wistar rats, that represent two of the three species subsequently used for in vivo pharmacology experimentation (Table 2). Plasma exposure reached Tmax around 15–30 min following both i.p and p.o. administration, and the compound was cleared relatively fast, with a half-life ranging from 0.7 to 1.8 h. Exposure appeared to be linear at the lower dose levels, as shown by relatively constant AUC∞/D up to the 3 mg·kg−1 dose level; however, at the highest tested dose of 10 mg·kg−1 i.p., exposure increased substantially, which may be due to inhibition of metabolism or saturation of a clearance route. NS9283 penetrates very well into the brain with a brain to plasma ratio of approximately 1 or higher. Brain exposure and time profiles appeared to correlate with plasma concentrations (data not shown), indicating that plasma concentrations are a relevant measure for brain exposure at all time points. However, half-lives (T½) for brain are slightly longer than plasma, indicating a prolonged exposure to brain. Two important points emerge from these data: (1) Exposure of NS9283 appears to be largely insensitive to the administration route, as seen by the similar data obtained with i.p and p.o. administration. (2) Exposure furthermore appears comparable across the two strains of rats employed, as seen by the constant AUC∞/D in Sprague–Dawley and Wistar rats. Pharmacokinetic parameters for NS9283 have also been determined in female NMRI mouse (data not shown). Generally, data for mouse correlate well with rat data, although with a slightly faster clearance (T½∼30 min) and hence somewhat lower total exposure. Overall, these data are supportive of using NS9283 to explore centrally mediated α4β2 nACh receptor PAM effects.
Table 2.
Pharmacokinetic properties for NS9283 in rats, calculated using WinNonLin non-compartmental analysis
| Strain1and gender | Dose (mg·kg−1) | Route | Cmax2 (ng·mL−1) | Cmax,brain3 (µmol·kg−1tissue) | t½ (h) | AUC∞/D4 (h·ng·mL−1·mg−1·kg) | B/P5 |
|---|---|---|---|---|---|---|---|
| Sprague–Dawley, male | 0.1 | i.p. | 19.3 | NA | 0.7 | 417 | NA |
| 1 | i.p. | 397 | 1.1 | 0.8 | 578 | 1.1 | |
| 10 | i.p. | 6120 | 24.7 | 1.8 | 2200 | 1.0 | |
| Wistar, male | 1 | p.o | 549 | 1.9 | 0.8 | 785 | 1.4 |
| 3 | i.p. | 649 | 5.76 | 1.6 | 534 | 2.87 |
NS9283 was administered rats i.p and p.o and plasma as well as brain concentrations were determined at various time points (n= 3 at each point (n= 2 for Wistar i.p.)) as described in the Methods section.
Maximal plasma concentrations (Cmax). Determined as the highest measured plasma concentration following i.p. or p.o. dosing.
Maximal brain concentrations (Cmax,brain). Determined as the highest measured brain concentration following i.p. or p.o. dosing and expressed as µmol·kg−1tissue.
AUC∞normalized to dose.
Brain-to-plasma distribution ratios (B/P) were determined as AUC∞,brain/AUC∞,plasma.
Brain concentration at 1 h time point.
Brain-to-plasma distribution ratio (B/P) was determined as Cbrain/Cplasma.
General pharmacology
Acute maximal tolerated dose of NS9283 (10, 30 and 60 mg·kg−1, p.o.) in mice and rats is estimated to be >> 60 mg·kg−1, as no serious adverse effects were noted within a 2–3 h time frame, except mild stimulation at the higher tested doses. Furthermore, NS9283 (3, 10 and 30 mg·kg−1, p.o.) had no effect on locomotor activity in NMRI mice previously habituated to the test box in a 120 min observation time-frame as determined by one-way anova (F(3,27)= 0.18, P= 0.9). Mean values ± SEM for data summated over 120 min were 131.4 ± 14.9, 128.5 ± 12.9, 141.7 ± 18.4 and 120.9 ± 23.4 for the groups of mice treated with vehicle, 3, 10 and 30 mg·kg−1 respectively. This indicate no or at least very limited stimulant effects of the compound at these doses. Finally, no effects of NS9283 (3, 10 and 30 mg·kg−1, p.o., −30 min) could be observed on rectal body temperature. Mean values ± SEM were 37.8 ± 0.28, 38.1 ± 0.07, 37.9 ± 0.06 and 37.1 ± 0.2 °C for the groups of mice treated with vehicle, 3, 10 and 30 mg·kg−1 NS9283, respectively, and P > 0.05 for all treatment groups as compared with vehicle treatment using one-way anova on ranks.
Cognitive pharmacology assessment
The cognitive psychopharmacology of NS9283 was investigated across models spanning a range of different cognitive domains classically associated with nicotinic receptor pharmacology, including episodic memory, attentional processes and reference long-term memory. Moreover, the effect of NS9283 on pre-attentional sensory information processing was investigated using a paradigm of PCP-disrupted pre-pulse inhibition in rat. In these experiments, NS9283 was observed to reverse the PCP-induced impairment and thus to normalize pre-pulse inhibition. A detailed presentation of these data can be found under Supplementary Information Figure S1.
Social recognition task
The rat social recognition task is a model of short-term episodic memory. To investigate whether NS9283 (0.075, 0.75 and 7.5 mg·kg−1, i.p.) would improve recognition memory, the compound was administered to adult Sprague–Dawley rats immediately following initial exposure to a juvenile rat. Nicotine (0.1 mg·kg−1, i.p.) was also tested as an active comparator.
Statistical analysis of recognition ratios using one-way anova with drug treatment as factor revealed significant main effects of treatment (F(4,44)= 13.8, P < 0.0001). From post hoc multiple comparisons of vehicle with treatment groups, it was seen that NS9283 resulted in a significant decrease in time spent investigating the same juvenile on the second encounter (P < 0.05, P < 0.01 and P < 0.001 for 0.075, 0.75 and 7.5 mg·kg−1, respectively) (Figure 4). The effects of NS9283 were dose-dependent and maximal effect was seen at the highest dose of 7.5 mg·kg−1 at which the T2/T1 ratio of 0.30 indicates a very high degree of recognition. On the other hand, vehicle-treated rats exhibited a T2/T1 ratio of 1.05 basically indicating no recognition of the juvenile. Nicotine used as positive control in this study also displayed a significant effect compared with vehicle (P < 0.001), and the T2/T1 ratio of 0.56 is comparable to that observed with NS9283 and fully consistent with an improvement of short-term episodic memory.
Figure 4.
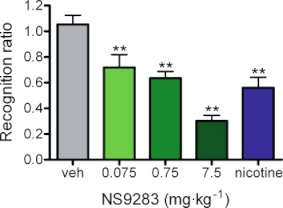
NS9283 improves performance in rat social recognition. Groups of nine adult male Sprague–Dawley rats were administered NS9283 (0.075, 0.75 and 7.5 mg·kg−1, i.p.) or nicotine (0.1 mg·kg−1, i.p.) immediately following initial juvenile exposure (T1), and changes in duration of subsequent juvenile interaction (T2) were assessed 2 h later. Vehicle control animals did not display recollection of the first encounter, as suggested by a recognition index of ∼1 (left bar). However, NS9283 significantly reduced the recognition index in a dose-dependent manner (middle bars), suggesting an improvement in recognition memory, equivalent to that observed for nicotine (right bar). Data are shown as mean ± SEM (n= 9) and significance levels *: P < 0.05, **: P < 0.01 and ***: P < 0.001.
Five choice serial reaction time task
The five-choice serial reaction time task is an operant model of sustained attention. Previous studies have demonstrated, that challenging the vigilance and attentional functions, by, for example lengthening ITI and shortening the stimulus duration, may increase the chances for observing cognitive enhancing effects of non-selective nACh receptor agonists such as nicotine (e.g. Mirza and Stolerman, 1998). Consequently, ITI was set to 15 s and stimulus duration to 0.5 s in these experiments. In a pilot study using a cohort of eight PVG hooded rats, NS9283 showed small but significant increases in correct responding as well as latency to response accuracy, and this was further accompanied with dose-dependent (albeit not significant) decreases in errors of omissions and premature responding (data not shown). Optimal doses appeared to be in the range of 1–3 mg·kg−1, and therefore a similar dosing regimen of NS9283 (0.3, 1 and 3 mg·kg−1, i.p., −30 min) was chosen for a new study in a larger cohort of Lister hooded rats. To enable comparisons, nicotine (0.03, 0.1 and 0.3 mg·kg−1, s.c., −10 min) was tested in the same cohort of rats following a wash-out period.
Statistical analysis using one-way repeated-measures anova, with drug treatment as factor and study animal identity as repeated measure revealed significant main effects of NS9283 on the following parameters: errors of omissions (F(3,65)= 4.7; P < 0.001); premature responses (F(3,62)= 5.2; P= 0.004); perseverative responses (F(3,63)= 2.9; P= 0.043); head entries (F(3,66)= 3.7; P= 0.018); and completed trials (F(3,64)= 4.2; P= 0.01); although this latter measure is linked to the pattern of errors of omissions. From post hoc multiple comparisons, it was seen that NS9283 resulted in a significant increase in alertness and vigilance as shown by a dose-related reduction of errors of omissions with maximal effect at 3 mg·kg−1 (P < 0.001) (Figure 5A2). This was accompanied by a non-significant marginal increase in response accuracy, where doses of 0.3, 1 and 3 mg·kg−1 resulted in mean values of 87.1%, 89.2% and 88.7%, respectively, as compared with 86.8 % after vehicle treatment (Figure 5A1). Furthermore, vigilance enhancing and mild stimulant effects of NS9283 were supported by dose-related and significant increases in head entries, where doses of 1 and 3 mg·kg−1 resulted in significant increases (P= 0.019 and P= 0.002, respectively) (Figure 5A8). Somewhat unexpectedly, administration of NS9283 at doses of 0.3 and 1 mg·kg−1 resulted in significantly increased premature responding (P < 0.001 and P= 0.03, respectively) (Figure 5A3), which indicates pro-impulsive effects at these doses. However, on this parameter the dose–response relationship was inverted, as the highest dose of 3 mg·kg−1 did not differ from vehicle levels (P= 0.48). Moreover, the number of premature responses generated under the influence of vehicle in this study was low (mean = 4.5 ± 0.9) in comparison with the norm seen for this cohort of rats (around 9–10). This could suggest that this increase may not reflect a true effect of the compound but rather an exceptionally low baseline in this study specifically. Assuming a more normal vehicle level revealed a dose-related decrease of premature responses by NS9283, which is consistent with the pilot study and observations with nicotine. Compulsive behaviour is commonly inferred by the number of perseverant responses. NS9283 caused an increase in this parameter, which only reached statistical significance for 1 mg·kg−1 dose (P= 0.005) (Figure 5A7). Response latencies (correct latency and incorrect latency) as well as magazine latency were not affected by NS9283 (Figure 5A4–A6).
Figure 5.
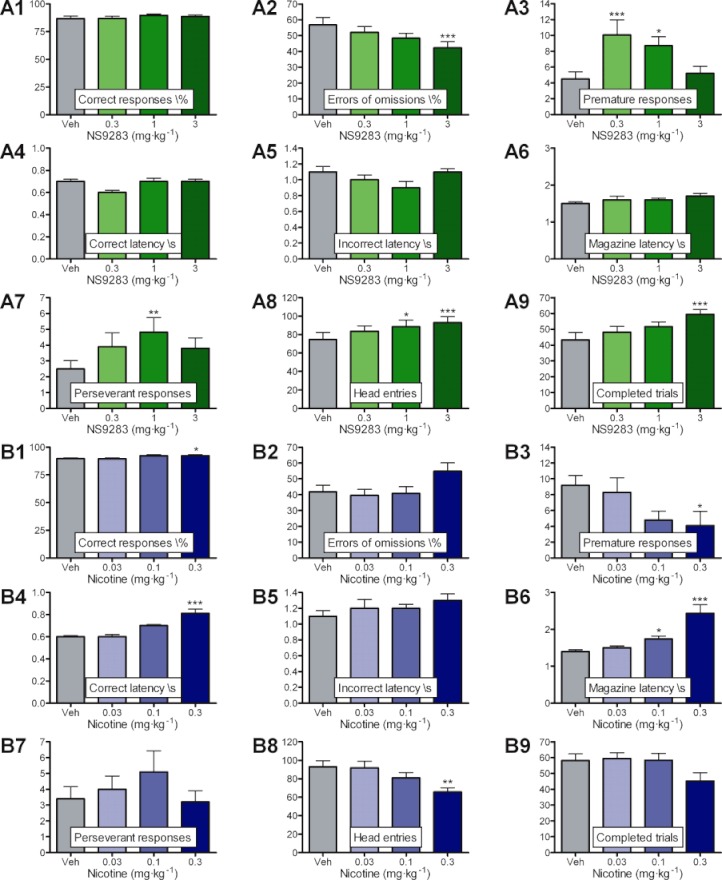
NS9283 and nicotine improves attention performance in the rat five-choice serial reaction time task. Fifteen water-deprived male Lister hooded rats were trained to discriminate a brief visual cue randomly presented in one out of five nose-poke units and upon correct responding they received a water reward. ITI was set very high (15 s) in these experiments in order to challenge attentional processes maximally. (A1–A9) NS9283 enhance attentional functions by increasing vigilance. NS9283 (0.3, 1 and 3 mg·kg−1, i.p., −30 min) significantly and dose-dependently affected errors of omissions (A2), completed trials (A9), perseverant responses (A7) and head entries (A8) but had no effects on any of the latencies (A4–A6). Minor improvements were seen on correct responses (A1) and premature responding (A3) was increased albeit with reverse dose-dependency. (B1–B9) Nicotine enhances attentional functions by increasing sustained attention in conjunction with mild stimulant effects. Nicotine (0.03, 0.1 and 0.3 mg·kg−1, s.c., −10 min) significantly and dose-dependently affected correct responses (B1), premature responding (B3), head entries (B8) correct latency (B4) and magazine latency (B6). Errors of omissions (B2), incorrect latency (B5), perseverant responses (B7) and completed trials (B9) were not significantly affected by the compound. For ease of reading the y-axis titles are placed within the graphs. Results are presented as mean ± SEM (n= 15) and significance levels *: P < 0.05, **: P < 0.01, ***: P < 0.001.
Statistical analysis of a similar dose–response study revealed significant main effects of nicotine on the following parameters: response accuracy (F(3,57)= 3.3; P= 0.029); premature responses (F(3,55)= 2.9; P= 0.048); correct latency (F(3,37)= 16.1; P < 0.001); magazine latency (F(3,56)= 15.7; P < 0.001); and head entries (F(3,57)= 3.3; P= 0.03). From the post hoc multiple comparisons, it was seen that nicotine significantly increased response accuracy at the highest dose of 0.3 mg·kg−1 (P= 0.02) (Figure 5B1). Although a trend for an increase was evident, there was no significant effect on the errors of omissions at the three doses tested (Figure 5B2). In accordance with this, there was a non-significant reduction in the number of trials completed (Figure 5B9). A dose-related decrease in premature responses and number of head entries reached significance at the highest dose of 0.3 mg·kg−1 (P= 0.02, P= 0.008 respectively) (Figures 5B3 and 4B8). Latency to correct response as well as magazine latency were both increased by nicotine (Figures 5B4 and 4B6). For latency to correct response, statistical significance was seen at the 0.3 mg·kg−1 dose (P < 0.001), while a dose-related increase in magazine latency reached significance at doses of 0.1 and 0.3 mg·kg−1 (P= 0.039 and P < 0.001 respectively).
Morris water maze task
The Morris's water maze task measures hippocampal-dependent long-term spatial memory. To establish an adequate signal window for observing cognitive improvement, task performance was impaired by sub-chronic administration of PCP (1.5 mg·kg−1, s.c., −30 min), a procedure optimized to result in a considerable impairment in task acquisition, as reflected by a prolongation in swimming distance, increased percentage of trials where the platform is not found and time in thigmotaxis (stereotyped swimming behaviour) but with no significant impact on swimming speed (Figure 6A1–4, day 1). In two pilot dose–response experiments, NS9283 gave significant and reproducible ameliorations of PCP deficits within the dose range 0.1 to 3 mg·kg−1 (data not shown). Based on these studies, the lowest fully efficacious dose of NS9283 (0.3 mg·kg−1, p.o., −30 min) was selected for the present experiment designed to demonstrate target engagement at the nACh receptors in conjunction with mecamylamine (3 mg·kg−1, s.c., −30 min).
Figure 6.
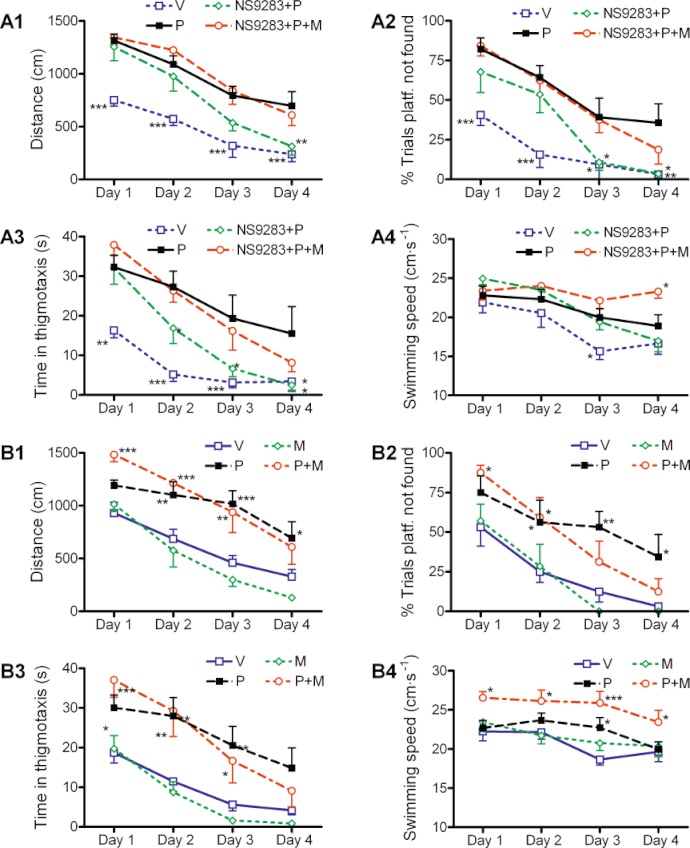
NS9283 improvements in the Morris water maze task were mediated by nACh receptors as evidenced by target engagement studies with mecamylamine. Adult rat acquisition of a visuo-spatial learning task in the Morris water maze was monitored by video over a 4 day period. Task acquisition was impaired considerably by a sub-chronic PCP (1.5 mg·kg−1 s.c., −30 min) administration all 4 days, as evidenced by the significant difference in distance, % trial platform not found and time in thigmotaxis between the vehicle and PCP groups at day 1. (A1–A4) Water maze improvements by NS9283 could be reversed with mecamylamine. NS9283 (0.3 mg·kg−1, p.o., −30 min) produced a significant alleviation of the PCP-deficits and improved task acquisition to nearly control levels at days 3 and 4 depending on behavioural parameter. This effect of NS9283 was completely abolished by co-administration of the non-selective nACh receptor antagonist mecamylamine (3 mg·kg−1, s.c., −30 min). A significant effect on swimming speed was only seen in two of the groups at days 3 and 4 and was generally considered minor. (B1–B4) Mecamylamine does not impair MWM performance or interact with PCP-induced deficits. Effects of mecamylamine (3 mg·kg−1, s.c., −30 min) treatment alone did not differ from the vehicle group at any behavioural parameter at any of the 4 days. Likewise, mecamylamine administration did not significantly affect the main impairments seen with PCP except for an increase in swimming speed at days 1 and 4 (P < 0.05). Results are presented as mean ± SEM (n= 7–8). Significance levels indicated in the graphs were versus the PCP group for A1–A4 (full line pattern) and versus vehicle for B1–B4 (full line pattern) and *: P < 0.05, **: P < 0.01, ***: P < 0.001.
Subjecting the data from this experiment to two-way repeated-measures anova, with drug treatment and days as factors, revealed clear effects at three key parameters: distance to platform, % trials platform not found and time in thigmotaxis (main treatment effect: F(3,119)= 15.8, P < 0.001, F(3,119)= 12.2; P < 0.001 and F(3,119)= 10.3; P < 0.001 respectively). From post hoc pairwise comparisons, this was, as expected, seen to reflect a significantly impaired performance at all three parameters in the group receiving PCP compared with vehicle treatment (Figure 6A1–3, Table 3). Administration of 0.3 mg·kg−1 NS9283 significantly ameliorated the PCP-induced deficits and improved performance towards vehicle levels starting from day 2 and fully reaching vehicle levels at day 4. Interestingly, co-administration with the non-selective nACh receptor antagonist mecamylamine completely blocked the ameliorative effects of NS9283 as treatment with NS9283 + PCP + mecamylamine did not statistically differ from treatment with PCP alone. Contrary to the other parameters, swimming speed was only marginally affected by sub-chronic PCP treatment. Although there was a main effect of treatment (F(3,119)= 4.2, P= 0.015), this did not translate into a significant pair wise effect of PCP versus vehicle treatment, but statistical significance was reached at day 3 when analysing within days (Figure 6A4, Table 3). Similarly, analysing within days revealed a significantly increased swimming speed for the NS9283 + PCP + mecamylamine group versus the PCP group at day 4 (P= 0.014). Upon inspecting the curves (Figure 6A4), these findings do not seem to reflect specific trends and generally swimming speed per se is not likely to significantly affect the overall cognitive performance conclusions as parameters such as distance travelled and time in thigmotaxis are not directly influenced by swimming speed.
Table 3.
Statistical P-values for the Morris water maze task
| Parameter | Veh versus PCP | NS9283+PCP versus PCP | NS9283+PCP+Mec. versus PCP | ||
|---|---|---|---|---|---|
| Treatment1 | Within day2 | Treatment | Within day | Treatment | |
| Distance to platform | <0.001 | <0.001 | 0.037 | n.s. | 0.74 |
| <0.001 | n.s. | ||||
| <0.001 | n.s. | ||||
| <0.001 | 0.009 | ||||
| % Trials platform not found | <0.001 | 0.001 | 0.007 | n.s. | 0.53 |
| <0.001 | n.s. | ||||
| 0.016 | 0.026 | ||||
| 0.009 | 0.013 | ||||
| Time in thigmotaxis | <0.001 | 0.002 | 0.016 | n.s. | 0.67 |
| 0.001 | 0.043 | ||||
| 0.001 | 0.014 | ||||
| 0.016 | 0.012 | ||||
| Swimming speed | 0.09 | 0.61 | 0.88 | 0.24 | 0.11 |
| 0.31 | 0.52 | ||||
| 0.015 | 0.75 | ||||
| 0.2 | 0.29 | ||||
| Parameter | PCP versus.Veh | PCP+Mec. versus Veh | Mec. versus Veh | ||
|---|---|---|---|---|---|
| Treatment | Within day | Treatment | Within day | Treatment | |
| Distance to platform | 0.001 | n.s. | 0.001 | <0.001 | 0.4 |
| 0.009 | 0.001 | ||||
| <0.001 | 0.003 | ||||
| 0.02 | n.s. | ||||
| % Trials platform not found | 0.005 | n.s. | 0.025 | 0.016 | 0.85 |
| 0.028 | 0.016 | ||||
| 0.005 | n.s. | ||||
| 0.028 | n.s. | ||||
| Time in thigmotaxis | 0.007 | 0.04 | 0.009 | 0.001 | 0.64 |
| 0.003 | 0.002 | ||||
| 0.008 | 0.045 | ||||
| n.s. | n.s. | ||||
| Swimming speed | 0.22 | 0.8 | <0.001 | 0.01 | 0.67 |
| 0.36 | 0.016 | ||||
| 0.014 | <0.001 | ||||
| 0.85 | 0.022 | ||||
Statistical evaluation was performed by two-way repeated-measures anova, and P-values are from Fisher's LSD post hoc pair wise comparisons with treatment as factor.
P-values from pair wise comparisons with treatment within day as factor are listed for days 1–4 in consecutive order;, n.s., not significant.
High doses of mecamylamine have been reported to impair water maze performance (i.e. Riekkinen and Riekkinen, 1994). This could potentially confound the interpretation of the present NS9283 data. Consequently, another study was conducted under identical conditions to investigate the effect of mecamylamine per se as well as the effect of the combination of mecamylamine and PCP. Two-way anova revealed significant main effect of treatment on distance to platform, % trials platform not found and time in thigmotaxis (F(3,123)= 12.5, P < 0.001, F(3,123)= 5.3; P= 0.005 and F(3,123)= 6.3, P= 0.002 respectively). From post hoc comparisons with the vehicle group, this was again seen to be due to significant decreased performance in the group treated with PCP but also the one treated with PCP + mecamylamine (Figure 6B1–3, Table 3). Importantly, at these parameters co-administration of PCP + mecamylamine did not further impair or improve performance as compared with PCP treatment alone (P within a range of 0.5 to 0.95), and there was no impairing effect of mecamylamine per se as compared with vehicle treatment. Swimming speed was also influenced by treatment (F(3,123)= 5.9; P= 0.003). As observed in the previous study, no pairwise treatment effect was seen for PCP compared with vehicle, but a significant effect could be observed at a single day (Figure 6B4, Table 3). In comparison with vehicle treatment, there was no effect of mecamylamine administration alone. However, the combination PCP + mecamylamine resulted in significantly increased swimming speed compared with vehicle treatment, which was evident on all days. In fact, the PCP + mecamylamine-treated group also had significantly higher swimming speed than that of the PCP-treated group (P= 0.015). The significance of this is difficult to interpret with the current data set; however, as mentioned above, it is unlikely that swimming speed could alter the overall conclusions.
Discussion and conclusions
The present study identifies the compound NS9283 as an efficacious and potent PAM at α2- and α4-containing nACh receptors. Appreciable plasma and brain exposure was demonstrated after systemic dosing of NS9283 to rodent animal species and in subsequent behavioural pharmacology experiments, NS9283 was observed to improve and/or alleviate pharmacological deficits across a variety of paradigms, taxing a spectrum of cognitive modalities, including sensory gating (PPI), vigilance, attention (5-CSRTT), episodic and reference memory (social recognition and Morris's water maze).
In terms of selectivity profile, NS9283 was highly selective for the 3α : 2β over the 2α : 3β stoichiometry of α4β2 nACh receptors. Furthermore, it produced modulation of α2β2, α2β4 and α4β4 when expressed in 3α : 2β stoichiometries but not α3-containing or any 2α : 3β stoichiometry nACh receptors. The high 3α : 2β > 2α : 3β selectivity of NS9283 suggests an impressive ability to discriminate between two different subunit compositions and, yet, NS9283 is unable to distinguish β2/β4-containing nACh receptors and α2/α4-containing nACh receptors. These observations could be reconciled by hypothesizing that only the 3α : 2β (and not the 2α : 3β) subunit stoichiometry present the binding site for NS9283. This matter is presently the subject of further investigation, and the results thereof will be presented in a subsequent paper. Interestingly, the data with NS9283 indicate that it is a universal feature of heteromeric nACh receptors to have the ability to assemble in different stoichiometries.
Mechanistically, NS9283 allosteric modulation was attributed to an increase in functional agonist potency, whereas the levels of maximal receptor activation and receptor desensitization properties appeared unchanged. The magnitude of the leftward shifts in the nicotine or ACh concentration–response relationships ranged from 50 to 500, and the potency as a modulator ranged from 0.5 to 5 µM, depending on the assays. Whilst functional agonist potency was enhanced by NS9283, there was no effect of the compound on radioligand binding affinity (Kd for [3H]-cytisine). In this respect, the PAM profile of NS9283 is reminiscent of the classical benzodiazepine-type PAMs of GABAA receptors (Macdonald and Olsen, 1994) and somewhat distinct from the profile of previously reported PAMs of the α4β2 nACh receptor (Weltzin and Schulte, 2010).
From the brain slice recordings on thalamocortical neurons, it is seen that ACh-evoked DHβE-sensitive currents could be augmented by NS9283, whereas the compound by itself was unable to evoke a detectable current. This provides strong evidence that NS9283 does in fact modulate native rat β2*-nACh receptors and further suggests that α4β2 receptors of the 3α : 2β stoichiometry are present in thalamocortical neurons, consistent with the findings of a previous report (Gotti et al., 2008).
NS9283 was rapidly absorbed systemically following administration in rats, with a relatively linear plasma AUC∞/D across the doses used in the behavioural experiments, and further penetrates well into the brain with a brain to plasma ratio of approximately 1. Importantly, exposure appears largely unaffected by administration route and animal species. In contrast to nicotine, no adverse effects of the compound were noted in doses up to 30 mg·kg−1 in study types traditionally sensitive to α4β2 nACh receptor agonists. These behavioural monitoring experiments included observations for seizures, locomotor activity and core body temperature.
The in vivo pharmacology data presented in the present study generally align well with those obtained from previous studies on the cognitive pharmacology of nicotine and agonists selective for the α4β2 nACh receptor. Pharmacological disruption of PPI has previously been demonstrated to be sensitive to modulation by nicotinic agonists (Acri et al., 1994). Although no significant effects were observed with NS9283 treatment in naïve animals (pilot studies), the present studies demonstrated clear dose-dependent amelioration of acute PCP-induced deficits in mouse PPI performance (see Supplementary Information Figure S1). The social recognition paradigm used in this study has also previously been reported to be sensitive to improvement by nicotinic agonists (Bitner et al., 2007), and while this was reproduced with nicotine, NS9283 also proved to have quite comparable dose-dependent efficacy. Nicotine has been reported to enhance sustained attention in the five-choice serial reaction time task in particular under conditions set to challenge vigilance (Mirza and Stolerman, 1998; Grottick et al., 2003). These findings were reproduced in the present study where nicotine enhanced sustained attention, as shown by increased accuracy at 0.3 mg·kg−1. At the same time, 0.3 mg·kg−1 nicotine reduced impulsivity, as measured by a reduction of premature responses; however, magazine latency as well as the number of total head entries was also affected, indicating non-specific and non-cognitive effects starting to influence behaviour at this dose. Allosteric modulation of nACh receptors seems to have a somewhat different fingerprint as the present data suggest vigilance-enhancing properties of NS9283, as shown by the dose-related reduction of errors of omission. Mild stimulant effects of the compound can be speculated due to the increased number of head entries and increased perseverant responses. However, NS9283 did not affect any of the response latencies, a hallmark of stimulants such as d-amphetamine (Cole and Robbins, 1987), strengthening the interpretation of vigilance enhancing effects rather that stimulant effects. Nicotine and various selective α4β2 nACh receptor agonists have previously been reported to revert pharmacologically-induced deficits in the Morris water maze model of reference memory (Decker et al., 1992; Socci et al., 1995). Furthermore, allosteric modulators of the nACh α7 receptor have also shown effects in acquisition of water maze learning tasks (Timmermann et al., 2007). In the present study, a daily sub-chronic administration of 1.5 mg·kg−1 PCP significantly impaired rat task acquisition in two independent animal cohorts. Treatment with 0.3 mg·kg−1 NS9283 ameliorated the PCP-induced deficits basically to vehicle levels at days 3 and 4 and, importantly, these ameliorative effects could be fully reversed by blocking nACh receptors with mecamylamine. Given that the 3 mg·kg−1 mecamylamine dose chosen in itself showed no significant effects on the major parameters, this strongly suggests that NS9283's actions are mediated through nACh receptors.
Considering the efficacious dose range across the different behavioural models, minimally efficacious doses spanned a range from ∼0.1 mg·kg−1 (social recognition model) to 3 mg·kg−1 (five-choice task). By extrapolation from the pharmacokinetic data available for i.p. administration, this would correspond to peak brain concentrations of 0.11–3.3 µM. On comparison with the in vitro data, this concentration range would fall within a range up to EC50 PAM activity, conferring plausibility that nACh receptors are the primary target of NS9283. Further to this coherent pharmacokinetic–pharmacodynamic association, several other lines of evidence clearly point to (α4)3(β2)2 nACh receptors as mediators of the behavioural pharmacology of NS9283: (i) No effects have been observed at other targets in binding assays or functionally. (ii) ACh-evoked and NS9283-modulated current in native neurons could be blocked with DHβE. (iii) Ameliorative effects of NS9283 in the water maze studies could be blocked by mecamylamine. (iv) Combination treatment of ABT-594 (a full agonist at (α4)3(β2)2 receptors) and NS9283 in pain models (Lee et al., 2011; Zhu et al., 2011) significantly lowered minimal effective doses of the agonist.
Nevertheless, the question remains whether the PAM activity of NS9283 on α2 and/or β4 nACh receptors contributes to its pharmacology. Within the mammalian brain, the β4-subunit is almost exclusively expressed in the medial habenula, most likely in the form of α3β4 nACh receptors (Zoli et al., 1995; Han et al., 2000), and α4β4 nACh receptors would therefore not be expected to play a significant role in the efficacy of NS9283. Studies of the α2* nACh receptors have indicated high levels of expression and potential substitution of α4β2 by α2β2 in the primate brain (Han et al., 2000), still, α2* nACh receptors only display limited expression in the rodent brain (Wada et al., 1989; Whiteaker et al., 2009). Therefore, considering the distribution pattern of other nACh receptor subtypes, at which NS9283 is active, it appears highly likely that the in vivo pharmacology of NS9283 should mainly be attributed to allosteric modulation of (α4)3(β2)2 nACh receptors in rodents whereas modulation of (α2)3(β2)2 could be important in primates.
To the knowledge of the authors, this report is the first direct demonstration of in vivo pro-cognitive efficacy mediated via allosteric modulation of α4β2 nACh receptors. Previously, agonists selective for the (α4)2(β2)3 nACh receptor (e.g. ispronicline/TC-1734) have been demonstrated to confer cognitive augmentation preclinically and clinically (Gatto et al., 2004; Dunbar et al., 2006; 2007a, b), but the observation that the (α4)3(β2)2 receptor is also involved in mediating cognitive operations is novel. The limited information available on the physiological distribution patterns of 2α : 3β versus 3α : 2β form α4β2 nACh receptors suggest both forms are present in, for example, the thalamus and cerebral cortex (Gotti et al., 2008), which is fully consistent with the present data.
As mentioned, NS9283 was recently reported to augment the anti-hyperalgesic effects of α4β2 agonists in pain models but was ineffective when administered alone. Interestingly, the pro-cognitive efficacy of NS9283 observed in the present study did not require co-administration of exogenous agonist, an observation that may suggest that the level of cholinergic tone in the cognitive circuitry is sufficient to drive PAM efficacy. On comparing the effects of the different agonists, it appears that allosteric modulation has the potential to give efficacy in the same range as nicotine and certainly offer better selectivity. Therefore, the present findings with NS9283 suggest that allosteric modulation of α4β2 nACh receptors offer new promise as a treatment strategy in clinical management of cognitive dysfunction.
Acknowledgments
The authors would like to acknowledge the technical contributions of Aino Munch, Katrine S Hansen, Lene G Larsen, Camilla Irlind, Claus Nørgaard Johansson, Hanne Nord Søndergaard and Henrik Marcher.
Glossary
- nACh
nicotinic acetylcholine, NS9283, 3-[3-(3-pyridyl)-1,2,4-oxadiazol-5-yl]benzonitrile
- PAM
positive allosteric modulator
- PCP
phencyclidine
- PPI
pre-pulse inhibition
Conflicts of interest
Several authors are (or were) employees of NeuroSearch A/S (DBT, KS-N, TD, MS, A-MJ, EØN, MG, JKC, MLJ, DP, GMO and PKA) or Abbott Laboratories (KK). No further conflicts of interests.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 NS9283 reverses sensory gating deficits. PCP induced PPI deficits in mice can be reversed by NS9283. The ability of weak acoustic prepulse stimuli to decrease reflexive startle responses produced by a high intensity second pulse stimulus were recorded. PPI of the acoustic startle response in mice was disrupted by administration of PCP (1.5 mg·kg−1, s.c., −10 min). Data from pre-pulse intensities of 4, 8, 16 and 24 db were pooled. Co-administration of NS9283 (1, 3 and 10 mg·kg−1, p.o., −30 min) led to a significant reversal of the PCP-deficit. Results are presented as mean ± S.E.M. (n = 9–10) and analyzed using two-way repeated measures ANOVA with Fisher LSD pair wise multiple comparisons.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Acri JB, Morse DE, Popke EJ, Grunberg NE. Nicotine increases sensory gating measured as inhibition of the acoustic startle reflex in rats. Psychopharmacology (Berl) 1994;114:369–374. doi: 10.1007/BF02244861. [DOI] [PubMed] [Google Scholar]
- Albrecht BK, Berry V, Boezio AA, Cao L, Clarkin K, Guo W, et al. Discovery and optimization of substituted piperidines as potent, selective, CNS-penetrant alpha4beta2 nicotinic acetylcholine receptor potentiators. Bioorg Med Chem Lett. 2008;18:5209–5212. doi: 10.1016/j.bmcl.2008.08.080. [DOI] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, Bunnelle WH, Anderson DJ, Briggs CA, Buccafusco J, Curzon P, et al. Broad-spectrum efficacy across cognitive domains by alpha7 nicotinic acetylcholine receptor agonism correlates with activation of ERK1/2 and CREB phosphorylation pathways. J Neurosci. 2007;27:10578–10587. doi: 10.1523/JNEUROSCI.2444-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, Gubbins EJ, Marks MJ, Putman CB, Thimmapaya R, Meyer MD, et al. Untranslated region-dependent exclusive expression of high-sensitivity subforms of alpha4beta2 and alpha3beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2006;70:227–240. doi: 10.1124/mol.105.020198. [DOI] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 1987;91:458–466. doi: 10.1007/BF00216011. [DOI] [PubMed] [Google Scholar]
- Decker MW, Majchrzak MJ, Anderson DJ. Effects of nicotine on spatial memory deficits in rats with septal lesions. Brain Res. 1992;572:281–285. doi: 10.1016/0006-8993(92)90485-r. [DOI] [PubMed] [Google Scholar]
- Dunbar G, Demazieres A, Monreal A, Cisterni C, Metzger D, Kuchibhatla R, et al. Pharmacokinetics and safety profile of ispronicline (TC-1734), a new brain nicotinic receptor partial agonist, in young healthy male volunteers. J Clin Pharmacol. 2006;46:715–726. doi: 10.1177/0091270006288730. [DOI] [PubMed] [Google Scholar]
- Dunbar G, Boeijinga PH, Demazieres A, Cisterni C, Kuchibhatla R, Wesnes K, et al. Effects of TC-1734 (AZD3480), a selective neuronal nicotinic receptor agonist, on cognitive performance and the EEG of young healthy male volunteers. Psychopharmacology (Berl) 2007a;191:919–929. doi: 10.1007/s00213-006-0675-x. [DOI] [PubMed] [Google Scholar]
- Dunbar GC, Inglis F, Kuchibhatla R, Sharma T, Tomlinson M, Wamsley J. Effect of ispronicline, a neuronal nicotinic acetylcholine receptor partial agonist, in subjects with age associated memory impairment (AAMI) J Psychopharmacol. 2007b;21:171–178. doi: 10.1177/0269881107066855. [DOI] [PubMed] [Google Scholar]
- Faghih R, Gopalakrishnan SM, Gronlien JH, Malysz J, Briggs CA, Wetterstrand C, et al. Discovery of 4-(5-(4-chlorophenyl)-2-methyl-3-propionyl-1H-pyrrol-1-yl)benzenesulfonamide (A-867744) as a novel positive allosteric modulator of the alpha7 nicotinic acetylcholine receptor. J Med Chem. 2009;52:3377–3384. doi: 10.1021/jm9003818. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, et al. Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol. 1999;55:432–443. [PubMed] [Google Scholar]
- Gatto GJ, Bohme GA, Caldwell WS, Letchworth SR, Traina VM, Obinu MC, et al. TC-1734: an orally active neuronal nicotinic acetylcholine receptor modulator with antidepressant, neuroprotective and long-lasting cognitive effects. CNS Drug Rev. 2004;10:147–166. doi: 10.1111/j.1527-3458.2004.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, et al. Partial deletion of the nicotinic cholinergic receptor alpha 4 or beta 2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha 4 and beta 2 subunits. Mol Pharmacol. 2008;73:1796–1807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Haman M, Wyler R, Higgins GA. Reversal of a vigilance decrement in the aged rat by subtype-selective nicotinic ligands. Neuropsychopharmacology. 2003;28:880–887. doi: 10.1038/sj.npp.1300102. [DOI] [PubMed] [Google Scholar]
- Han ZY, Le Novere N, Zoli M, Hill JA, Jr, Champtiaux N, Changeux JP. Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci. 2000;12:3664–3674. doi: 10.1046/j.1460-9568.2000.00262.x. [DOI] [PubMed] [Google Scholar]
- Harpsøe K, Ahring PK, Christensen JK, Jensen ML, Peters D, Balle T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J Neurosci. 2011;31:10759–10766. doi: 10.1523/JNEUROSCI.1509-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst RS, Hajos M, Raggenbass M, Wall TM, Higdon NR, Lawson JA, et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Schrimpf MR, Sippy KB, Bunnelle WH, Li T, Anderson DJ, et al. Synthesis and structure-activity relationship studies of 3,6-diazabicyclo[3.2.0]heptanes as novel alpha4beta2 nicotinic acetylcholine receptor selective agonists. J Med Chem. 2007;50:5493–5508. doi: 10.1021/jm070755h. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khiroug SS, Khiroug L, Yakel JL. Rat nicotinic acetylcholine receptor alpha2beta2 channels: comparison of functional properties with alpha4beta2 channels in Xenopus oocytes. Neuroscience. 2004;124:817–822. doi: 10.1016/j.neuroscience.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Lee CH, Zhu C, Malysz J, Campbell T, Shaughnessy T, Honore P, et al. alpha4beta2 neuronal nicotinic receptor positive allosteric modulation: an approach for improving the therapeutic index of alpha4beta2 nAChR agonists in pain. Biochem Pharmacol. 2011;82:959–966. doi: 10.1016/j.bcp.2011.06.044. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Stolerman IP. Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 1998;138:266–274. doi: 10.1007/s002130050671. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, et al. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitrile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–968. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Ng HJ, Whittemore ER, Tran MB, Hogenkamp DJ, Broide RS, Johnstone TB, et al. Nootropic alpha7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci USA. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Riekkinen M, Riekkinen P., Jr Effects of THA and physostigmine on spatial navigation and avoidance performance in mecamylamine and PCPA-treated rats. Exp Neurol. 1994;125:111–118. doi: 10.1006/exnr.1994.1014. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Duan WR, Thomas J, Nothaft W, Backonja MM. A randomized, double-blind, placebo-controlled trial evaluating the efficacy and safety of ABT-594 in patients with diabetic peripheral neuropathic pain. Pain. 2009;146:245–252. doi: 10.1016/j.pain.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiol Aging. 1995;16:857–860. doi: 10.1016/0197-4580(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Springer SK, Woodin KS, Berry V, Boezio AA, Cao L, Clarkin K, et al. Synthesis and activity of substituted carbamates as potentiators of the alpha4beta2 nicotinic acetylcholine receptor. Bioorg Med Chem Lett. 2008;18:5643–5647. doi: 10.1016/j.bmcl.2008.08.092. [DOI] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Timmermann DB, Grønlien JH, Kohlhaas KL, Nielsen EØ, Dam E, Jørgensen TD, et al. An allosteric modulator of the alpha7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Weltzin MM, Schulte MK. Pharmacological characterization of the allosteric modulator desformylflustrabromine and its interaction with alpha4beta2 neuronal nicotinic acetylcholine receptor orthosteric ligands. J Pharmacol Exp Ther. 2010;334:917–926. doi: 10.1124/jpet.110.167684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteaker P, Wilking JA, Brown RW, Brennan RJ, Collins AC, Lindstrom JM, et al. Pharmacological and immunochemical characterization of alpha2* nicotinic acetylcholine receptors (nAChRs) in mouse brain. Acta Pharmacol Sin. 2009;30:795–804. doi: 10.1038/aps.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KD, Liu Q, Wu J, Lukas RJ. Kinetics of desensitization and recovery from desensitization for human alpha4beta2-nicotinic acetylcholine receptors stably expressed in SH-EP1 cells. Acta Pharmacol Sin. 2009;30:805–817. doi: 10.1038/aps.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–9015. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CZ, Chin CL, Rustay NR, Zhong C, Mikusa J, Chandran P, et al. Potentiation of analgesic efficacy but not side effects: co-administration of an alpha4beta2 neuronal nicotinic acetylcholine receptor agonist and its positive allosteric modulator in experimental models of pain in rats. Biochem Pharmacol. 2011;82:967–976. doi: 10.1016/j.bcp.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Zoli M, Le Novere N, Hill JA, Jr, Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci. 1995;15:1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


